DEAR EDITOR,
Since the epoch-making observations of circadian rhythm in Mimosaceae plants, sleep has been investigated for centuries (de Mairan, 1729; Du Monceau, 1758). As a natural and reversible state, sleep is marked by reduced responsiveness to external stimuli, relative inactivity, and loss of consciousness. Although reduced responsiveness could potentially introduce significant danger to survival, nearly all animals in nature sleep. This strongly implies an adaptive role of sleep in increasing overall fitness of an organism. Research has shown that sleep is responsible for many vital physiological functions, including tissue repair (Oswald, 1980), skin function (Rechtschaffen, 1998), thermoregulation (Parmeggiani, 1986; Rechtschaffen, 1998), energy saving (Siegel, 2005), insulin release and responsiveness (Spiegel et al., 1999), metabolic regulation (Sharma & Kavuru, 2010), immunological enhancement (Besedovsky et al., 2012; Imeri & Opp, 2009), synaptic plasticity (Benington & Frank, 2003), neuron viability (Zhang et al., 2014a), and memory formation (Rasch & Born, 2013; Walker & Stickgold, 2006). Sleep therefore plays an essential role in human health and is vital for physical and psychological performance (Halson & Juliff, 2017; Thun et al., 2015; Vitale & Weydahl, 2017).
Given its indispensable functions, insufficiency in sleep can cause a cascade of negative consequences for general health, as well as cardiovascular, metabolic, mental, and immunological health, and in cancer, pain, and all-cause mortality (Parekh et al., 2015; Scullin & Bliwise, 2015; Vgontzas et al., 2013; Watson et al., 2015a, 2015b, 2015c, 2015d). Sleep is also equally important in behavioral and physical performance. For example, sleep deprivation and extension are negatively and positively associated with athletic performance, respectively (Thun et al., 2015), suggesting that chronotype effects should be considered during the scheduling of training sessions (Vitale & Weydahl, 2017). According to the third edition of the International Classification of Sleep Disorders (ICSD), there are seven major sleep disorders, including insomnia, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep-wake disorders, sleep-related movement disorders, parasomnias, and other sleep disorders (Sateia, 2014).
Although human subjects are an ideal choice for studies on sleep and sleep disorders, animal-based research has played a fundamental role in the elucidation of the mechanisms that underlie sleep, as well as its regulation and disorders, and is essential for validating sleep mechanisms and testing therapeutics for sleep disorders (Singh et al., 2017; Toth & Bhargava, 2013). Sleep deprivation experiments have been successfully performed in rats (Rechtschaffen et al., 1989), mice (Mackiewicz et al., 2007; Maret et al., 2007), fruit-flies (Shaw et al., 2002), and roundworms (Sanders et al., 2017). Non-human primates (NHPs) are among the best-studied animal models, in large part because of their close phylogenetic relatedness to humans (Nunn & Samson, 2018; Zhang et al., 2014b). Likewise, sleep also plays a dominant role in NHP health, behavior, and ecology, and can exert a significant influence on daily activity schedules (Anderson, 1998, 2000; Qiu et al., 2019). Thus, NHPs are especially valuable for comparative studies on sleep, with tremendous potential to provide critical improvement in our understanding of human sleep and associated disorders (Fruth et al., 2018; Nunn et al., 2010).
Electroencephalography (EEG) is commonly used in sleep research and can provide an objective and functional marker of sleep (Feinberg et al., 1967; Roffwarg et al., 1964). Although EEG can be performed in restrained or freely moving animals under controlled laboratory conditions, this technique is invasive, involving implantation of electrodes in the brain or subcutaneously. Implantation usually involves drilling holes in the skull to place electrodes directly on the brain. Compared with a single-channel EEG, polysomnogram (PSG) is considered the gold standard to objectively assess sleep (Boulos et al., 2019; O'Donnell et al., 2018). PSG integrates both normal and abnormal physiological indicators during sleep, including brain activity (EEG), eye movements (EOG: electrooculography), muscle activity (EMG: electromyography), heart rhythm, respiratory effort, airflow through the mouth and nose, and audible snoring. However, a significant limitation of PSG is that it requires electrodes attached to the scalp and skin surface, and sensors to collect data (Lucey et al., 2016). These are difficult or impossible to apply in freely moving monkeys. Even though subcutaneous implantations are minimally invasive, PSG is expensive and burdensome to obtain and may be difficult to use with longer recording intervals. This greatly limits the use of PSG or EEG, especially for long-term research involving a large sample size.
