DEAR EDITOR,
Rhesus monkeys (Macaca mulatta) are valuable experimental animals for studies on neurodegenerative diseases due to their evolutionarily close relationship to humans (Zhang et al., 2014). Rhesus monkeys also display similar hallmarks of aging and neurodegeneration as humans, including formation of senile plaques in the brain (Beckman et al., 2019; Paspalas et al., 2018). However, changes in formaldehyde (FA) levels in the cerebrospinal fluid (CSF) of rhesus monkeys with aging have not been reported. Additionally, whether changes in CSF FA are correlated with changes in amyloid-β (Aβ) concentrations have not yet been explored. Here, the CSF levels of Aβ40, Aβ42, and FA were measured in 56 rhesus monkeys of different ages, ranging from 4 to 26 years old. Results revealed significant declines in Aβ40 and Aβ42, and an increase in FA with age. Interestingly, the increase in FA levels was negatively correlated with Aβ40 and Aβ42 concentrations in aged rhesus monkeys but not in young and middle-aged monkeys. These results appear to parallel changes seen within human aging, i.e., decreased levels of CSF Aβ and increased levels of FA in normal aged adults and Alzheimer’s disease (AD) patients. These findings further indicate that rhesus monkeys are a reliable model for studying age-related neurological disorders such as AD and suggest that FA is an important factor in AD development and may be used as a diagnostic indicator of such disease.
Aβ is a secreted peptide of unknown physiological function that is produced by sequential cleavage of β-amyloid precursor protein (APP) by β-secretase and γ-secretase (Vassar, 2005). Most Aβ is produced in the brain, but it also effluxes into the CSF and plasma, appearing in relatively high and low concentrations, respectively. Aβ occurs in multiple forms ranging from 38 to 43 amino acids in length (Perrin et al., 2009). Among these, Aβ40 is the most abundant species, but Aβ42 is essential for initiating Aβ aggregation and is considered central to the amyloid cascade hypothesis of AD (Hardy and Selkoe, 2002). Soluble Aβ (including monomers and a few oligomers and protofibrils) in CSF can be used as a diagnostic indicator for certain neurological diseases. For example, AD patients exhibit a significant decrease in soluble Aβ in their CSF, indicating a portion of soluble Aβ is deposited in brain tissue to form senile plaques (Fagan et al., 2006; Irie, 2020; Lana et al., 2019; Mattsson et al., 2009; Shaw et al., 2009).
There is compelling evidence that suggests FA is related to AD pathology, both in vivo and in vitro. Several studies have found that FA concentration in the human body increases with age, and concentrations of FA in urine, blood, CSF, and brain tissue of AD patients are significantly higher than those in the control group at the same age (He et al., 2010; Tong et al., 2013). In addition, FA concentration in the urine of AD patients is negatively correlated with cognitive level (Tong et al., 2017; Tong et al., 2011). Therefore, FA is considered to be closely related to the occurrence and development of AD (Tulpule and Dringen, 2013; Wang et al., 2019). In rodent studies, elevated FA can lead to memory impairment, Tau protein hyperphosphorylation, and neuronal loss; rodent animal models of AD also show an imbalance in FA metabolism and elevated FA in vivo (Qiang et al., 2014; Tong et al., 2013; Yang et al., 2014a). In non-human primate (NHP) studies, elevated FA levels not only lead to impaired memory, but also to the occurrence of all the pathological hallmarks of human AD in the brain, including senile plaques, neurofibrillary tangles, neuronal loss, and glial proliferation (Yang et al., 2014b; Zhai et al., 2018). Studies have shown that very low concentrations of FA can promote the aggregation of Aβ and the formation of structures similar to senile plaques in vitro (Chen et al., 2006; Rizak et al., 2014). Co-incubation of FA and Tau protein can increase the diameter of Tau protein particles, and the increase in participle size is positively correlated with FA concentration and incubation time extension (Nie et al., 2005). FA can also induce the formation of hyperphosphorylated Tau protein and neurofibrillary tangles in vitro (He et al., 2017).
