Abstract
The cytotoxic activity of myeloid cells is regulated by a balance of signals that are transmitted through inhibitory and activating receptors. The Cluster of Differentiation 47 (CD47) protein, expressed on both healthy and cancer cells, plays a pivotal role in this balance by delivering a “don’t eat me signal” upon binding to the Signal-regulatory protein alpha (SIRPα) receptor on myeloid cells. In this Perspective, we describe the current state of knowledge on the role of the CD47-SIRPα axis in physiological tissue homeostasis and as a promising therapeutic target in, amongst others, oncology, fibrotic diseases, atherosclerosis and stem cell therapies. In addition, we highlight where additional insight will be beneficial to allow optimal exploitation of this myeloid cell checkpoint as a target in human disease.
Introduction
The immune system has evolved to detect and destroy target cells that are diseased, such as pathogen-infected or cancer cells that would potentially be harmful when not inactivated. In addition, the immune system plays a central role in tissue homeostasis by removing unnecessary cells or, in some cases, by modifying their interactions with surrounding cells. Target cell clearance or modification mediated by myeloid effector cells, including macrophages, neutrophils and microglia, is regulated by receptors that either transmit activating or inhibitory signals upon ligand binding. The latter class of inhibitory receptors, also known as immune checkpoints, includes a series of immunoreceptor tyrosine-based inhibitory (ITIM)- and immunoreceptor tyrosine-based switch motif (ITSM)-containing receptors such as SIRPα, Leukocyte Immunoglobulin-like Receptor B1 (LILRB1), Siglec-10 and Programmed-cell Death Protein 1 (PD-1), which can bind CD47, MHC class I, CD24 and Programmed Death Ligand-1 (PD-L1) on target cells, respectively (Barkal et al., 2019; 2017; Gordon et al., 2017; Oldenborg et al., 2000).
Work over the past decades has demonstrated that the CD47-SIRPα interaction regulates the outcome of myeloid cell-target cell crosstalk in a number of homeostatic processes. In particular, signaling through the CD47-SIRPα axis has been shown to influence erythrocyte, platelet and hematopoietic stem cell (HSC) maintenance, and to regulate synaptic pruning during neuronal development (Jaiswal et al., 2009; Lehrman et al., 2018; Lutz and Bogdanova, 2013; Yamao et al., 2002). Next to an important role in these homeostatic processes, CD47 expression on cancer cells has been shown to inhibit myeloid cell-mediated elimination in a manner that bears a superficial resemblance to the inhibition of intratumoral T cell activity by the PD-1/PD-L1 immune cell checkpoint (Sun et al., 2018).
Based on its role as a regulator of tumor cell fate, a series of molecules that block the CD47-SIRPα axis is currently in clinical development in oncological indications, with an encouraging clinical signal in some settings. In addition, a number of preclinical studies have provided compelling evidence for the potential value of the targeting of this axis in stem cell transplantation, and possibly also diseases such as atherosclerosis and fibrosis (Kojima et al., 2016; Wernig et al., 2017)
In this Perspective, we discuss the structural and functional properties of the CD47-SIRPα axis and highlight the deficits in our understanding of the, possibly context-dependent, mechanisms that regulate CD47-SIPRα signaling strength and that can be expected to influence the activity and toxicity of therapeutic targeting.
Molecular wiring of the CD47-SIRPα axis
The CD47 protein (also known as IAP, MER6 or OA3) consists of a single extracellular V-set IgSF domain, a presenilin domain with five membrane-spanning sections and a short cytoplasmic domain that is subject to alternative splicing, thereby giving rise to four isoforms (Campbell et al., 1992; Mushegian, 2002; Reinhold et al., 1995). In addition to binding SIRPα and its family member SIRPγ, CD47 also interacts with integrins and secreted glycoprotein extracellular matrix protein thrombospondin-1 (TSP-1) (reviewed in (Brown and Frazier, 2001) and (Oldenborg, 2013)). In contrast to the more restricted expression pattern of SIRPα (see below), CD47 is broadly expressed across different cell types in the body, with varying expression of the CD47 protein isoforms in different tissues (Reinhold et al., 1995).
The SIRP family of proteins consists of five members; SIRPα, SIRPß1, SIRPß2, SIRPγ and SIRPδ. Two of these, SIRPα (also known as PTPNS1, SHPS1, CD172A and P84) and SIRPγ (also known as CD172b), are known to bind CD47. SIRPα contains an extracellular region with three immunoglobulin superfamily (IgSF) domains, including a NH2-terminal ligand binding V-domain. Allelic variants with polymorphisms in the ligand binding domain have been reported in the African, Japanese, Chinese and Caucasian populations, three of which (SIRPαV1, SIRPαV2 and SIRPαV8) are the most prominent haplotypes among the human population, jointly covering approximately 90% (Takenaka et al., 2007; Voets et al., 2019). The observation of significant polymorphism, including alleles that are substantially divergent in the ligand binding domain, is suggestive of evolutionary pressure. However, the observations that the polymorphic residues in the NH2-terminal domain of SIRPα are distant from the CD47 binding site, and do not influence CD47 binding capacity for the at least 5 variants for which this was tested, argues against selective pressure at the level of CD47-SIRPα signaling strength (Hatherley et al., 2014). The intracellular region of SIRPα contains both ITIM and ITSM motifs that are essential for inhibitory activity of the receptor (Fujioka et al., 1996; Kharitonenkov et al., 1997). The extracellular domain of SIRPγ is similar to SIRPα, but binds to CD47 with a ten-fold lower affinity (KD ~23 μM for SIRPγ versus ~2 μM for SIRPα)(Brooke et al., 2004). The cytoplasmic domain of SIRPγ consists of only 4 amino acids without an apparent signaling function (Brooke et al., 2004; Ichigotani et al., 2000).
SIRPα is expressed on all myeloid cell types, including monocytes, macrophages, neutrophils, a subset of dendritic cells and microglia (Adams et al., 1998; Seiffert et al., 1999; Veillette et al., 1998). In addition, SIRPα expression has also been observed in brain tissue (Uhlén et al., 2015)(http://www.proteinatlas.org), and on a subset of CD8+ T cells during chronic infection (Myers et al., 2019). Signaling through ITIM- or ITSM-containing receptors such as SIRPα serves to counteract the cellular activation that occurs when an activating – frequently an ITAM-containing – receptor is triggered. In other words, in the absence of such an activating signal, triggering of ITIM/ITSM receptors is considered a null event. Inhibition of signaling through activating immune receptors by SIRPα engagement requires the phosphorylation of the tyrosine residues within the cytoplasmic ITIM and ITSM sequences, resulting in the recruitment and activation of the SH2-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2 (Fujioka et al., 1996; Kharitonenkov et al., 1997). In addition, some evidence has been obtained for association of SIRPα with the inhibitory protein kinase Csk and, to a lesser extent, the Grb-2 adaptor molecule (Veillette et al., 1998). Although our understanding of the direct downstream targets of the SHP-1 and -2 phosphatases upon SIRPα signaling is likely to be incomplete, deactivation of motor protein myosin IIA, and decreased cytotoxic activity of myeloid effector cells, have been shown as downstream consequences of SIRPα ligation (Tsai and Discher, 2008). Over the past years, SIRPα signaling has been shown to counteract signals that myeloid cells receive through a variety of activating membrane receptors, including 1) Fc receptors (FcRs) that bind the Fc domain of target-opsonizing antibodies, 2) complement receptors, and 3) the lipoprotein-related protein (LRP) that binds calreticulin (CRT) (Chao et al., 2010b; Oldenborg et al., 2001). As a side note, the fact that FcRs are one class of activating receptors of which the activity is inhibited by SIRPα ligation implies that studies that utilize CD47 blocking molecules with intact FcR binding capacity should be interpreted with caution, as the intervention simultaneously removes an inhibitory signal and introduces an activating signal (see further below) (Ingram et al., 2017; Weiskopf et al., 2013).
