Abstract
Ewing sarcoma (ES) is an aggressive, high-grade neuroectodermal neoplasm that frequently manifests in children and young adults. Although ES without osseous involvement most commonly involves paravertebral regions of the spine, it very rarely presents as a primary intracranial tumor. This report discusses a unique presentation of an adult extraosseous ES arising from the pineal region with extension into the third and fourth ventricles and multiple drop metastases to the spine. This case demonstrates the application of current chemotherapeutic and adjuvant management and offers insight into possible treatment modalities for metastasis in an atypical extraosseous ES involving the brain and spine.
Keywords: Chemotherapy, Ewing sarcoma, pineal region tumor, radiation therapy
Ewing sarcoma (ES) is an aggressive neuroectodermal neoplasm that frequently manifests in children and young adults. ES tumors without osseous involvement are currently classified as extraosseous ES and were previously designated as primitive neuroectodermal tumors, World Health Organization grade IV.1,2 Histopathological identification of extraosseous ES tumors relies on the presence of a balanced translocation involving chromosomes 11 and 22 or 21 and 22. Extraosseous ES cells characteristically display dense expression of gene product MIC-2 membrane protein (CD99).3 Approaches to the chemotherapeutic treatment, radiation, and overall prognosis are thought to differ greatly between extraosseous ES and other embryonal tumors.4 Here we present a case of adult extraosseous ES arising from the pineal region with third and fourth ventricular invasion and evidence of leptomeningeal spread.
CASE PRESENTATION
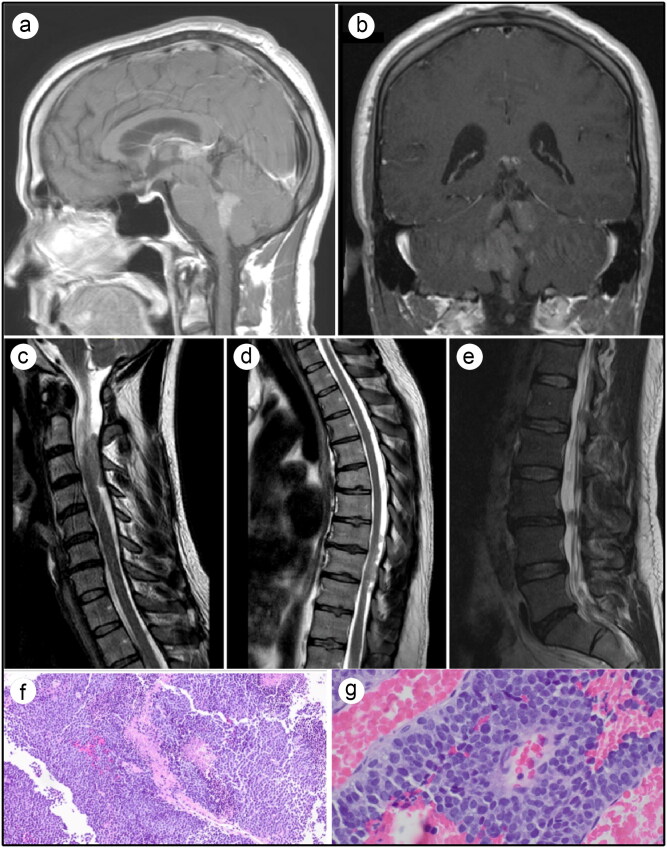
A 37-year-old man with no significant past medical history presented with a 1-month history of dizziness associated with headache, nausea, vomiting, and tinnitus. Magnetic resonance imaging (MRI) of the brain revealed multiple homogenously enhancing masses of the pineal region, third ventricle, and fourth ventricle, with extension into the bilateral foramen of Luschka, interpeduncular cistern, and dorsal pons (Figure 1a,b). MRI of the cervical, thoracic, and lumbar spine revealed multiple enhancing intradural lesions at various levels consistent with drop metastases (Figure 1c–e). Computed tomography of the chest, abdomen, and pelvis was unremarkable. Cerebrospinal fluid analysis was negative for human chorionic gonadotropin, alpha-fetoprotein, alkaline phosphatase, infection, and malignant cytology. The patient underwent stereotactic endoscopic third ventriculostomy with intraventricular tumor biopsy. The tumor tissue consisted of small round blue cells with an increased nuclear to cytoplasmic ratio and high mitotic activity (Figure 1f,g). Fluorescence in situ hybridization analysis reported rearrangement of the EWSR1 gene at 22q12, and immunohistochemistry reflected CD99 positivity. A final pathology report from the Mayo Clinic revealed ES, World Health Organization grade IV.
