Abstract
Antibody phage display is regarded as a critical tool for the development of monoclonal antibodies for infectious diseases. The different classes of antibody libraries are classified based on the source of repertoire used to generate the libraries. Immune antibody libraries are generated from disease infected host or immunization against an infectious agent. Antibodies derived from immune libraries are distinct from those derived from naïve libraries as the host's in vivo immune mechanisms shape the antibody repertoire to yield high affinity antibodies. As the immune system is constantly evolving in accordance to the health state of an individual, immune libraries can offer more than just infection-specific antibodies but also antibodies derived from the memory B-cells much like naïve libraries. The combinatorial nature of the gene cloning process would give rise to a combination of natural and un-natural antibody gene pairings in the immune library. These factors have a profound impact on the coverage of immune antibody libraries to target both disease-specific and non-disease specific antigens. This review looks at the diverse nature of antibody responses for immune library generation and discusses the extended potential of a disease-specified immune library in the context of phage display.
Keywords: Monoclonal antibodies, Phage display, Immune libraries, Repertoire, Infectious disease
1. Introduction
The threat posed by emerging and re-emerging infectious diseases has highlighted the need to develop novel prophylactic and therapeutic strategies to overcome these infections. Moreover, emergence of multidrug-resistant organisms has spurred the need to develop alternate strategies to the standard antibiotics therapy regime. One such strategy is the use of human monoclonal antibodies (mAbs) that can function both in a prophylactic and therapeutic manner [1]. The breadth of mAb application in this area has seen a rapid increase of mAb trials over the past decade for a wide range of pathologies including infectious diseases [2]. The development of antibody display technologies such as phage display [[3], [4], [5]], yeast display [6], mRNA display [7], ribosome display [8], bacterial display [[9], [10], [11]] and mammalian cell surface display [12] have aided the rapid development of new mAbs. The basic principle of these in vitro selection technologies stems from the physical link between phenotype (displayed antibody construct) and genotype (antibody genes) tethered to the carrier particle [13]. Even with the availability of different display methods, phage display is widely regarded as the preferred approach for antibody display.
The general requirement for a successful mAb selection process is the existence of a diverse combinatorial repertoire of antibody genes from which to select. This collection of antibody genes is commonly referred to as an antibody library [14]. Antibody libraries can be distinguished by the source of antibody genes used for display. This ranges from naïve (healthy individuals), immune (infected or immunized individuals), synthetic (chemically synthesized) and semi-synthetic (a mixture of natural immune and chemically synthesized genes) libraries [[14], [15], [16]]. The development of human antibody libraries requires B-cells obtained from human donors which sometimes represents a bottleneck due to the specific characteristics of the samples needed such as source of the B-cells, and the strict regulation in terms of human biological samples usage in research. Apart from humans, animals are also a valuable source of B-cells for antibody library generation. In the context of infectious diseases, immune libraries are very attractive options as they are designed to mirror the immune response of an infected individual or immunized animal, reflecting the biased repertoire of antibody genes specific to that infection. This is only true in the case where the B-cells can elicit an immune response to the infection. Therefore, the nature of the immune response to a particular infection is the key to the design of an immune antibody library and shapes the utility, quality, and versatility of the library [17]. However, immune antibody library repertoires constructed in vitro by combinatorial mixing of immunoglobulin genes may not entirely reflect the true nature of the natural antibody repertoire as the random pairing of heavy and light chains may result in non-functional antibody clones with incorrect folding [18]. Here we review how the immune response to different infections can influence the identification of anti-infectives from an immune library perspective and refines the considerations of repertoire representation by immune antibody libraries for phage display.