Cell types under control of the CD47-SIRPα axis
Early work has provided compelling evidence that the CD47-SIRPα axis can regulate the activity of a variety of myeloid cell types. For example, a role for macrophages in tumor control has been shown with anti-CD47 mouse IgG1 blocking antibodies in various NSG cancer models that lack components of the adaptive immune system (Chao et al., 2010a; Majeti et al., 2009). In the case of neutrophils, antibody-dependent cellular cytotoxicity (ADCC) towards target cells has been shown to be enhanced upon pathway blockade in vitro (Zhao et al., 2011). In addition, in a human Fc alpha receptor (FcαR) transgenic mouse model, the tumor cell killing that was induced by IgA1 anti-Her2 antibodies and that was boosted by pathway inhibition was primarily dependent on neutrophils (Logtenberg et al., 2019).
Over the past years, a growing body of evidence has emerged that suggests that the adaptive immune system, and in particular CD8+ T cells, can also contribute to the tumor control that is seen upon SIRPα-CD47 pathway inhibition. This observation may either be explained by a direct influence of the SIRPα-CD47 pathway on T cell activity, or by an indirect mechanism in which pathway blockade influences the capacity of myeloid cells to boost CD8+ T cell reactivity. In an adoptive transfer model, ovalbumin (OVA)-specific CD8+ T cells displayed enhanced killing capacity towards OVA-positive targets upon injection of macrophages that had been co-cultured with antigen-expressing target cells in the presence of anti-CD47 IgG (Tseng et al., 2013). These data suggest that the increased tumor control that is seen upon in vivo CD47 blockade may also in part be explained by improved APC function. In another study, enhanced antigen-presentation function of DCs, rather than macrophages, was proposed to be required for the observed anti-tumor effects of low dose intratumoral anti-CD47 treatment in syngeneic mouse models, as depletion of either CD11c+ cells or CD8+ T cells severely impaired the therapeutic effect (X. Liu et al., 2015). In a study by Sockolosky et al., anti-PD-L1 treatment synergized with antibodies targeting CD47 and the mouse melanoma antigen tyrosinase-related protein 1 (TRP-1), an observation that was also interpreted as evidence for a dominant role of T cells upon CD47 blockade, possibly via increased DC acquisition of CD47-opsonized tumor cells and enhanced antigen presentation (Sockolosky et al., 2016). As a side note, PD-1 is expressed in myeloid cells in tumor-bearing mice, and the observed synergy between PD-L1 and CD47 blockade may in theory also be explained by a role of PD-1 as an inhibitory receptor in myeloid cells, as shown in recent mouse experiments (Strauss et al., 2020). Finally, work by Li et al. has demonstrated that vaccination with CD47-deficient tumor cells induced expansion of CD11c+ DC, and that therapy-induced tumor control was abrogated upon CD11c+ cell depletion (Li et al., 2020).
While the observations in the above studies are likely to be explained by enhanced antigen-presentation upon pathway blockade, and hence do not provide substantial evidence for a direct effect of this pathway on T cell function, several other studies do make a somewhat stronger case for such a model. First, in a pooled genetic screen, CD47 deletion was shown to increase the sensitivity of melanoma cells to GVAX/ anti-PD-1 combination immunotherapy, and this effect was dependent on T cells (Manguso et al., 2017). It may be argued that this sensitizing effect of CD47 loss could reflect increased activation of myeloid cells and a resulting boosting of T cell activity. However, this would then have to assume a highly localized regulation of T cell activity by myeloid cells, as selective loss of CD47 negative cells in a sea of CD47 positive cells would otherwise not be expected. As a second ‘myeloid centric’ explanation, one could propose that while T cell activity is critical for tumor control, ultimate tumor cell killing may not be due to T cell activity but due to myeloid cell effector function. As an alternative explanation for the observed effect of CD47 loss in this study it may be proposed that the CD47-SIRPα axis may not only regulate T cell function by influencing APCs but can also influence T cell activity directly. Some support for such a model comes from the observation that SIRPα is induced on a subset of virus-specific CD8+ T cells in mouse models of chronic viral infection and, while restricted to a smaller T cell subset, parallels PD-1 expression (Myers et al., 2019).
Regulation of tissue homeostasis and tissue remodeling by the CD47-SIRPα axis
The CD47-SIRPα axis regulates homeostatic processes by controlling myeloid cell-mediated removal of ageing cells, erythrocytes, HSCs and neuronal synapses (Figure 1). The first evidence demonstrating a protective role for CD47 came from in vivo experiments that showed rapid elimination of CD47-deficient erythrocytes by splenic macrophages when transferred into wild-type recipients (Oldenborg et al., 2000). Furthermore, increased clearance of CD47-deficient erythrocytes was abrogated in SIRPA-mutant mice lacking most of the SIRPα intracellular signaling domain (Ishikawa-Sekigami, 2006), thereby implying a role for both components of the CD47-SIRPα axis in the process. Over the past years, a number of factors have been identified that may influence the susceptibility of erythrocytes to macrophage-mediated elimination. Work by Khandelwal et al. has demonstrated that aged erythrocytes show a reduced CD47 expression (Khandelwal et al., 2007). In addition, increased display of a number of activating signals, such as increased phosphatidylserine (PS) exposure and auto-antibody binding to band 3 (Boas et al., 1998; Kay, 1984; Lutz and Bogdanova, 2013), might also contribute to the enhanced clearance of aged erythrocytes. The latter type of auto-antibody binding might also be of relevance in Autoimmune Hemolytic Anemia (AIHA), a syndrome characterized by accelerated clearance of erythrocytes (Barcellini, 2015), and spontaneous AIHA in nonobese diabetic (NOD) mice is substantially accelerated by CD47 deficiency (Oldenborg et al., 2002; 2001). In line with these data, SIRPα signaling has been shown to inhibit Fcγ- or complement receptor-dependent clearance of antibody- and complement-opsonized erythrocytes by macrophages (Oldenborg et al., 2001).
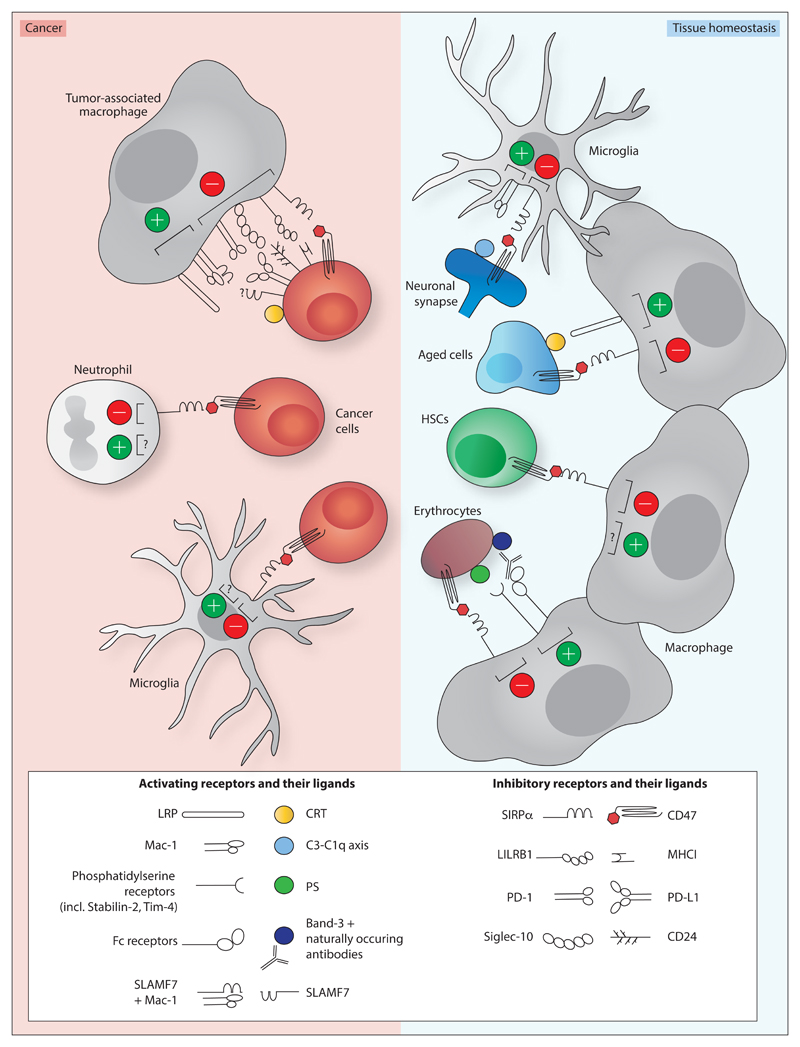
Figure 1. Activating receptors on myeloid cells that are influenced by the CD47-SIRPα axis in tissue homeostasis and in cancer.