Figure 1.
Pretreatment MRI and histology findings. (a,b) T1-weighted brain MRI with contrast showing multiple homogenous enhancing masses of the third ventricle and posterior fossa. (c,d,e) T2-weighted MRI of cervical, thoracic, and lumbar spines, respectively, showing multiple enhancing intradural lesions at various levels. (f,g) Histopathologic features of Ewing sarcoma/peripheral primitive neuroectodermal tumor. Hematoxylin and eosin staining revealed various sheets of small round blue cells with an increased nuclear to cytoplasmic ratio and high mitotic activity.
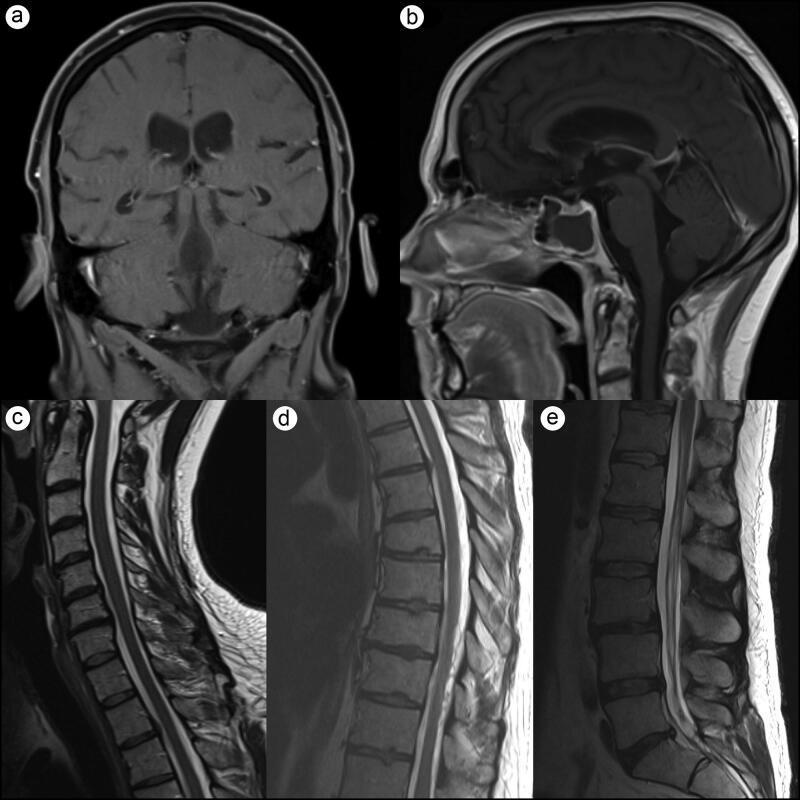
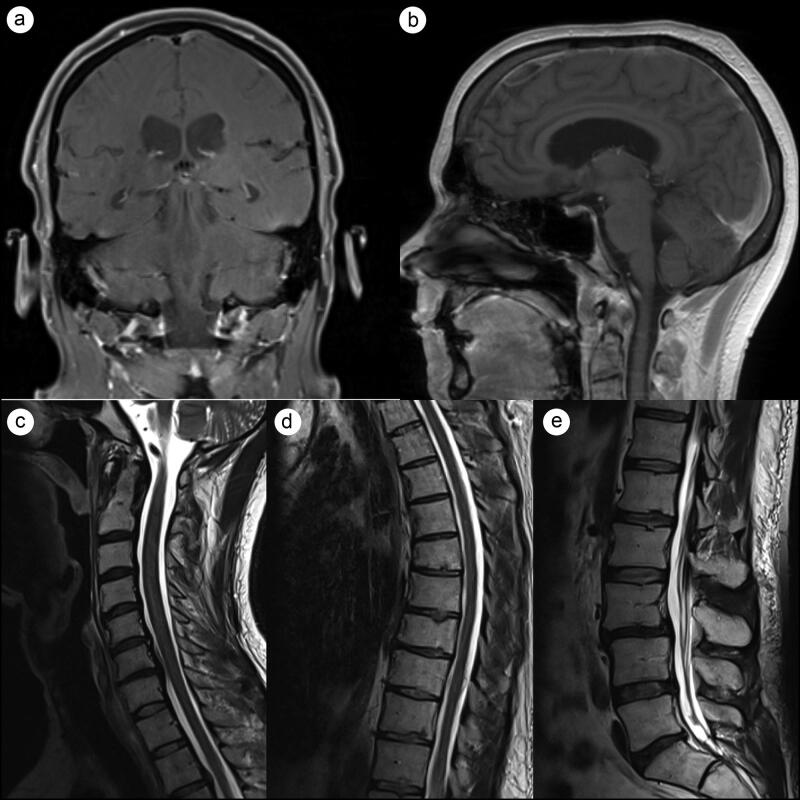
The treatment plan consisted of induction with 6 weeks of weekly vincristine infusion with daily adjuvant craniospinal irradiation of 36 Gy in 21 fractions followed by a boost up to 54 Gy to identifiable gross disease. After four cycles of vincristine infusions, the patient developed Aspergillus pneumonia and chemotherapy was withdrawn, while the radiotherapy regimen was continued to completion. A 3-month follow-up MRI revealed marked tumor regression with no residual intracranial or cervical enhancing lesions (Figure 2). A 7-month follow-up MRI revealed no disease recurrence (Figure 3).