Animal models play a major role in defining critical disease-related mechanisms and exploring potential therapeutic approaches in neurodegenerative diseases such as AD (Heuer et al., 2012). Due to their evolutionarily close relationship to humans, NHPs are essential for the study of age-associated changes in the brain and other central nerve system diseases (Chu et al., 2014; Feng et al., 2019; Qin et al., 2013; Zhang et al., 2014). Studies have indicated that CSF levels of Aβ40 and Aβ42 decrease significantly with age in cynomolgus and vervet monkeys (Chen et al., 2018; Yue et al., 2014). However, no study has investigated the relationship of FA concentrations in CSF samples from different aged rhesus monkeys or the correlation between FA and Aβ levels in CSF samples. In this study, FA and Aβ levels in CSF samples were measured in different aged rhesus monkeys, and the correlations between FA and Aβ in different age groups were investigated.
Fifty-six rhesus monkeys (Macaca mulatta) were selected for CSF collection based on restrictive conditions (age: 4–26 years old; healthy and without any prior experimental operations). The monkeys were divided into three groups depending on age: i.e., young (4–7 years old, n=15), middle-aged (10–15 years old, n=22), and aged (19–26 years old, n=19) group (Supplementary Materials and Methods). For CSF collection, the monkeys were anesthetized with 10 mg/kg body weight of ketamine by intramuscular injection. A 22-gauge spinal needle was inserted into the lumbar interspace at the same level as the palpated iliac crest, and approximately 1.5 mL of CSF (divided into three 0.5 mL fractions) was withdrawn through a lumbar puncture (Supplementary Materials and Methods). The CSF samples were then immediately frozen in liquid nitrogen and stored in a −80°C freezer until analysis.
The levels of Aβ40 and Aβ42 in CSF were measured using commercial ELISA kits (Aβ42 and Aβ40 Assay Kits, Cat. No. KHB3441 and KHB3481, respectively, Life Technologies, USA) according to the manufacturer’s instructions. Each sample was tested in duplicate. For measurement of FA levels, after centrifugation (20 000 g, 4 °C, 15 min), the resulting supernatant fractions were used for analysis of FA by high-performance liquid chromatography with fluorescence detection (Fluo-HPLC), as described in previous study (Supplementary Materials and Methods) (Tong et al., 2011). For statistical analysis, intergroup differences were evaluated by one-factor analysis of variance followed by Least Square Difference (LSD) tests. The relationships between FA and Aβ concentrations were subsequently analyzed by linear regression. A value of P<0.05 was considered significant in all analyses. All statistical analyses were conducted using GraphPad Prism 8 (San Diego, USA).
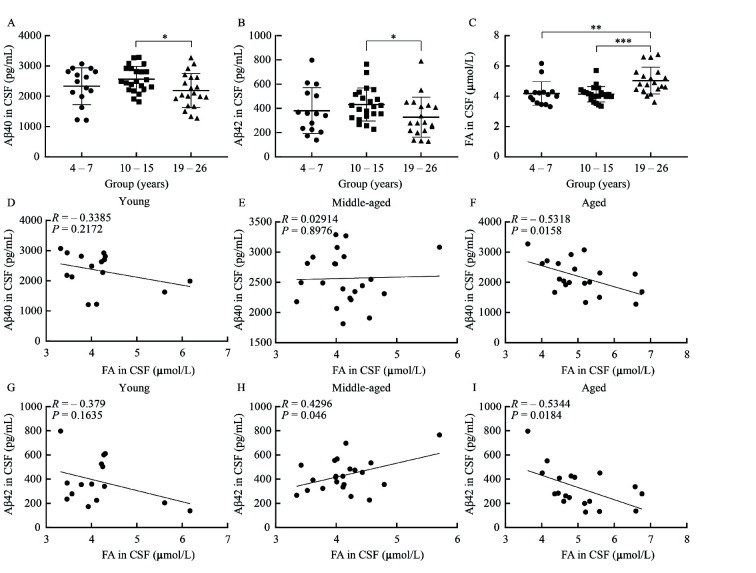
The concentrations of Aβ40 (P=0.011, Figure 1A) and Aβ42 (P=0.044, Figure 1B) in the CSF of aged monkeys were markedly decreased compared to that in middle-aged subjects. These results are in agreement with prior studies, which report a significant decrease in soluble Aβ in the CSF of AD patients and aged monkeys, indicating a portion of soluble Aβ is deposited in brain tissue to form senile plaques (Fagan et al., 2006; Yue et al., 2014). APP, which can be cleaved into Aβ by β-secretase and γ-secretase, is completely homologous between humans