The emergence of videography has offered convenience for observing behaviors in naturally sleeping monkeys (Chen et al., 2017; Kripke et al., 1968; Weitzman et al., 1965). The video technique avoids the need for surgery and electrode implantation, and most importantly, it is inexpensive and noninvasive, allowing for large sample size and long-term study by means of specific behavioral criteria defined for each state. Nevertheless, manual video analysis presents some limitations, including greater subjectivity and considerable human labor and time requirements. In comparison, actigraphy provides a more objective measure than videography, and can be considered as an alternative sleep assessment method in research (Ancoli-Israel et al., 2003; Littner et al., 2003; Morgenthaler et al., 2007; Sadeh et al., 1995; Sadeh & Acebo, 2002; Thorpy et al., 1995). The use of actigraph accelerometers for detection of movement is a reliable, noninvasive method for monitoring activity (Andersen et al., 2010, 2012; Mann et al., 2005). Despite the increase in studies utilizing actigraphy, no comparisons in sleep scoring based on actigraphy and videography have been conducted in NHPs.
The present study was designed to compare videographic and actigraphic sleep scoring in 10 cynomolgus monkeys over seven nights of simultaneous behavioral recordings, and to validate the use of actigraphy in sleep measurement.
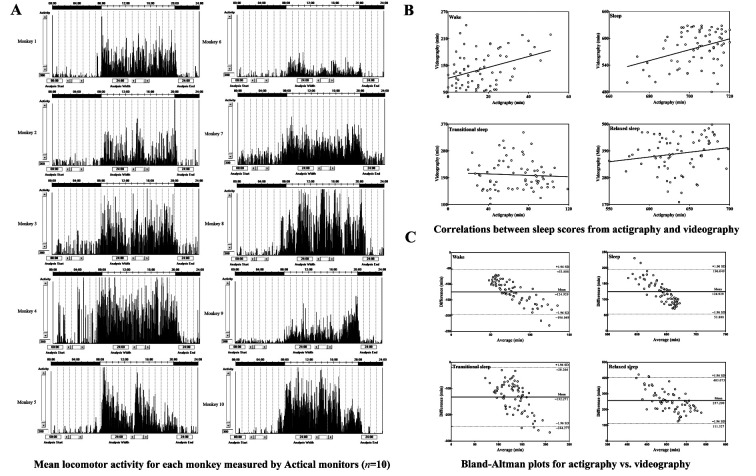
Mean locomotor activity for each monkey was measured by Actical monitors attached to the monkeys’ necks (n=10). Data collected by actigraphy over the seven days were presented as means of that time period. As shown in Figure 1A, the monkeys displayed consistently high activity during light time (0800 h–2000 h) and low activity during dark time (2000 h–0800 h).
Figure 1. Locomotor activity, correlations, and Bland-Altman plots.
A: Mean locomotor activity for each monkey measured by Actical monitors (n=10). Activity data were collected over a seven-day period and are presented as means over that period. Lights were on at 0800 h and off at 2000 h. B: Correlations between sleep scores from actigraphy and videography. Four states were scored by actigraphy (X-axis: Actigraphy) and videography analysis (Y-axis: Videography), respectively, including wake, sleep, transitional sleep, and relaxed sleep. C: Bland-Altman plots for actigraphy vs. videography. X axes represent average of two methods ((actigraphy+videography)/2) and Y axes show differences between two paired measurements (actigraphy–videography), including wake, sleep, transitional sleep, and relaxed sleep. Solid lines show mean differences between actigraphy and videography measurements; dotted lines represent 95% limits of agreement, from –1.96 SD (standard deviation) to +1.96 SD.
Figure 1B shows the correlations between the mean time spent in each state per night obtained from actigraphy and videography analysis. Results showed a positive correlation between the two methodologies for scoring the state of wake (r=0.368 P=0.002) and sleep (r=0.368, P=0.002). When measuring the state of transitional sleep (r=–0.058, P=0.631) and relaxed sleep (r=0.174, P=0.149), no significant correlation was observed.