2. Immune antibody library repertoires
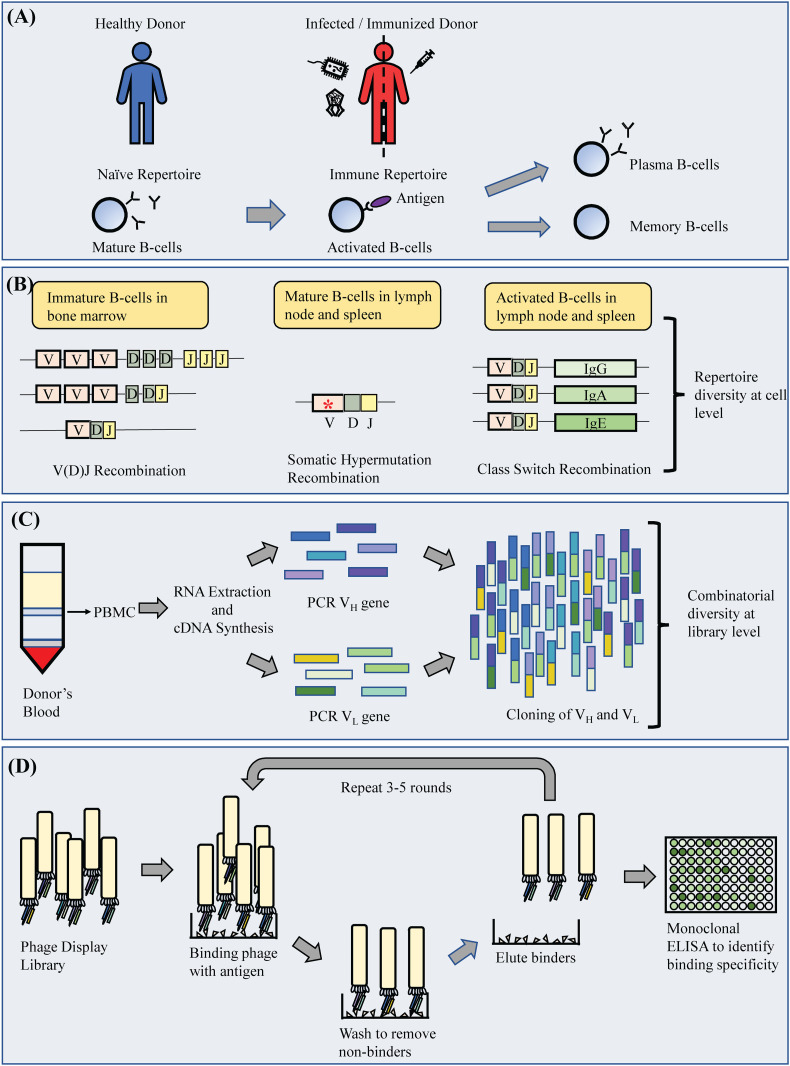
B-cells, as a major component of the immune system safeguarding our body from harmful antigens, are constantly at work, producing antibodies prior to and following the occurrence of infection. The repertoire of antibodies in a healthy state is diverse enough to generate a response against new infections as well as to remember old encounters. This principal feature of B-cells is possible with the diverse repertoire attained from two primary mechanisms, being V(D)J recombination of the variable (V), diversity (D) and joining (J) gene segments and somatic hypermutation (SHM) [19,20]. This includes to a lesser extent unconventional secondary mechanisms that also increase diversity of the antibody repertoire including non-standard recombination that breaches the 12/23 rule of recombination, SHM-associated genetic insertions and deletions, direct antigen contact by non-complementarity determining regions (non-CDRs) of antibody, post-translational modifications, conformational heterogeneity and employment of non-protein cofactors [20,21]. These mechanisms together contribute to the diverse variations within the antibody CDRs, which form the primary antigen binding site. The events leading up to the generation of a mature antibody gene are also multifaceted as the recombination of multiple variable genes furnishes a large combinatorial diversity to start with and is further expanded with varying heavy (VH) and light (VL) chain combinations (Fig. 1 ) [21]. Since SHM and related mechanisms are elicited upon encountering an antigen, exposure to an infection would influence the resulting repertoire as the antibody repertoire would be biased and shaped to combat the invading pathogen.
Fig. 1.
Schematic diagram of monoclonal antibody generation from sampling to isolation of antibodies. (A) The antibody from mature B-cells of a healthy donor is referred for a naïve repertoire. Upon infection or immunization, the mature B-cells is activated to produce plasma B-cells and memory B-cells. This antibody pool is referred to as immune repertoire. (B) The natural antibody repertoire in a healthy individual is diversified via V(D)J recombination, and this represents a naïve antibody library repertoire. Upon infection or immunization, the natural antibody repertoire undergoes somatic hypermutation (SHM), class switch recombination (CSR) and a series of secondary mechanisms to form an immune antibody repertoire. (C) While the recombination of immunoglobulin genes in the variable region are primarily responsible for the diversity of the antibody repertoire, random combination of the heavy (VH) and light (VL) chain during library construction further enlarges the diversity of a library repertoire. (D) The diverse VH/VL gene pairs are then displayed on phages to form an antibody library. The phages are exposed to antigen immobilized on various surfaces for selection, usually subjected to 3–5 rounds of selection process. The final pool of antibodies is analyzed through monoclonal ELISA to isolate out monoclonal antibodies with high specificity.
