Abstract
Exosomes are small vesicles secreted by all cell types in the brain and play a role in cell-cell communication through the transfer of cargo or encapsulation. Exosomes in the brain have considerable impact on neuronal development, activation, and regeneration. In addition, exosomes are reported to be involved in the onset and propagation of various neurodegenerative diseases. In this review, we discuss the content of exosomes derived from major cell types in the brain, and their function under physiological and pathological conditions.
Keywords: Exosomes, Brain, Neurodegenerative diseases, Cell-cell communication
INTRODUCTION
Brain function is critically dependent on proper intercellular communication. Cells interact with the extracellular environment and other cells in various ways. Extracellular vesicles (EVs) are emerging as a novel form of substance exchange within the nervous system (Caruso Bavisotto et al., 2019). EVs are lipid bilayer structures formed and released by budding from cell’s plasma membrane, with diverse sizes ranging from 100 nm to 1 μm in diameter (Tkach & Théry, 2016). EVs commonly bear surface molecules that allow them to induce signaling transduction via ligand-receptor binding. Exosome content, such as proteins, lipids, and nuclear acids, can be internalized into target cells via endocytosis and/or phagocytosis, even by direct fusion of exosomes with the plasma membrane, thereby modifying the physiological state of recipient cells (Abels & Breakefield, 2016). Exosomes are a type of small vesicle (<150 nm in diameter) and are enriched in endosome-derived components (Colombo et al., 2014). In this review, we highlight and discuss the most recent studies on exosomes and their regulatory roles in the brain.
EXOSOME BIOGENESIS AND CONTENT
Exosomes are produced from the in-budding of endosomes, which later form multivesicular bodies (MVBs) that contain intraluminal vesicles (ILVs). MVBs can either follow a degradation pathway by fusing with lysosomes, where their contents are degraded and recycled within the cell, or alternatively proceed to the cytoplasmic side of the plasma membrane, where they fuse with the cell membrane and ILVs are released as exosomes into the extracellular space by an exocytic step (Cocucci & Meldolesi, 2015). Exosomes are produced by all cell types in the brain, including neurons (Fauré et al., 2006; Lachenal et al., 2011), astrocytes (Bianco et al., 2009; Taylor et al., 2007), oligodendrocytes (Bakhti et al., 2011; Krämer-Albers et al., 2007), as well as microglia (Potolicchio et al., 2005). Exosomes derived from various central nervous system (CNS) cell types have emerged as an important form of intercellular communication.
Exosome content is determined at the budding endosome compartment and endosomal sorting complex (ESCRT) is required to package cargo. Particularly, exosomes contain lipids, such as cholesterol and sphingomyelin, and proteins related to their biogenesis, such as Alix (ALGA2 interacting protein X) and Tsg101 (tumor susceptibility gene 101), which commonly serve as marker proteins of exosomes. Exosomes also contain distinct cytosolic proteins, such as heat-shock proteins, and certain membrane proteins, such as tetraspanins and integrins. In addition, exosomes also carry nucleic acids, such as DNA, mtDNA, and coding and non-coding RNA (Théry, 2011; Théry et al., 2002). This horizontal transfer of genetic information has gained increasing attention recently as it provides a way in which to regulate gene expression in both recipient and donor cells. Of note, exosome number and content change dynamically in response to physiological and environmental conditions (Zappulli et al., 2016).
Neuron-derived exosomes
Neurons, as the basic unit of the CNS, rapidly receive and transmit impulses via chemical or electrical synapses. Neuronal exosomes are primarily localized to soma and dendrites, as indicated in cell-type-specific exosome reporter mice (Men et al., 2019). Exosome release is induced by neuronal activation (Fauré et al., 2006; Lachenal et al., 2011), commonly from post-synaptic soma or dendrites (Men et al., 2019). However, other studies have reported that exosomes are also secreted from pre-synaptic cells, which control postsynaptic retrograde signaling (Korkut et al., 2013). Neuron-derived exosomes contain synaptic proteins, such as L1 cell adhesion molecule (L1CAM), glycosylphosphatidylinositol (GPI)-anchored prion protein, and glutamate receptor subunit GluR2/3 (Fauré et al., 2006), suggesting a regulatory role of exosomes at synapses. In addition to synaptic proteins, exosomes also carry Wnt1, a Wnt signaling pathway ligand, and therefore induce Wnt signaling in target cells (Korkut et al., 2009; Gross et al., 2012). These neuron-secreted exosomes are not only taken up by neighboring neurons, but also by other cell types, such as glia (Chivet et al., 2014; Men et al., 2019). On the one hand, neurons can exploit the exosomal pathway to maintain homeostasis based on a lysosome-independent mechanism (Fauré et al., 2006). On the other hand, neuron-derived exosomes can be captured by neighboring cells and elicit a series of downstream events (Korkut et al., 2009).
