Abstract
This editorial refers to ‘Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice’†, by C.-L. Liu et al., on page 2456.
The discovery of therapies to tackle the burden of abdominal aortic aneurysm (AAA) disease remains an unmet clinical need in cardiovascular medicine. AAAs are silent killers, as they remain mostly asymptomatic until complications transpire. The identification of an AAA is commonly an accidental finding, when imaging is, for example, being performed to investigate unrelated abdominal symptoms. However, over the past decade, several ultrasound-based screening programmes have been implemented by several Western world countries.1,2 Interestingly, AAAs share a lot of similarities when it comes to modifiable as well as non-modifiable risk factors with atherosclerosis and its related diseases (such as coronary artery disease; CAD). Still, AAAs should not be considered as a variant of atherosclerosis, as key mechanisms triggering aortic dilation appear to be distinctly different. However, similar to atherosclerosis, most previous studies in experimental models and human pathology samples indicate a major role for chronic aortic inflammation in AAA pathogenesis.3
Modifiable risk factors for AAA include cigarette smoking (potentially also e-cigarettes4), arterial hypertension, hypercholesterolaemia, and concomitant existence of other atherosclerotic diseases, such as coronary heart disease, peripheral arterial occlusive disease (PAOD), and ischaemic forms of stroke. The non-modifiable risk profile of individuals suffering from an AAA includes advanced age (disease onset usually not before the sixth life decade), male sex, and a positive family history or genetic association.5 In addition, race seems to play a role, as lower risks are present in Afro-American, Asian, and Hispanic patients as opposed to Caucasians. An active lifestyle together with a healthy diet seems to lower the risk for aortic dilation. The role of obesity as a risk factor for AAA was previously suggested, but like many other features in developing aneurysms remains poorly understood (and understudied). Golledge et al.6 were able to identify that obesity is independently associated with AAA. In their study, serum level concentrations of resistin, which is an interesting linker between obesity, insulin resistance, and diabetes, were more strongly associated with aortic diameter than adipokines. However, unlike other atherosclerosis-related disease, patients with diabetes mellitus are less likely to develop an aortic aneurysm.7 Whether this is due to hyperglycaemia and its effect in the aorta itself, or potentially through medications being commonly used in diabetic patients (metformin is one candidate8,9), remains to be clarified.
Atherosclerotic changes in the arterial tree are the origin of advanced and unstable plaque formation and luminal stenosis. The molecular mechanisms that, apart from the atherosclerotic component, promote aneurysm formation in susceptible sites of arteries (such as the abdominal aorta) remain largely elusive. Most commonly AAAs are defined as an increase of the infrarenal diameter of ≥3 cm upon imaging (ultrasonography or computed tomography). Potentially due to a decline in cigarette smoking, AAA prevalence seems to have shrunk in recent years to ∼2.2% in a cohort of 65-year-old men.2 However, the fatal risk of acute ruptures is still looming. Untreated AAA ruptures have a mortality rate as high as 97.2%. Fatal outcomes can nowadays be effectively decreased to 24.7% (when using endovascular aortic repair; EVAR) or 37.3% (via open surgical repair; OSR).10
The current standard of care includes surveillance and rupture prevention (by EVAR or OSR). To date, no effective drug therapy is available to limit AAA progression, which has certainly triggered a substantial interest in understanding the pathogenesis of the disease.3 There might be one much-debated exception though: a previously published meta-analysis by Salata et al. suggests that statins are associated with a reduction in AAA progression and rupture risk, and lower rates of peri-operative mortality upon elective repair.11
Researchers utilized rodent models, as well as genetic and epidemiological studies, in the recent past to identify the therapeutic potential of various approaches, including antihypertensive agents, medications to correct dyslipidaemias, anti-thrombotics, and various anti-inflammatory drugs.12 Thus, comprehensive AAA screening in populations at risk, surveillance of identified clinically relevant AAA cases, and anticipatory surgical repair at the point when rupture risk outweighs the surgical risk (currently recommended at ≥5 cm for women and ≥5.5 cm for men) appears crucial.13 It is still suggested to initiate and pursue the best possible medical treatment regimen, which should include (based on the individual patient) statins, platelet aggregation inhibitors, as well as antihypertensive agents to amend the overall cardiovascular risk profile.14 Although these drugs cannot prevent aneurysm progression and acute rupture, they will lower the risk for other cardiovascular events (e.g. stroke or myocardial infarction) from which the majority of patients with a small AAA will probably die before they require surgical or interventional aneurysm correction.