Despite that the repertoire of an immune antibody library offers better prospects in isolating disease-specific binders, the majority of the current phage-displayed antibody approaches focus primarily on the use of naïve repertoires to generate mAbs against infectious diseases, as evident in the majority of the US Food and Drug Administration (FDA) and European Medicines Agency (EMA)-approved phage display-derived mAbs [22]. The reason is mainly attributed to technical and cost implications rather than immunological characteristics. A naïve library, due to the unbiased nature of its repertoire, makes it ideal for the generation of antibodies against virtually any target molecule [15,23,24]. From a utility point of view, naïve library is a preferred single-pot library type for antibody development as it can be ‘recycled’ for multiple diseases as opposed to a disease-specific immune library which would have to be generated for each new disease. Further, the construction of multiple disease-specific libraries would face the additional challenge of obtaining proper clinical samples for each disease in addition to the constraints on the number of new libraries that can be reasonably generated. The affinity of antibodies obtained from naïve libraries has been reported to be weaker compared to immune libraries due to the lack of in vivo affinity maturation [25]. This would mean further downstream in vitro affinity maturation processes would have to be carried out. Considering the cost implication, time and effort to generate sub-libraries for a particular clone, having a lower affinity antibody from the naïve library is seen as a small compromise for the broader specificity contained therein.
2.1. Isotype-specific repertoire
The major challenge for library construction is the actual design strategy used for antibody generation. Since the majority of libraries are generated using a primer combination targeting the variable gene segment and the junction gene segment [26,27], no actual discrimination in terms of isotype responses is made unless isotype-specific reverse primers are used for repertoire generation. However, there is a clear benefit to the use of isotype-specific repertoires for immune library generation [28,29]. Analyzing this concept from an immunological standpoint: as B-cells are activated, class-switch recombination (CSR) occurs to allow switching of antibody production in an immature B-cell from IgM or IgD to isotypes IgG, IgA or IgE. IgG is further classified into four subclasses (IgG1, IgG2, IgG3 and IgG4) while IgA is further classified into two subclasses (IgA1 and IgA2) [30]. The CSR event is dependent on the T helper (Th) cell response, which is dependent on the nature of antigen as well as the primary invasion path [31]. To efficiently combat invading antigens, each of the isotypes possess a distinct role and distribution site in the body [32]. IgG is the most abundant isotype in plasma which is responsible for protection against extracellular infection [32]. IgA, the second most abundant immunoglobulin in plasma, also predominant in mucous secretions, may reflect the primary infection at mucosal surfaces [[33], [34], [35]]. IgE is the least abundant immunoglobulin in plasma which bound strongly to mast cells predominate under skin and mucosa layer [32]. Increase in IgE level is associated to parasitic infections such as helminth and protozoan infections [36,37]. The isotypes and subclasses can also reflect the progression and stage of infection, as demonstrated in the changes of the antibody population in early and late infection of measles [38,39] and human herpes virus 6 (HHV-6) [40,41]. Therefore, the choice of immunoglobulin isotype for repertoire generation based on the infection pathway and the infection stage are important aspects for immune library design as isotype-specific gene sampling enables the collection of the most effective antibody repertoire.
2.2. Combinatorial repertoire
An immune antibody library can be constructed either in a non-combinatorial or combinatorial manner. A non-combinatorial library uses the original VH/VL pairing in a single B-cell whereas a combinatorial library relies heavily on random combinatorial mixing of the heavy and light chain repertoire [18]. The antibody gene repertoire available in an immune setting would provide a cocktail of sequences present during pre- and post-infection with a higher degree of gene complexity post-infection due to affinity maturation [42]. The repertoire from a post-infection environment would result in a repertoire that is biased against the infection. However, the combinatorial strategy will add complexity to the actual enriched gene representation by random pairing of heavy and light chain proteins to yield a final immune library repertoire [43]. Such an approach would lead to the generation of natural as well as unnatural VH/VL combinations which could result in functional or non-functional folding of clones within the library. In the context of antibody discovery, having both VH/VL gene pairings that are natural and unnatural in combination may expand the functional diversity of the library. Alternately, unnatural VH/VL pairing and CDR packing can also result in misfolding that can lead to aggregation of the antibody, rendering them ineffective [15].