The protected RNase-free environment of exosomes provides an advantage for RNA transport. Neuron-secreted exosomes contain a variety of miRNAs, a class of noncoding RNAs 22 nucleotides in length, that typically suppress gene expression at the post-transcriptional level. Of note, the miRNA profile of secreted exosomes is distinct from that of neurons (Men et al., 2019). This is because the neurite-restricted decrease in miRNA expression is typically accompanied by an increase in secreted exosome expression (Goldie et al., 2014), thus suggesting that a subset of miRNA is selectively packed into exosomes and released upon depolarization. Exosomal miRNAs can be internalized into target cells directly by exosome uptake, thereby influencing gene expression in recipient neurons in an activity-dependent manner (Pastuzyn et al., 2018). In addition, exosome-mediated transfer of miRNA can increase levels of glutamate transporter (GLT1) in target cultured astrocytes as well as glutamate uptake in the brain (Morel et al., 2013). In addition to miRNAs, exosomes also carry mRNAs such as Arc to target cells, where Arc mRNA undergoes activity-dependent translation (Pastuzyn et al., 2018).
Treatment of exosomes isolated from human-induced pluripotent stem cell (hiPSC)-derived neurons onto cultured neurons can lead to an increase in neurogenesis in cultured neurons by promoting cell proliferation and neuronal differentiation (Sharma et al., 2019). Specifically, MECP2, a protein carried in exosomes, is responsible for these changes, suggesting that secreted exosomes influence cell fate in developing neural circuits (Sharma et al., 2019). Synaptic pruning can change synaptic connections dynamically in response to the environment (Bahrini et al., 2015). Neuronal exosomes can facilitate synaptic pruning via microglia-mediated phagocytosis (Bahrini et al., 2015). Ephrin-Eph signaling is deeply involved in axon guidance (Klein & Kania, 2014). Specifically, both ligand ephrin and receptor Eph can be secreted by primary neurons in exosomes, which are then subsequently taken up by neighboring cells and induce neuronal growth cone collapse. This suggests that in addition to canonical cell-cell contact, ephrin-Eph can signal at a distance via secreted exosomes (Gong et al., 2016).
Astrocyte-derived exosomes
Astrocytes communicate with neurons and other glial cells through the release of neuroactive substances, including neurotransmitters and other metabolic and trophic factors (Verkhratsky et al., 2016). Secretory vesicles, particularly exosomes, are implicated in glia-neuron communication (Frühbeis et al., 2013b). Astrocyte-derived exosomes are secreted in response to stress, resulting in an increase in the release of exosomes containing neuroprotective factors, such as synapsin I (a synaptic vesicle-associated protein implicated in neural development), heat shock protein 70 (HSP70), and apolipoprotein D (ApoD), eventually promoting neurite outgrowth and neuronal survival (Pascua-Maestro et al., 2018; Taylor et al., 2007; Wang et al., 2011).
Importantly, astrocyte-derived exosomes can also carry miRNAs. Treatment of IL-1β or TNFα in astrocytes can lead to the release of exosomes enriched with miRNAs that target neurotrophic signaling in neurons (Chaudhuri et al., 2018). Increased exosomal miR-34a under lipopolysaccharide (LPS)-induced stress improves the vulnerability of neurons against toxins (Mao et al., 2015). Exposure of astrocytes to morphine and HIV protein Tat can result in an increase in the release of miR-29 in astroglia-derived exosomes, leading to direct repression of trophic factor platelet-derived growth factor B (PDGF-B) at the post-transcriptional level in target neurons (Hu et al., 2012).
Oligodendrocyte-derived exosomes
Mature oligodendrocytes ensheath axons with an insulating myelin sheath, facilitating electric impulse propagation in the brain. Release of exosomes from oligodendrocytes is triggered by glutamate, a neurotransmitter released by electrically active neurons (Frühbeis et al., 2013a). Exposure of neurons to oligodendrocyte-derived exosomes not only increases their action potential firing rate, but also has a beneficial effect on neurons under stress conditions (Fröhlich et al., 2014; Frühbeis et al., 2013b). Oligodendrocyte-secreted exosomes have the potential to influence neuronal physiology across a broad spectrum, as demonstrated by extensive neuronal gene expression changes (Fröhlich et al., 2014). Oligodendrocyte-derived exosomes can also be transferred to microglia and internalized by a subpopulation of unstimulated cells via a micropinocytosis mechanism (Fitzner et al., 2011). Microglia, therefore, participate in the macropinocytotic clearance process to degrade oligodendroglial membranes.