Macrophage-, neutrophil- and microglia-mediated cytotoxicity towards cancer cells (left panel) is inhibited by CD47-SIRPα signalling. Macrophage-mediated cytotoxicity has also been shown to be negatively influenced by additional inhibitory receptor-ligand interactions, including leukocyte immunoglobulin-like receptor 1 (LILRB1)/ major histocompatibility complex I (MHC-I) (Barkal et al. 2017), programmed cell death 1 (PD-1)/ programmed cell death 1 ligand 1 (PD-L1) (Gordon et al. 2017), and Siglec-10/CD24 (Barkal et al. 2019). The interaction between prolow-density lipoprotein receptor-related protein (LRP) and calreticulin (CRT) (Chao et al. 2010) has been identified as activating signal in myeloid-mediated cytotoxicity towards cancer cells.
In tissue homeostasis (right panel), synaptic pruning by microglia, and removal of aged cells, erythrocytes and hematopoietic stem cells (HSCs) by macrophages has been shown to be inhibited by CD47-SIRPα signalling (Boas et al. 1998; Kay et al. 1994; Lutz & Bogdanova 2013; Oldenborg et al. 2000; Jaiswal et al. 2009; Lehrman et al. 2018; Feng et al. 2018). C1q or C3 localization to neuronal synapses enhances microglia-dependent synaptic pruning by binding Mac-1 (Schafer et al. 2012; Stevens et al. 2007). CRT binding to LRP on macrophages stimulates the removal of ageing neutrophils (Feng et al. 2018). Binding of naturally occurring antibodies to band-3 and increased phosphatidylserine (PS) exposure may enhance macrophage-mediated removal of ageing erythrocytes (Boas et al. 1998; Kay et al. 1994; Lutz & Bogdanova 2013). Activating signals on HSCs remain unidentified.
CD47 levels also play a role in the regulation of platelet turnover (Olsson et al., 2005) and in regulating the fate of circulating hematopoietic stem cells. Circulating human HSCs have been documented to express higher CD47 levels as compared to bone-marrow resident HSCs, and CD47-deficient c-Kit+ HSCs are phagocytosed more efficiently by macrophages in vitro. Furthermore, in a parabiosis model in which the circulatory systems of wild-type and CD47-deficient mice were surgically joined, bone marrow HSC chimerism was observed in the CD47 deficient animals but not in the wild-type animals, suggesting that CD47-deficient HSCs are cleared from the blood compartment in vivo (Jaiswal et al., 2009). In patients with Hemophagocytic Lymphohistiocytosis (HLH), a syndrome characterized by increased phagocytosis of HSCs by bone marrow macrophages, CD47 expression on the CD34+CD38- HSC fraction is decreased relative to healthy subjects, reaching levels that are insufficient to prevent macrophage-mediated phagocytosis (Kuriyama et al., 2012). Activating signals on HSCs from healthy subjects or patients with HLH that promote this phagocytic process have not been identified to date, although CRT does not appear to be involved (Kuriyama et al., 2012).
As a final example of the role of CD47 as a physiological regulator of cell survival and cellular interactions, expression of CD47 has been shown to protect neuronal synapses from elimination during “synaptic pruning”, a developmental process in which microglia remove synaptic elements to form an organized brain circuity (Lehrman et al., 2018; Paolicelli et al., 2011). Complement components such as C1q and C3 localize to developing synapses to promote the microglial engulfment of synaptic inputs that is necessary for refinement, in a microglial C3 receptor (CR3 or Mac-1) dependent manner (Schafer et al., 2012; Stevens et al., 2007). Microscopic analysis of coronal sections during the peak of synaptic pruning has demonstrated that CD47 protein is enriched in the dorsal geniculate nucleus (dLGN) of the thalamus and that SIRPα is highly expressed by microglia. Notably, CD47- and SIRPα-deficient mice display increased microglial engulfment of retinal ganglion cell (RGC) inputs and enhanced synaptic pruning, leading to a sustained reduction in the number of synapses. Interestingly, microglia are known to preferentially engulf less active synaptic inputs, and the observed preferential localization of CD47 to active inputs suggests a mechanism in which local inhibitory ligand expression is used to convey activity-related information to microglia (Lehrman et al., 2018).
Next to its role in tissue homeostasis, there is emerging evidence for a role of the CD47-SIRPα axis in tissue remodeling in fibrotic diseases (Wernig et al., 2017), and in atherosclerosis (Kojima et al., 2016). The fibrotic response is a normal component of tissue repair processes, however this response can lead to life-threatening conditions when uncontrolled, such as in idiopathic pulmonary fibrosis (IPF), primary myelofibrosis and scleroderma. In lung samples obtained from patients with idiopathic lung fibrosis, CD47 was highly expressed on fibroblasts, whereas calreticulin was found to be expressed on macrophages and a subset of bronchoepithelial cells. Furthermore, fibrosis was shown to be reduced by anti-CD47 treatment in mouse models of fibrotic disease (Wernig et al., 2017). In the development of atherosclerosis, impaired cell clearance leads to the formation of pathogenic plaques that are composed of diseased vascular and apoptotic cells. As such, atherosclerotic lesions are at risk of rupture and obstruction, increasing the possibility of myocardial infarction and stroke (Kasikara et al., 2018; Schrijvers et al., 2005). CD47 was found to be upregulated in human atherosclerotic plaques as compared to healthy vascular tissue, possibly due to the enhanced TNFα levels in these lesions, and treatment with CD47 blocking antibodies was shown to reduce atherosclerosis formation in vivo (Kojima et al., 2016). In both these studies, CD47 blockade involved the use of antibodies with Fcγ-receptor activating capacity, and in the work by Kojima et al., increased erythrocyte clearance and compensatory reticulocytosis was observed. In view of the known side-effects of FcR binding anti-CD47 antibodies, this appears to be an issue that deserves further attention.
Regulation of cancer cell fate by the CD47-SIRPα axis
CD47 expression is frequently observed on cancer cells in both hematological and solid malignancies, including non-Hodgkin lymphoma (NHL), acute myeloid leukemia (AML), glioblastoma, ovarian, breast, colon, bladder, hepatocellular, and prostate cancer (Chao et al., 2010a; Majeti et al., 2009; Willingham et al., 2012). While one study has reported copy number alterations in the CD47 gene in 5% (15 of 316) of patients with ovarian serous cystadenocarcinoma (Brightwell et al., 2016), in most human tumors CD47 levels are only modestly increased relative to surrounding healthy tissue (Willingham et al., 2012; Zhao et al., 2011). The lack of frequent overexpression of CD47 on tumor cells could either imply that the strength of myeloid cell inhibition is not boosted by a further increase in CD47 expression, or that the axis is not a substantial modifier of cancer cell fate during natural disease progression, an issue that requires further study.
