Abstract
Severe coronavirus disease 2019 (COVID-19) symptoms, including systemic inflammatory response and multisystem organ failure, are now affecting thousands of infected patients and causing widespread mortality. Coronavirus infection causes tissue damage, which triggers the endoplasmic reticulum stress response and subsequent eicosanoid and cytokine storms. Although proinflammatory eicosanoids, including prostaglandins, thromboxanes, and leukotrienes, are critical mediators of physiological processes, such as inflammation, fever, allergy, and pain, their roles in COVID-19 are poorly characterized. Arachidonic acid–derived epoxyeicosatrienoic acids could alleviate the systemic hyperinflammatory response in COVID-19 infection by modulating endoplasmic reticulum stress and stimulating the resolution of inflammation. Soluble epoxide hydrolase (sEH) inhibitors, which increase endogenous epoxyeicosatrienoic acid levels, exhibit potent anti-inflammatory activity and inhibit various pathologic processes in preclinical disease models, including pulmonary fibrosis, thrombosis, and acute respiratory distress syndrome. Therefore, targeting eicosanoids and sEH could be a novel therapeutic approach in combating COVID-19. In this review, we discuss the predominant role of eicosanoids in regulating the inflammatory cascade and propose the potential application of sEH inhibitors in alleviating COVID-19 symptoms. The host-protective action of omega-3 fatty acid–derived epoxyeicosanoids and specialized proresolving mediators in regulating anti-inflammation and antiviral response is also discussed. Future studies determining the eicosanoid profile in COVID-19 patients or preclinical models are pivotal in providing novel insights into coronavirus-host interaction and inflammation modulation.
Early in the coronavirus disease 2019 (COVID-19) pandemic, it was observed that morbidity and mortality were associated with a drastic systemic inflammatory response caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1, 2, 3 This was initially observed in the lung, with many patients experiencing respiratory distress, pneumonia, fever, and multi-organ failure. Severe pathologies are now widely recognized in many organs throughout the human body, including the lung, heart, spleen, lymph nodes, brain, liver, kidneys, eyes, and vasculature, because of widespread inflammatory responses.1,4,5 The proinflammatory cytokine storm is a critical driving force in severe COVID-19 infection and may lead to multisystem organ failure.2,3,5,6 One of the first descriptions of the cytokine storm was detailed on graft-versus-host disease in 1993.7 Since then, cytokine storms and associated hyperinflammatory mechanisms have been characterized in studies of viral infection [influenza, SARS-associated coronavirus (SARS-CoV), and SARS-CoV-2], macrophage activation syndrome, immunotherapy, chemotherapy, rheumatic diseases, traumatic injury, and acute respiratory disease and may thus be utilized as potential diagnostic markers of inflammatory disease progression.8, 9, 10, 11, 12
Both SARS-CoV and the influenza virus induce apoptosis and necrosis of various cell types, including T cells, endothelial cells, and epithelial cells, through proinflammatory cytokine- and chemokine-mediated vascular leakage and suboptimal T-cell responses.6,13, 14, 15, 16 Moreover, viral clearance may be impaired by a macrophage-derived proinflammatory cytokine storm and thus targeting viral replication may not be sufficient to promote host survival.16 Locally and systemically elevated proinflammatory cytokines, including IL-1β, interferon-γ, interferon-γ–inducible protein 10, IL-1, IL-6, and monocyte chemoattractant protein 1, have been detected in select critically ill patients with COVID-19 and found to correlate with disease severity.2,6 Interestingly, this hyperinflammatory innate host response to SARS-CoV may additionally correlate with death in large animal models (eg, primates) more directly than virus titers.17 Thus, a therapeutic approach to stimulating the resolution of the host inflammatory response, including the cytokine storm, may be as critical as preventing viral replication for patient survival.3