Microglia-derived exosomes
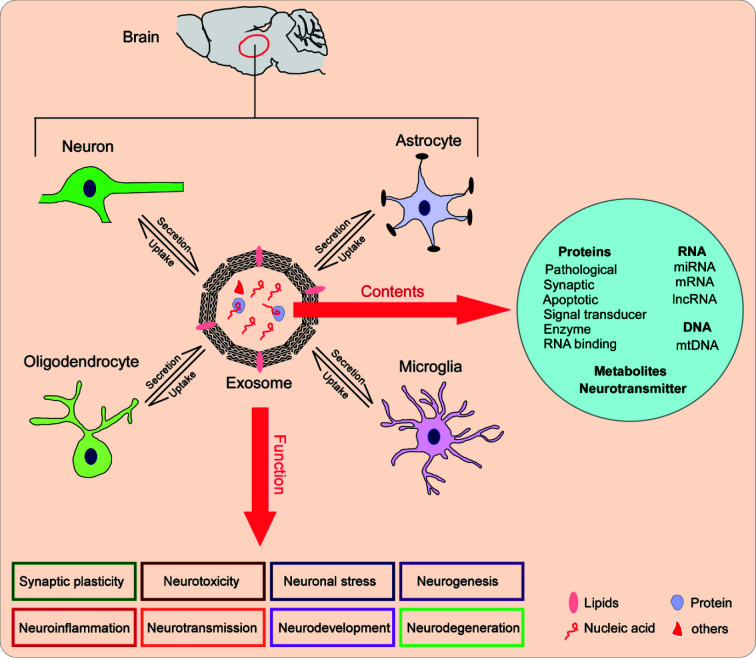
Microglia are innate immune cells in the brain and largely rely on vesicles to propagate cytokine-mediated inflammatory responses. Microglia-derived exosomes improve synaptic transmission by increasing the production of ceramide and sphingosine in neurons (Antonucci et al., 2012). These exosomes also inhibit presynaptic transmission in target GABAergic neurons via enclosed endocannabinoids (Gabrielli et al., 2015). Stimulation with LPS can significantly increase exosome release in microglia, which are enriched in proinflammatory cytokines, such as IL-1β, and ultimately induce inflammation propagation and progressive neuroinflammatory response (Kumar et al., 2017). Microglial exosomes also contain miRNAs, as increased miR-124-3p in microglial exosomes after traumatic brain injury (TBI) inhibits neuronal inflammation and promotes neurite outgrowth (Huang et al., 2018). Microglia are activated upon treatment with α-synuclein, resulting in the release of high levels of exosomes containing TNF-α and ultimately an increase in apoptosis (Chang et al., 2013). A schematic of exosome-mediated cell-cell communication is illustrated in Figure 1.
Figure 1. Schematic of exosome-mediated cell-cell communication and function in the brain.
EXOSOME AND NEURODEGENERATIVE DISEASES
Alzheimer’s disease (AD)
AD is a neurodegenerative disease and the most common type of dementia. AD is characterized by severe impairment of cognitive function and other mental disorders. Accumulation of amyloid β (Aβ) peptides and over-phosphorylation of tau protein can lead to Aβ plaque and neurofibrillary tangles (NFT), which are both pathological markers of AD (Cummings, 2004). Exosome signaling may facilitate the spread of pathogenic protein aggregates in neurodegenerative conditions. For example, exosomes have been proposed to transfer pathogens, such as amyloid precursor protein (APP), leading to amyloid deposition in the brain (Vella et al., 2008; Vingtdeux et al., 2007). Aβ and phosphorylated tau, key pathological proteins of AD, are secreted via an exosome-dependent mechanism (Rajendran et al., 2006; Saman et al., 2012). In support of this, exosomal marker proteins accumulate in amyloid plaque in AD brains (Rajendran et al., 2006). When exosome secretion is inhibited by GW4869, a chemical targeting the key regulatory enzyme (neutral sphingomyelinase 2, nSMase2) in exosome production, AD model mice (5xFAD) exhibit reduced Aβ42 accumulation in the brain (Dinkins et al., 2014). Similar observations have been obtained in nSMase2-deficient 5XFAD mice, which also show reduced tau phosphorylation and glial activation and, more importantly, improved cognitive function (Dinkins et al., 2016). In contrast, enhanced exosome secretion from neuronal cells is reported to facilitate Aβ uptake into microglia and therefore reduce extracellular Aβ levels (Yuyama et al., 2012). Continuous intracerebral infusion of exogenous neuroblastoma-generated exosomes can lead to reduced Aβ levels and amyloid deposition via a microglial phagocytosis mechanism in APP transgenic mice (Yuyama et al., 2014). These findings thus support the notion that the balance that determines whether exosomes drive pathological spread or pathological molecule degradation is likely dependent on how efficiently exosomes are removed from the brain parenchyma (Yuyama et al., 2014). In addition to amyloid peptide levels, tau levels in exosomes derived from tau transgenic mouse brains are significantly increased (Polanco et al., 2016). Exosomal tau is phosphorylated to a different extent, even lower than the cells from which they are derived. Despite this, exosomal tau is considered a seeding tau, and its transmission is essential for AD progression (Polanco et al., 2016).