and rhesus monkeys (Podlisny et al., 1991). β-amyloid cleaving enzyme-1 (β-secretase, or BACE-1) activity increases significantly with age in mouse, monkey, and human brains (Fukumoto et al., 2004). This causes the production of Aβ to increase with age. However, during the onset of AD in old age, Aβ is deposited in the brain to form senile plaques, and CSF Aβ levels are significantly reduced (Fagan et al., 2006). Soluble Aβ in the CSF of young monkeys should account for all Aβ, as there should be no Aβ deposition in the brain tissue (Kimura et al., 2003). Furthermore, the production of Aβ should also increase with the increase in age. However, after brain tissue deposition or receptor interaction increases (Lustbader et al., 2004), soluble Aβ in CSF decreases instead. That is probably why significant age-associated declines in CSF Aβ40 (Figure 1A) and Aβ42 (Figure 1B) were found between the middle-aged and aged monkeys, but not between the young monkeys and other groups.
Figure 1. Intergroup analyses of Aβ40, Aβ42, and FA concentrations in CSF, and correlations between CSF Aβ and FA concentrations in young, middle-aged, and aged rhesus monkeys .
A: Intergroup analyses of Aβ40 concentrations. B: Intergroup analyses of Aβ42 concentrations. C: Intergroup analyses of FA concentrations. D: Correlation between CSF Aβ40 and FA concentrations in young group (R=–0.3385, P=0.2172). E: Correlation between CSF Aβ40 and FA concentrations in middle-aged group (R=0.02914, P=0.8976). F: Correlation between CSF Aβ40 and FA concentrations in aged group (R=–0.5318, P=0.0158). G: Correlation between CSF Aβ42 and FA concentrations in young group (R=–0.379, P=0.1635). H: Correlation between CSF Aβ42 and FA concentrations in middle-aged group (R=0.4296, P=0.046). I: Correlation between CSF Aβ42 and FA concentrations in aged group (R=–0.5344, P=0.0184). Error bars indicate mean± standard deviation (SD). *: P<0.05, **:P<0.01, ***:P<0.001. Aβ: β-amyloid; CSF: Cerebrospinal fluid.
The concentrations of FA in CSF samples of aged monkeys showed a marked elevation compared with that in young (P=0.001) and middle-aged (P<0.001) monkeys (Figure 1C). The significant increase in FA concentration in the CSF of monkeys with age is consistent with results from human studies (Tong et al., 2015). There are multiple factors that contribute to the endogenous accumulation of FA, including environmental pollution (Clejan and Cederbaum, 1993; Takeuchi et al., 2007), FA-generating enzyme disorders (del Mar Hernandez et al., 2005; Ferrer et al., 2002), and FA-degrading enzyme deficiencies (Ohta and Ohsawa, 2006; Wang et al., 2008). Rhesus monkeys and humans show the same FA metabolism pathway; thus, aging in rhesus monkeys and humans may produce abnormal FA metabolism, leading to an increase in FA in the body, and possibly to an increase in pathology (Liesivuori and Savolainen, 1991; Tulpule and Dringen, 2013; Zhai et al., 2016). Furthermore, a strong causative connection between FA and AD-like pathology and cognitive impairment has been proposed based on our previous studies. Elevated FA not only causes the aggregation of Aβ peptides, Tau hyperphosphorylation, and Tau protein polymerization in vitro (Lu et al., 2013; Rizak et al., 2014), but also causes pathological and cognitive impairment similar to AD in laboratory animals (Yang et al., 2014a, 2014b; Zhai et al., 2018).