2.3. Single variable domain antibody repertoire
As antibodies from mice and humans consist of two variable domains, the same combinatorial diversity is not represented in immune libraries generated from camelid and sharks as the antibodies are in single variable domain antibody (sdAb) format, i.e. VHH for camelid [44] and VNAR for shark [45]. These sdAbs also termed as nanobodies, contains only the VH domain and exhibits some advantages in terms of solubility, stability, and target accessibility due mainly to their small size [[46], [47], [48]]. However, the absence of the VL domain has limited repertoire diversification in comparison to humans as combinatorial pairing of VH and VL is not achievable. Instead, single variable domain immune library repertoire is depended solely on the inherent germline diversification mechanism upon immunization [49]. Even so, the available repertoire would generally consist of a hyperimmune repertoire with a combination of existing natural repertoires of the immunized host animal. Therefore, the diversity available although seen smaller in comparison to mammals is still adequate to yield high affinity antibodies. The single domain nature of sdAb would also mean that only a relatively smaller sized library is sufficient to adequately represent the immune repertoire making it less cumbersome to generate [50]. Nonetheless, to compensate for the shortcoming of the limited domain repertoire, efficient methods to construct high quality immune libraries are important as the host animal would also yield a mixed repertoire specific to a target as well as other exposed antigens. A classical restriction in antibody library generation processes is the cloning procedure. The quality of a sdAb immune library can be improved through alternative cloning strategies such as Golden Gate Cloning and negative selection using lethal ccdB gene [51]. In fact, these strategies could also be applicable to other libraries construction to maximize the library repertoire.
As the immune system is constantly surveilling and producing antibodies, this would mean that an infection-biased repertoire of an individual or host animal would also harbor remnants of antibodies against previously encountered antigens through memory B-cells [52]. Thus, immune repertoires arising from the memory of previous infections co-exist with repertoires from newly encountered infections. The extracted antibody repertoire would then reflect, at any given time, multiple infections derived from both plasma and memory B-cells collectively. In mammals, the bigger diversity caused by the random combination of antibody genes will lead to the generation of new VH/VL gene combinations. This could stretch the value of the constructed repertoire in an immune antibody library for lead candidate discovery. However, the breadth of the antibody repertoire generated by a combinatorial approach can be further augmented by sampling immunoglobulin genes from multiple individuals or host animals to yield a repertoire of higher diversity. This will result in a diverse repertoire that could rival the naïve repertoire, allowing immune libraries to extend its application beyond a specific disease.
3. Application of immune antibody libraries for infectious diseases
The antibodies derived from immune antibody libraries have the potential to be applied as diagnostic reagents or therapeutic agents. Although immune libraries created from recovered patients are ideal for therapeutic mAbs, immune libraries can also be created from immunized animals such as mice, chicken, llama, alpaca, camel, sheep, shark and non-human primates (detailed in Table 1 ) for other applications. In Table 1 we listed some of the immune libraries developed for infectious diseases, with the details of the format and donor of the library.
Table 1.
Summary of some infectious disease-specific immune libraries from different hosts, constructed in different formats and displayed on phage display platform.
| Type of infection | Species | Targeting site | Antibody format | Donor | Reference |
|---|---|---|---|---|---|
| Bacterial | Bacillus anthracis | Live spore | scFv | Mouse | [89] |
| S-layer protein EA1 | VHH | Llama | [90] | ||
| Lethal factor (LF) | scFv | Macaque | [91] | ||
| Edema toxin (EF) | Fab | Chimpanzee | [92] | ||
| Brucella melitensis | Whole cell | scFv | Mouse | [93] | |
| Clostridium botulinum | Botulinum neurotoxin serotype A (BoNT/A) | scFv | Human | [54] | |
| BoNT/A, BoNT/B | scFv | Macaque | [55] | ||
| Clostridium difficile | Toxin A (TcdA) | VHH | Llama | [94] | |
| Clostridium tetani | Tetanus neurotoxin (TeNT) | scFv | Human | [95] | |
