Abstract
Sarcoidosis is a chronic inflammatory multisystem disease. The stomach is the most commonly involved gastrointestinal organ. Symptomatic appendicular sarcoidosis is extremely rare. We present a case of a 49-year-old woman with abdominal pain. An ultrasound of the abdomen was suggestive of acute appendicitis. Laparoscopic appendectomy was performed and the pathology revealed nonnecrotizing granulomas. Biopsy of the mediastinal lymph nodes suggested noncaseating granulomas. She was treated with steroid therapy followed by mycophenolate mofetil. Our case demonstrates the importance of considering appendiceal sarcoid among the differentials in a patient with systemic sarcoidosis presenting with an acute abdomen.
Keywords: Acute appendicitis, appendiceal neoplasm, appendiceal sarcoidosis, gastrointestinal sarcoidosis
Sarcoidosis is a chronic inflammatory multisystem disease.1 Organs typically affected include the lungs, lymph nodes, and skin.2 Gastrointestinal (GI) involvement, although not uncommon, is asymptomatic 99% of the time.3 The stomach is the most commonly involved GI organ, but the esophagus, appendix, colon, and rectum have also been implicated.4 Fewer than 10 cases of appendiceal sarcoidosis have been reported in the English language literature. Symptomatic appendiceal sarcoidosis is extremely rare.
CASE DESCRIPTION
A 49-year-old black woman with recurrent chronic low back pain and kidney stones presented to the emergency department with worsening abdominal pain for 3 days. The pain was sharp, located in the right iliac fossa, and radiated to the periumbilical region.
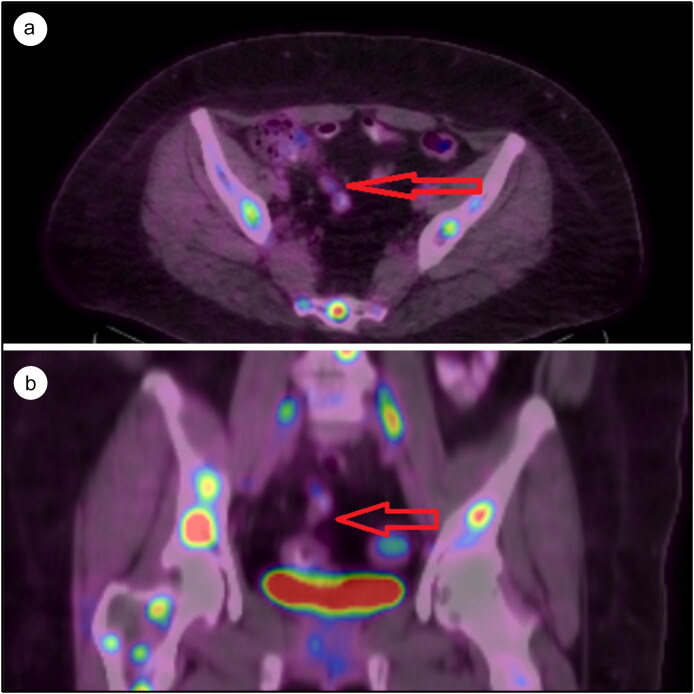
The patient was hemodynamically stable on arrival with a blood pressure of 120/70 mm Hg and a heart rate of 103 beats/minute. Palpation of the right iliac fossa disclosed tenderness. One hour after admission, the patient had an episode of nonbilious vomiting containing food particles. Her hemoglobin level was 11.2 g/dL; sedimentation rate, 9 mm/h; and white blood cell count, 4.2 × 103/µL. An ultrasound of the abdomen was suggestive of acute appendicitis with McBurney’s point tenderness. A computed tomography (CT) scan of the abdomen showed an appendicular mass. Magnetic resonance imaging (MRI) of the spine done just prior to this admission (for back pain) showed hypoechoic lesions concerning for vertebral metastases. A fluorodeoxyglucose–positron emission tomography scan done at the same time revealed extensive metastatic lesions with nodular uptake in the right upper lobe of the lung, numerous mediastinal lymph nodes, hypermetabolic foci in the axial skeleton, and focal hypermetabolism in the mid to distal appendix (standardized uptake value 3.8), concerning for an appendiceal neoplasm (Figure 1).
Figure 1.
Fluorodeoxyglucose positron emission tomography scan of the abdomen: (a) axial section, with the arrow pointing to the appendiceal mass, and (b) coronal section, with the arrow pointing to the appendiceal mass.
