DEAR EDITOR,
Intestinal biopsy is a basic experimental method for studying pathological changes in the intestinal tract during human immunodeficiency virus (HIV) infection. In this study, jejunal resection and anastomosis were successfully performed in 12 Chinese rhesus macaques (Macaca mulatta). The sampled gut tissues were then examined by hematoxylin and eosin (H&E) staining, electron microscopy, flow cytometry, immunofluorescence detection, and RNA quality analysis to ensure suitability for histological, physiological, pathological, and immunological detection, as well as mechanistic analysis at the cellular and molecular level. Importantly, the surgery did not affect the ratio or number of immune cells in peripheral blood or the concentration of lipids, proteins, and vitamins in plasma, which are important indicators of nutritional status. Our results thus indicated that jejunal resection and anastomosis are feasible, and that immune homeostasis and intestinal barrier integrity are not altered by surgery. All macaques recovered well (except for one), with no postoperative complications. Therefore, this animal surgery may be applicable for longitudinal intestinal research related to diseases such as acquired immunodeficiency syndrome (AIDS).
The intestinal mucosa is among the earliest and most serious targets of HIV infection, even in individuals receiving antiretroviral therapy (ART). The gut contains the largest proportion of lymphoid tissue (up to 85%) and lymphocytes (up to 90%) in the body (Wong & Yukl, 2016). In addition, approximately 60%–80% of memory CD4+ T cells in the gastrointestinal tract are depleted within days of HIV and simian immunodeficiency virus (SIV) infection (Veazey, 2019). Both HIV/SIV infections are also associated with structural and functional impairment of gut mucosal tissue (Song et al., 2018; Zhang et al., 2016; Zhang et al., 2019). For instance, during infection, intestinal villi become flat and sucrase activity is reduced (Brenchley & Douek, 2008), with disruption of the intestinal epithelial barrier leading to microbial translocation and chronic immune activation (Somsouk et al., 2015), which can further accelerate and exacerbate HIV infection.
Studies on intestinal-related diseases are limited by material availability. The most used intestinal tissue in AIDS research is obtained through autopsy, which can result in serious defects in intestinal integrity and cell viability. In addition, obvious heterogeneity exists in both HIV-infected patients and macaques with AIDS, with HIV infection consisting of four distinct phases, i.e., acute infection, incubation, pre-AIDS, and typical AIDS periods. Hence, precise biopsies are more suitable for longitudinal HIV-related intestinal studies. In recent years, biopsy has not only been used for the detection of intestinal immunity during HIV progression, but also as a unique technique to assess HIV persistence or reservoirs in gut tissue during ART (Cattin et al., 2019; Gosselin et al., 2017).
As described in earlier studies, both endoscopy (Chai, 2015; Song et al., 2019) and resection and anastomosis (Edghill-Smith et al., 2002) can be used to perform intestinal biopsy. We previously reported on the sampling method and biopsy quality obtained by endoscopy (Song et al., 2019). Here, to determine the feasibility of intestinal resection and anastomosis in longitudinal sampling studies of non-human primate models, we obtained 12 Chinese rhesus macaques (Supplementary Table S1) from the Kunming Primate Research Center, Chinese Academy of Sciences, China. The monkeys were housed in a facility with animal care and use programs accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures were performed according to the guidelines approved by the Ethics Committee of the Kunming Institute of Zoology (approval number: KPRC-PF20-03-V4.0).