| Corynebacterium diphtheriae | Diptheria toxin (DT) | scFv | Human | [57] | |
| Escherichia coli | Shiga toxin (Stx) | VHH | Alpaca | [96] | |
| Haemophilus influenzae | Capsular polysaccharides | Fab | Human | [97] | |
| Helicobacter pylori | Cell lysate, urease | scFv | Human | [98] | |
| Mycobacterium avium | Cell lysate | scFv | Sheep | [99] | |
| Mycobacterium tuberculosis | α-Crystalline | scFv | Human | [100] | |
| Staphylococcus aureus | Staphylococcal enterotoxin B (SEB) | scFv | Mouse | [101] | |
| Viral | Dengue virus | – | Fab | Chimpanzee | [60] |
| Ebola virus | Envelope glycoprotein | Fab | Human | [61] | |
| VP40 | scFv | Mouse | [62] | ||
| Nucleoprotein | VNAR | Shark | |||
| Foot-and-mouth disease virus | 3ABC | scFv | Chicken | [102] | |
| Hantavirus | Nucleoprotein | VHH | Llama | [103] | |
| Envelope G2 protein | Fab | Human | [104] | ||
| Hepatitis A virus | Capsid | Fab | Chimpanzee | [105] | |
| Hepatitis B virus | Surface antigen | Fab | Human | [63,64] | |
| Surface antigen | IgG | Human | [65] | ||
| Hepatitis C virus | Core protein, envelope E2 protein | scFv | Human | [106] | |
| core protein | Fab | Human | [107] | ||
| Hepatitis E virus | ORF2 | Fab | Chimpanzee | [108] | |
| Herpes simplex virus | Glycoprotein, virus lysate | Fab | Human | [109,110] | |
| Human immunodeficiency virus Type 1 (HIV-1) | gp120 | Fab | Human | [66] | |
| gp120 | scFv | Human | [68] | ||
| gp140 | Fab | Human | [69] | ||
| gp140 | scFv | Human | [111] | ||
| gp140 | VHH | Llama | [70] | ||
| Human immunodeficiency virus Type 2 (HIV-2) | gp125 | Fab | Human | [67] | |
| Influenza A | Hemagglutinin (HA) glycoprotein | scFv | Human | [71] | |
| HA | Fab | Human | [112] | ||
| Japanese encephalitis | Envelope protein | Fab | Human | [113] | |
| Marburg virus | Glycoprotein | scFv | Macaque | [114] | |
| Measles virus | Measles virus protein | Fab | Human | [72] | |
| Polio virus | Capsid protein | Fab | Chimpanzee | [115] | |
| aRabies virus | Glycoprotein | scFv | Human | [73] | |
| Respiratory syncytial virus | F glycoprotein | Fab | Human | [74] | |
| SARS-CoV | Spike protein | scFv | Chicken | [116] | |
| S1 protein | scFv | Human | [117] | ||
| S and M protein | Fab | Human | [118] | ||
| VEEV | TC83 | scFv | Mouse | [119] | |
| West Nile virus | Envelope protein | scFv | Human | [120] | |
| Envelope protein | Fab | Human | [121] | ||
| Parasitic | Brugia malayi | BmR1 | scFv | Human | [83] |
| Brugia malayi | BmSXP | scFv | Human | [84] | |
| Plasmodium falciparum | Pfs48/45 | scFv | Human | [79] | |
| MSP-1 | scFv | Human | [80] | ||
| Plasmodium vivax | DBP | scFv | Human | [122] | |
| Taenia solium | TS14 | VHH | Camel | [81] | |
| Toxoplasma gondii | TgMIC2 | scFv | Mouse | [82] |
3.1. Bacterial infections
Historically, bacterial infections were treated with anti-serum from animals and later with antibiotics. The evolution of bacteria has led to the surge of drug-resistant strains that are dampening the efficacy of antibiotic-based therapeutic strategies. As such, mAbs are touted as potential alternatives for antibiotic resistant bacterial infections. In general, bacterial toxins play a major role in enhancing infection. The tetanus toxoid immunized library is one of the first combinatorial immune antibody libraries constructed from the peripheral B-cell repertoire of an immunized human, from which multiple mAbs were successfully isolated but not evaluated for their neutralizing potential [53]. Since then, several immune libraries have been generated for studies against bacterial infections. An immune library for Clostridium botulinum reported antibodies that identified two key epitopes that were shown to prolong the time to neuroparalysis [54]. Another set of immune library-derived antibodies against the botulinum toxins possessed neutralizing activity in a phrenic nerve-hemidiaphragm assay [55]. An immune antibody library for Pseudomonas aeruginosa was reported to generate neutralizing antibodies targeting the Psl exopolysaccharide [56]. A recent report highlighted the development of neutralizing diphtheria antibodies from an immune library [57]. Raxibacumab, obiltoxaximab and bezlotixumab are exotoxins neutralizing antibodies approved by FDA for treatment of bacterial infections. However, mAbs currently under clinical trials apply a different approach by targeting cell surface proteins or polysaccharides. The antibody-bacteria complex formed will promote antibody-mediated opsonophagocytosis and antibody-dependent complement activation for bactericidal effect [58,59]. Of the three FDA-approved mAbs only raxibacumab is isolated by phage display platform while obiltoxaximab and bezlotixumab are chimeric- and hybridoma-derived mAbs, respectively.