As will be discussed in the next section, the CD47-SIRPα axis does form a critical regulator of tumor cell fate at the moment tumor cells are decorated with activating signals as a consequence of therapeutic intervention. In addition, cellular changes associated with malignant transformation may in some cases by themselves already lead to the expression of ligands for activating immune receptors by cancer cells. Only in such situations, where an endogenous activating signal is present, single agent blockade of CD47 is likely to suffice to induce tumor cell killing, and hence it is important to understand the nature and prevalence of such activating signals (Figure 1). As a first example of such an endogenous activating signal, CRT, the ligand of the myeloid cell receptor LRP-1, is present at high levels on several human cancers, and blockade of CRT binding to LRP can prevent the in vitro phagocytosis of certain cancer cell lines that is induced by anti-CD47 antibodies (Chao et al., 2010b). Of note, the CRT pool at the tumor cell surface need not originate from an endogenous CRT pool that translocates to the cell surface (Garg et al., 2012). Specifically, CRT has been shown to be secreted by macrophages in the tumor microenvironment, and secreted CRT can bind cancer cells that display specific patterns of membrane glycans (Feng et al., 2018). A similar binding of extracellular CRT has been described as a mechanism behind the clearance of ageing neutrophils (Feng et al., 2018). Next to CRT, myeloid cells may recognize other activating signals on cancer cells. Specifically, on cancer cell lines and hematopoietic cells that were phagocytosed equally efficient by wild-type and LRP-1-deficient macrophages upon CD47 blockade, the interaction between myeloid cell-expressed signaling lymphocytic activation molecule family 7 (SLAMF7; also known as CRACC, CS1 and CD319) and myeloid cell-expressed Mac-1 was shown to provide an activating signal (Chen et al., 2017). As SLAMF7 is a homotypic receptor, it was proposed that binding of myeloid cell-expressed SLAMF7 to SLAMF7 on cancer cells would be required for cancer cell recognition and phagocytosis, however this model has recently been contradicted (He et al., 2019). Additional evidence for the presence of endogenous activating signals on cancer cells comes from the increase in in vitro phagocytosis of cancer cell lines that is induced by anti-CD47 Fab fragments (Chao et al., 2010a). It is noted though that in these experiments, phagocytosis was significantly enhanced when anti-CD47 treatment was combined with Rituximab, suggesting that the endogenous activating signal is weak relative to the activating signal induced by antibody opsonization. In line with the notion that endogenous activating signals are frequently insufficient to allow significant CD47 blockade monotherapy activity, treatment with anti-CD47 F(ab’)2 fragments only increased neutrophil-mediated in vitro killing of tumor cells in the presence of anti-Her2/Neu antibody (Zhao et al., 2011). Furthermore, treatment with a high affinity SIRPα monomer only slowed lymphoma growth slightly and had no effect on breast cancer growth in vivo, whereas the combination with rituximab (in the case of lymphoma) or trastuzumab (in the case of breast cancer) resulted in a major reduction in tumor growth (Weiskopf et al., 2013). By the same token, even localized secretion of CD47 blocking agents that lacked Fc domains was shown not to affect growth of melanoma tumors in vivo (Ingram et al., 2017), and genetic deletion of CD47 only minimally affected tumor growth in a B16 melanoma model (Barkal et al., 2017). Finally, also treatment with anti-SIRPα antibodies has been shown to be insufficient to induce myeloid cell-dependent tumor cell killing (Ring et al., 2017; Sim et al., 2019). Collectively, these data make a rather compelling case that the fraction of human tumors that intrinsically carry a sufficiently strong activating signal is limited, or that additional inhibitory signals are present on these cells that prevent the activity of single agent SIRPα-CD47 blockade. Future studies in this area would benefit substantially from efforts that would allow one to not only measure CD47 levels, but to also measure the presence of activating signals. Much like the presence of antigen can be used to predict the efficiency of T cell recognition at the moment inhibitory receptors are blocked, the quantification of molecules and/or tumor cell states that report on the presence of activating myeloid cell ligands could be used to improve our capacity to predict the consequence of CD47 pathway inhibition.
Regulation of CD47-SIRP α signaling strength
The importance of the CD47-SIRPα checkpoint in tissue homeostasis and remodeling, and its potential value in cancer therapy, are both evident. In view of this, it is surprising that relatively little is known on the parameters that regulate the strength of this signaling axis. As a general thought, an inhibitor of immune cell activation that would provide the same negative signal under all circumstances would not seem to provide any means of regulation, and simply making the activating receptor less sensitive would appear a more straightforward solution. For this reason, the presence of inhibitory receptor systems is likely to indicate a need to provide contextual information, and two conceptually different classes of such regulators may be distinguished. 1) In a “differential threshold system” a given target cell type or tissue may always have the same expression level of an inhibitory receptor ligand, and a given immune cell type may always have the same level of expression and signaling capacity of the corresponding inhibitory receptor. In such a setting, the same activating signal will lead to a differential response when present at a site that either shows low or high expression of the inhibitory receptor ligand. By the same token, the activating signal present on a given target cell type may lead to differential activation of distinct immune cell types, depending on their inhibitory receptor expression. In other words, in this setting, the expression level of inhibitory receptors and their ligands does not show dynamic regulation, but rather serve as a code to control the outcome of different cellular interactions. 2) On the contrary, in a “dynamic system”, inhibitory receptor-ligand interactions are not primarily used to regulate which immune cell types respond to which targets, but rather to have the same immune cell type respond differently, depending on environmental cues such as inflammation. A well-understood example of the latter type of systems is the PD-1/PD-L1 signaling axis that controls T cell activity (Sun et al., 2018), and, based on more recent data, in some cases also myeloid cell activity (Strauss et al., 2020). Specifically, expression of the PD-1 immune checkpoint on T cells is induced by T cell receptor triggering, thereby leading to a lowering of T cell sensitivity under conditions of chronic antigen encounter. Similarly, PD-L1 expression on tumor cells and other target cells can be increased many-fold by the T cell effector molecule IFNγ, thereby also creating a negative feedback loop by receptor regulation on the target cell side. What is the evidence for a role of the CD47-SIRPα axis as either a “differential threshold regulator”, or as a “dynamic regulator” of immune cell activity?
In at least some situations, such as during mobilization of human HSCs where CD47 expression levels are increased approximately 4-fold, environmental regulation of axis activity appears to take place. Notably, this modest change in CD47 expression level does seem physiologically relevant, as HSCs from mice that lack a single CD47 allele, and that show a 2-fold reduction in CD47 expression levels, display a significant increase in susceptibility to macrophage-mediated phagocytosis in vivo (Jaiswal et al., 2009). Dynamic regulation of CD47-SIRPα signaling strength also appears relevant for synaptic pruning in brain development (Lehrman et al., 2018), where increased localization of CD47 to active synapses, thereby preventing engulfment by microglial cells, has been shown.
With respect to the mechanistic basis for alterations in CD47 expression or localization, in vitro studies have provided evidence that in a subset of cell lines, and most prominently in the MCF7 breast cancer cell line, CD47 expression levels are boosted through TNFα- and NF-κB signaling, and the NF-κB transcription factor was shown to directly bind to a super enhancer (SE) site near the CD47 gene that promotes CD47 gene transcription (Betancur et al., 2017). In addition, blockade of TNFα signaling was both shown to reduce CD47 expression and to induce increased phagocytosis of MCF7 cancer cells. Furthermore, tumor cells with active constituent enhancers in SEs near the CD47 gene had increased CD47 levels compared to tumor cells lacking these (Betancur et al., 2017). Interestingly, cytokine stimulation may in certain settings also reduce CD47 expression levels, as observed in HLH patients. Incubation of CD34+CD38- HSCs with a cytokine cocktail containing IL-6, M-CSF, IFNγ and TNFα at concentrations similar to those observed in patient serum led to a modest decrease in CD47 expression, to approximately 75% of control values, whereas CD47 levels on CD34+CD38+ progenitor or unfractionated mature cells were unaffected (Kuriyama et al., 2012). Similarly, TNFα was shown to induce a slight decrease in CD47 levels on malignant T cells (but not non-transformed T cells) from patients with Sézary syndrome, whereas CD47 levels were increased upon incubation with IL-13, IL-7 and IL-4 (Johnson et al., 2019). Finally, CD47 expression levels in cancer cells have also been shown to be regulated by miR-133a (Suzuki et al., 2012), miR-192 (Yang et al., 2015), the MYC oncogene (Casey et al., 2016), by HIF-1 in a subset of breast cancer cells when exposed to hypoxic conditions (Zhang et al., 2015), and by ERK signaling via nuclear respiratory factor 1 (NRF-1) in melanoma cells (F. Liu et al., 2017).