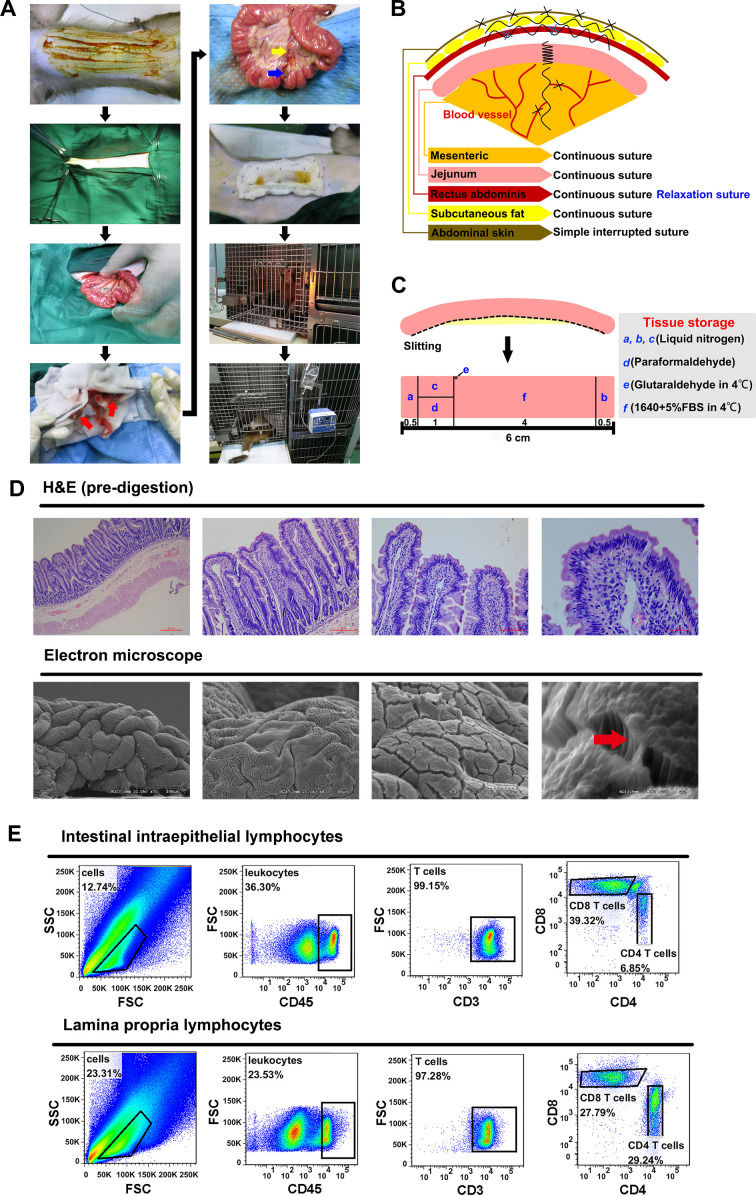
Intestinal resection and anastomosis were performed in a dedicated procedure room. After 24 h of fasting, the animals were anesthetized for surgery via inhalation of isoflurane. Fluctuations in vital signs were carefully monitored throughout the procedure. Firstly, each macaque was fixed to the operating table and held in the supine position. After shaving and disinfecting, sequential incisions in the abdominal skin, subcutaneous fat layer, and rectus abdominis muscle were made to open the abdominal wall. Subsequently, the position of the proximal end of the jejunum was located (about 10 cm below the duodenum). The mesentery was exposed, followed by ligation of the mesenteric vessels that supply the 6–7 cm section of the jejunum. The intestinal contents in the 6 cm section of the jejunum were extruded proximally and distally, with the cut ends then held with intestinal forceps wrapped with saline-soaked gauze (Figure 1A). Finally, the intestinal tissue between the intestinal clamps was resected, and the incisions of the bowel ends, mesentery, and all abdominal wall structures were joined sequentially using absorbable sutures (Figure 1B). Briefly, a single layer of continuous sutures was used on the gut ends, mesentery, and mesenteric bleeding sites. The intestine was placed back into the abdominal cavity and the rectus abdominis received single-layer continuous sutures and two tension sutures. The adipose tissue was sutured in a continuous single layer, and the abdominal skin was sutured intermittently. After the skin wound was sterilized with iodine, a layer of gauze was sewn to the abdomen to cover the skin (this was only performed in the first operation as the monkey removed the gauze immediately after waking from surgery, damaging the wound; as such, subsequent surgeries did not have this step). Drying of the exposed mesentery and gut was prevented by continuous spraying with saline solution. In addition, total parenteral nutrition was performed through an intravenous indwelling needle during surgery. Monkeys were given the following doses per 10 kg of body weight (body weight of monkeys during surgery is shown in Supplementary Table S1): A, metronidazole sodium chloride injection (30 mL); B, 0.9% sodium chloride injection (70 mL)+cefoperazone sodium sulbactam sodium (1.0 g); C, 5% glucose injection (150 mL)+adenosine triphosphate injection (20 mg)+coenzyme A (100 units); D, compound amino acid injection (18AA, 30 mL). Recovery observation and wound disinfection (iodophor, 5±0.5 g/L) were performed daily after surgery.