Endocytic pathway abnormality characterized by enlargement of early endosomes and enhanced early endosome marker Rab5 has been observed in neurons of AD brains (Nixon, 2005). More importantly, this impairment promotes the release of ganglioside (GM1)-associated exosomes, which induce the production of Aβ fibrillogenesis (Yuyama et al., 2008). Neuron-derived exosomes contain full-length APP, APP metabolites, and key enzymes for APP processing, suggesting that exosomes are one of the sites where APP cleavage takes place, and onsite Aβ production is also responsible for lesion spread (Rajendran et al., 2006; Vingtdeux et al., 2007). In support of this conclusion, exosomes isolated from the mouse brain also contain full-length APP, along with other metabolites (Perez-Gonzalez et al., 2012). In addition to neuron-derived exosomes, secretion of astroglia-derived exosomes is also promoted by surrounding amyloid plaque in AD. These exosomes contain more prostate apoptosis response 4 (PAR4) and ceramide, which, in turn, induce apoptosis in neighboring astrocytes via direct uptake (Wang et al., 2012). Levels of plasma exosomal Aβ and tau are higher in AD patients than in controls, concordant with their levels in cerebrospinal fluid (CSF), thus suggesting that plasma and CSF exosomes could serve as biomarkers for AD diagnosis (Jia et al., 2019).
Apolipoprotein E (ApoE) is the predominant carrier for cholesterol transport from astrocytes to neurons in the brain (Zhang & Liu, 2015). There are three major alleles of the ApoE gene in humans, coding for ApoE2, ApoE3, and ApoE4, respectively. ApoE4 is a major risk factor for AD (Zhang et al., 2019). The ApoE4 genotype leads to reduced exosome production, not only in post-mortem brains, but also in ApoE-humanized mice, in an age-dependent manner (Peng et al., 2019). Consequently, an impaired endosomal-exosomal-lysosomal system results in deficits in the degradation of toxic material, contributing to AD pathology (Peng et al., 2019).
Parkinson’s disease (PD)
PD is the most common motor disorder of the CNS. The main clinical manifestations are static tremor and muscle stiffness. One of the neuropathological hallmarks of PD is abnormal aggregation of the synaptic protein alpha-synuclein (α-syn), termed Lewy bodies (LBs) in inherited and sporadic forms of PD (Kalia & Lang, 2015). The transmission of aggregated α-syn across cells has been reported in earlier research (Lee et al., 2012). Previous study has also identified α-syn in exosomes released from neuronal cells with α-syn overexpression, which can be transferred across cells, with negative impact on the viability of recipient neuronal cells (Emmanouilidou et al., 2010). Importantly, when brain homogenates containing α-syn aggregates are injected into mice, the mice show both aggregation of α-syn in their brains and the onset of clinical symptoms (Henderson et al., 2019). The spread mechanism of α-syn aggregates could be attributed to exosome-mediated transport.
To degrade α-syn deposits, neuronal exosomes can be taken up by astrocytes or microglia (Russo et al., 2012). However, excessive uptake of α-syn can produce glial inclusions and trigger inflammatory responses, which are both hallmarks of PD pathogenesis (Russo et al., 2012). Therefore, exosomes may be involved in neuroinflammation via modulating neuron-glia communication, or during the propagation of the inflammatory response through glia-glia communication.
Thus, exosomes have complicated roles in neurodegenerative diseases, as they can be beneficial by discarding accumulated and toxic substances, such as α-synuclein and Aβ. However, they can also contribute to the transport and extracellular build-up of toxic material, leading to disease progression.