Figure 2.
Three-month follow-up T1-weighted brain MRI and T2-weighted spine MRI. (a,b) Marked tumor regression with no residual intracranial or (c) cervical enhancing lesions. (d) One area of minimal enhancement remained in the thoracic spine. (e) The lumbar spine was significantly improved with only minor residual thickening of the cauda equina.
Figure 3.
Seven-month follow-up T1-weighted brain MRI and T2-weighted spine MRI. (a,b) Stable tumor regression with no residual intracranial lesions. (c-e) Marked regression of metastatic spinal lesions with no enhancement in (d) thoracic or (e) lumbar spine.
DISCUSSION
Due to the rare occurrence of primary intracranial ES, there remains limited evidence regarding optimal management strategies. Although primary intracranial extraosseous ES with leptomeningeal dissemination has been rarely described in the literature, no other cases share the extent of central nervous system metastasis present in this patient.5–7 Radiation and chemotherapeutic management strategies for primary intracranial ES with metastasis are illustrated in Table 1.5–9
Table 1.
Management of primary intracranial Ewing sarcoma with metastasis
| Author, year | Age/sex | Tumor location | Location of metastasis | Resection | Radiation (Gy) | Chemotherapy regimen | Follow-up (mo) |
|---|---|---|---|---|---|---|---|
| Present case, 2020 | 37, M | Pineal region | Spinal cord | Biopsy | 36 | 4 weeks vincristine | No disease (7) |
| Chen et al, 20186 | 23, M | L TP, DB | Spinal cord | STR | 55 | None | Died (6) |
| Chen et al, 20186 | 22, F | R T | Skull | GTR | 55 | VAC | Died (38) |
| Chen et al, 20186 | 12, F | R T, L FP, DB | Diffuse metastasis | STR | 50 | VIDE | Died (20) |
| Alqahtani et al, 20177 | 3, M | PF | Spinal cord | GTR | NS | VIDE + C for 3 mo then VTI | Recurrence (8) |
| Tanboon et al, 20128 | 22, F | Frontal DB | Diffuse metastasis | GTR | None | None | Died (6 postop) |
| Mobley et al, 20069 | 21, M | O | Multiple vertebrae | Biopsy | 54 | Dactinomycin, VAC | Recurrence (18) |
| Jay et al, 19965 | 4, M | PF | Spinal cord | GTR | CSI, dose NS | VCE then ICE | Progression of LMS |
CSI indicates craniospinal irradiation; DB, dural-based; F, frontal; FP, frontoparietal; GTR, gross total resection; ICE, ifosfamide, carboplatin, etoposide, mesna; L, left; LMS, leptomeningeal spread; NS, not specified; O, occiput; PF, posterior fossa; R, right; STR, subtotal resection; T, temporal; TP, temporoparietal; VAC, vincristine, doxorubicin, cyclophosphamide; VCE, vincristine, cyclophosphamide, epirubicin; VIDE + C, vincristine, ifosfamide, doxorubicin, etoposide plus cyclophosphamide; VIDE, vincristine, ifosfamide, doxorubicin, etoposide; VTI, vincristine, temozolomide, irinotecan.
A study of 14 cases from a single institution revealed that patients who underwent adjuvant radiotherapy had statistically significant improvement in 1- and 2-year survival and median survival time compared to patients without radiotherapy.6,10 Radiation regimens are typically initiated postoperatively with the standard craniospinal irradiation protocol of 36 Gy in 21 fractions followed by a boost to 54 Gy.6 In this case, craniospinal irradiation served as the primary method of treatment due to the mid-cycle discontinuation of chemotherapy. This case illustrates how adjuvant radiotherapy is a cornerstone of extraosseous ES treatment, specifically in the context of subtotal resection and/or evidence of metastasis.