Other putative mechanisms that may control pathway strength by regulation of CD47 levels or function include alternative polyadenylation (ApA) of CD47 transcripts and posttranslational modification of the CD47 protein. With respect to the former, the transport of CD47 protein to the membrane is regulated by the length of the 3’ untranslated region (3’UTR) of mRNA transcripts. Specifically, ApA leads to the generation of two CD47 mRNA isoforms that either contain a short or long 3’UTR. The long 3’UTR is rich in potential binding sites for the HuR RNA-binding protein that has been shown to mediate CD47 protein localization to the plasma membrane via SET and active RAC1. Unable to bind these proteins, the CD47 protein that is translated from the short 3’UTR version is translocated into the endoplasmic reticulum (Berkovits and Mayr, 2015). Cells that express the CD47 protein translated from the short 3’UTR mRNA display increased susceptibility to macrophage-mediated phagocytosis even when total cellular CD47 levels are the same. The ability to create functionally distinct pools of CD47 protein by spatial separation may perhaps play a role in the CD47-mediated regulation of synaptic pruning, where local activity of the CD47-SIRPα axis in the cell membrane is thought to be important. Next to the regulation of CD47 protein localization, the activity of the CD47 protein has also been shown to depend on post-translational modification. Specifically, the amino-terminus of the CD47 protein contains a pyroglutamate (pGlu) residue that is essential to create a high affinity SIRPα binding site, and this modification has been shown to depend on the glutaminyl-peptide cyclotransferase-like (QPCTL) protein (Logtenberg et al., 2019). In line with the role of the CD47 pGlu in SIRPα binding, both genetic deletion of QPCTL and pharmacological inhibition of QPCTL suppresses inhibitory signaling via the CD47-SIRPα axis. At present, no evidence has been obtained indicating that regulation of either QPCTL, or of enzymes that may remove pGlu residues, is used as a physiological mechanism to tune pathway strength. Nevertheless, pharmacological manipulation of QPCTL activity using small molecule inhibitors forms a conceptually attractive strategy to manipulate pathway strength for therapeutic purposes.
With respect to the regulation of SIRPα levels on myeloid cells, stimulation of macrophages with the TLR ligands LPS and poly(I:C) has been shown to induce SIRPα downregulation (Dong et al., 2008; Kong et al., 2007). Furthermore, work by Zen et al. has provided evidence that inflammation can also reduce SIRPα signaling in polymorphonuclear (PMN) cells by inducing cleavage of the intracellular domain of the receptor (Zen et al., 2013). SIRPα proteins isolated from PMNs, but not monocytes, from donors with various inflammatory conditions consisted of a mixture of the full-length and a truncated protein, the latter lacking the ITIM/ITSM-containing cytoplasmic domains. Furthermore, the cleavage of SIRPα intracellular domains correlated with IL-17 levels in a dextran sulphate sodium (DSS)-induced colitis model, and could be blocked by anti-IL-17 antibody (Zen et al., 2013). As a separate form of regulation of SIRPα activity by proteolytic cleavage, the generation of a soluble extracellular domain of SIRPα following exposure of monocytic THP-1 cells to LPS and TNFα has been reported (Londino et al., 2015). By the same token, the generation of soluble extracellular SIRPα has also been reported in synapses in response to neuronal activity, presumably contributing to presynaptic maturation (Toth et al., 2013).
To conclude, while our understanding of the regulation of CD47-SIRPα axis signaling strength is presently limited, evidence indicating spatial or temporal control of CD47 and/or SIRPα expression level or function is starting to emerge. In addition, it is noted that regulation of signalling strength need not necessarily occur through changes in either receptor expression level or localization but could, for instance, also be achieved by altered intracellular signal transduction, and a further effort to understand potential regulation at this level would appear warranted.
Therapeutic manipulation of the CD47-SIRPα axis
Inhibition of the CD47-SIRPα axis can be considered attractive in all settings where clearance of intended target cells can be promoted while clearance of other vital cell types is avoided (Figure 2). At present, 3 classes of inhibitors of the CD47-SIRPα signaling pathway that are in varying stages of development may be distinguished: Molecules that inhibit pathway activity by blocking the CD47 molecules on target cells, molecules that inhibit pathway activity by blocking the SIRPα molecules on immune effector cells, and inhibitors of the QPCTL enzyme that is required for CD47 maturation. With respect to the first class of molecules, which includes not only anti-CD47 antibodies but also SIRPα-Fc fusions, it is important to distinguish molecules that do and do not lead to substantial FcR triggering. Specifically, the clinical value of CD47 binding molecules that retain substantial FcR activating capacity (e.g. human IgG1) is likely to be capped by on-target – off-tumor toxicity, such as depletion of erythrocytes (Ingram et al., 2017). Indeed, many of the efforts in the clinical development of anti-CD47 antibodies currently focus on molecules with decreased FcγR-binding capacity, such as IgG4, although it is noted that most of these IgG4 CD47-targeting molecules still induce substantial anemia in non-human primates or in cancer patients (Advani et al., 2018; Weiskopf et al., 2013). Interestingly, TTI-621, a protein consisting of the CD47-binding domain of SIRPα fused to the human IgG1 Fc domain appears to only minimally bind human erythrocytes and induce hemagglutination in vitro, in spite of the presence of the activating Fc domain (Petrova et al., 2017). This reduced toxicity may be due to the less effective clustering of CD47 on erythrocytes because of the stringent association with the cytoskeleton (Mouro-Chanteloup et al., 2003; Subramanian et al., 2006). To what extent the differential dependency on receptor clustering of this molecule would also influence the occurrence of any (other) major on-target off-tumor toxicities requires further understanding. However, clinical benefit with a decrease in dominant malignant T cells has been reported in four out of five patients with Sézary syndrome (Johnson et al., 2019). In more recent work, agents that simultaneously target CD47 and tumor-specific antigens have been tested in preclinical and clinical studies, with the aim to restrict CD47 blockade to the tumor antigen-positive cell population (Dheilly et al., 2017; Fischer et al., 2015; Ma et al., 2020; Piccione et al., 2015; 2016; Ponce et al., 2017). As these molecules contain active Fc domains, successful use of such bispecifics will require minimal binding to non-malignant CD47-positive cells, something that may be achieved by lowering the affinity of the CD47 targeting arm (Buatois et al., 2018; Piccione et al., 2016). Whether such molecules that carry a low affinity CD47 targeting arm allow sufficient blockade of CD47 molecules in the clinic remains to be established. In addition, this approach is expected not to be effective for tumor antigens that are expressed at low levels relative to CD47.