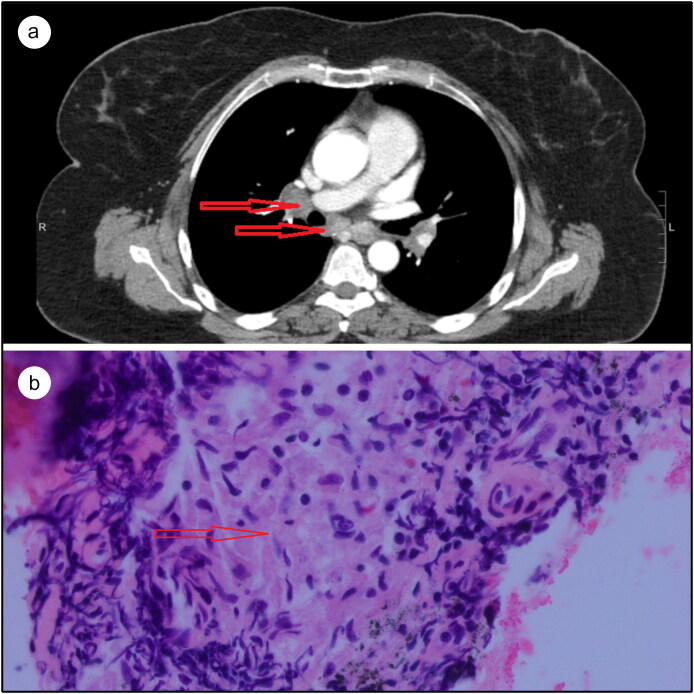
Due to concern for perforation of the appendicular mass and abscess formation in the setting of appendicitis, a laparoscopic appendectomy was performed and the histologic study revealed nonnecrotizing granulomas. After surgery, the patient’s abdominal pain resolved. A CT scan of the chest was performed to further delineate the mediastinal lymphadenopathy (Figure 2a). Subsequently, a bronchoscopic ultrasound-guided biopsy of the lymph nodes revealed granulomas without active inflammation (Figure 2b). Serum angiotensin-converting enzyme levels were elevated (68 U/L).
Figure 2.
(a) CT of the chest, with the arrows pointing to mediastinal lymphadenopathy. (b) Lymph node biopsy with hematoxylin and eosin stain showing noncaseating granuloma.
Oral corticosteroids were initiated at 30 mg daily (0.5 mg/kg/day) prior to discharge. Her back pain improved over a few months. A repeat MRI of her spine after 6 months of treatment showed a decrease in the size of the vertebral lesions. Steroids were slowly tapered by 5 mg per week. At her 6-month visit, mycophenolate mofetil was initiated at 500 mg twice daily and the dose was increased by 500 mg weekly as tolerated. Currently, she remains symptom free on mycophenolate (1500 mg twice daily) and prednisone (5 mg daily).
DISCUSSION
Fewer than 10 cases of sarcoid involving the appendix have been reported, of which <4 cases reported nonnecrotizing granulomas without acute inflammation.5 The present case of appendiceal sarcoidosis causing acute right iliac fossa pain without evidence of acute inflammation appears to be the fifth with similar findings.6 In our patient, granulomas identified after appendectomy provided one of the major clues for the diagnosis. Her pain completely resolved postoperatively.
Although there are no specific guidelines for the treatment of GI sarcoidosis, typically systemic sarcoidosis is treated for at least a year and then imaging is repeated to determine the disease status. For most cases of GI sarcoidosis, steroids are the standard immunosuppressants and are usually initiated at 0.5 mg/kg/day. They can be titrated to 10 mg/day over 6 months based on the clinical response. Steroid-sparing agents like methotrexate, azathioprine, leflunomide, and mycophenolate mofetil can be started at low doses and titrated up.7–9
In conclusion, GI sarcoidosis may present with symptoms of acute appendicitis without inflammation. Our case demonstrates the importance of considering appendiceal sarcoid among the differentials in a patient with systemic sarcoidosis presenting with an acute abdomen. It is important to operate on these patients emergently to avoid the risk of perforation.6 Steroid therapy and or steroid-sparing immunosuppressants should be considered in the postoperative period.
References
- 1.Van Gundy K, Sharma OP. Pathogenesis of sarcoidosis. West J Med. 1987;147(2):168–174. [PMC free article] [PubMed] [Google Scholar]
- 2.O’Regan A, Berman JS. Sarcoidosis. Ann Intern Med. 2012;156:ITC5. [DOI] [PubMed] [Google Scholar]
- 3.Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med. 1963;35:67. [DOI] [PubMed] [Google Scholar]
- 4.Vahid B, Spodik M, Braun KN, et al. Sarcoidosis of gastrointestinal tract: a rare disease. Dig Dis Sci. 2007;52:3316–3320. doi: 10.1007/s10620-006-9448-y. [DOI] [PubMed] [Google Scholar]
- 5.Hunjan T, Chaudery M, Zaidi A, Beggs AD. Appendicular sarcoidosis mimicking acute appendicitis. BMJ Case Rep. 2012;2012:bcr2012006825. doi: 10.1136/bcr-2012-006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker ON, Healy V, Jeffers M, Keane F. Granulomatous appendicitis. Surgeon. 2003;1(5):286–289. [DOI] [PubMed] [Google Scholar]
- 7.Cremers JP, Drent M, Bast A, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. 2013;19:545–561. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(1):43–48. [DOI] [PubMed] [Google Scholar]
- 9.Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014;108(11):1663–1669. doi: 10.1016/j.rmed.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]