The benefit of adjuvant chemotherapy in extraosseous ES treatment remains controversial. In adults, chemotherapy is never used in isolation but rather in combination with resection and/or radiation.6 However, isolated chemotherapy has been used in pediatric cases when radiation cannot be tolerated.6,11 Multiagent therapy has largely been utilized in patients with nonresectable or metastatic tumors. The current regimen for ES includes vincristine, doxorubicin, cyclophosphamide, and dactinomycin alternating with ifosfamide and etoposide.12,13 This multiagent therapy has shown great efficacy in the treatment of ES of bone; however, its application to intracranial extraosseous ES remains unclear. In the case presented, the chemotherapeutic regimen was largely based on the medulloblastoma protocol known as the “Packer protocol”: vincristine weekly for 6 weeks during radiation, then 6 cycles of cisplatin, cyclophosphamide, and vincristine every 28 days.14 In patients with primary intracranial ES, the efficacy of ES vs medulloblastoma protocols has yet to be investigated. This patient did not complete the induction-phase regimen but showed marked tumor regression without recurrence after 7 months with radiotherapy alone. Therefore, the full benefits of chemotherapy as an adjuvant therapy remain unproven in this patient.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: IARC Press; 2016. [Google Scholar]
- 3.Ladanyi M, Lewis R, Garin-Chesa P, et al. EWS rearrangement in Ewing’s sarcoma and peripheral neuroectodermal tumor. Molecular detection and correlation with cytogenetic analysis and MIC2 expression. Diagn Mol Pathol. 1993;2:141–146. [PubMed] [Google Scholar]
- 4.Pekala JS, Gururangan S, Provenzale JM, et al. Central nervous system extraosseous Ewing sarcoma: radiologic manifestations of this newly defined pathologic entity. AJNR Am J Neuroradiol. 2006;27(3):580–583. [PMC free article] [PubMed] [Google Scholar]
- 5.Jay V, Zielenska M, Lorenzana A, Drake J. An unusual cerebellar primitive neuroectodermal tumor with t(11;22) translocation: pathological and molecular analysis. Fetal & Pediatric Path. 1996;16(1):119–128. doi: 10.3109/15513819609168668. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Jiang Q, Zhang Y, et al. Clinical features and long-term outcome of primary intracranial Ewing sarcoma/peripheral primitive neuroectodermal tumors: 14 cases from a single institution. World Neurosurg. 2019;122:e1606–e1614. doi: 10.1016/j.wneu.2018.11.151. [DOI] [PubMed] [Google Scholar]
- 7.Alqahtani A, Amer R, Bakhsh E. Primary occipital Ewing’s sarcoma with subsequent spinal seeding. Case Rep Pediatr. 2017;2017:1–4. doi: 10.1155/2017/1521407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanboon J, Sitthinamsuwan B, Paruang T, Marrano P, Thorner PS. Primary intracranial Ewing sarcoma with an unusually aggressive course: a case report and review of the literature. Neuropathology. 2012;32(3):293–300. doi: 10.1111/j.1440-1789.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- 9.Mobley BC, Roulston D, Shah GV, Bijwaard KE, McKeever PE. Peripheral primitive neuroectodermal tumor/Ewing’s sarcoma of the craniospinal vault: case reports and review. Hum Pathol. 2006;37(7):845–853. doi: 10.1016/j.humpath.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Asri ACE, Benzagmout M, Chakour K, et al. Primary intracranial pPNET/Ewing sarcoma: diagnosis, management, and prognostic factors dilemma–a systematic review of the literature. World Neurosurg. 2018;115:346–356. doi: 10.1016/j.wneu.2018.04.164. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Srivastava AK, Pal L, et al. Extraosseous primary intracranial Ewing sarcoma/peripheral primitive neuroectodermal tumor: series of seven cases and review of literature. Asian J Neurosurg. 2018;13(2):288–296. doi: 10.4103/1793-5482.228570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juergens C, Weston C, Lewis I, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47(1):22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 13.Craft A, Cotterill S, Malcolm A, et al. Ifosfamide-containing chemotherapy in Ewing’s sarcoma: the second United Kingdom Children’s Cancer Study Group and the Medical Research Council Ewing’s Tumor Study. JCO. 1998;16(11):3628–3633. doi: 10.1200/JCO.1998.16.11.3628. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg HS, Chamberlain MC, Glantz MJ, Wang S. Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol. 2001;3(1):29–34. doi: 10.1093/neuonc/3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]