Figure 1C shows the differences and limits of agreement between actigraphy and videography measurements. Results indicated that, compared with videographic analysis, actigraphy underestimated the durations of wake and transitional sleep by an average of 124.929 min and 132.271 min, respectively, but overestimated the durations of sleep and relaxed sleep by 124.929 min and 257.200 min, respectively.
Total durations of time spent in each state for each monkey based on videography and actigraphy are illustrated in Supplementary Table S1. When comparing the differences for each state between the two methods, the durations of time spent in each state obtained from actigraphy were significantly different from those obtained by videography (wake: actigraphy vs. videography=16.629±1.404 vs. 141.557±4.652 min per night, P=1.771×10-54; sleep: actigraphy vs. videography=703.371±1.404 vs. 578.443±4.652 min per night, P=1.771×10-54; transitional sleep: actigraphy vs. videography=67.157±2.915 vs. 199.429±6.009 min per night, P=3.626×10-42; relaxed sleep: actigraphy vs. videography=636.214±3.889 vs. 379.014±8.706 min per night, P=7.009×10-57).
The epoch-by-epoch analysis results are presented in Supplementary Table S2 and Table 1 and relate to the duration of epochs and percentages for all dark period recordings in all monkeys. The maximum percentage agreement between the two methods relative to the state of sleep reached 99.852%. Of the 40 491 epochs scored for sleep by videography, 40 431 (99.852%) were correctly scored by actigraphy analysis and 60 (0.148%) were allocated to the state of wake. Of the 9 909 wake epochs, however, only 1 104 (11.141%) were correctly scored by actigraphy, with 8 805 epochs (88.859%) scored as sleep. This demonstrates that actigraphic analysis can correctly score the state of sleep but may not correctly score the state of wakefulness.
Table 1. Epoch-by-epoch analysis for each state (including wake, transitional sleep, and relaxed sleep) showing total duration (min) and corresponding percentage (%) of epochs scored in agreement (diagonal from left to right) between videography (rows) and actigraphy (columns) methods and those scored differently by actigraphy analysis.
| State | Min | % | |||||
| Wake | Transitional sleep | Relaxed sleep | Wake | Transitional sleep | Relaxed sleep | ||
| Wake | 1 104 | 3 290 | 5 515 | 11.141 | 33.202 | 55.656 | |
| Transitional sleep | 26 | 800 | 13 134 | 0.186 | 5.731 | 94.083 | |
| Relaxed sleep | 34 | 611 | 25 886 | 0.128 | 2.303 | 97.569 | |
To further determine whether actigraphy is suitable for scoring states of sleep. As shown in Table 1, actigraphic analysis was preferable for scoring the state of relaxed sleep. Of the 26 531 relaxed sleep epochs, 25 886 (97.569%) were correctly scored using actigraphy, with 34 (0.128%) and 611 epochs (2.303%) respectively scored as the state of wake and transitional sleep. Actigraphy also correctly scored 800 epochs (5.731%) as transitional sleep out of the 13 960 epochs of transitional sleep obtained from videographic analysis. Of the remaining inconsistently scored transitional sleep epochs, 13 134 (94.083%) were allocated to relaxed sleep and 26 (0.186%) were allocated to wakefulness.
In the present study, actigraphy constituted a reliable method for scoring the state of sleep in monkeys (Andersen et al., 2013; Berro et al., 2016; Golub & Hogrefe, 2016; Kantha & Suzuki, 2006), showing a significant correlation in comparison with states obtained by videography. Epoch-by-epoch analysis provided an exact measure of the percentage agreement between the two methods, which showed that actigraphy could accurately score the state of sleep (99.852%). This is consistent with results previously obtained in humans, with actigraphy demonstrating 93% and 94% sensitivity in measuring sleep compared with PSG (Niel et al., 2019; Yavuz-Kodat et al., 2019). In four species of lemur, Cramer’s V correlation between actigraphy-classified sleep and videography-classified sleep revealed highly consistent results (Melvin et al., 2019). Here, although the sensitivity of actigraphy in detecting sleep was very high, it performed poorly in detecting wakefulness, with only 11.141% of epochs correctly identified. This accords with previous research comparing actigraphy and polysomnography in older adults (Sivertsen et al., 2006). These results suggest that actigraphy may show poor sensitivity in detecting wakefulness during the sleep-period (Terrill et al., 2010). This may be because the monitors were mounted to the monkeys’ necks, and small movements may not be detected, such as hand movements involving no changes of position. As a result, 8 805 epochs (88.859%) were wrongly scored as the state of sleep.
