Abstract
Acute kidney injury (AKI) requiring dialysis is becoming more common. Several types of renal replacement therapies have been used, including continuous, intermittent, and prolonged intermittent renal replacement therapy (PIRRT). There is no clear difference between those therapies in terms of patient survival. The aim of this study was to describe a form of PIRRT (shift continuous veno-venous hemodialysis [CVVHD]) and the results of this technique in a population of patients with AKI requiring dialysis in a tertiary care center. We studied 302 patients with AKI requiring dialysis over a 3-year period. All patients were treated in the intensive care unit. There were 1709 treatments in the study. Shift CVVHD was done for 8 h daily using NxStage machines, with a bicarbonate base dialysate at a rate of 5 L/h. Demographics and laboratory data were obtained from the electronic medical record. Dialysis data were obtained from the dialysis run sheets. Patient mortality was 51.3%.The dialysis time was close to 8 h and the blood flow was 310 (± 43) mL/min. The mean arterial pressure was stable before and after the dialysis. The total ultrafiltration averaged 2934 mL per treatment; the ultrafiltration rate was 4.1 (± 3.1) mL/kg/h, and the ultrafiltration per hour was 359 (± 257.8) mL/h. The average dialysate potassium used was 2.9 mEq/L. The dose of dialysis was 57 (± 19) mL/kg/h. The urea reduction ratio was 48% (± 15%), the standardized KT/V (a measure of dialysis dose obtained by urea kinetic modeling) was 3.5 (± 0.9), and the equivalent renal urea clearance (EKR) was 9.8 (± 4.1) mL/min. The method produced a consistent reduction in the levels of blood urea nitrogen, creatinine, potassium, and phosphorous. The delivered dose of dialysis was stable during the observation period. In conclusion, shift CVVHD is effective in treating patients with AKI requiring dialysis and has a survival similar to that of continuous therapies with less intensive use of resources.
Keywords: Acute kidney injury, continuous renal replacement therapy, hybrid dialysis, technical hemodialysis
The incidence of acute kidney injury (AKI) requiring dialysis is increasing, mostly because of the type of patients and the severity of illness. Most dialysis treatments are currently done in the intensive care unit (ICU) setting in patients with multiorgan failure. The only treatment for severe AKI is renal replacement therapy (RRT). The best method to provide RRT has not yet been determined. Continuous therapies (24 h, 7 days a week), intermittent dialysis (every other day), and hybrid therapies (prolonged intermittent renal replacement therapy [PIRRT]) have been tried. Multiple clinical trials have been completed, and no clear benefit can be seen between the methods.1,2 The purpose of this study is to describe a form of PIRRT consisting of short continuous veno-venous hemodialysis (CVVHD) (shift) treatments. This study also describes the specifics regarding the therapy as well as results on the population studied.
METHODS
All patients requiring RRT for AKI in a tertiary care center between 2010 and 2013 were included. Demographics, laboratory data, and clinical data were obtained from the electronic medical record. Dialysis details were obtained from the dialysis monitoring sheets for each treatment. Dialysis dose data were obtained by standard methods: urea reduction ratio, KT/V (dialysis dose, single, pool, and standardized), dose of CVVHD as mL/kg/h, and equivalent renal urea clearance (EKR). Shift CVVHD consisted of an 8-hour treatment, 5 L per hour dialysate (bicarbonate base), with an electrolyte composition (potassium and calcium) determined by patient characteristics. The dialysis nurse set up the equipment, initiated the dialysis, and monitored the patient every hour. The ICU nurses were instructed on alarm troubleshooting and notified the dialysis nurse if no resolution of the alarm could be achieved.
Blood flow was between 300 and 400 mL/min, and ultrafiltration was hemodynamically tolerated. Vascular access was obtained through catheters in the internal jugular position and femoral position. The equipment included an NxStage System One cycler with acute care cartridge (including a 1.6 m2 polyethersulfone dialyzer, Purema H). Six to seven treatments were given per week. Pre and post blood urea nitrogen (BUN) values were obtained for each dialysis treatment. Dialysis parameters recorded included dialysate potassium, blood flow, number of treatments per patient, dialysis time, total ultrafiltration, ultrafiltration per hour, and ultrafiltration per kilogram of weight per hour. A standard observation for the first 10 treatments was done and included the following measurements: pre and post BUN and predialysis creatinine, potassium, calcium, and phosphorous. Data are reported as mean and standard deviation. Statistical analysis was done using SPSS 13.0 with a P < 0.05 for significance.