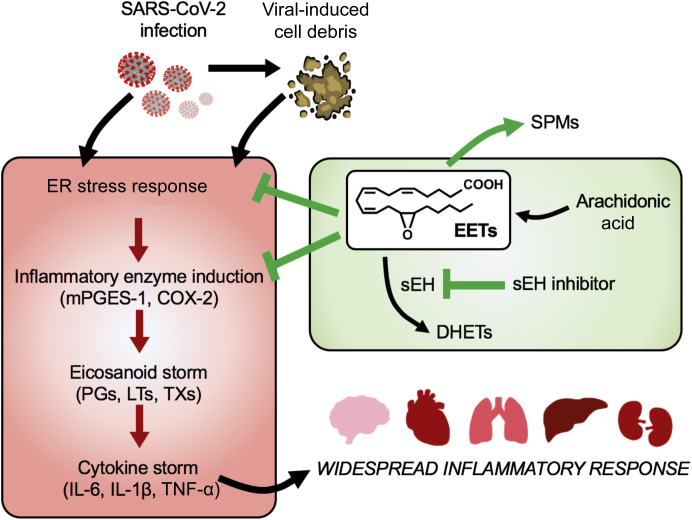
Cell death, including apoptosis, is induced by host defense mechanisms in response to infections.18 However, cell death (debris) can trigger an eicosanoid and cytokine storm in inflammatory diseases.10 Virus-induced inflammatory proteins and cell debris trigger a cellular endoplasmic reticulum (ER) stress response, which mediates the virus-host interaction in infected cells.19 Normally, an ER stress response down-regulates global protein synthesis to diminish further viral replication in infected cells. However, prolonged responses initiate proinflammatory and later pro-apoptotic programs of ER stress in host cells, which cause elevated cytokine production during infection. The spike protein, which mediates SARS-CoV binding to angiotensin-converting enzyme 2 for cellular entry, has been shown to activate ER stress transducer X-box binding protein 1 and up-regulate ER-resident protein Herpud1 and chemokine Cxcl2 in infected murine fibroblast L cells.20 In addition, overexpression of SARS-CoV open reading frame 8b protein elevates ER stress effector C/EBP homologous protein (CHOP), which leads to cell apoptosis in HeLa cells.21 Coronavirus-induced tissue damage and accumulation of cellular debris (ie, cell fragments and proteins) has further been implicated in stimulating cell apoptosis and boosting inflammation by regulating other cellular pathways (c-Jun N-terminal kinases/p38, B-cell lymphoma 2/Bcl-2 associated X-protein, inflammasome, and NF-κB) with specific ER stress sensor arms.19 Manipulations of the coronavirus-induced ER stress response upstream of the devastating cytokine storm could be a powerful approach in combating COVID-19 pathogenesis in patients with severe pneumonia or multi-organ failure (Figure 1).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leads to severe tissue damage, which releases cell debris. Both primary infection and the accumulation of cell debris initiate the endoplasmic reticulum (ER) stress response and up-regulate inflammatory enzymes, including microsomal prostaglandin E synthase-1 (mPGES-1) and prostaglandin-endoperoxide synthase 2 [cyclooxygenase 2 (COX-2)], which subsequently produce eicosanoids, including prostaglandins (PGs), leukotrienes (LTs), and thromboxanes (TXs). These proinflammatory lipid autacoids induce cytokine storms that mediate widespread inflammatory responses and organ damage in severe coronavirus disease 2019 (COVID-19) patients. By contrast, epoxyeicosatrienoic acids (EETs), which are stabilized by inhibition of their metabolizing enzyme, soluble epoxide hydrolase (sEH), are anti-inflammatory and proresolving mediators that promote the termination (resolution) of inflammation by suppressing the ER stress response, inflammatory enzyme induction, and proinflammatory cytokine production. EETs also shift arachidonic acid metabolism to favor the production of specialized proresolving mediators (SPMs), which initiate downstream anti-inflammatory and proresolving programs. EETs and sEH inhibitors may counterregulate the unabated systemic inflammatory response and organ failure associated with COVID-19 infection. DHET, dihydroxyeicosatrienoic acid; TNF-α, tumor necrosis factor-α.