3.2. Viral infections
Immune antibody libraries have also been utilized extensively to discover neutralizing mAbs against viral infections such as dengue fever [60], Ebola virus disease [61,62], hepatitis B [[63], [64], [65]], human immunodeficiency virus (HIV) infection [[66], [67], [68], [69], [70]], influenza [71], measles [72], rabies [73] and respiratory syncytial virus (RSV) [74]. The common and effective strategy for majority of commercial anti-viral mAb development is focused on inactivating the virus via binding of antibodies to the virus surface receptor such as envelope glycoprotein (gp), spike protein and the receptor binding domain (RBD) at the initial stage of infection so that internalization of the virus into host cells can be inhibited [75]. The diversity of the antibody repertoire of an immune antibody library can also be leveraged for the isolation of broadly neutralizing mAbs. Studies on mAbs from immune antibody repertoires demonstrate cross-reactivity against conserved epitopes on multiple viruses from the same family or subtype, as evident in a study of H5N1 influenza, where more than 300 monoclonal antibodies isolated from a combinatorial immune library created from H5N1 influenza survivors are able to neutralize H1 and H5 subtype of influenza viruses [76]. This illustrates the application of a combinatorial immune library for the selection of antibodies against closely related antigens that carry similar epitopes. Immune libraries are also used extensively in HIV studies, mainly to isolate neutralizing antibodies against the glycoprotein gp120 and gp41 [66,69,77]. The panel of antibodies generated from these HIV-1 immune libraries demonstrated broadly neutralizing characteristics against different subtypes [70,77]. The use of an immune library for other disease has also been demonstrated. HIV-1 positive patients are often associated with opportunistic infections. Therefore, an immune library constructed from HIV-1 patients was successfully used to isolate neutralizing antibodies against RSV FG glycoprotein [74]. Collectively, these studies illustrate the utility of combinatorial immune antibody library repertoires towards isolating functional antibodies with inter-species and inter-strain specificity beyond the original infection. This is especially true in the case of viral infections as viruses undergoes rapid mutation, often resulting in multiple subtypes that share a certain degree of conserved regions or mechanism of infection. The identification of broadly neutralizing mAbs from combinatorial immune libraries demonstrates diversity of the antibody repertoire used in the library that is able to provide coverage for closely related antigenic motifs.
3.3. Parasitic infections
Parasitic diseases caused by protozoa, helminths and ectoparasites are also a major healthcare burden. One of the most common examples is the mosquito-borne protozoa, Plasmodium, that causes malaria [78]. Immune libraries have been generated to isolate mAbs against Plasmodium falciparum Pfs48/45 gamete surface protein [79] and Block 2 region of P. falciparum merozoite surface protein-1 [80] as well as other parasites such as Taenia solium Ts14 glycoprotein [81] and Toxoplasma gondii MIC2 protein [82]. Only the mAbs targeting gametocyte surface protein of P. falciparum blocked development of the parasite in mosquito upon bloodmeal ingestion [79]. This showed the potential use of immune library-derived antibodies to block transmission of parasitic disease. The other mAbs developed were only evaluated based on the binding capacity to the target antigen. Another immune antibody library derived from lymphatic filariasis (LF)-infected donors was developed to isolate mAbs against LF-related antigens, BmR1 and BmSXP [83,84]. A parallel experiment using immune and naïve libraries resulted in more unique clones with better binding from the immune library, suggesting the ability of immune libraries to better generate mAbs against targets of a specific infection [83].
Currently, raxibacumab, a naïve antibody library-derived mAb against Bacillus anthracis is the only anti-infective approved by the FDA for market [85]. Raxibacumab functions as a neutralizing antibody that prevents binding of the protective antigen (PA) of anthrax toxin to host receptors, curbing the subsequent release of lethal factor (LF) and edema factor (EF) into the cell, thereby halting disease progression [85]. A human immune single-chain variable fragment (scFv) library isolated antibody against rabies virus glycoprotein antigenic site III, named foravirumab (CR4098), is currently under phase II clinical trial in the form of a cocktail with rafivirumab (CR57), a mAb derived from somatic cell hybridization targeting rabies virus glycoprotein antigenic site I [73,86]. Despite that the only anti-infective approved clinically to date was derived from a naïve phage display library, the growing number of neutralizing antibodies described from immune libraries in research laboratories and the clinical trial of foravirumab suggests that immune library derived mAbs will soon be making an impact in the pharmaceutical industry. Nonetheless, the cost of mAbs as passive immunotherapy, mainly incurred by the perishable characteristic and complicated administration, is remained as the chief obstacle for widespread usage of mAbs especially in low income families and countries. With the maturity of mAbs discovery technologies and availability of more mAbs, it would hopefully provide a more cost-effective solution for infectious diseases with no effective drug treatment [87,88].