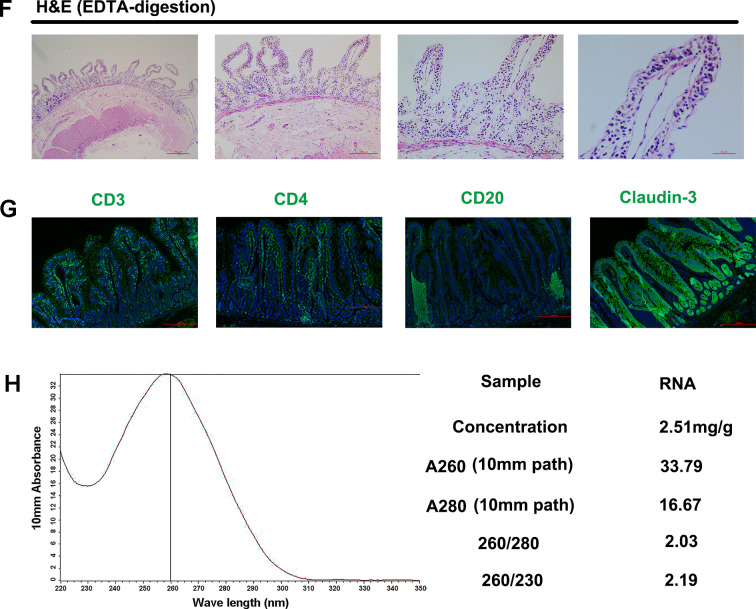
Figure 1. Surgical procedure and schematics and availability verification of intestinal samples.
A: Intestinal resection and anastomosis surgical procedure: red arrow is end of intestinal incision; blue arrow is anastomotic intestinal tube; yellow arrow is mesenteric suture. B: Schematic of suture methods for various tissues and organs during intestinal resection and anastomosis. C: Schematic of biopsy preservation. D: Intestinal villus and fundamental structure observed by H&E and scanning electron microscope, arrow shows small intestine microvilli. E: Subsets and proportion of epithelial lymphocytes and lamina propria lymphocytes analyzed by flow cytometry. F: Structure of intestinal villi after EDTA digestion. G: Antigenic epitopes observed by immunofluorescence staining. H: RNA quality analysis.
After removing the respiratory anesthesia machine, animals recovered within 0.5 h and stayed awake during the whole night after surgery. Macaque number 00034 died within 10 h of surgery. Subsequently, a systematic nursing strategy was performed for the other animals (Table 1). Firstly, for 3 d after surgery, the macaques received parenteral nutrition via the posterior tibial vein. Animals were then fed with daily incremental liquid food and water for the following 7 d. Normal defecation was observed in all animals within 48 h after feeding, suggesting recovery of intestinal motor function. Finally, macaques started to accept solid foods on day 10, with solid food then provided from day 11. Animal condition, including defecation and inflammation of the wound, was observed during the surgical and nursing stages. None of the remaining 11 macaques showed significant abnormal performance during the observation period (Supplementary Table S1).
Table 1. Nursing care after intestinal resection and anastomosis.
| Stage | Days after surgery | Food and drink (one monkey) | Parenteral nutrition support | Wound disinfection | Note |
| "(–)" indicates item no longer needed for this stage. | |||||
| Fasting period | 1–3 | Fasting food and water | Monkeys given drugs intravenously based on following doses per 10 kg of body weight once a day: A: Metronidazole sodium chloride injection 30 mL; B: 0.9% sodium chloride injection 70 mL+cefoperazone sodium sulbactam sodium 1.0 g; C: 5% glucose injection 150 mL+adenosine triphosphate injection 20 mg+coenzyme A 100 units; D: Compound amino acid injection (18 AA) 30 mL | Once a day: A: Intramuscular injection of 400 000 units of penicillin; B: Wound cleaned with iodine (5±0.5 g/L) | Observe monkey water intake, diet, mental state, defecation. and wound recovery every day |
| Liquid diets period | 4–5 | Once a day A: 50 g millet cooked in boiling water for 30 min; Twice a day B: 50 g apple cooked in boiling water for 30 min; Once a day C: 0.8 g lactobacillus tablets; D: clean drinking water | (–) | Once every two days: A: Intramuscular injection of 400 000 units of penicillin; B: Wound cleaned with iodine (5±0.5 g/L) | |
| 6–7 | Once a day A: 100 g millet cooked in boiling water for 30 min; Twice a day B: 100 g apple cooked in boiling water for 30 min; Once a day C: 0.8 g lactobacillus tablets; D: clean drinking water | (–) | |||
| 8–9 | Once a day A: 100 g millet cooked in boiling water for 30 min; Twice a day B: 150 g apple cooked in boiling water for 30 min; Once a day C: 0.8 g lactobacillus tablets; D: Clean drinking water | (–) | |||
| 10 | Once a day A: 100 g millet cooked in boiling water for 30 min; Twice a day B: 150 g apple cooked in boiling water for 30 min; Once a day C: 0.8 g lactobacillus tablets; D: Clean drinking water; E:50 g apple; F: 50 g solid fodder | (–) | |||
| Euphagia | 10 days later | Twice a day A: 150 g solid fodder; Once a day B: 150 g apple; C: Clean drinking water | (–) | (–) | |
The biopsied tissues were preserved (Figure 1C) and used as follows. In brief, tissues a, b, and c were stored in liquid nitrogen for RNA extraction. Tissue d was fixed in 4% paraformaldehyde and used for H&E and immunofluorescence staining. Tissue e was stored in glutaraldehyde and used for scanning electron microscopy. Tissue f was stored in RPMI1640 with 5% fetal bovine serum (FBS) for separation of lymphocytes, as described previously (Ling et al., 2010; Steenholt et al., 2017).