Other neurodegenerative diseases
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease. The symptoms of ALS include progressive motor system dysfunction, muscle paralysis, atrophy, and eventually respiratory failure. Aggregation of TDP-43 is a typical hallmark of ALS pathology (Neumann et al., 2006). The TDP-43 nuclear protein plays a role in regulating transcription, pre-mRNA splicing, and translation (Mackenzie et al., 2010). Mutated TDP-43 is translocated to the cytosol, where it forms aggregates (Scotter et al., 2015). TDP-43 oligomers are loaded in exosomes and taken up by the neuronal soma and synaptic cleft, and therefore contribute to disease pathology (Feiler et al., 2015). Importantly, secretion of exosomal TDP-43 is enhanced in ALS brains. This secretion is cell-type dependent, with TDP-43 primarily secreted in exosomes derived from neurons, but not from astrocytes or microglia (Iguchi et al., 2016). Exosomal TDP-43 not only contributes to the transmission and propagation of TDP-43, but also serves as a key means for the clearance of TDP-43 aggregates (Iguchi et al., 2016). Fused in sarcoma (FUS) is another RNA-binding protein that resides in the nucleus and is implicated in the pathology of ALS (Mackenzie et al., 2010). FUS is structurally and functionally similar to TDP-43, and mutated FUS is preferentially localized to the cytoplasm, where it induces stress granule-like structures (Mackenzie et al., 2010). FUS is also detected in secreted exosomes, particularly enriched in FUS-expressing cells (Kamelgarn et al., 2016). Dipeptide repeat proteins (DPRs), derived from aberrant hexanucleotide repeat expansions in the C9orf72 gene, are also detected in exosomes (Westergard et al., 2016). Similar to TDP-43, intercellular transmission of DRPs occurs through anterograde and retrograde transport in neurons, and also between neurons and astrocytes (Feiler et al., 2015). These findings underline the importance of exosomes in the propagation of ALS through spreading toxic proteins.
Huntington’s disease (HD) is an autosomal dominant hereditary disease caused by expanded repeats of the CAG sequence in the first exon of the HTT gene, resulting in the production of polyglutamine sequence (PolyQ) protein, which exhibits neuronal toxicity and is essential for aggregate formation (Zhang et al., 2016). Of note, both PolyQ and CAG-repeat RNA have been found in exosomes secreted by 293T cells, suggesting that exosome-loaded toxic protein and RNA can be transferred between cells (Zhang et al., 2016). In addition, exosomes also carry mutant huntingtin (mHtt), which triggers the manifestation of HD-related pathology (Jeon et al., 2016). Notably, mHtt is undetectable in astrocyte-derived exosomes, but suppresses the secretion of exosomes from astrocytes (Hong et al., 2017). Accumulation of mHtt significantly represses exosome secretion from astrocytes in HD model mice, whereas injection of astrocytic exosomes reduces the burden of mHtt aggregates (Hong et al., 2017). These findings suggest that exosomes play an essential role in HD pathology.
EXOSOMES AS POTENTIAL DIAGNOSTIC AND THERAPEUTIC TOOLS
The identification of exosomes in blood plasma opens new opportunities for biomarker discovery. Proteomic analysis of serum exosomes has identified 23 exosome-associated proteins that are differentially expressed in PD patients, which could potentially serve as biomarkers for PD diagnosis (Tomlinson et al., 2015). Furthermore, neuron- and astrocytic-origin exosomes can be separated, with neuron-derived exosomes serving as better biomarkers as they contain higher levels of signaling molecules related to cellular metabolism, survival, and repair (Mustapic et al., 2017). In fact, neuron-derived exosomes show good diagnostic and predictive performance for AD (Goetzl et al, 2016).