Arachidonic acid–derived lipid autacoids, including prostaglandins (PGs), thromboxanes, and leukotrienes, generated by cyclooxygenases and lipoxygenases, are collectively termed eicosanoids and are critical mediators of inflammation, resolution, and tissue homeostasis.22 Eicosanoids play pivotal roles in a broad range of physiological processes, such as inflammation, fever, allergy, and pain.22,23 On activation by cell debris or infection, the inositol-requiring enzyme 1α–X-box binding protein 1 branch of the ER stress pathway up-regulates expression of microsomal prostaglandin E synthase-1 and prostaglandin-endoperoxide synthase 2 [cyclooxygenase 2 (COX-2)], and accelerates the biosynthesis of prostaglandins (PGE2, PGD2, and PGF2α) and thromboxane B2 from arachidonic acid.24,25 This ensuing eicosanoid storm may be associated with production of a cytokine storm.10,26 Indeed, activated ER stress signaling alone only slightly increases the level of IL-6, whereas the presence of PGE2 and/or other cytokines (interferon-γ) with an activated ER stress response greatly enhances IL-6 production in glial cells.27 More important, this eicosanoid surge has not been adequately evaluated in the setting of COVID-19 infection.
Although elevated cytokine levels in COVID-19 have been identified as a major factor contributing to morbidity and mortality, the role of eicosanoids in COVID-19 as key mediators of both inflammation and its active resolution remains poorly characterized.3,28, 29, 30 More important, not all eicosanoids are proinflammatory as arachidonic acid and related fatty acids are also metabolized into anti-inflammatory and proresolution docosanoids in certain temporal situations.22,31, 32, 33 Infectious processes often activate inflammasome formation and the subsequent formation of an eicosanoid storm consisting of both proinflammatory and anti-inflammatory mediators, thereby disrupting the temporal progression of inflammation and its resolution.34 Namely, phospholipase A2 regulates eicosanoid class switching during inflammasome and caspase activation, triggering arachidonic acid to generate proresolution mediators, such as lipoxins.34,35 As increased proinflammatory cytokines may be a driving force in severe COVID-19, the balance of proinflammatory/anti-inflammatory eicosanoids and proresolution lipid mediators during the initiation and resolution of infection can regulate the cytokine storm.34 Thus, we hypothesize that SARS-CoV-2 may trigger a temporal production of an eicosanoid storm, including an imbalance of both proinflammatory and proresolution mediators.3,22
Although eicosanoids, such as prostaglandins and leukotrienes, are best known as products of arachidonic acid metabolism by cyclooxygenases and lipoxygenases, arachidonic acid is also a substrate for another enzymatic pathway; the cytochrome P450 system. This eicosanoid pathway consists of two main branches: ω-hydroxylases, which convert arachidonic acid to hydroxyeicosatetraenoic acids; and epoxygenases, which convert it to four regioisomeric epoxyeicosatrienoic acids (EETs; 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET).36 EETs regulate inflammation and vascular tone and are produced predominantly in the endothelium.36 More important, the epoxides of EETs are rapidly converted into dihydroxyeicosatrienoic acids by the soluble epoxide hydrolase (sEH) enzyme (Figure 1). Soluble epoxide hydrolase inhibitors, which raise endogenous EET levels, exhibit potent anti-inflammatory activity, including inhibiting proinflammatory cytokines in various pathologic diseases, including inflammatory bowel disease, atherosclerosis, pancreatitis, diabetes, hypertension, stroke, cerebral ischemia, dyslipidemia, pain, immunologic disorders, ocular diseases, neurologic diseases, renal disease (eg, acute kidney injury), organ damage, vascular remodeling, ischemia-reperfusion, lung disease (chronic obstructive pulmonary disease), fibrosis (eg, pulmonary and cardiac fibrosis), graft stenosis, and other medical conditions.37, 38, 39 Although EETs and sEH signaling play a key role in hyperinflammatory diseases, their role in COVID-19 should be explored.
EETs, which are generated from arachidonic acid by cytochrome P450 enzymes, promote the active termination (resolution) of inflammation through mediating a broad array of anti-inflammatory and proresolving mechanisms, including mitigation of the cytokine storm.3,40,41 In an endotoxemia mouse model, increased EET biosynthesis suppresses the endotoxin-induced surge of proinflammatory cytokines (IL-6 and IL-1β), chemokines (monocyte chemoattractant protein 1 and epithelial-derived neutrophil-activating peptide 78), adhesion molecules (E-selectin), and NF-κB activation in lung.42 Similarly, treatment with 14,15-EET inhibits activation of ER stress effectors (phosphorylated eukaryotic initiation factor 2 alpha, CHOP, and glucose-regulated protein 78) by cigarette smoke and suppresses injury-induced oxidative stress and cell apoptosis in human bronchial epithelial cells.43 Reduction of the ER stress response by EETs and promoting the activity of anti-inflammatory, proresolving mediators may represent a promising new therapeutic avenue in COVID-19 treatment.