The H&E staining results (Figure 1D, top) showed that the intestinal villi and epithelial barrier of the biopsied tissue acquired by intestinal resection and anastomosis were intact. In addition, intact small intestinal microvilli from the jejunum tissue were also observed by scanning electron microscopy (Figure 1D, bottom). Results suggested that the biopsy obtained by intestinal resection and anastomosis did not affect the fundamental structure of the intestine. In addition, flow cytometry was used to identify CD4+ and CD8+ T cells in the intestinal intraepithelial lymphocytes and lamina propria lymphocytes. We found that the proportion of CD8+ T cells in the epithelial layer was significantly higher than that of CD4+ T cells, whereas no significant differences were observed in the proportion of these two cells in the lamina propria (Figure 1E), consistent with previous research (Steenholt et al., 2017). These data imply that the epithelial and lamina propria lymphocytes were divided effectively and that the isolation method was reliable for studying the proportion and function of distinct intestinal cells. These findings were also confirmed by H&E staining of tissues before and after ethylenediaminetetraacetic acid (EDTA) digestion (Figure 1D, F). Before digestion, the intestine contained a complete epithelial layer and lamina propria, whereas only lamina propria cells were observed after EDTA digestion. Subsequently, the locations of molecular markers, i.e., CD3 (T cells), CD4 (T cells), CD20 (B cells), and tight junction proteins (claudin-3), were detected by immunofluorescence (Figure 1G). Results showed that the biopsy retained intact antigenic epitopes, which are crucial for studying antigen localization and intercellular interactions. High purity RNA was extracted by RNAisoPlus (Figure 1H) for RNA sequence analysis.
Four months after surgery, the immune status of the macaques was detected to evaluate the impact of surgical stress on their immune response. Although the number and percentage of monocytes significantly decreased post-surgery, surgery did not affect the counts or ratios of B cells, T cells, CD4+ T cells, or CD8+ T cells (Supplementary Figure S1A). In addition, the concentrations of sCD14 and FABP2, which are the peripheral biomarkers of microbial translocation and intestinal epithelial injury, showed no significant differences before or after the operation (Supplementary Figure S1B), demonstrating that intestinal integrity and permeability were not altered by surgery.
The body weight of animals showed a significant decrease post-surgery. However, the macaques maintained their post-surgery weight until SIV infection (Supplementary Figure S2A). Plasma levels of lipids, proteins, and vitamins were further tested to evaluate the effects of surgery on intestinal nutrient absorption and metabolism. The levels of triglycerides (TG), cholesterol (CHOL), and low-density lipoprotein cholesterol (LDL-CH) did not significantly change post-surgery (Supplementary Figure S2B), indicating that surgery did not cause abnormal lipid metabolism in macaques. There were no obvious differences in the concentrations of transferrin and albumin pre- or post-surgery (Supplementary Figure S2C), suggesting that macaques did not exhibit nutritional deficiencies, anemia, or infectious diseases after surgery. Vitamin A plays an important role in all stages of wound healing (Polcz & Barbul, 2019; Zinder et al., 2019), especially in stimulating epithelial growth. However, vitamin A deficiency is common during infection (Polcz & Barbul, 2019). In addition to antioxidant effects, immunomodulatory effects of vitamin E have been observed in animal and human models under normal and disease conditions (Lee & Han, 2018; Szymańska et al., 2017). Therefore, we also tested vitamin content in plasma post-surgery. No significant differences were found in vitamin A or vitamin E before or after surgery (Supplementary Figure S2D), indicating normal intestinal absorption and no vitamin deficiency after operation. Taken together, these data suggest that animals recovered well after surgery, with no significant postoperative complications.