Further epoch-by-epoch analysis indicated that actigraphy was more suitable for scoring the state of relaxed sleep, with 97.569% of relaxed sleep (25 866 epochs) correctly identified in comparison with videography. Only 34 (0.128%) and 611 epochs (2.303%) were differently interpreted as wake and transitional sleep, respectively, compared with videographic analysis. One of the most important reasons for this result is that relaxed sleep was scored when the monkeys exhibited neither body nor limb movements and the activity monitor was highly sensitive to detecting the state of rest. When the monitor did not detect any movements within 1 min, this minute was scored as relaxed sleep. In contrast, the actigraphy method could not easily discriminate between the state of relaxed and transitional sleep (800 epochs, i.e., 5.731%). Although a monkey may have exhibited one or two movements within 1 min, the monitor could not detect such movement, and thus, 94.083% of transitional sleep (13 134 epochs) was incorrectly allocated to the state of relaxed sleep. Also, a tiny fraction of transitional sleep was interpreted as wakefulness (26 epochs, i.e., 0.186%).
In view of the differences between the two sleep scoring methods, compared with videographic analysis, actigraphy underestimated the durations of wake and transitional sleep by 88.253% and 66.325%, respectively, but overestimated the durations of sleep and relaxed sleep by 21.597% and 67.860%, respectively. One reason for this is because the neck-attached monitors showed poor sensitivity to slight movements by the monkeys. Most of the time, the monitors could not detect any movements, and thus interpreted the state of wakefulness (≥3 movements within 1 min) and transitional sleep (1–2 movements in 1 min) as either sleep or relaxed sleep. A wrist-attached monitor would likely provide more accurate results and is a commonly used site for sleep monitoring (Mathie et al., 2004). Periods of sleep are usually accompanied by minimal movement, with relatively more movements occurring during the periods of wakefulness, which can be better discriminated by wrist actigraphy (Slater et al., 2015; Tryon, 1996). However, certain populations, such as children with neurodevelopmental disorders, may have difficulty tolerating wrist placement (Adkins et al., 2012). In this case, other locations have also been utilized to measure sleep, including the leg (Middelkoop et al., 1997), waist (Enomoto et al., 2009; Paavonen et al., 2002), shoulder (Adkins et al., 2012), and hip (Zinkhan et al., 2014). Although most validation studies comparing actigraphy to the gold standard measure of polysomnography have used non-dominant wrist placement, other locations can be used if the wrist is not available or subjects are unable to tolerate wrist placement. In the present study, the neck location was utilized to avoid possible adverse effects on daily routine and allow for longer-term wear. The new generation of accelerometers provides high temporal and intensity resolution, allowing the detection of tiny body movements and identification of pulse waves when attached to the wrist (Zschocke et al., 2019). Even when worn on the wrist or hip, these devices provide data that can be used to track respiratory rate throughout the night (Zinkhan & Kantelhardt, 2016). As such, these high-resolution accelerometers should detect tiny motions, even when attached to the neck, and triaxial recordings may be able to distinguish whether the monkey is in an upright position or lying down. Algorithms are especially important for the deduction of sleep measures. At present, however, most algorithms have been developed and validated for wrist measurements. When accelerometers are placed at the hip, mean PSG bias is larger, and there is a distinct dependency in differences of magnitude of respective sleep measures compared to wrist measurements (Zinkhan & Kantelhardt, 2016). Thus, new algorithms need to be developed to adapt to the different placements of accelerometers.