RESULTS
A total of 302 patients were included in the analysis;1709 treatments were performed, with 1105 included in the analysis and 604 excluded due to lack of parameters or required information. The clinical parameters are shown in Table 1. Patient mortality was 51%. The average number of treatments was 6.5, and the average dialysis time was close to the prescribed time (8 h). Most patients required a potassium dialysate of 3 mEq/L. The blood flow was 310 mL/min for the 8-hour period. Blood pressure measurements were stable before and after dialysis.
Table 1.
Clinical parameters
| Variable | Mean (standard deviation) |
|---|---|
| Number of treatments | 6.5 (6.3) |
| Dialysis time (min) | 415.3 (12) |
| Dialysate potassium (mEq/L) | 2.9 (0.5) |
| Blood flow (mL/min) | 310 (43.8) |
| Predialysis mean arterial pressure (mm Hg) | 82.5 (16.9) |
| Postdialysis mean arterial pressure (mm Hg) | 82.9 (16.5) |
| Predialysis systolic blood pressure (mm Hg) | 122.9 (25.7) |
| Postdialysis systolic blood pressure (mm Hg) | 123 (26) |
| Predialysis diastolic blood pressure (mm Hg) | 62.4 (16) |
| Postdialysis diastolic blood pressure (mm Hg) | 63 (16) |
| Total ultrafiltration (mL) | 2934 (1664) |
| Ultrafiltration (mL/kg/h) | 4.1 (3.1) |
| Ultrafiltration (mL/h) | 358 (257.8) |
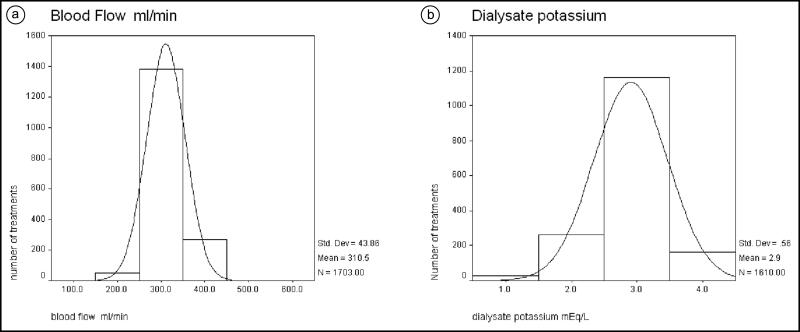
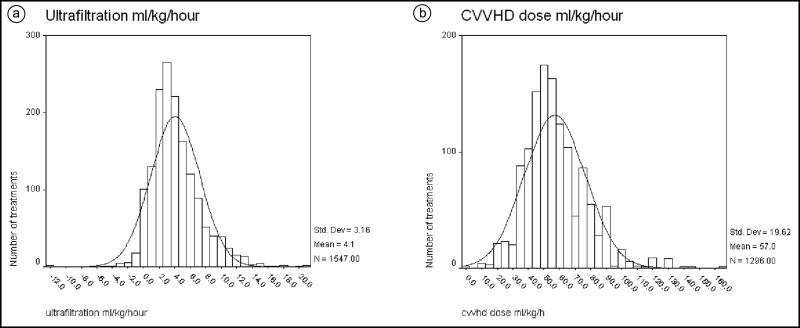
The distribution of the blood flow achieved is presented in Figure 1a. Most patients achieved an adequate blood flow for dialysis, with a mean of 310 mL/min. Most patients required a dialysate potassium of 3 (mean 2.9; standard deviation [SD] 0.5; Figure 1b). The mean ultrafiltration per treatment was 2934 mL (SD 1664). The ultrafiltration per hour was 358 mL/h (SD 257). The distribution of ultrafiltration per kilogram of weight per hour is presented in Figure 2a. The mean was 4.1 mL/kg/h (SD 3.1).