Although inflammation can induce sEH expression,36,37 COVID-19 infection may further stimulate sEH levels throughout the body in various tissues. Stabilizing the anti-inflammatory, proresolving EETs by using sEH inhibitors (sEHIs) has been shown to have a valuable therapeutic application in various preclinical models and human trials, including sepsis, cardiovascular disease, neuroinflammatory disease, and cancer.44, 45, 46 More important, administration of sEHIs suppresses pulmonary cytokine expression and neutrophil infiltration, thereby alleviating pulmonary inflammation and edema and decreasing mortality in murine models of endotoxin-induced acute respiratory distress syndrome.47 Similar anti-inflammatory activity of sEHIs has been observed in other models of chronic obstructive pulmonary disease, asthma, and pulmonary fibrosis.48 In addition, sEHIs dramatically reduce NF-κB induction of inflammatory enzymes (ie, COX-2), as well as the downstream production of proinflammatory mediators, such as PGE2.24
These omega-3 fatty acid–derived epoxyeicosanoids exhibit anti-inflammatory activity in various inflammatory diseases, including in the lung, heart, ocular angiogenesis, and pain.49 Omega-3 supplementation may also synergize with sEH inhibition to suppress inflammation.50 Omega-3 epoxides, stabilized via inhibition of sEH, are important regulators of inflammation and autophagy in metabolic diseases.51 Dietary eicosapentaenoic acid/docosahexaenoic acid supplementation causes a profound shift of the endogenous cytochrome P450–eicosanoid profile from arachidonic acid– to eicosapentaenoic acid– and docosahexaenoic acid–derived metabolites, increasing, in particular, the plasma and tissue levels of 17,18-epoxyeicosatetraenoic acid and 19,20-epoxydocosapentaenoic acid. COVID-19 is characterized by increased angiogenesis demonstrated in autopsy samples of lungs from patients who died from COVID-19 compared with influenza patients.52 Inhibition of sEH prevents angiogenic diseases, such as diabetic retinopathy.53 Cytochrome P450–derived lipid metabolites epoxydocosapentaenoic acids and epoxyeicosatetraenoic acids also dampen choroidal angiogenesis.54
Moreover, EETs shift arachidonic acid metabolism to stimulate the production of specialized proresolving mediators, such as lipoxins, which stimulate clearance of inflammatory cellular debris and counter proinflammatory cytokine production without being immunosuppressive.24,48 Pharmacological enhancement of resolution via resolvins at nanogram doses per day or omega-3 fatty acid supplementation restores endogenous specialized proresolving mediators.55, 56, 57 Stimulation of resolution of inflammation by immunoresolvent agonists, including resolvins, is host protective during infection and sepsis.58, 59, 60, 61 The anti-inflammatory agent dexamethasone may have activity in COVID-19 patients.62 Dexamethasone stimulates specialized proresolving mediators to stimulate resolution of airway inflammation.63 The ER stress response can be reduced by dexamethasone by promoting protein folding and degradation of misfolded proteins from the ER.64