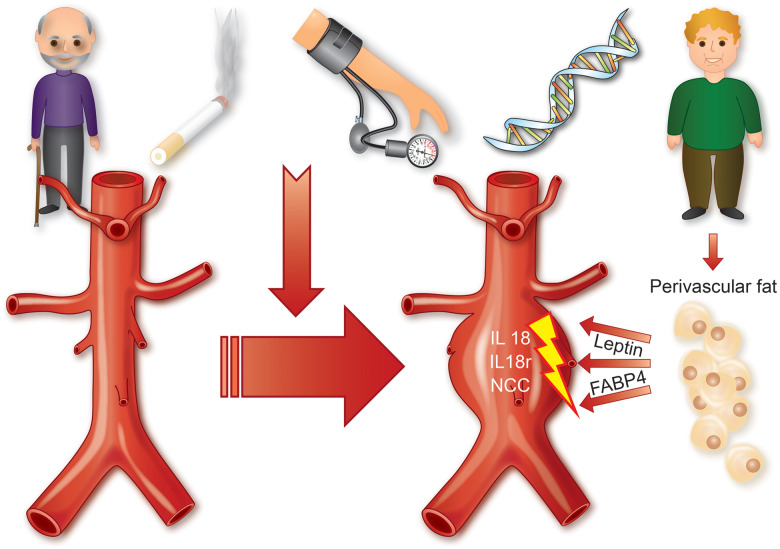
In their manuscript published in this issue of the European Heart Journal, Liu and colleagues unravel a connection between perivascular adipose tissue and progressing AAAs via interleukin-18 (IL-18) and its receptors IL18r and the Na–Cl co-transporter (NCC).15 IL-18 has been previously established by the same laboratory for its role in stimulating macrophage activity in atherosclerosis,16 and for being increased in human coronary lesions upon acute coronary syndrome.17 A similar role has now been described for this cytokine in experimental aortic dilation, where IL-18 not only activates macrophages, but further influences T-cell stimulation, smooth muscle cell (SMC) apoptosis, as well as endothelial dysfunction and inflammation (via up-regulation of IL18r and NCC). Important adipokine-derived stimulators in this intriguing concept are leptin, the ‘hunger hormone’ being mainly released from adipose cells and enterocytes to regulate the body’s energy balance, as well as fatty acid-binding protein 4 (FABP4), a highly conserved, cytoplasmic transporter and regulator with previously described relevance not only in metabolism but also in cardiovascular diseases, such as CAD (see Take home figure). Interestingly, perivascular implantation of adipose tissue from either diet-induced obese or lean mice, but not that from leptin-deficient ob/ob mice aggravated angiotensin II-induced AAA development in the respective recipient animals. The authors were further able to show that combined and single deletion of NCC and/or IL18r was capable of limiting murine AAA progression, which implies that both receptors contribute independently to the disease pathogenesis. However, lesion macrophage and T-cell contents, matrix remodelling and metalloproteinase (MMP) activity, angiogenesis, SMC depletion, and inflammatory cytokine expression were all increased in single receptor knockout animals in comparison with the double knockouts. Overall it seems likely that both the IL18r and NCC receptor can act autonomously during AAA development and disease progression. Numerous pathways and variables of importance to aortic inflammation and matrix remodelling (indicated by changes in MMP activity and cathepsin expression) appear to be substantially regulated by IL-18 during experimental aortic dilation.
Take home figure.
Role of interleukin IL-18 during abdominal aortic aneurysm formation. Different modifiable and non-modifiable risk factors contribute to abdominal aortic aneurysm disease. Adipocyte-derived leptin and fatty acid-binding protein 4 (FABP4) trigger increased interleukin-18 (IL-18) binding to its two receptors in vascular cells: the interleukin 18 receptor (IL18r) and Na–Cl co-transporter (NCC) promoting experimental aneurysm formation.
Of importance, not only this current experimental AAA study in rodents indicates an important contributory role for IL-18 and its receptors in aortic inflammation and vessel expansion. Previous studies in tissue specimens from patients undergoing elective open AAA repair also confirm an up-regulation for IL-18 and Il18r. Both findings further emphasize how important IL-18 is in acting in the context of AAA development and progression (as shown in murine models), as well as in end-stage human disease when a patient’s aneurysm has reached a critical threshold (probably ≥5.5 cm) for which surgical intervention is currently our only treatment option.
In conclusion, targeting IL-18 and its receptors within the aortic wall could be a promising therapeutic strategy to limit AAA progression and the risk of acute rupture in patients. As discussed by the authors of this current manuscript, several antibodies as well as drugs already exist that either target IL-18 (GSK1070806) and IL18r (anti-IL-1RAcPL) or inhibit NCC (thiazide diuretics such as chlorothiazide). Additional experiments in pre-clinical large animal models, which reflect some of the pathological aspects of a dilating aorta better than murine models, will have to be performed before well-designed clinical studies can tell us whether IL-18 blockade has a therapeutic benefit in managing AAA patients.