Figure 1.
Distribution (mean and standard deviation) of (a) blood flow (mL/min) and (b) dialysate potassium (mEq/L) used for dialysis treatment.
Figure 2.
Distribution (mean and standard deviation) of (a) ultrafiltration (fluid removal) per kilogram of weight per hour per treatment and (b) short continuous veno-venous hemodialysis dose (mL/kg/h) per treatment.
Table 2 summarizes the dialysis dose measured based on pre and post BUN and the frequency of dialysis. The average CVVHD dose (mL/kg/h) was 57 (SD 16), which is higher than the recommended dose (20–25 mL/kg) for continuous therapy (24 h). However, the duration of the treatment was only 8 h. The distribution of the CVVHD dose is presented in Figure 2b. The standardized KT/V was 3.5 (SD 0.9), and the EKR was 9.8 mL/min (SD 4.1) for an average of six treatments per week.
Table 2.
Dialysis dose
| Variable | Mean (SD) |
|---|---|
| Short continuous veno-venous hemodialysis dose (mL/kg/h) | 57 (19.6) |
| Urea reduction ratio (%) | 48.6 (15.6) |
| Standardized KT/V* | 3.5 (0.9) |
| Equivalent renal urea clearance (mL/min) | 9.8 (4.1) |
Measure of dialysis dose obtained by urea kinetic modeling, no units.
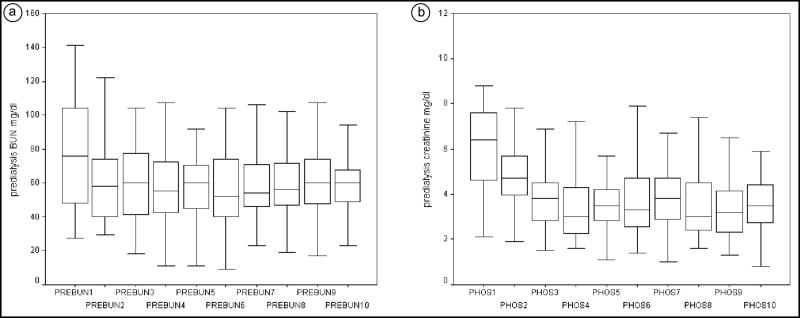
The distribution of predialysis serum BUN and phosphorous levels obtained during the first 10 dialysis treatments is presented in Figure 3. There was an initial decrease but no significant fluctuations between treatments afterwards. The most significant decline was observed in phosphorous levels. The distribution of the dialysis dose as mL/kg/h, standardized KT/V, and EKR did not change (data not shown).
Figure 3.
Predialysis values, during the first 10 dialysis treatments, of (a) blood urea nitrogen (mg/dL) and (b) phosphorous (mg/dL).
DISCUSSION
Multiple therapy options exist for patients with AKI requiring dialysis, including continuous RRT, intermittent hemodialysis, and PIRRT. The outcomes are comparable using these therapies.1,2 Several conclusions were drawn from this study using shift CVVHD on a group of patients. First, shift CVVHD is simple and can be accommodated in hospitals that do not have continuous RRT due to personnel or economic limitations. A single dialysis nurse can supervise the treatment of several patients in the ICU. (The average in our institution was 6 to 8 patients.) This also has an economic impact. The second conclusion relates to the operational characteristics of shift CVVHD. Both shift CVVHD and continuous RRT use a higher potassium in the dialysate due to the prolonged nature of the therapy. Shift CVVHD, however, allows for a higher blood flow than continuous RRT, which offers an advantage in terms of clearance. Third, shift CVVHD uses equipment that is simple and reliable enough to be operated by ICU nurses. The equipment has been used in the ICU for other treatments, such as continuous RRT or shorter dialysis therapy.3,4 The dialysate flow used in this study was 83 mL/min. This dialysate flow is higher than the usual 33 mL/min (2 L/h) and was chosen to improve the clearance and simulate the previous “pulse” hemofiltration therapy described by Ratanarat.5 The dialysate flow was chosen to compensate for the shorter duration of shift CVVHD (8 h vs 24 h with continuous RRT). Fourth, the clinical parameters showed that patients were able to tolerate the therapy. Mean arterial pressure before and after the procedure was reasonable. This result is similar to results previously reported in PIRRT.6–8 Finally, the ultrafiltration results are similar to those of previous reported studies, with an average of 3 L of fluid removal per day. Moreover, the ultrafiltration rate was below the recently suggested rates of 13 mL/kg/h or 800 mL/h to avoid long-term consequences for the end-stage renal disease population and probably apply to AKI patients.9,10