4. Conclusion
Antibody gene repertoire is the vital parameter for the success of any antibody library generated. The ability to extract the antibody gene repertoire of an individual post recovery and display it for functional binding permits the discovery of target-specific antibodies for therapeutic and diagnostic applications. This disease-constrained repertoire can be an important source of high affinity antibodies for disease-specific antigens. Immune libraries have the added potential of generating cross-reactive antibodies with homologs of protein from the same family and closely related diseases like viral disease that are caused by different strains of the same virus. Therefore, in the context of immune antibody library repertoires, one should understand that it is a collection of preferentially expressed, disease preferring antibodies resulting from gene segment rearrangements or random combination of heavy and light chain sequences in mammals. Several considerations should be put in place when designing an immune antibody library. This includes the isotype source as a reflection of infection route, agent or stage, and recombination as a potential source of novel reactivity. In conclusion, the potential of immune antibody libraries transcends its ability to just generate high affinity disease specific mAbs but also has the diversity to produce mAbs against other target proteins making it an indispensable alternative to naïve libraries in antibody phage display development laboratories.
Author statement
Jing Yi Lai drafted and edited the manuscript.
Theam Soon Lim conceptualised the article, reviewed and edited the manuscript.
Funding information
Jing Yi Lai acknowledges the support by the Graduate Assistant Scheme from Universiti Sains Malaysia.
Theam Soon Lim acknowledges the support by Malaysian Ministry of Higher Education through the Fundamental Research Grant Scheme [Grant No. 203/CIPPM/6711658 (FRGS/1/2018/STG05/USM/02/2)].
Author contributions
JYL and TSL wrote the manuscript.
Funding
This work was supported by the Malaysian Ministry of Higher Education through the Fundamental Research Grant Scheme [Grant No. 203/CIPPM/6711658 (FRGS/1/2018/STG05/USM/02/2)]. JYL would like to acknowledge support from Graduate Assistant Scheme from Universiti Sains Malaysia.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Marston H.D., Paules C.I., Fauci A.S. Monoclonal antibodies for emerging infectious diseases — borrowing from history. N. Engl. J. Med. 2018;378(16):1469–1472. doi: 10.1056/NEJMp1802256. [DOI] [PubMed] [Google Scholar]
- 2.Wagner E.K., Maynard J.A. Engineering therapeutic antibodies to combat infectious diseases. Curr. Opin. Chem. Eng. 2018;19:131–141. doi: 10.1016/j.coche.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCafferty J., Griffiths A.D., Winter G., Chiswell D.J. Phage antibodies: filamentous phage displaying antibody variable domains. nature. 1990;348(6301):552. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 4.Breitling F., Dübel S., Seehaus T., Klewinghaus I., Little M. A surface expression vector for antibody screening. Gene. 1991;104(2):147–153. doi: 10.1016/0378-1119(91)90244-6. [DOI] [PubMed] [Google Scholar]
- 5.Clackson T., Hoogenboom H.R., Griffiths A.D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 6.Boder E.T., Wittrup K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997;15(6):553. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 7.Roberts R.W., Szostak J.W. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. 1997;94(23):12297–12302. doi: 10.1073/pnas.94.23.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanes J., Plückthun A. In vitro selection and evolution of functional proteins by using ribosome display. Proc. Natl. Acad. Sci. 1997;94(10):4937–4942. doi: 10.1073/pnas.94.10.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelson P., Hansson M., Ahlborg N., Andréoni C., Götz F., Bächi T., Nguyen T.N., Binz H., Uhlen M., Ståhl S. Cell surface display of recombinant proteins on Staphylococcus carnosus. J. Bacteriol. 1995;177(6):1470–1476. doi: 10.1128/jb.177.6.1470-1476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiou G., Stathopoulos C., Daugherty P.S., Nayak A.R., Iverson B.L., Iii R.C. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat. Biotechnol. 1997;15(1):29–34. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 11.Mazor Y., Van Blarcom T., Mabry R., Iverson B.L., Georgiou G. Isolation of engineered, full-length antibodies from libraries expressed in Escherichia coli. Nat. Biotechnol. 2007;25(5):563. doi: 10.1038/nbt1296. [DOI] [PubMed] [Google Scholar]