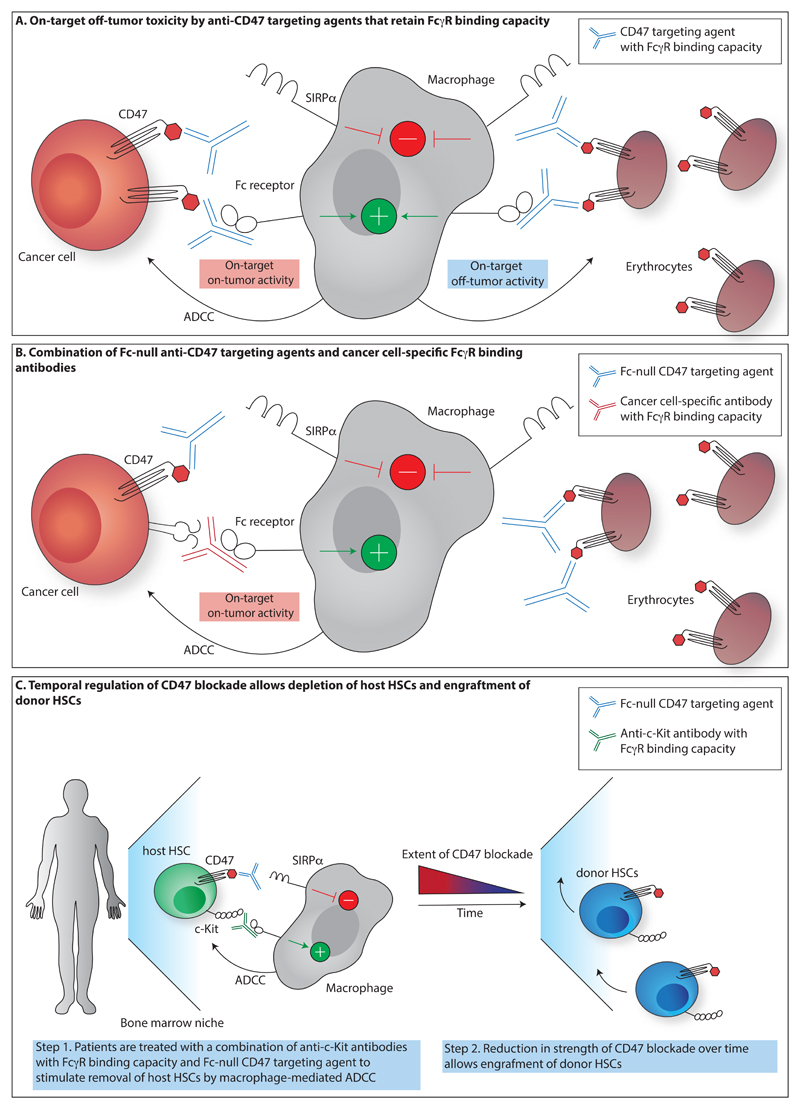
Figure 2. Strategies to exploit CD47 blockade.
A. Blocking CD47 targeting agents that retain FcγR binding capacity prevent inhibitory signalling through SIRPα, but simultaneously decorate CD47+ cells with an activating signal, thereby inducing antibody-dependent cytotoxicity (ADCC) by, for example, macrophages. Use of such agents can result in on-target off-tumor toxicity towards healthy CD47-expressing cells, such as erythrocytes. B. Fc-null CD47 targeting agents, such as the high-affinity SIRPα-Fc fusion protein ALX148 that contains an inactive Fc domain, prevent inhibitory signalling through SIRPα, but without the simultaneous delivery of an activating signal. Combination of such CD47 blocking agents with cancer cell-specific antibodies that retain FcγR binding capacity forms a strategy to direct ADCC activity towards cancer cells while minimizing on-target off-tumor toxicity. C. Selective depletion of defined cell pools may be achieved by temporal control over CD47 blockade. For example, as shown by Chhabra et al., recipient hematopoietic stem cells (HSCs) can be targeted for removal by the combination of blocking agents targeting CD47 and opsonizing anti-c-Kit antibodies (Chhabra et al. 2016). Transplantation of donor HSCs at a time when the strength of CD47 inhibition has diminished then allows donor HSC engraftment.
Biologicals that target SIRPα and small molecule inhibitors of QPCTL are currently being pursued in parallel to CD47 targeting agents. In vitro and in vivo data suggest that, much like Fc-null CD47 blocking antibodies, SIRPα-blocking agents can effectively induce anti-tumor control when used in combination with tumor-opsonizing antibodies (Ho et al., 2015; Ring et al., 2017; Voets et al., 2019; Zhao et al., 2011). In addition, as SIRPα is only expressed on a restricted number of cell types, targeting of the CD47-SIRPα axis with SIRPα-blocking agents does not suffer from the antigen sink issue that has complicated the use of CD47 targeting molecules. With respect to the targeting of the CD47-SIRPα axis via the CD47 modifier QPCTL, at present in vivo data on small molecule inhibition of this enzyme are lacking. However, small molecule inhibition of the pathway may be attractive because of the expected high tissue penetrance of small molecule inhibitors and potential for oral bioavailability. Furthermore, CD47 molecules that newly arrive at the cell surface upon QPCTL inhibition lack pyroglutamate modification and, unlike antagonistic molecules that bind to CD47 or SIRPα, competition by the natural binding partners in the tumor microenvironment will thus not occur.
As discussed above, inhibition of the CD47-SIRPα axis will in many cases require simultaneous provision of an activating signal that marks a target cell population for destruction. Semi-selective marking of tumor cells may be achieved with antibodies against proteins such as CD20 and EGFR that opsonize the intended target cell population. However, sufficiently tumor-selective antibodies are lacking for most human malignancies. As a second strategy that does not rely on such opsonizing antibodies, activating signals may potentially be induced by cytotoxic therapies such as chemotherapy and low-dose radiation, with the implicit assumption that induction of stress signals would be biased towards the (tumor) cells that require clearing. The fact that CRT can be translocated to the cell surface upon induction of immunogenic cancer cells death by some chemotherapies (anthracyclines) and radiotherapies (reviewed in (Galluzzi et al., 2017)) provides some evidence for this model.
As a third strategy to achieve a sufficient level of selectivity, strategies that can provide either spatial or temporal control over CD47 pathway blockade could be considered. Spatial separation may, for instance, be attempted using technologies that allow local uncapping of antibodies in the tumor microenvironment (reviewed in (Autio et al., 2019)). Conceivably, uncapping of anti-CD47 or anti-SIRPα agents could be used to bias pathway inhibition to that site, while in the case of CD47 blockade also remedying the previously described antigen sink issue. Alternatively, selective recognition of cells at the tumor site may theoretically be achieved through the use of capped opsonizing antibodies. With respect to the use of temporal control of pathway inhibition to achieve an increased level of specificity, two strategies have been developed (Figure 2). As a first strategy, in order to ameliorate the erythrocyte depletion seen upon CD47 blockade, a priming strategy has been developed in which patients are first treated at a low dose, in order to remove the ageing erythrocytes that are more prone to display prophagocytic signals, thereby inducing compensatory hematopoiesis (Advani et al., 2018). Second, as shown by Chhabra et al, temporal control over pathway inhibition may also be used to facilitate cell transplants. The transplantation of allogeneic hematopoietic stem cells that is used to treat hematologic malignancies requires the elimination of host HSCs. To avoid the toxicities associated with the currently used host conditioning regimens, a strategy has been developed in which in vivo HSC depletion is achieved by co-administration of CD47 antagonists with anti-c-Kit antibodies that opsonize HSCs and their downstream progenitors (Chhabra et al., 2016; George et al., 2019). The resulting depletion of host HSCs then allows engraftment of donor HSCs, provided obviously that HSC opsonization/ CD47-SIRPα pathway inhibition no longer occurs at that point. Optimal temporal control over pathway inhibition may be achieved using antibodies that display a short in vivo half -life or, perhaps preferably, with small molecule QPCTL inhibitors, as such inhibitors will not affect the preformed CD47 molecules on incoming cells. In future work, the concept of enhancing cell engraftment by transient targeting of an endogenous cell pool may conceivably be extended towards other (stem) cell therapies.
Clinical data that evaluate the potential of CD47 blockade have started to emerge. Evaluation of the combination of CD47 blockade with anti-CD20 treatment has yielded particularly promising data in patients with anti-CD20 refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma, with a 50% objective response rate, and a 36% complete response rate (Advani et al., 2018). In addition, early results suggest that the combination of CD47 blockade with azacitidine leads to significant activity in myelodysplastic syndrome (Chao et al., 2020). Next to the use of CD47 pathway inhibitors in oncology, cellular therapies, and possibly fibrosis and atherosclerosis, interventions that enhance pathway activity may also be of value in certain settings. Specifically, overexpression of CD47 on induced pluripotent stem cells (iPSCs), in combination with MHC class I and class II gene inactivation, was shown to render cells “hypoimmunogenic”, thereby enabling cell transplantation across MHC barriers (Deuse et al., 2019). These data provide the first tantalizing evidence indicating that enhancement of the CD47-SIRPα axis is likely to have biomedical value, and use in e.g. off-the-shelf CAR T cells would seem of potential interest.