To determine the relationship between Aβ and FA levels in CSF, we explored the correlations among CSF Aβ40 and Aβ42 concentrations with FA levels (Figure 1D–I). At a nominal significance threshold (P=0.05), Aβ40 was correlated with FA concentration in the aged group (P=0.0158, Figure 1F), and Aβ42 was correlated with FA concentration in the middle-aged (P=0.046, Figure 1H) and aged groups (P=0.0184, Figure 1I). Each regression coefficient was negative in the aged group (Figure 1F, I); that is, higher concentrations of Aβ40 and Aβ42 were associated with lower FA levels. However, each regression coefficient was positive in the middle-aged group (Figure 1H); that is, higher concentrations of Aβ42 were associated with higher FA levels. Aβ-binding alcohol dehydrogenase (ABAD) is the main alcohol dehydrogenase in mitochondria and is also one of the metabolic enzymes of FA. Combining Aβ with ABAD will inhibit ABAD activity, resulting in mitochondrial dysfunction, which may be one of the reasons for the decrease in FA removal rate in AD patients and in middle-aged monkeys here (Lustbader et al., 2004; Yao et al., 2011). Therefore, to some extent, the increase in Aβ led to FA elevation (Figure 1H). Thus, the significant negative correlation between FA increase and Aβ decrease in the CSF of the aged group only, the time when AD typically develops, indicates that the increase in FA in the brain may also be related to the onset of AD. Such correlations were not observed in the young or middle-aged groups, again suggesting that the increase in endogenic FA is likely related to the development of AD.
In conclusion, for the first time, we described an increase in FA in the CSF of rhesus monkeys with aging and a negative correlation between FA and Aβ concentrations in aged rhesus monkeys. These results not only indicate that rhesus monkeys are good model animals for studying AD but also suggest that FA is an important factor in AD development and may be a diagnostic indicator of such disease.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.H.L., X.P.H., X.T.H., and R.Q.H. designed the study. Z.H.L. and X.P.H. performed the experiment. Z.H.L., H.L., and X.T.H. wrote the manuscript with other authors’ input. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Long-Bao Lv, Zheng-Fei Hu, and Yun Wang from the Kunming Primate Research Center, Chinese Academy of Sciences, for their support during sample collection.
Funding Statement
This study was supported by the National Key R&D Program of China (2018YFA0801403), Key Realm R&D Program of GuangDong Province (2019B030335001), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32060200), the National Natural Science Foundation of China (81941014, 81471312, 81771387, 81460352, 81500983, 31700897, 31700910, 31800901, 31960178, 91732302), the Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (2017FB109, 2018FB052, 2018FB053, 2019FA007), China Postdoctoral Science Foundation (2018M631105) and CAS “Light of West China” Program
Contributor Information
Rong-Qiao He, Email: xthu@mail.kiz.ac.cn.
Xin-Tian Hu, Email: xthu@mail.kiz.ac.cn.