In this study, jejunal resection and anastomosis were successfully performed in 12 Chinese rhesus macaques. Monkey 00034 was found to have severe abdominal distension during laparotomy and died one day after surgery. All other monkeys were able to eat and defecate normally. In addition, the biopsied tissues could be used for histological, physiological, pathological, and immunological detection during HIV/SIV infection, as well as for mechanistic analysis at the cellular and molecular level. Four months post-surgery, animals showed no significant changes in counts or ratios of B cells, CD4 T, and CD8 T cells, except that counts of monocytes significantly declined, thus implying no immune system disorder following surgery. Importantly, there was no obvious microbial translocation in the plasma after surgery, proving that the intestinal barrier was restored. Animal weight showed a significant decrease post-surgery. However, this was not likely caused by abnormal intestinal absorption function after surgery as all animals exhibited a good nutritional status post-surgery. The weight loss may be in response to winter conditions (when the surgery was carried out), with animals expending more body energy to maintain thermogenesis. In addition, the macaques maintained their body weight for a long period until viral challenge, implying that the absorption and metabolism remained stable after operation. Although resection and anastomosis are complicated and significantly increase tissue trauma and surgical pressure (Perret-Gentil et al., 2000; Shogan et al., 2014), we found that nursing care after surgery improved the survival rate of animals, accelerated intestinal recovery, and reduced the experimental period.
Intestinal endoscopy and resection and anastomosis are the most commonly used methods of intestinal sampling. Endoscopy is generally considered to be easier and safer than resection and anastomosis. Therefore, AIDS-related research focusing on the intestinal immune system mostly uses biopsy tissue obtained through endoscopy. However, laparoscopic surgery requires advanced skills and can be hindered by operational difficulty within a small gut area, inadequate collection of mucosal lymphocytes, and incomplete intestinal smooth muscle sampling, which are serious limitations of endoscopic biopsies. Due to the physiological curvature of the intestine, endoscopy is generally performed in the duodenum and colon. In addition, the number of lymphocytes obtained by endoscopy is approximately 3×106 (Brenchley et al., 2008). In contrast, compared with endoscopy, the duodenum, jejunum, ileum, and colon can be more easily sampled following the method described in this study. More importantly, intestinal resection and anastomosis can obtain a 6 cm section of intestinal tissue, resulting in the collection of approximately 5×106–20×106 intestinal epithelial lymphocytes and 2×107–5×107 lamina propria lymphocytes (Veazey et al., 1997). Furthermore, this biopsy sampling acquires adequate resources for longitudinal study of animal intestines. Therefore, the successful implementation of intestinal resection and anastomosis may be important for research related to the pathogenesis and treatment of diseases such as HIV infection.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.T.Z. and X.H.W. conceived and designed the experiments. X.H.W., T.Z.S., and L.L. performed the experiments. L.L., C.R.T., T.L., and R.R.T. helped with sample collection. X.H.W. performed data analyses. X.H.W., T.Z.S., and Y.T.Z. wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was partly supported by the National Natural Science Foundation of China (U1802284, 81671627, 81601808) and 13th Five-Year Key Scientific and Technological Program of China (2017ZX10202102-001-005, 2018ZX10301406-003, 2018ZX10301101-002)
References
- 1.Brenchley JM, Douek DC HIV infection and the gastrointestinal immune system. Mucosal Immunology. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattin A, Wiche Salinas TR, Gosselin A, Planas D, Shacklett B, Cohen EA, Ghali MP, Routy JP, Ancuta P HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS. 2019;33(8):1293–1306. doi: 10.1097/QAD.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai N Endoscopy and endosurgery in nonhuman primates. Veterinary Clinics of North America: Exotic Animal Practice. 2015;18(3):447–461. doi: 10.1016/j.cvex.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Edghill-Smith YY, Aldrich K, Zhao J, Pinczewski J, Kalyanaraman VS, Johnson M, Heyliger A, Perrin RP, Woodward R, Robert-Guroff M Effects of intestinal survival surgery on systemic and mucosal immune responses in SIV-infected rhesus macaques. Journal of Medical Primatology. 2002;31(6):313–322. doi: 10.1034/j.1600-0684.2002.01018.x. [DOI] [PubMed] [Google Scholar]