Although the actigraphic approach showed poor sensitivity for detecting wakefulness in the current study, it could play a supportive role in the elucidation of sleep differences between control and specific disease model monkeys. In comparison with EEG, this method does not require deep or subcutaneous implantation of electrodes, and thus is almost noninvasive, which could help reduce adverse effects on monkeys, such as infection from surgery. In addition, EEG and PSG equipment is relatively expensive and may be difficult to use under long recording intervals and large sample sizes. Although videography is noninvasive and can allow observations of behaviors in naturally sleeping monkeys (Kripke et al., 1968; Weitzman et al., 1965), it requires greater subjectivity and considerable human labor and time costs. In consideration of the above-mentioned factors and the results reported in this study, the use of actigraphy for scoring sleep (especially relaxed sleep) shows potential for future research on sleep in NHPs. The behavioral criteria and approach were validated in this study and could be considered as a complementary technique to conventional EEG and/or videography analysis for sleep studies in NHPs.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
X.T.H., L.X., Y.L., and Y.C.C. designed the study. D.D.Q. and S.F.F. performed the experiments, analyzed the data, and drafted the initial manuscript. D.D.Q., S.F.F., W.J.S., and Y.Z. acquired the behavioral data. F.Y.Z., N.W., T.F.X., X.L.X., X.T.Y., X.Z., and X.Z. conducted the behavioral analysis. All authors read and approved the final version of the manuscript.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31700897, 31960178), Yunnan Provincial Natural Science Foundation of China (2018FB053, 2019FA007), China Postdoctoral Science Foundation (2018M631105), Post-Doctoral Training Program in Yunnan Province, and Key Realm R&D Program of Guangdong Province (2019B030335001)
Contributor Information
Yun Liu, Email: liuyun@etyy.cn.
Yong-Chang Chen, Email: chenyc@lpbr.cn.
References
- 1.Adkins KW, Goldman SE, Fawkes D, Surdyka K, Wang L, Song YN, Malow BA A pilot study of shoulder placement for actigraphy in children. Behavioral Sleep Medicine. 2012;10(2):138–147. doi: 10.1080/15402002.2011.596598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 3.Andersen ML, Diaz MP, Murnane KS, Howell LL Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology. 2013;227(1):101–107. doi: 10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology. 2010;210(3):439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen ML, Sawyer EK, Carroll FI, Howell LL Influence of chronic dopamine transporter inhibition by rti-336 on motor behavior, sleep, and hormone levels in rhesus monkeys. Experimental and Clinical Psychopharmacology. 2012;20(2):77–83. doi: 10.1037/a0026034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JR Sleep, sleeping sites, and sleep‐related activities: Awakening to their significance. American Journal of Primatology. 1998;46(1):63–75. doi: 10.1002/(SICI)1098-2345(1998)46:1<63::AID-AJP5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JR Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Medicine Reviews. 2000;4(4):355–373. doi: 10.1053/smrv.2000.0105. [DOI] [PubMed] [Google Scholar]
- 8.Benington JH, Frank MG Cellular and molecular connections between sleep and synaptic plasticity. Progress in Neurobiology. 2003;69(2):71–101. doi: 10.1016/S0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 9.Berro LF, Andersen ML, Tufik S, Howell LL Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys. Experimental and Clinical Psychopharmacology. 2016;24(2):142–146. doi: 10.1037/pha0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besedovsky L, Lange T, Born J Sleep and immune function. Pflügers Archiv-European Journal of Physiology. 2012;463(1):121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulos MI, Jairam T, Kendzerska T, Im J, Mekhael A, Murray BJ Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. The Lancet Respiratory Medicine. 2019;7(6):533–543. doi: 10.1016/S2213-2600(19)30057-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Yu JH, Niu YY, Qin DD, Liu HL, Li G, Hu YZ, Wang JJ, Lu Y, Kang Y, Jiang Y, Wu KH, Li SG, Wei JK, He J, Wang JB, Liu XJ, Luo YP, Si CY, Bai RX, Zhang KS, Liu J, Huang SY, Chen ZZ, Wang S, Chen XY, Bao XH, Zhang QP, Li FX, Geng R, Liang AB, Shen DG, Jiang TZ, Hu XT, Ma YY, Ji WZ, Sun YE Modeling rett syndrome using talen-edited MECP2 mutant cynomolgus monkeys. Cell. 2017;169(5):945–955.e10. doi: 10.1016/j.cell.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mairan JJO. 1729. Observation botanique. Histoire de l'Académie royale des sciences Paris.