Acknowledgements
Research in Lars Maegdefessel’s laboratories is sponsored by an ERC Starting Grant (acronym: NORVAS), the German Center for Cardiovascular Research (DZHK Junior Research Grant and Translational Research Project), the German Research Council (DFG; Heisenberg Professorship, SFB1123 ‘Novel Targets in Atherosclerosis’, TRR267 ‘Non-coding RNAs in the cardiovascular system’), the Free State of Bavaria, Ministry of Health (DigiMed Bayern), the Swedish Research Council (Vetenkapsrådet), and the Swedish Heart and Lung Foundation (Hjärt-Lungfonden).
Conflict of interest: none declared.
Footnotes
† doi:10.1093/eurheartj/ehz856.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Oliver-Williams C, Sweeting MJ, Turton G, Parkin D, Cooper D, Rodd C, Thompson SG, Earnshaw JJ, Gloucestershire, Swindon Abdominal Aortic Aneurysm Screening Programme. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg 2018;105:68–74. [DOI] [PubMed] [Google Scholar]
- 2. Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A.. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118–1123. [DOI] [PubMed] [Google Scholar]
- 3. Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol 2019;16:225–242. [DOI] [PubMed] [Google Scholar]
- 4. Wagenhauser MU, Schellinger IN, Yoshino T, Toyama K, Kayama Y, Deng A, Guenther SP, Petzold A, Mulorz J,, Mulorz P, Hasenfuss G, Ibing W, Elvers M, Schuster A, Ramasubramanian AK, Adam M, Schelzig H, Spin JM, Raaz U, Tsao PS.. Chronic nicotine exposure induces murine aortic remodeling and stiffness segmentation—implications for abdominal aortic aneurysm susceptibility. Front Physiol 2018;9:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Pan C, Zhang S, Spin JM, Deng A, Leung LLK, Dalman RL, Tsao PS, Snyder M.. Decoding the genomics of abdominal aortic aneurysm. Cell 2018;174:1361–1372. [DOI] [PubMed] [Google Scholar]
- 6. Golledge J, Clancy P, Jamrozik K, Norman PE.. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation 2007;116:2275–2279. [DOI] [PubMed] [Google Scholar]
- 7. Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G.. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010;52:539–548. [DOI] [PubMed] [Google Scholar]
- 8. Itoga NK, Rothenberg KA, Suarez P, Ho TV, Mell MW, Xu B, Curtin CM, Dalman RL.. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg 2019;69:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golledge J, Morris DR, Pinchbeck J, Rowbotham S, Jenkins J, Bourke M, Bourke B, Norman PE, Jones R, Moxon JV.. Editor’s Choice—Metformin prescription is associated with a reduction in the combined incidence of surgical repair and rupture related mortality in patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2019;57:94–101. [DOI] [PubMed] [Google Scholar]
- 10. Salata K, Syed M, Hussain MA, de Mestral C, Greco E, Mamdani M, Tu JV, Forbes TL, Bhatt DL, Verma S, Al-Omran M.. Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IMPROVE Trial Investigators, Powell JT, Hinchliffe RJ, Thompson MM, Sweeting MJ, Ashleigh R, Bell R, Gomes M, Greenhalgh RM, Grieve RJ, Heatley F, Thompson SG, Ulug P.. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 2014;101:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golledge J, Moxon JV, Singh TP, Bown MJ, Mani K, Wanhainen A.. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med 2019;in press. [DOI] [PubMed] [Google Scholar]
- 13. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM,, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW.. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2–77. [DOI] [PubMed] [Google Scholar]
- 14. Bahia SS, Vidal-Diez A, Seshasai SR, Shpitser I, Brownrigg JR, Patterson BO, Ray KK, Holt PJ, Thompson MM, Karthikesalingam A.. Cardiovascular risk prevention and all-cause mortality in primary care patients with an abdominal aortic aneurysm. Br J Surg 2016;103:1626–1633. [DOI] [PubMed] [Google Scholar]
- 15. Liu C-LRen J, Wang Y, Zhang X, Sukhova GK, Liao M, Santos M, Luo S, Yang D, Xia M, Inouye K, Hotamisligil GS, Lu G, Upchurch GR, Libby P, Guo J, Zhang J, Shi G-P. Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur Heart J 2020;41:2456–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, Shi MA, He A, Zhou Y, Sukhova GK, Chen H, Cheng XW, Kuzuya M, Murohara T, Zhang J, Cheng X, Jiang M, Shull GE, Rogers S, Yang CL, Ke Q, Jelen S, Bindels R, Ellison DH, Jarolim P, Libby P, Shi GP.. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na–Cl co-transporter. Nat Med 2015;21:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akerblom A, James SK, Lakic TG, Becker RC, Cannon CP, Steg PG, Himmelmann A, Katus HA, Storey RF, Wallentin L, Weaver WD, Siegbahn A; PLATO investigators. Interleukin-18 in patients with acute coronary syndromes. Clin Cardiol 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]