Targeting eicosanoid metabolism could be a promising new approach in COVID-19 infection given the critical role that eicosanoids play in both the initiation and resolution of inflammation.22 Although current therapeutic strategies are aimed at inhibiting individual inflammatory cytokines (ie, IL-1 and/or IL-6) or viral cell entry and intracellular processing, these strategies neglect the critical upstream role that ER stress and eicosanoids play between generation of cell fragments and proteins released during viral-induced death (debris) and the downstream cytokine production in coronavirus infection (Figure 1). Nonsteroidal anti-inflammatory drugs (NSAIDs), the pan-COX inhibitors that block prostaglandin biosynthesis, are a routine and effective choice for the relief of influenza-caused fever and pain.23,37 There may be minimal to no benefit for severe SARS-CoV-2 infections with NSAIDs, which also increase the risk of gastrointestinal and cardiovascular complications.65 Initially during infection, the classic mediators of inflammation, including prostaglandins and leukotrienes, are produced, leading to the initiation of inflammation.66 Subsequently, prostaglandin E2 and prostaglandin D2 induce a lipid mediator class switching of eicosanoid production by neutrophils from leukotriene B4 and 5-lipoxygenase pathways to lipoxins, resolvins, and protectins to promote resolution.33,66, 67, 68, 69 Thus, NSAIDs should be used with caution as they may block subsequent activity of prostaglandins, which are essential for the resolution of inflammation.22 NSAIDs are currently recommended to be on an individual basis because of the insufficient evidence in clinical trials and their well-known gastrointestinal and cardiovascular toxicities.70
Interestingly, sEHIs synergize with COX inhibitors in reducing inflammation and blocking the gastrointestinal erosion and cardiovascular events associated with these drugs.40,71,72 Shifting arachidonic acid metabolism via sEH inhibition also alters the ER stress response toward a more homeostatic role in cell maintenance and promotes the production of anti-inflammatory, proresolving lipids, thus representing an attractive new approach to controlling inflammation in COVID-19. Indeed, sEHIs have been demonstrated to suppress the inflammatory response and inflammation-driven diseases in lung, heart, liver, kidney, and vasculature in numerous preclinical models.37,40 Recently, sEHIs have proved nontoxic at high doses in clinical development (phase 2A trials) and synergistic with other well-established anti-inflammatory medications, such as NSAIDs, to promote anti-inflammatory programs and reduce the gastrointestinal adverse effects.71 Interestingly, risk factors for COVID-19 are similar to idiopathic pulmonary fibrosis, and previous coronavirus outbreaks have been characterized by fibrosis.73 Pharmacologic or genetic inhibition of soluble epoxide hydrolase prevents inflammation and fibrosis in various tissues, including lung, heart, liver, and kidney.74, 75, 76, 77, 78, 79, 80 Thus, the antifibrotic activity of sEHIs may additionally be useful in speeding recovery after COVID-19 infection.40 Temporal data on eicosanoid levels in patients are critical to further establish the role of a putative eicosanoid storm in COVID-19, and additional preclinical and clinical studies are needed to test the safety and efficacy of sEHIs in COVID-19 patients. Herein, we propose sEHIs alone or in combination with other agents, such as COX inhibitors or omega-3 lipids, are envisioned to be of benefit in blocking or moderating the inflammatory cascade when the patient's condition first appears to be deteriorating in COVID-19.
Footnotes
Supported by NIH grants, including National Institute of Environmental Health Science Superfund Research Program P42 ES004699 (B.D.H. and D.P.); National Institute of Environmental Health Science River Award R35ES030443 (B.D.H.); the Credit Unions Kids at Heart Team; the CJ Buckley Pediatric Brain Tumor Fund; and the Joe Andruzzi Foundation (D.P.).
Disclosures: None declared.
Contributor Information
Bruce D. Hammock, Email: bdhammock@ucdavis.edu.
Dipak Panigrahy, Email: dpanigra@bidmc.harvard.edu.