Concluding remarks
The CD47-SIRPα axis is a key regulator of cell fate in a number of conditions and hence an attractive therapeutic target. To optimally exploit this axis as therapeutic target, it will be valuable to increase our understanding on the nature of the activating signals that are counterbalanced by the CD47-SIRPα axis in different cell systems. In addition, it will be important to determine by which mechanisms the strength of the SIRPα-mediated inhibitory signal is regulated. Finally, it will be critical to design therapeutic strategies that modify the relative strength of activating signals and the SIRPα-mediated inhibitory signal in either a time-dependent, location-specific, or cell type-specific manner. Strategies that manipulate the CD47-SIRPα axis are starting to show an intriguing clinical signal, and an improved understanding how to steer the balance between this axis and the activating signals that cells receive is likely to increase the scope of clinical application.
Acknowledgements
The authors would like to thank L. Meyaard, K. Franke, A. van der Leun and A. Sahillioglu for input and insightful discussions. This work was supported by ERC AdG SENSIT to T.N.S, Leiden University Medical Center fellowship to F.A.S. and KWF grant 12629 to F.A.S.
Footnotes
Author contributions
M.E.W.L researched data for the article, M.E.W.L., F.A.S and T.N.S jointly discussed data and co-wrote the article.
Competing interests
M.E.W.L., F.A.S., and T.N.S. are inventors of IP related to blockade of the CD47-SIRPα axis, T.N.S. is advisor to and shareholder in Scenic Biotech.
References
- Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Döpp EA, Dijkstra CD, Simmons DL, van den Berg TK. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio KA, Boni V, Humphrey RW, Naing A. Probody Therapeutics: An Emerging Class of Therapies Designed to Enhance On-target Effects with Reduced Off-tumor Toxicity for Use in Immuno-Oncology. Clinical Cancer Research clincanres. 2019 doi: 10.1158/1078-0432.CCR-19-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellini W. New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia. Transfus Med Hemother. 2015;42:287–293. doi: 10.1159/000439002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, Weissman IL. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;138:286. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, McKenna KM, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2017;373:1033. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovits BD, Mayr C. Alternative 3' UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015;522:363–367. doi: 10.1038/nature14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y, Leeper NJ, Ho P, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8 doi: 10.1038/ncomms14802. 14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proceedings of the National Academy of Sciences. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightwell RM, Grzankowski KS, Lele S, Eng K, Arshad M, Chen H, Odunsi K. The CD47 “don't eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol. 2016;143:393–397. doi: 10.1016/j.ygyno.2016.08.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke G, Holbrook JD, Brown MH, Barclay AN. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol. 2004;173:2562–2570. doi: 10.4049/jimmunol.173.4.2562. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Buatois V, Johnson Z, Salgado-Pires S, Papaioannou A, Hatterer E, Chauchet X, Richard F, Barba L, Daubeuf B, Cons L, Broyer L, et al. : Preclinical Development of a Bispecific Antibody that Safely and Effectively Targets CD19 and CD47 for the Treatment of B-Cell Lymphoma and Leukemia. Mol Cancer Ther. 2018;17:1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–5420. [PubMed] [Google Scholar]
- Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, Felsher DW. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010a;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Science Translational Medicine. 2010b;2 doi: 10.1126/scitranslmed.3001375. 63ra94–63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, Volkmer J-P, Weissman IL, Majeti R. Therapeutic Targeting of the Macrophage Immune Checkpoint CD47 in Myeloid Malignancies. Front Oncol. 2020;9:225–9. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhong M-C, Guo H, Davidson D, Mishel S, Lu Y, Rhee I, Pérez-Quintero L-A, Zhang S, Cruz-Munoz M-E, Wu N, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–497. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A, Ring AM, Weiskopf K, Schnorr PJ, Gordon S, Le AC, Kwon H-S, Ring NG, Volkmer J, Ho PY, Tseng S, et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aae0501. 351ra105–351ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;116:1346. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheilly E, Moine V, Broyer L, Salgado-Pires S, Johnson Z, Papaioannou A, Cons L, Calloud S, Majocchi S, Nelson R, Rousseau F, et al. Selective Blockade of the Ubiquitous Checkpoint Receptor CD47 Is Enabled by Dual-Targeting Bispecific Antibodies. Mol Ther. 2017;25:523–533. doi: 10.1016/j.ymthe.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L-W, Kong X-N, Yan H-X, Yu L-X, Chen L, Yang W, Liu Q, Huang D-D, Wu M-C, Wang H-Y. Signal regulatory protein alpha negatively regulates both TLR3 and cytoplasmic pathways in type I interferon induction. Mol Immunol. 2008;45:3025–3035. doi: 10.1016/j.molimm.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Feng M, Marjon KD, Zhu F, Weissman-Tsukamoto R, Levett A, Sullivan K, Kao KS, Markovic M, Bump PA, Jackson HM, Choi TS, et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05211-7. 3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Elson G, Magistrelli G, Dheilly E, Fouque N, Laurendon A, Gueneau F, Ravn U, Depoisier J-F, Moine V, Raimondi S, et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat Commun. 2015;6 doi: 10.1038/ncomms7113. 6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJM, Annaert W, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George BM, Kao KS, Kwon H-S, Velasco BJ, Poyser J, Chen A, Le AC, Chhabra A, Burnett CE, Cajuste D, Hoover M, et al. Antibody Conditioning Enables MHC-Mismatched Hematopoietic Stem Cell Transplants and Organ Graft Tolerance. Stem Cell. 2019;25:1–12. doi: 10.1016/j.stem.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatherley D, Lea SM, Johnson S, Barclay AN. Polymorphisms in the human inhibitory signal-regulatory protein α do not affect binding to its ligand CD47. J Biol Chem. 2014;289:10024–10028. doi: 10.1074/jbc.M114.550558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Bouwstra R, Wiersma VR, de Jong M, Jan Lourens H, Fehrmann R, de Bruyn M, Ammatuna E, Huls G, van Meerten T, Bremer E. Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat Commun. 2019;10 doi: 10.1038/s41467-018-08013-z. 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CCM, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Ozkan E, Mori Y, Weissman IL, Garcia KC. “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem. 2015;290:12650–12663. doi: 10.1074/jbc.M115.648220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichigotani Y, Matsuda S, Machida K, Oshima K, Iwamoto T, Yamaki K, Hayakawa T, Hamaguchi M. Molecular cloning of a novel human gene (SIRP-B2) which encodes a new member of the SIRP/SHPS-1 protein family. J Hum Genet. 2000;45:378–382. doi: 10.1007/s100380070013. [DOI] [PubMed] [Google Scholar]
- Ingram JR, Blomberg OS, Sockolosky JT, Ali L, Schmidt FI, Pishesha N, Espinosa C, Dougan SK, Garcia KC, Ploegh HL, Dougan M. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci USA. 2017;114:10184–10189. doi: 10.1073/pnas.1710776114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Sekigami T. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LDS, Banerjee S, Kruglov O, Viller NN, Horwitz SM, Lesokhin A, Zain J, Querfeld C, Chen R, Okada C, Sawas A, et al. Targeting CD47 in Sézary syndrome with SIRPαFc. Blood Adv. 2019;3:1145–1153. doi: 10.1182/bloodadvances.2018030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128:2713–2723. doi: 10.1172/JCI97950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MM. Localization of senescent cell antigen on band 3. Proceedings of the National Academy of Sciences. 1984;81:5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal S, van Rooijen N, Saxena RK. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion. 2007;47:1725–1732. doi: 10.1111/j.1537-2995.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Volkmer J-P, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X-N, Yan H-X, Chen L, Dong L-W, Yang W, Liu Q, Yu L-X, Huang D-D, Liu S-Q, Liu H, Wu M-C, et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med. 2007;204:2719–2731. doi: 10.1084/jem.20062611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama T, Takenaka K, Kohno K, Yamauchi T, Daitoku S, Yoshimoto G, Kikushige Y, Kishimoto J, Abe Y, Harada N, Miyamoto T, et al. Engulfment of hematopoietic stem cells caused by down-regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood. 2012;120:4058–4067. doi: 10.1182/blood-2012-02-408864. [DOI] [PubMed] [Google Scholar]
- Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, Walker AJ, Heller MD, Umemori H, Chen C, Stevens B. CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron. 2018;100:120–134.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang M, Wang X, Liu W, Wang H, Yang Y-G. Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice. Nat Commun. 2020;11 doi: 10.1038/s41467-019-14102-4. 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Jiang CC, Yan XG, Tseng H-Y, Wang CY, Zhang YY, Yari H, La T, Farrelly M, Guo ST, Thorne RF, et al. BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget. 2017;8:69477–69492. doi: 10.18632/oncotarget.17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu Y-X, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg MEW, Jansen JHM, Raaben M, Toebes M, Franke K, Brandsma AM, Matlung HL, Fauster A, Gomez-Eerland R, Bakker NAM, Schot S, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019;8:1–27. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londino JD, Gulick D, Isenberg JS, Mallampalli RK. Cleavage of Signal Regulatory Protein α (SIRPα) Enhances Inflammatory Signaling. J Biol Chem. 2015;290:31113–31125. doi: 10.1074/jbc.M115.682914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhu M, Gai J, Li G, Chang Q, Qiao P, Cao L, Chen W, Zhang S, Wan Y. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J Nanobiotechnology. 2020;18:12–15. doi: 10.1186/s12951-020-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, Weiss SA, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouro-Chanteloup I, Delaunay J, Gane P, Nicolas V, Johansen M, Brown EJ, Peters LL, Van Kim CL, Cartron JP, Colin Y. Evidence that the red cell skeleton protein 4.2 interacts with the Rh membrane complex member CD47. Blood. 2003;101:338–344. doi: 10.1182/blood-2002-04-1285. [DOI] [PubMed] [Google Scholar]
- Mushegian A. Refining structural and functional predictions for secretasome components by comparative sequence analysis. Proteins: Structure, Function, and Genetics. 2002;47:69–74. doi: 10.1002/prot.10073. [DOI] [PubMed] [Google Scholar]
- Myers LM, Tal MC, Torrez Dulgeroff LB, Carmody AB, Messer RJ, Gulati G, Yiu YY, Staron MM, Angel CL, Sinha R, Markovic M, et al. A functional subset of CD8+ T cells during chronic exhaustion is defined by SIRPα expression. Nat Commun. 2019;10 doi: 10.1038/s41467-019-08637-9. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg P-A. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013;2013:614619–19. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg P-A, Gresham HD, Chen Y, Izui S, Lindberg FP. Lethal autoimmune hemolytic anemia in CD47-deficient nonobese diabetic (NOD) mice. Blood. 2002;99:3500–3504. doi: 10.1182/blood.v99.10.3500. [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg P-A. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Petrova PS, Viller NN, Wong M, Pang X, Lin GHY, Dodge K, Chai V, Chen H, Lee V, House V, Vigo NT, et al. TTI-621 (SIRPαFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clinical Cancer Research. 2017;23:1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, Wang L, Weiskopf K, Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–956. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione EC, Juarez S, Tseng S, Liu J, Stafford M, Narayanan C, Wang L, Weiskopf K, Majeti R. SIRPα-Antibody Fusion Proteins Selectively Bind and Eliminate Dual Antigen-Expressing Tumor Cells. Clinical Cancer Research. 2016;22:5109–5119. doi: 10.1158/1078-0432.CCR-15-2503. [DOI] [PubMed] [Google Scholar]
- Ponce LP, Fenn NC, Moritz N, Krupka C, Kozik J-H, Lauber K, Subklewe M, Hopfner K-P. SIRPα-antibody fusion proteins stimulate phagocytosis and promote elimination of acute myeloid leukemia cells. Oncotarget. 2017;8:11284–11301. doi: 10.18632/oncotarget.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108(Pt 11):3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer J-P, George BM, Lietzenmayer M, McKenna KM, Naik TJ, McCarty A, Zheng Y, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114:E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GRY, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, Brown EJ, Ullrich A, Bühring HJ. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–3643. [PubMed] [Google Scholar]
- Sim J, Sockolosky JT, Sangalang E, Izquierdo S, Pedersen D, Harriman W, Wibowo AS, Carter J, Madan A, Doyle L, Harrabi O, et al. Discovery of high affinity, pan-allelic, and pan-mammalian reactive antibodies against the myeloid checkpoint receptor SIRPα. MAbs. 2019;11:1036–1052. doi: 10.1080/19420862.2019.1624123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockolosky JT, Dougan M, Ingram JR, Ho CCM, Kauke MJ, Almo SC, Ploegh HL, Garcia KC. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA. 2016;113:E2646–54. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, Pal R, Yuan M, Asara J, Patsoukis N, Boussiotis VA. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5:eaay1863. doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Tsai R, Sen S, Dahl KN, Discher DE. Membrane mobility and clustering of Integrin Associated Protein (IAP, CD47)--major differences between mouse and man and implications for signaling. Blood Cells Mol Dis. 2006;36:364–372. doi: 10.1016/j.bcmd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Yokobori T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28:465–472. doi: 10.3892/or.2012.1831. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JCY, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Toth AB, Terauchi A, Zhang LY, Johnson-Venkatesh EM, Larsen DJ, Sutton MA, Umemori H. Synapse maturation by activity-dependent ectodomain shedding of SIRPα. Nat Neurosci. 2013;16:1417–1425. doi: 10.1038/nn.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. The Journal of Cell Biology. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng D, Volkmer J-P, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. 1260419–1260419. [DOI] [PubMed] [Google Scholar]
- Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- Voets E, Paradé M, Lutje Hulsik D, Spijkers S, Janssen W, Rens J, Reinieren-Beeren I, van den Tillaart G, van Duijnhoven S, Driessen L, Habraken M, et al. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint. J Immunother Cancer. 2019;7:340. doi: 10.1186/s40425-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf K, Ring AM, Ho CCM, Volkmer J-P, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, Garcia KC. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Chen S-Y, Cui L, Van Neste C, Tsai JM, Kambham N, Vogel H, Natkunam Y, Gilliland DG, Nolan G, Weissman IL. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017;114:4757–4762. doi: 10.1073/pnas.1621375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Volkmer J-P, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H, Hayashi Y, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–39839. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- Yang SY, Choi SA, Lee JY, Park A-K, Wang K-C, Phi JH, Koh EJ, Park W-Y, Park S-H, Hwang DW, Jung HW, et al. miR-192 suppresses leptomeningeal dissemination of medulloblastoma by modulating cell proliferation and anchoring through the regulation of DHFR, integrins, and CD47. Oncotarget. 2015;6:43712–43730. doi: 10.18632/oncotarget.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Guo Y, Bian Z, Lv Z, Zhu D, Ohnishi H, Matozaki T, Liu Y. Inflammation-induced proteolytic processing of the SIRPα cytoplasmic ITIM in neutrophils propagates a proinflammatory state. Nat Commun. 2013;4 doi: 10.1038/ncomms3436. 2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–23. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, Finetti P, Van Egmond M, Matozaki T, Kraal G, Birnbaum D, et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA. 2011;108:18342–18347. doi: 10.1073/pnas.1106550108. [DOI] [PMC free article] [PubMed] [Google Scholar]