References
- 1.Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, Morrison JH Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(52):26239–26246. doi: 10.1073/pnas.1902301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JA, Fears SC, Jasinska AJ, Huang A, Al-Sharif NB, Scheibel KE, Dyer TD, Fagan AM, Blangero J, Woods R, Jorgensen MJ, Kaplan JR, Freimer NB, Coppola G Neurodegenerative disease biomarkers Aβ1-40, Aβ1-42, tau, and p-tau181 in the vervet monkey cerebrospinal fluid: Relation to normal aging, genetic influences, and cerebral amyloid angiopathy . Brain and Behavior. 2018;8(2):e00903. doi: 10.1002/brb3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K, Maley J, Yu PH Potential implications of endogenous aldehydes in β-amyloid misfolding, oligomerization and fibrillogenesis. Journal of Neurochemistry. 2006;99(5):1413–1424. doi: 10.1111/j.1471-4159.2006.04181.x. [DOI] [PubMed] [Google Scholar]
- 4.Chu XX, Rizak JD, Yang SC, Wang JH, Ma YY, Hu XT A natural model of behavioral depression in postpartum adult female cynomolgus monkeys (Macaca fascicularis) . Zoological Research. 2014;35(3):174–181. doi: 10.11813/j.issn.0254-5853.2014.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clejan LA, Cederbaum AI Stimulation by paraquat of microsomal and cytochrome P-450-dependent oxidation of glycerol to formaldehyde . Biochemical Journal. 1993;295(3):781–786. doi: 10.1042/bj2950781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Mar Hernandez M, Esteban M, Szabo P, Boada M, Unzeta M Human plasma semicarbazide sensitive amine oxidase (SSAO), β-amyloid protein and aging. Neuroscience Letters. 2005;384(1-2):183–187. doi: 10.1016/j.neulet.2005.04.074. [DOI] [PubMed] [Google Scholar]
- 7.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of Neurology. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 8.Feng XL, Che HL, Ning X, Ba XY, Li J, Zhang JF, Wang Y, Hu ZF, Hu XT, Ren XF Direct sunlight exposure reduces hair cortisol levels in rhesus monkeys (Macaca mulatta) . Zoological Research. 2019;40(6):583–586. doi: 10.24272/j.issn.2095-8137.2019.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer I, Lizcano JM, Hernández M, Unzeta M Overexpression of semicarbazide sensitive amine oxidase in the cerebral blood vessels in patients with Alzheimer's disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neuroscience Letters. 2002;321(1-2):21–24. doi: 10.1016/S0304-3940(01)02465-X. [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC β-secretase activity increases with aging in human, monkey, and mouse brain. The American Journal of Pathology. 2004;164(2):719–725. doi: 10.1016/S0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy J, Selkoe DJ The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 12.He RQ, Lu J, Miao JY Formaldehyde stress. Science China Life Sciences. 2010;53(12):1399–1404. doi: 10.1007/s11427-010-4112-3. [DOI] [PubMed] [Google Scholar]
- 13.He XP, Li ZH, Rizak JD, Wu SH, Wang ZB, He RQ, Su M, Qin DD, Wang JK, Hu XT Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Frontiers in Neuroscience. 2017;10:598. doi: 10.3389/fnins.2016.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuer E, Rosen RF, Cintron A, Walker LC Nonhuman primate models of Alzheimer-like cerebral proteopathy. Current Pharmaceutical Design. 2012;18(8):1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irie K New diagnostic method for Alzheimer's disease based on the toxic conformation theory of amyloid β. Bioscience, Biotechnology, and Biochemistry. 2020;84(1):1–16. doi: 10.1080/09168451.2019.1667222. [DOI] [PubMed] [Google Scholar]
- 16.Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y Age-related changes of Alzheimer's disease-associated proteins in cynomolgus monkey brains. Biochemical and Biophysical Research Communications. 2003;310(2):303–311. doi: 10.1016/j.bbrc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Lana E, Gellerbring A, Jung S, Nordberg A, Unger Lithner C, Darreh-Shori T Homomeric and heteromeric aβ species exist in human brain and CSF regardless of alzheimer's disease status and risk genotype. Frontiers in Molecular Neuroscience. 2019;12:176. doi: 10.3389/fnmol.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liesivuori J, Savolainen AH Methanol and formic acid toxicity: biochemical mechanisms. Pharmacology & Toxicology. 1991;69(3):157–163. doi: 10.1111/j.1600-0773.1991.