- 14.Du Monceau D. 1758. La Physique Des Arbres, Où Il Est Traité de L'anatomie des Plantes et de L'économie Végétale: Pour Servird'introduction au Traité Complet des Bois & des Forests: Avec Unedissertation sur L'utilité des Méthodes de Botanique; & une Explicationdes Termes Propres à Cette Science, & Qui Sont en Usage Pour L'exploitationdes Bois & des Forêts. Paris: H. L. Guérin & L.F. Delatour.
- 15.Enomoto M, Endo T, Suenaga K, Miura N, Nakano Y, Kohtoh S, Taguchi Y, Aritake S, Higuchi S, Matsuura M, Takahashi K, Mishima K Newly developed waist actigraphy and its sleep/wake scoring algorithm. Sleep and Biological Rhythms. 2009;7(1):17–22. doi: 10.1111/j.1479-8425.2008.00377.x. [DOI] [Google Scholar]
- 16.Feinberg I, Koresko RL, Heller N EEG sleep patterns as a function of normal and pathological aging in man. Journal of Psychiatric Research. 1967;5(2):107–144. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- 17.Fruth B, Tagg N, Stewart F Sleep and nesting behavior in primates: a review. American Journal of Physical Anthropology. 2018;166(3):499–509. doi: 10.1002/ajpa.23373. [DOI] [PubMed] [Google Scholar]
- 18.Golub MS, Hogrefe CE Sleep disturbance as detected by actigraphy in pre-pubertal juvenile monkeys receiving therapeutic doses of fluoxetine. Neurotoxicology and Teratology. 2016;55:1–7. doi: 10.1016/j.ntt.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halson SL, Juliff LE Sleep, sport, and the brain. Progress in Brain Research. 2017;234:13–31. doi: 10.1016/bs.pbr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Imeri L, Opp MR How (and why) the immune system makes us sleep. Nature Reviews Neuroscience. 2009;10(3):199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantha SS, Suzuki J Sleep quantitation in common marmoset, cotton top tamarin and squirrel monkey by non-invasive actigraphy. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2006;144(2):203–210. doi: 10.1016/j.cbpa.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Kripke DF, Reite ML, Pegram GV, Stephens LM, Lewis OF Nocturnal sleep in rhesus monkeys. Electroencephalography and Clinical Neurophysiology. 1968;24(6):581–586. doi: 10.1016/0013-4694(68)90047-3. [DOI] [PubMed] [Google Scholar]
- 23.Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Lucey BP, McLeland JS, Toedebusch CD, Boyd J, Morris JC, Landsness EC, Yamada K, Holtzman DM Comparison of a single-channel eeg sleep study to polysomnography. Journal of Sleep Research. 2016;25(6):625–635. doi: 10.1111/jsr.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI Macromolecule biosynthesis: a key function of sleep. Physiological Genomics. 2007;31(3):441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mann TM, Williams KE, Pearce PC A novel method for activity monitoring in small non-human primates. Laboratory Animals. 2005;39(2):169–177. doi: 10.1258/0023677053739783. [DOI] [PubMed] [Google Scholar]
- 27.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O'Hara BF, Franken P, Tafti M Homer1a is a core brain molecular correlate of sleep loss. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathie MJ, Coster ACF, Lovell NH, Celler BG Accelerometry: providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiological Measurement. 2004;25(2):R1–R20. doi: 10.1088/0967-3334/25/2/R01. [DOI] [PubMed] [Google Scholar]
- 29.Melvin E, Samson D, Nunn CL Eulerian videography technology improves classification of sleep architecture in primates. Primates. 2019;60(5):467–475. doi: 10.1007/s10329-019-00744-x. [DOI] [PubMed] [Google Scholar]
- 30.Middelkoop HAM, van Dam EM, Smilde-van den Doel DA, Van Dijk G 45-hour continuous quintuple-site actimetry: relations between trunk and limb movements and effects of circadian sleep-wake rhythmicity. Psychophysiology. 1997;34(2):199–203. doi: 10.1111/j.1469-8986.1997.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 31.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson Jr A, Coleman J, Lee-Chiong T, Pancer J, Swick TJ Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 32.Niel K, LaRosa KN, Klages KL, Merchant TE, Wise MS, Witcraft SM, Hancock D, Caples M, Mandrell BN, Crabtree VM. 2019. Actigraphy versus polysomnography to measure sleep in youth treated for craniopharyngioma. Behavioral Sleep Medicine, doi: 10.1080/15402002.2019.1635133.