The characteristics and manageability of exosomes make them potential candidates for delivering active molecules, particularly therapeutic drugs to specific target cell types. Engineered exosomes that carry selected cargo, such as drugs and/or other therapeutic proteins, can transport cargo across the blood-brain barrier and deliver it to target tissues/cells (Alvarez-Erviti et al., 2011). Exosomes containing neprilysin, which targets and degrades Aβ, can reduce both secreted and intracellular Aβ levels (Katsuda et al., 2013). Additionally, exosome-mediated short interfering RNA (siRNA) delivery also has therapeutic potential for AD treatment, as demonstrated by strong knockdown of BACE1, a protease that cleaves amyloid precursor and generates Aβ peptides (Alvarez-Erviti et al., 2011). Exosomes derived from adipose stem cells not only reduce mHtt aggregates, but also ameliorate abnormal apoptotic protein levels in HD models (Lee et al., 2016). However, with all the advantages that exosome-based therapy might offer, potential problems need to be solved before these therapeutic strategies can be implemented safely. The loading capacity of exosomes and the half-life of the cargo need to be determined and administered accordingly. Assessment of systematic administration of exosomes and their biodistribution in target and non-target tissues/cells should be performed before potential clinical use. Finally, the requirement of bioengineered ligands in exosomes for efficient delivery of cargo needs to be assessed. Further development of exosome-mediated therapeutic strategies will require continuous investigation into exosome biology.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
X.L., J.Z., and Q.L. conceived the review. J.Z., and Q.L. prepared the draft. D.L. designed the figure. X.L., J.Z., W.W., Z.X., and Q.L. contributed to discussions. All authors read and approved the final version of the manuscript.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31871082, 91849101, 81601221), The Strategic Priority Research Program of the Chinese Academy of Sciences (XDB39000000), Key Research Program of Frontier Sciences of CAS (QYZDB-SSW-SMC035), the Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology (2018CXFX005), the Fundamental Research Funds for the Central Universities, and China Postdoctoral Science Foundation (2019M662178). The Open Fund of State Key Laboratory of Tea Plant Biology and Utilization (SKLTOF20150101)
Contributor Information
Zhong-Wen Xie, Email: zhongwenxie@ahau.edu.cn.
Qiang Liu, Email: liuq2012@ustc.edu.cn.
References
- 1.Abels ER, Breakefield XO Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Yin HF, Betts C, Lakhal S, Wood MJA Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 3.Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. The EMBO Journal. 2012;31(5):1231–1240. doi: 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrini I, Song JH, Diez D, Hanayama R Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Scientific Reports. 2015;5:7989. doi: 10.1038/srep07989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhti M, Winter C, Simons M Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. Journal of Biological Chemistry. 2011;286(1):787–796. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO Journal. 2009;28(8):1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso Bavisotto C, Scalia F, Marino Gammazza A, Carlisi D, Bucchieri F, Conway de Macario E, Macario AJL, Cappello F, Campanella C Extracellular vesicle-mediated cell-cell communication in the nervous system: focus on neurological diseases. International Journal of Molecular Sciences. 2019;20(2):434. doi: 10.3390/ijms20020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CW, Lang HJ, Geng N, Wang J, Li N, Wang XL Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neuroscience Letters. 2013;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC Jr, Hao HP, Witwer KW, Haughey NJ TNFα and il-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death & Disease. 2018;9(3):363. doi: 10.1038/s41419-018-0369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. Journal of Extracellular Vesicles. 2014;3(1):24722. doi: 10.3402/jev.v3.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocucci E, Meldolesi J Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends in Cell Biology. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Colombo M, Raposo G, Théry C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL Alzheimer's disease. The New England Journal of Medicine. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 14.Dinkins MB, Dasgupta S, Wang GH, Zhu G, Bieberich E Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiology of Aging. 2014;35(8):1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinkins MB, Enasko J, Hernandez C, Wang GH, Kong JN, Helwa I, Liu YT, Terry AV Jr, Bieberich E Neutral sphingomyelinase-2 deficiency ameliorates Alzheimer's disease pathology and improves cognition in the 5XFAD mouse. Journal of Neuroscience. 2016;36(33):8653–8667. doi: 10.1523/JNEUROSCI.1429-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. Journal of Neuroscience. 2010;30(20):6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R Exosomes are released by cultured cortical neurones. Molecular and Cellular Neuroscience. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li DY, Thal DR, Walther P, Ludolph AC, Danzer KM, Weishaupt JH TDP-43 is intercellularly transmitted across axon terminals. Journal of Cell Biology. 2015;211(4):897–911. doi: 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. Journal of Cell Science. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 20.Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Krämer-Albers EM Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1652):20130510. doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers EM Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biology. 2013a;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM Extracellular vesicles as mediators of neuron-glia communication. Frontiers in Cellular Neuroscience. 2013b;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Reports. 2015;16(2):213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM, Boxer A, Miller BL Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. The FASEB Journal. 2016;30(12):4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldie BJ, Dun MD, Lin MJ, Smith ND, Verrills NM, Dayas CV, Cairns MJ Activity-associated miRNA are packaged in map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Research. 2014;42(14):9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong JY, Körner R, Gaitanos L, Klein R Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. Journal of Cell Biology. 2016;214(1):35–44. doi: 10.1083/jcb.201601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross JC, Chaudhary V, Bartscherer K, Boutros M Active Wnt proteins are secreted on exosomes. Nature Cell Biology. 2012;14(10):1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 28.Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan RJ, Sandler RM, Bassett DS, Trojanowski JQ, Lee VMY Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nature Neuroscience. 2019;22(8):1248–1257. doi: 10.1038/s41593-019-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Y, Zhao T, Li XJ, Li SH Mutant huntingtin inhibits αB-crystallin expression and impairs exosome secretion from astrocytes. Journal of Neuroscience. 2017;37(39):9550–9563. doi: 10.1523/JNEUROSCI.1418-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, Cheney PD, Fox HS, Buch S Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death & Disease. 2012;3(8):e381. doi: 10.1038/cddis.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Ge XT, Yu JW, Han ZL, Yin ZY, Li Y, Chen FL, Wang HC, Zhang JN, Lei P Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons . The FASEB Journal. 2018;32(1):512–528. doi: 10.1096/fj.201700673r. [DOI] [PubMed] [Google Scholar]
- 32.Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, Kawai K, Takagi S, Yoshida M, Katsuno M, Sobue G, Julien JP Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139(12):3187–3201. doi: 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon I, Cicchetti F, Cisbani G, Lee S, Li ED, Bae J, Lee N, Li L, Im W, Kim M, Kim HS, Oh SH, Kim TA, Ko JJ, Aubé B, Oueslati A, Kim YJ, Song J Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathologica. 2016;132(4):577–592. doi: 10.1007/s00401-016-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia LF, Qiu QQ, Zhang H, Chu L, Du YF, Zhang JW, Zhou CK, Liang FR, Shi SL, Wang S, Qin W, Wang Q, Li FY, Wang QG, Li Y, Shen LX, Wei YP, Jia JP Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s & Dementia. 2019;15(8):1071–1080. doi: 10.1016/j.jalz.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Kalia LV, Lang AE Parkinson's disease. The Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 36.Kamelgarn M, Chen J, Kuang LS, Arenas A, Zhai JJ, Zhu HN, Gal J Proteomic analysis of FUS interacting proteins provides insights into FUS function and its role in ALS. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016;1862(10):2004–2014. doi: 10.1016/j.bbadis.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific Reports. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein R, Kania A Ephrin signalling in the developing nervous system. Current Opinion in Neurobiology. 2014;27:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless . Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korkut C, Li YH, Koles K, Brewer C, Ashley J, Yoshihara M, Budnik V Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039–1046. doi: 10.1016/j.neuron.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, Nave KA, Schild H, Trotter J Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics: Clinical Applications. 2007;1(11):1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. Journal of Neuroinflammation. 2017;14(1):47. doi: 10.1186/s12974-017-0819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and Cellular Neuroscience. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Lee M, Liu T, Im W, Kim M Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model . European Journal of Neuroscience. 2016;44(4):2114–2119. doi: 10.1111/ejn.13275. [DOI] [PubMed] [Google Scholar]
- 45.Lee SJ, Desplats P, Lee HJ, Spencer B, Masliah E. 2012. Cell-to-cell transmission of α-synuclein aggregates. In: Sigurdsson EM, Calero M, Gasset M. Amyloid Proteins: Methods and Protocols. USA: Humana Press, 347–359.