One problematic issue with AKI RRT is the measurement of the dialysis dose. There is no general agreement as to what method to use or what values to use. In this study, the standard measurement of dialysate flow and ultrafiltration were used to calculate the dose of dialysis in mL/kg/h. Our protocol requires measuring pre and post BUN in all dialysis treatments. Based on these values, we calculated the usual measures used for chronic hemodialysis, which include urea reduction ratio, single pool and standardized KT/V, and EKR. The CVVHD dose expressed in mL/kg/h was higher than the accepted 20 to 25 mL/kg/h, but the treatment was short, reflecting also the higher dialysate flow described above. In term of the usual urea reduction ratio and single pool KT/V, the average value obtained was similar to previous studies that showed benefit.11 Extrapolating the urea kinetics for more frequent dialysis, the standardized KT/V value was acceptable and higher than the number recommended for more frequent dialysis in end-stage renal disease patients. This study also calculated the EKR,12 which is used to compare small solute clearance between continuous and intermittent therapies to achieve metabolic control. The values obtained were consistent with those of previous studies.
To ensure the therapy delivery was consistent, the first 10 dialysis treatments per patient were observed and showed a consistent decline in the measured parameters (urea, creatinine, potassium). One important observation is the significant decline in phosphorous levels, as described also in continuous RRT.13 This indicates the efficiency of the method but also calls for cautious monitoring of phosphorous levels when using this therapy. Changes in the dose measurements showed consistency when done on a daily basis. This confirms the applicability of the method.
In conclusion, shift CVVHD is an alternative for continuous therapies and achieves similar results in terms of metabolic control, dose, and hemodynamic stability. Shift CVVHD offers advantages for hospitals that cannot afford continuous therapies or have limited personnel to support a continuous dialysis program.
References
- 1.Mehta RL, McDonald B, Gabbai FB, et al. Collaborative Group for Treatment of ARF in the ICU. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60(3):1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 2.Vinsonneau C, Camus C, Combes A, et al. ; Hemodiafe Study Group. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 3.Clark WR, Turk JE Jr.. Approaches to quotidian dialysis: the NxStage System One. Semin Dial. 2004;17(2):167–170. doi: 10.1111/j.0894-0959.2004.17220.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark WR, Ronco C. Continuous renal replacement techniques. Contrib Nephrol. 2004;144:264–277. doi: 10.1159/000078894. [DOI] [PubMed] [Google Scholar]
- 5.Ratanarat R, Brendolan A, Piccinni P, et al. Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care. 2005;9(4):R294–R302. doi: 10.1186/cc3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fieghen HE, Friedrich JO, Burns KE, et al. The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11(1):32. doi: 10.1186/1471-2369-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kron J, Kron S, Wenkel R, et al. Extended daily on-line high-volume haemodiafiltration in septic multiple organ failure: a well-tolerated and feasible procedure. Nephrol Dial Transplant. 2012;27(1):146–152. doi: 10.1093/ndt/gfr269. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two-year single center report. Int J Artif Organs. 2004;27(5):371–379. doi: 10.1177/039139880402700505. [DOI] [PubMed] [Google Scholar]
- 9.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 11.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346(5):305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 12.Casino FG, Marshall MR. Simple and accurate quantification of dialysis in acute renal failure patients during either urea non-steady state or treatment with irregular or continuous schedules. Nephrol Dial Transplant. 2004;19(6):1454–1466. doi: 10.1093/ndt/gfh218. [DOI] [PubMed] [Google Scholar]
- 13.Kraus MA. Selection of dialysate and replacement fluids and management of electrolyte and acid-base disturbances. Semin Dial. 2009;22(2):137–140. doi: 10.1111/j.1525-139X.2008.00558.x. [DOI] [PubMed] [Google Scholar]