- 33.Nunn CL, McNamara P, Capellini I, Preston BT, Barton RA. 2010. Primate sleep in phylogenetic perspective. In: McNamara P, Barton RA, Nunn CL. Evolution of Sleep: Phylogenetic and Functional Perspectives. Cambridge: Cambridge University Press, 123–145.
- 34.Nunn CL, Samson DR Sleep in a comparative context: investigating how human sleep differs from sleep in other primates. American Journal of Physical Anthropology. 2018;166(3):601–612. doi: 10.1002/ajpa.23427. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell S, Beaven CM, Driller MW From pillow to podium: a review on understanding sleep for elite athletes. Nature and Science of Sleep. 2018;10:243–253. doi: 10.2147/NSS.S158598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oswald I Sleep as a restorative process: human clues. Progress in Brain Research. 1980;53:279–288. doi: 10.1016/S0079-6123(08)60069-2. [DOI] [PubMed] [Google Scholar]
- 37.Paavonen EJ, Fjällberg M, Steenari MR, Aronen ET Actigraph placement and sleep estimation in children. Sleep. 2002;25(2):235–237. doi: 10.1093/sleep/25.2.235. [DOI] [PubMed] [Google Scholar]
- 38.Parekh PJ, Oldfield IV EC, Challapallisri V, Ware CJ, Johnson DA Sleep disorders and inflammatory disease activity: chicken or the egg? American Journal of Gastroenterology. 2015;110(4):484–488. doi: 10.1038/ajg.2014.247. [DOI] [PubMed] [Google Scholar]
- 39.Parmeggiani PL Interaction between sleep and thermoregulation: An aspect of the control of behavioral states. Sleep. 1986;10(5):426–435. doi: 10.1093/sleep/10.5.426. [DOI] [PubMed] [Google Scholar]
- 40.Qiu PY, Jiang J, Liu Z, Cai YJ, Huang T, Wang Y, Liu QM, Nie YH, Liu F, Cheng JM, Li Q, Tang YC, Poo MM, Sun Q, Chang HC Bmal1 knockout macaque monkeys display reduced sleep and psychiatric disorders. National Science Review. 2019;6(1):87–100. doi: 10.1093/nsr/nwz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasch B, Born J About sleep's role in memory. Physiological Reviews. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rechtschaffen A Current perspectives on the function of sleep. Perspectives in Biology and Medicine. 1998;41(3):359–390. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- 43.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12(1):68–87. [PubMed] [Google Scholar]
- 44.Roffwarg HP, Dement WC, Fisher C. 1964. Preliminary observations of the sleep-dream pattern in neonates, infants, children and adults. In: Harms E. Problems of Sleep and Dreams in Children. Vol II. New York: Pergamon.
- 45.Sadeh A, Acebo C The role of actigraphy in sleep medicine. Sleep Medicine Reviews. 2002;6(2):113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 46.Sadeh A, Hauri PJ, Kripke DF, Lavie P The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 47.Sanders J, Scholz M, Merutka I, Biron D Distinct unfolded protein responses mitigate or mediate effects of nonlethal deprivation of C. Elegans sleep in different tissues. BMC Biology. 2017;15(1):67. doi: 10.1186/s12915-017-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sateia MJ International classification of sleep disorders-third edition. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 49.Scullin MK, Bliwise DL Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspectives on Psychological Science. 2015;10(1):97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma S, Kavuru M Sleep and metabolism: an overview. International Journal of Endocrinology. 2010;2010:270832. doi: 10.1155/2010/270832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417(6886):287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 52.Siegel JM Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Bedi O, Bhakuni GS, Bansal PK. 2017. Animal models of sleep disorder. In: Bansal PK, Deshmukh R. Animal Models of Neurological Disorders: Principle and Working Procedure for animal Models of Neurological Disorders. Singapore: Springer, 181–194.