References
- 1.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh Across Speciality Collaboration UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortes-Puch I., Sime P.J., Phipps R.P., Serhan C.N., Hammock B.D. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39:337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Bruggen M.C., O'Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara J.L., Abhyankar S., Gilliland D.G. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25:1216–1217. [PubMed] [Google Scholar]
- 8.Cron R.Q., Chatham W.W. The rheumatologist's role in COVID-19. J Rheumatol. 2020;47:639–642. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 9.Filippou P.S., Karagiannis G.S. Cytokine storm during chemotherapy: a new companion diagnostic emerges? Oncotarget. 2020;11:213–215. doi: 10.18632/oncotarget.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartung A., Yang J., Sukhatme V.P., Bielenberg D.R., Fernandes D., Chang J., Schmidt B.A., Hwang S.H., Zurakowski D., Huang S., Kieran M.W., Hammock B.D., Panigrahy D. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci U S A. 2019;116:1698–1703. doi: 10.1073/pnas.1803999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulciner M.L., Serhan C.N., Gilligan M.M., Mudge D.K., Chang J., Gartung A., Lehner K.A., Bielenberg D.R., Schmidt B., Dalli J., Greene E.R., Gus-Brautbar Y., Piwowarski J., Mammoto T., Zurakowski D., Perretti M., Sukhatme V.P., Kaipainen A., Kieran M.W., Huang S., Panigrahy D. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215:115–140. doi: 10.1084/jem.20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold S., Steinmueller M., von Wulffen W., Cakarova L., Pinto R., Pleschka S., Mack M., Kuziel W.A., Corazza N., Brunner T., Seeger W., Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogner K., Wolff T., Pleschka S., Plog S., Gruber A.D., Kalinke U., Walmrath H.D., Bodner J., Gattenlohner S., Lewe-Schlosser P., Matrosovich M., Seeger W., Lohmeyer J., Herold S. Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9:e1003188. doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigue-Gervais I.G., Labbe K., Dagenais M., Dupaul-Chicoine J., Champagne C., Morizot A., Skeldon A., Brincks E.L., Vidal S.M., Griffith T.S., Saleh M. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe. 2014;15:23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung T.S., Huang M., Liu D.X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus-host interactions. Virus Res. 2014;194:110–123. doi: 10.1016/j.virusres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Versteeg G.A., van de Nes P.S., Bredenbeek P.J., Spaan W.J. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J Virol. 2007;81:10981–10990. doi: 10.1128/JVI.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D., Dubois R.N. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelzer K.R., Kubala L., Newman J.W., Kim I.H., Eiserich J.P., Hammock B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopra S., Giovanelli P., Alvarado-Vazquez P.A., Alonso S., Song M., Sandoval T.A., Chae C.S., Tan C., Fonseca M.M., Gutierrez S., Jimenez L., Subbaramaiah K., Iwawaki T., Kingsley P.J., Marnett L.J., Kossenkov A.V., Crespo M.S., Dannenberg A.J., Glimcher L.H., Romero-Sandoval E.A., Cubillos-Ruiz J.R. IRE1alpha-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science. 2019;365:eaau6499. doi: 10.1126/science.aau6499. [DOI] [PubMed] [Google Scholar]
- 26.von Moltke J., Trinidad N.J., Moayeri M., Kintzer A.F., Wang S.B., van Rooijen N., Brown C.R., Krantz B.A., Leppla S.H., Gronert K., Vance R.E. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosoi T., Honda M., Oba T., Ozawa K. ER stress upregulated PGE(2)/IFNgamma-induced IL-6 expression and down-regulated iNOS expression in glial cells. Sci Rep. 2013;3:3388. doi: 10.1038/srep03388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. 2020;51:282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regidor P.A. Covid-19 management with inflammation resolving mediators? perspectives and potential. Med Hypotheses. 2020;142:109813. doi: 10.1016/j.mehy.2020.109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur J Clin Pharmacol. 2020;25:1–4. doi: 10.1007/s00228-020-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R., Chiang N., Oh S.F., Serhan C.N. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011 doi: 10.1002/0471142735.im1426s95. Chapter 14:Unit 14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan C.N. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Hong S., Gronert K., Devchand P.R., Moussignac R.L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 34.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris P.C., Gosselin D., Reichart D., Glass C.K., Dennis E.A. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:12746–12751. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeldin D.C. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 37.Imig J.D., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H.C. Soluble epoxide hydrolase inhibitors: a patent review. Expert Opin Ther Pat. 2010;20:941–956. doi: 10.1517/13543776.2010.484804. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Zhu J., Lyu F., Panigrahy D., Ferrara K.W., Hammock B., Zhang G. Omega-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113-115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner K.M., McReynolds C.B., Schmidt W.K., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017;180:62–76. doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilroy D.W., Edin M.L., De Maeyer R.P., Bystrom J., Newson J., Lih F.B., Stables M., Zeldin D.C., Bishop-Bailey D. CYP450-derived oxylipins mediate inflammatory resolution. Proc Natl Acad Sci U S A. 2016;113:E3240–E3249. doi: 10.1073/pnas.1521453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Y., Edin M.L., Theken K.N., Schuck R.N., Flake G.P., Kannon M.A., DeGraff L.M., Lih F.B., Foley J., Bradbury J.A., Graves J.P., Tomer K.B., Falck J.R., Zeldin D.C., Lee C.R. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu G., Zeng X., Wang H., Hou Q., Tan C., Xu Q., Wang H. 14,15-Epoxyeicosatrienoic acid suppresses cigarette smoke extract-induced apoptosis in lung epithelial cells by inhibiting endoplasmic reticulum stress. Cell Physiol Biochem. 2015;36:474–486. doi: 10.1159/000430113. [DOI] [PubMed] [Google Scholar]
- 44.Kodani S.D., Hammock B.D. The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain. Drug Metab Dispos. 2015;43:788–802. doi: 10.1124/dmd.115.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazaar A.L., Yang L., Boardley R.L., Goyal N.S., Robertson J., Baldwin S.J., Newby D.E., Wilkinson I.B., Tal-Singer R., Mayer R.J., Cheriyan J. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol. 2016;81:971–979. doi: 10.1111/bcp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belyanskaya S.L., Ding Y., Callahan J.F., Lazaar A.L., Israel D.I. Discovering drugs with DNA-encoded library technology: from concept to clinic with an inhibitor of soluble epoxide hydrolase. Chembiochem. 2017;18:837–842. doi: 10.1002/cbic.201700014. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y., Liu T., Duan J.X., Li P., Sun G.Y., Liu Y.P., Zhang J., Dong L., Lee K.S.S., Hammock B.D., Jiang J.X., Guan C.X. Soluble epoxide hydrolase inhibitor attenuates lipopolysaccharide-induced acute lung injury and improves survival in mice. Shock. 2017;47:638–645. doi: 10.1097/SHK.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono E., Dutile S., Kazani S., Wechsler M.E., Yang J., Hammock B.D., Douda D.N., Tabet Y., Khaddaj-Mallat R., Sirois M., Sirois C., Rizcallah E., Rousseau E., Martin R., Sutherland E.R., Castro M., Jarjour N.N., Israel E., Levy B.D., National Heart, Lung, and Blood Institute's Asthma Clinical Research Network Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190:886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schunck W.H., Konkel A., Fischer R., Weylandt K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol Ther. 2018;183:177–204. doi: 10.1016/j.pharmthera.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Xia R., Sun L., Liao J., Li H., You X., Xu D., Yang J., Hwang S.H., Jones R.D., Hammock B., Yang G.Y. Inhibition of pancreatic carcinoma growth through enhancing omega-3 epoxy polyunsaturated fatty acid profile by inhibition of soluble epoxide hydrolase. Anticancer Res. 2019;39:3651–3660. doi: 10.21873/anticanres.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Vicario C., Alcaraz-Quiles J., Garcia-Alonso V., Rius B., Hwang S.H., Titos E., Lopategi A., Hammock B.D., Arroyo V., Claria J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A. 2015;112:536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J., Dziumbla S., Lin J., Bibli S.I., Zukunft S., de Mos J., Awwad K., Fromel T., Jungmann A., Devraj K., Cheng Z., Wang L., Fauser S., Eberhart C.G., Sodhi A., Hammock B.D., Liebner S., Muller O.J., Glaubitz C., Hammes H.P., Popp R., Fleming I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552:248–252. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasegawa E., Inafuku S., Mulki L., Okunuki Y., Yanai R., Smith K.E., Kim C.B., Klokman G., Bielenberg D.R., Puli N., Falck J.R., Husain D., Miller J.W., Edin M.L., Zeldin D.C., Lee K.S.S., Hammock B.D., Schunck W.H., Connor K.M. Cytochrome P450 monooxygenase lipid metabolites are significant second messengers in the resolution of choroidal neovascularization. Proc Natl Acad Sci U S A. 2017;114:E7545–E7553. doi: 10.1073/pnas.1620898114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panigrahy D., Gartung A., Yang J., Yang H., Gilligan M.M., Sulciner M.L., Bhasin S.S., Bielenberg D.R., Chang J., Schmidt B.A., Piwowarski J., Fishbein A., Soler-Ferran D., Sparks M.A., Staffa S.J., Sukhatme V., Hammock B.D., Kieran M.W., Huang S., Bhasin M., Serhan C.N., Sukhatme V.P. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J Clin Invest. 2019;129:2964–2979. doi: 10.1172/JCI127282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barden A.E., Mas E., Mori T.A. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol. 2016;27:26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 57.Souza P.R., Marques R.M., Gomez E.A., Colas R.A., De Matteis R., Zak A., Patel M., Collier D.J., Dalli J. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ Res. 2020;126:75–90. doi: 10.1161/CIRCRESAHA.119.315506. [DOI] [PubMed] [Google Scholar]
- 58.Norris P.C., Arnardottir H., Sanger J.M., Fichtner D., Keyes G.S., Serhan C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2018;138:81–89. doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiang N., Fredman G., Backhed F., Oh S.F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalli J., Chiang N., Serhan C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21:1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 63.Pyrillou K., Chairakaki A.D., Tamvakopoulos C., Andreakos E. Dexamethasone induces omega3-derived immunoresolvents driving resolution of allergic airway inflammation. J Allergy Clin Immunol. 2018;142:691–695.e4. doi: 10.1016/j.jaci.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Das I., Png C.W., Oancea I., Hasnain S.Z., Lourie R., Proctor M., Eri R.D., Sheng Y., Crane D.I., Florin T.H., McGuckin M.A. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J Exp Med. 2013;210:1201–1216. doi: 10.1084/jem.20121268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.FitzGerald G.A. Misguided drug advice for COVID-19. Science. 2020;367:1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- 66.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 68.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yousefifard M., Zali A., Zarghi A., Madani Neishaboori A., Hosseini M., Safari S. Non-steroidal anti-inflammatory drugs in management of COVID-19: a systematic review on current evidence. Int J Clin Pract. 2020;00:e13557. doi: 10.1111/ijcp.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goswami S.K., Wan D., Yang J., Trindade da Silva C.A., Morisseau C., Kodani S.D., Yang G.Y., Inceoglu B., Hammock B.D. Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. J Pharmacol Exp Ther. 2016;357:529–536. doi: 10.1124/jpet.116.232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh R., Goswami S.K., Feitoza L., Hammock B., Gomes A.V. Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. Int J Cardiol. 2016;223:923–935. doi: 10.1016/j.ijcard.2016.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J., Imig J.D., Yang J., Hammock B.D., Padanilam B.J. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014;307:F971–F980. doi: 10.1152/ajprenal.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiang C.W., Lee H.T., Tarng D.C., Kuo K.L., Cheng L.C., Lee T.S. Genetic deletion of soluble epoxide hydrolase attenuates inflammation and fibrosis in experimental obstructive nephropathy. Mediators Inflamm. 2015;2015:693260. doi: 10.1155/2015/693260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C.H., Zheng L., Gui L., Lin J.Y., Zhu Y.M., Deng W.S., Luo M. Soluble epoxide hydrolase inhibition with t-TUCB alleviates liver fibrosis and portal pressure in carbon tetrachloride-induced cirrhosis in rats. Clin Res Hepatol Gastroenterol. 2018;42:118–125. doi: 10.1016/j.clinre.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Deng W., Zhu Y., Lin J., Zheng L., Zhang C., Luo M. Inhibition of soluble epoxide hydrolase lowers portal hypertension in cirrhotic rats by ameliorating endothelial dysfunction and liver fibrosis. Prostaglandins Other Lipid Mediat. 2017;131:67–74. doi: 10.1016/j.prostaglandins.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Dong X.W., Jia Y.L., Ge L.T., Jiang B., Jiang J.X., Shen J., Jin Y.C., Guan Y., Sun Y., Xie Q.M. Soluble epoxide hydrolase inhibitor AUDA decreases bleomycin-induced pulmonary toxicity in mice by inhibiting the p38/Smad3 pathways. Toxicology. 2017;389:31–41. doi: 10.1016/j.tox.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Zhou C., Huang J., Li Q., Zhan C., He Y., Liu J., Wen Z., Wang D.W. Pharmacological inhibition of soluble epoxide hydrolase ameliorates chronic ethanol-induced cardiac fibrosis by restoring autophagic flux. Alcohol Clin Exp Res. 2018;42:1970–1978. doi: 10.1111/acer.13847. [DOI] [PubMed] [Google Scholar]
- 80.Harris T.R., Bettaieb A., Kodani S., Dong H., Myers R., Chiamvimonvat N., Haj F.G., Hammock B.D. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–111. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]