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Miao JY, Su T, Liu Y, He RQ Formaldehyde induces hyperphosphorylation and polymerization of Tau protein both in vitro and in vivo . Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(8):4102–4116. doi: 10.1016/j.bbagen.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu SM, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H ABAD directly links Aß to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jonhagen ME, Minthon L, Winblad B, Blennow K CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 22.Nie CL, Zhang W, Zhang D, He RQ Changes in conformation of human neuronal tau during denaturation in formaldehyde solution. Protein & Peptide Letters. 2005;12(1):75–78. doi: 10.2174/0929866053405931. [DOI] [PubMed] [Google Scholar]
- 23.Ohta S, Ohsawa I Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer's disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. Journal of Alzheimer's Disease. 2006;9(2):155–166. doi: 10.3233/JAD-2006-9208. [DOI] [PubMed] [Google Scholar]
- 24.Paspalas CD, Carlyle BC, Leslie S, Preuss TM, Crimins JL, Huttner AJ, van Dyck CH, Rosene DL, Nairn AC, Arnsten AFT The aged rhesus macaque manifests Braak stage III/IV Alzheimer's-like pathology. Alzheimer’s & Dementia. 2018;14(5):680–691. doi: 10.1016/j.jalz.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin RJ, Fagan AM, Holtzman DM Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podlisny MB, Tolan DR, Selkoe DJ Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. The American Journal of Pathology. 1991;138(6):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang M, Xiao R, Su T, Wu BB, Tong ZQ, Liu Y, He RQ A novel mechanism for endogenous formaldehyde elevation in SAMP8 mouse. Journal of Alzheimer's Disease. 2014;40(4):1039–1053. doi: 10.3233/JAD-131595. [DOI] [PubMed] [Google Scholar]
- 28.Qin DD, Dominic Rizak J, Feng XL, Chu XX, Yang SC, Li CL, Lv LB, Ma YY, Hu XT Social rank and cortisol among female rhesus macaques (Macaca mulatta) . Zoological Research. 2013;34(E2):E42–E49. doi: 10.3724/SP.J.1141.2013.E02E42. [DOI] [PubMed] [Google Scholar]
- 29.Rizak JD, Ma YY, Hu XT Is formaldehyde the missing link in AD pathology? The differential aggregation of amyloid-beta with APOE isoforms in vitro. Current Alzheimer Research. 2014;11(5):461–468. doi: 10.2174/1567205011666140425112043. [DOI] [PubMed] [Google Scholar]
- 30.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VMY, Trojanowski JQ Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of Neurolog. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi A, Takigawa T, Abe M, Kawai T, Endo Y, Yasugi T, Endo G, Ogino K Determination of formaldehyde in urine by headspace gas chromatography. Bulletin of Environmental Contamination and Toxicology. 2007;79(1):1–4. doi: 10.1007/s00128-007-9172-0. [DOI] [PubMed] [Google Scholar]
- 32.Tong ZQ, Han CS, Luo WH, Wang XH, Li H, Luo HJ, Zhou JQ, Qi JS, He RQ Accumulated hippocampal formaldehyde induces age-dependent memory decline. AGE. 2013;35(3):583–596. doi: 10.1007/s11357-012-9388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong ZQ, Han CS, Qiang M, Wang WS, Lv JH, Zhang SZ, Luo WH, Li H, Luo HJ, Zhou JN, Wu BB, Su T, Yang X, Wang XM, Liu Y, He RQ Age-related formaldehyde interferes with DNA methyltransferase function, causing memory loss in Alzheimer's disease. Neurobiology of Aging. 2015;36(1):100–110. doi: 10.1016/j.neurobiolaging.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Tong ZQ, Wang WS, Luo WH, Lv JH, Li H, Luo HJ, Jia JP, He RQ Urine formaldehyde predicts cognitive impairment in post-stroke dementia and alzheimer's disease. Journal of Alzheimer's Disease. 2017;55(3):1031–1038. doi: 10.3233/JAD-160357. [DOI] [PubMed] [Google Scholar]
- 35.Tong ZQ, Zhang JL, Luo WH, Wang WS, Li FX, Li H, Luo HJ, Lu J, Zhou JN, Wan Y, He RQ Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiology of Aging. 2011;32(1):31–41. doi: 10.1016/j.neurobiolaging.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Tulpule K, Dringen R Formaldehyde in brain: an overlooked player in neurodegeneration? Journal of Neurochemistry. 2013;127(1):7–21. doi: 10.1111/jnc.12356. [DOI] [PubMed] [Google Scholar]
- 37.Vassar R. 2005. β-Secretase, APP and Aβ in Alzheimer's disease. In: Harris JR, Fahrenholz F. Alzheimer’s Disease. Boston: Springer, 79-103.