- 54.Sivertsen B, Omvik S, Havik OE, Pallesen S, Bjorvatn B, Nielsen GH, Straume S, Nordhus IH A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29(10):1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 55.Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR Assessing sleep using hip and wrist actigraphy. Sleep and Biological Rhythms. 2015;13(2):172–180. doi: 10.1111/sbr.12103. [DOI] [Google Scholar]
- 56.Spiegel K, Leproult R, Van Cauter E Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 57.Terrill PI, Mason DG, Wilson SJ. 2010. Development of a continuous multisite accelerometry system for studying movements during sleep. In: Proceedings of 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. Buenos Aires, Argentina: IEEE, 6150–6153.
- 58.Thorpy M, Chesson A, Derderian S, Kader G, Millman R, Potolicchio S, Rosen G, Strollo P Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995;18(4):285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- 59.Thun E, Bjorvatn B, Flo E, Harris A, Pallesen S Sleep, circadian rhythms, and athletic performance. Sleep Medicine Reviews. 2015;23:1–9. doi: 10.1016/j.smrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Toth LA, Bhargava P Animal models of sleep disorders. Comparative Medicine. 2013;63(2):91–104. [PMC free article] [PubMed] [Google Scholar]
- 61.Tryon WW Nocturnal activity and sleep assessment. Clinical Psychology Review. 1996;16(3):197–213. doi: 10.1016/0272-7358(95)00059-3. [DOI] [Google Scholar]
- 62.Vgontzas AN, Fernandez-Mendoza J, Liao DP, Bixler EO Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Medicine Reviews. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitale JA, Weydahl A Chronotype, physical activity, and sport performance: a systematic review. Sports Medicine. 2017;47(9):1859–1868. doi: 10.1007/s40279-017-0741-z. [DOI] [PubMed] [Google Scholar]
- 64.Walker MP, Stickgold R Sleep, memory, and plasticity. Annual Review of Psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 65.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E Joint consensus statement of the american academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015a;38(8):1161–1183. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E Joint consensus statement of the american academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Journal of Clinical Sleep Medicine. 2015b;11(8):931–952. doi: 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep. 2015c;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Twery M, Croft JB, Maher E, Barrett JA, Thomas SM, Heald JL Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Journal of Clinical Sleep Medicine. 2015d;11(6):591–592. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weitzman ED, Kripke DF, Pollak C, Dominguez J Cyclic activity in sleep of macaca mulatta. Archives of Neurology. 1965;12(5):463–467. doi: 10.1001/archneur.1965.00460290019003. [DOI] [PubMed] [Google Scholar]
- 70.Yavuz-Kodat E, Reynaud E, Geoffray MM, Limousin N, Franco P, Bourgin P, Schroder CM Validity of actigraphy compared to polysomnography for sleep assessment in children with autism spectrum disorder. Frontiers in Psychiatry. 2019;10:551. doi: 10.3389/fpsyt.2019.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Zhu Y, Zhan GX, Fenik P, Panossian L, Wang MM, Reid S, Lai D, Davis JG, Baur JA Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. Journal of Neuroscience. 2014a;34(12):4418–4431. doi: 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang XL, Pang W, Hu XT, Li JL, Yao YG, Zheng YT Experimental primates and non-human primate (NHP) models of human diseases in china: current status and progress. Zoological Research. 2014b;35(6):447–464. doi: 10.13918/j.issn.2095-8137.2014.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zinkhan M, Berger K, Hense S, Nagel M, Obst A, Koch B, Penzel T, Fietze I, Ahrens W, Young P, Happe S, Kantelhardt JW, Kluttig A, Schmidt-Pokrzywniak A, Pillmann F, Stang A Agreement of different methods for assessing sleep characteristics: a comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Medicine. 2014;15(9):1107–1114. doi: 10.1016/j.sleep.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Zinkhan M, Kantelhardt JW Sleep assessment in large cohort studies with high-resolution accelerometers. Sleep Medicine Clinics. 2016;11(4):469–488. doi: 10.1016/j.jsmc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Zschocke J, Kluge M, Pelikan L, Graf A, Glos M, Müller A, Mikolajczyk R, Bartsch RP, Penzel T, Kantelhardt JW Detection and analysis of pulse waves during sleep via wrist-worn actigraphy. PLoS One. 2019;14(12):e0226843. doi: 10.1371/journal.pone.0226843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.