- 6.Gosselin A, Wiche Salinas TR, Planas D, Wacleche VS, Zhang YW, Fromentin R, Chomont N, Cohen ÉA, Shacklett B, Mehraj V, Ghali MP, Routy JP, Ancuta P HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy . AIDS. 2017;31(1):35–48. doi: 10.1097/QAD.0000000000001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GY, Han SN The role of vitamin e in immunity. Nutrients. 2018;10(11):1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling BH, Mohan M, Lackner AA, Green LC, Marx PA, Doyle LA, Veazey RS The large intestine as a major reservoir for simian immunodeficiency virus in macaques with long-term, nonprogressing infection. The Journal of Infectious Diseases. 2010;202(12):1846–1854. doi: 10.1086/657413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perret-Gentil MI, Sinanan MN, Dennis MB, Anderson DM, Pasieka HB, Weyhrich JT, Birkebak TA Videoendoscopic techniques for collection of multiple, serial intra-abdominal biopsy specimens in HIV-negative and HIV-positive pigtail macaques (Macaca nemestrina) . Journal of Investigative Surgery. 2000;13(4):181–195. doi: 10.1080/089419300416474. [DOI] [PubMed] [Google Scholar]
- 10.Polcz ME, Barbul A The role of vitamin A in wound healing. Nutrition in Clinical Practice. 2019;34(5):695–700. doi: 10.1002/ncp.10376. [DOI] [PubMed] [Google Scholar]
- 11.Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014;2:35. doi: 10.1186/2049-2618-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29(1):43–51. doi: 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song TZ, Zhang HD, Zuo Z, Zheng YT Successful implementation of intestinal endoscopy in non-human primates prompts the possibility of achieving AIDS longitudinal intestinal research. Journal of Medical Primatology. 2019;48(3):176–178. doi: 10.1111/jmp.12404. [DOI] [PubMed] [Google Scholar]
- 14.Song TZ, Zhang MX, Xia YJ, Xiao Y, Pang W, Zheng YT Parasites may exit immunocompromised northern pig-tailed macaques (Macaca leonina) infected with SIVmac239 . Zoological Research. 2018;39(1):42–51. doi: 10.24272/j.issn.2095-8137.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steenholt JV, Nielsen C, Baudewijn L, Staal A, Rasmussen KS, Sabir HJ, Barington T, Husby S, Toft-Hansen H The composition of T cell subtypes in duodenal biopsies are altered in coeliac disease patients. PLoS One. 2017;12(2):e0170270. doi: 10.1371/journal.pone.0170270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szymańska R, Nowicka B, Kruk J Vitamin E - occurrence, biosynthesis by plants and functions in human nutrition. Mini Reviews in Medicinal Chemistry. 2017;17(12):1039–1052. doi: 10.2174/1389557516666160725094819. [DOI] [PubMed] [Google Scholar]
- 17.Veazey RS Intestinal CD4 depletion in HIV / SIV infection. Current Immunology Reviews. 2019;15(1):76–91. doi: 10.2174/1573395514666180605083448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clinical Immunology and Immunopathology. 1997;82(3):230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 19.Wong JK, Yukl SA Tissue reservoirs of HIV. Current Opinion in HIV and AIDS. 2016;11(4):362–370. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LT, Tian RR, Zheng HY, Pan GQ, Tuo XY, Xia HJ, Xia XS, Pang W, Zheng YT Translocation of microbes and changes of immunocytes in the gut of rapid- and slow-progressor Chinese rhesus macaques infected with SIVmac239. Immunology. 2016;147(4):443–452. doi: 10.1111/imm.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MX, Song TZ, Zheng HY, Wang XH, Lu Y, Zhang HD, Li T, Pang W, Zheng YT Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques (Macaca leonina) . Zoological Research. 2019;40(6):522–531. doi: 10.24272/j.issn.2095-8137.2019.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinder R, Cooley R, Vlad LG, Molnar JA Vitamin A and wound healing. Nutrition in Clinical Practice. 2019;34(6):839–849. doi: 10.1002/ncp.10420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.