- 46.Mackenzie IRA, Rademakers R, Neumann M TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. The Lancet Neurology. 2010;9(10):995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 47.Mao SS, Sun Q, Xiao H, Zhang CY, Li L Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein & Cell. 2015;6(7):529–540. doi: 10.1007/s13238-015-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Men YQ, Yelick J, Jin SJ, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, Yang YJ Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature Communications. 2019;10(1):4136. doi: 10.1038/s41467-019-11534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang YJ Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. Journal of Biological Chemistry. 2013;288(10):7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mustapic M, Eitan E, Werner JK Jr, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Frontiers in Neuroscience. 2017;11:278. doi: 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 52.Nixon RA Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiology of Aging. 2005;26(3):373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Pascua-Maestro R, Gonzalez E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Frontiers in Cellular Neuroscience. 2018;12:526. doi: 10.3389/fnins.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, Briggs JAG, Feschotte C, Shepherd JD The neuronal gene Arc encodes a repurposed retrotransposon gag protein that mediates intercellular RNA transfer . Cell. 2018;173(1-2):275–288. doi: 10.1016/j.cell.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng KY, Pérez-González R, Alldred MJ, Goulbourne CN, Morales-Corraliza J, Saito M, Saito M, Ginsberg SD, Mathews PM, Levy E Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019;142(1):163–175. doi: 10.1093/brain/awy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. Journal of Biological Chemistry. 2012;287(51):43108–43115. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polanco JC, Scicluna BJ, Hill AF, Götz J Extracellular vesicles isolated from the brains of rtg4510 mice seed tau protein aggregation in a threshold-dependent manner. Journal of Biological Chemistry. 2016;291(24):12445–12466. doi: 10.1074/jbc.M115.709485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potolicchio I, Carven GJ, Xu XN, Stipp C, Riese RJ, Stern LJ, Santambrogio L Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. The Journal of Immunology. 2005;175(4):2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 59.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(30):11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russo I, Bubacco L, Greggio E Exosomes-associated neurodegeneration and progression of Parkinson's disease. American Journal of Neurodegenerative Disease. 2012;1(3):217–225. [PMC free article] [PubMed] [Google Scholar]
- 61.Saman S, Kim WH, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NCY, Hall GF Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early alzheimer disease. Journal of Biological Chemistry. 2012;287(6):3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scotter EL, Chen HJ, Shaw CE TDP-43 proteinopathy and ALS: insights into disease mechanisms and therapeutic targets. Neurotherapeutics. 2015;12(2):352–363. doi: 10.1007/s13311-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma P, Mesci P, Carromeu C, McClatchy DR, Schiapparelli L, Yates III JR, Muotri AR, Cline HT Exosomes regulate neurogenesis and circuit assembly. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(32):16086–16094. doi: 10.1073/pnas.1902513116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Developmental Neurobiology. 2007;67(13):1815–1829. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 65.Théry C, Zitvogel L, Amigorena S Exosomes: composition, biogenesis and function. Nature Reviews Immunology. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 66.Théry C Exosomes: secreted vesicles and intercellular communications. F1000 Biology Reports. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tkach M, Théry C Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 68.Tomlinson PR, Zheng Y, Fischer R, Heidasch R, Gardiner C, Evetts S, Hu M, Wade-Martins R, Turner MR, Morris J, Talbot K, Kessler BM, Tofaris GK Identification of distinct circulating exosomes in Parkinson's disease. Annals of Clinical and Translational Neurology. 2015;2(4):353–361. doi: 10.1002/acn3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF The role of exosomes in the processing of proteins associated with neurodegenerative diseases. European Biophysics Journal. 2008;37(3):323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 70.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. The EMBO Journal. 2016;35(3):239–257. doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vingtdeux V, Hamdane M, Loyens A, Gelé P, Drobeck H, Bégard S, Galas MC, Delacourte A, Beauvillain JC, Buée L, Sergeant N Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. Journal of Biological Chemistry. 2007;282(25):18197–18205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- 72.Wang GH, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in alzheimer disease (AD) Journal of Biological Chemistry. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang SW, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. Journal of Neuroscience. 2011;31(20):7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westergard T, Jensen BK, Wen XM, Cai JL, Kropf E, Iacovitti L, Pasinelli P, Trotti D Cell-to-cell transmission of dipeptide repeat proteins linked to c9orf72-ALS/FTD . Cell Reports. 2016;17(3):645–652. doi: 10.1016/j.celrep.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuyama K, Sun H, Mitsutake S, Igarashi Y Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. Journal of Biological Chemistry. 2012;287(14):10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa JI, Fujitani N, Shinohara Y, Igarashi Y Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in alzheimer model mice. Journal of Biological Chemistry. 2014;289(35):24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuyama K, Yamamoto N, Yanagisawa K Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality: another putative pathway for GM1-induced amyloid fibril formation. Journal of Neurochemistry. 2008;105(1):217–224. doi: 10.1111/j.1471-4159.2007.05128.x. [DOI] [PubMed] [Google Scholar]
- 78.Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO Extracellular vesicles and intercellular communication within the nervous system. Journal of Clinical Investigation. 2016;126(4):1198–1207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang DF, Xu M, Bi R, Yao YG Genetic analyses of Alzheimer's disease in china: achievements and perspectives. ACS Chemical Neuroscience. 2019;10(2):890–901. doi: 10.1021/acschemneuro.8b00435. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Liu Q Cholesterol metabolism and homeostasis in the brain. Protein & Cell. 2015;6(4):254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington's disease: background and evaluation in cell culture. Cellular and Molecular Neurobiology. 2016;36(3):459–470. doi: 10.1007/s10571-016-0350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]