- 38.Wang BB, Wang J, Zhou SR, Tan SN, He X, Yang Z, Xie YC, Li S, Zheng CG, Ma X The association of mitochondrial aldehyde dehydrogenase gene (ALDH2) polymorphism with susceptibility to late-onset Alzheimer's disease in Chinese . Journal of the Neurological Sciences. 2008;268(1-2):172–175. doi: 10.1016/j.jns.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Chen DQ, Wu PP, Klein C, Jin CY Formaldehyde, epigenetics, and alzheimer's disease. Chemical Research in Toxicology. 2019;32(5):820–830. doi: 10.1021/acs.chemrestox.9b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang MF, Lu J, Miao JY, Rizak J, Yang JZ, Zhai RW, Zhou J, Qu JG, Wang JH, Yang SC, Ma YY, Hu XT, He RQ Alzheimer's disease and methanol toxicity (part 1): chronic methanol feeding led to memory impairments and tau hyperphosphorylation in mice. Journal of Alzheimer's Disease. 2014a;41(4):1117–1129. doi: 10.3233/JAD-131529. [DOI] [PubMed] [Google Scholar]
- 41.Yang MF, Miao JY, Rizak J, Zhai RW, Wang ZB, Huma T, Li T, Zheng N, Wu SH, Zheng YW, Fan XN, Yang JZ, Wang JH, Yang SC, Ma YY, Lü LB, He RQ, Hu XT Alzheimer's disease and methanol toxicity (part 2): lessons from four rhesus macaques (Macaca mulatta) chronically fed methanol . Journal of Alzheimer's Disease. 2014b;41(4):1131–1147. doi: 10.3233/JAD-131532. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Du H, Yan SQ, Fang F, Wang CD, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SD Inhibition of amyloid-β (Aβ) peptide-binding alcohol dehydrogenase-Aβ interaction reduces Aβ accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease. The Journal of Neuroscience. 2011;31(6):2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yue F, Lu CL, Ai Y, Chan P, Zhang ZM Age-associated changes of cerebrospinal fluid amyloid-β and tau in cynomolgus monkeys. Neurobiology of Aging. 2014;35(7):1656–1659. doi: 10.1016/j.neurobiolaging.2014.01.139. [DOI] [PubMed] [Google Scholar]
- 44.Zhai RW, Rizak J, Zheng N, He XP, Li ZH, Yi nY, Su T, He YG, He RQ, Ma YY, Yang MF, Wang ZB, Hu XT Alzheimer's disease-like pathologies and cognitive impairments induced by formaldehyde in non-human primates. Current Alzheimer Research. 2018;15(14):1304–1321. doi: 10.2174/1567205015666180904150118. [DOI] [PubMed] [Google Scholar]
- 45.Zhai RW, Zheng N, Rizak J, Hu XT Evidence for conversion of methanol to formaldehyde in nonhuman primate brain. Analytical Cellular Pathology. 2016;2016:4598454. doi: 10.1155/2016/4598454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XL, Pang W, Hu XT, Li JL, Yao YG, Zheng Y T Experimental primates and non-human primate (NHP) models of human diseases in China: current status and progress. Zoological Research. 2014;35(6):447–464. doi: 10.13918/j.issn.2095-8137.2014.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.