- 12.Ho M., Nagata S., Pastan I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl. Acad. Sci. 2006;103(25):9637–9642. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenzel A., Kügler J., Helmsing S., Meier D., Schirrmann T., Hust M., Dübel S. Designing human antibodies by phage display. Transfus. Med. Hemother. 2017;44(5):312–318. doi: 10.1159/000479633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogenboom H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005;23(9):1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 15.Ponsel D., Neugebauer J., Ladetzki-Baehs K., Tissot K. High affinity, developability and functional size: the holy grail of combinatorial antibody library generation. Molecules. 2011;16(5):3675–3700. doi: 10.3390/molecules16053675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim C.C., Choong Y.S., Lim T.S. IntechOpen; 2017. High Affinity Maturated Human Antibodies From Naïve and Synthetic Antibody Repertoires, Antibody Engineering. [Google Scholar]
- 17.Berry J.D., Popkov M., Sidhu S. Phage Display in Biotechnology and Drug Discovery. Vol. 3. 2005. Antibody libraries from immunized repertoires. [Google Scholar]
- 18.Beerli R.R., Rader C. Mining human antibody repertoires. mAbs. 2010;2(4):365–378. doi: 10.4161/mabs.2.4.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoehn K.B., Fowler A., Lunter G., Pybus O.G. The diversity and molecular evolution of B-cell receptors during infection. Mol. Biol. Evol. 2016;33(5):1147–1157. doi: 10.1093/molbev/msw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanyavuz A., Marey-Jarossay A., Lacroix-Desmazes S., Dimitrov J.D. Breaking the law: unconventional strategies for antibody diversification. Nat. Rev. Immunol. 2019;19(6):355–368. doi: 10.1038/s41577-019-0126-7. [DOI] [PubMed] [Google Scholar]
- 21.Briney B., Crowe J. Secondary mechanisms of diversification in the human antibody repertoire. Front. Immunol. 2013;4(42) doi: 10.3389/fimmu.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenzel A., Schirrmann T., Hust M. Phage display-derived human antibodies in clinical development and therapy. mAbs. 2016;8(7):1177–1194. doi: 10.1080/19420862.2016.1212149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kügler J., Wilke S., Meier D., Tomszak F., Frenzel A., Schirrmann T., Dübel S., Garritsen H., Hock B., Toleikis L., Schütte M., Hust M. Generation and analysis of the improved human HAL9/10 antibody phage display libraries. BMC Biotechnol. 2015;15(1):10. doi: 10.1186/s12896-015-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao A., Tohidkia M.R., Siegel D.L., Coukos G., Omidi Y. Phage antibody display libraries: a powerful antibody discovery platform for immunotherapy. Crit. Rev. Biotechnol. 2016;36(2):276–289. doi: 10.3109/07388551.2014.958978. [DOI] [PubMed] [Google Scholar]
- 25.Schirrmann T., Meyer T., Schütte M., Frenzel A., Hust M. Phage display for the generation of antibodies for proteome research, diagnostics and therapy. Molecules. 2011;16(1):412–426. doi: 10.3390/molecules16010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akamatsu Y., Cole M., Tso J., Tsurushita N. Construction of a human Ig combinatorial library from genomic V segments and synthetic CDR3 fragments. J. Immunol. 1993;151(9):4651–4659. [PubMed] [Google Scholar]
- 27.Frenzel A., Kügler J., Wilke S., Schirrmann T., Hust M. In: Human Monoclonal Antibodies: Methods and Protocols. Steinitz M., editor. Humana Press; Totowa, NJ: 2014. Construction of human antibody gene libraries and selection of antibodies by phage display; pp. 215–243. [DOI] [PubMed] [Google Scholar]
- 28.Rhyner C., Weichel M., Hübner P., Achatz G., Blaser K., Crameri R. Phage display of human antibodies from a patient suffering from coeliac disease and selection of isotype-specific scFv against gliadin. Immunology. 2003;110(2):269–274. doi: 10.1046/j.1365-2567.2003.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann N., Thormann V., Hu B., Köhler A.-B., Imai-Matsushima A., Locht C., Arnett E., Schlesinger L.S., Zoller T., Schürmann M., Kaufmann S.H., Wardemann H. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol. Med. 2016;8(11):1325–1339. doi: 10.15252/emmm.201606330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018;18(1):46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hjelholt A., Christiansen G., Sørensen U.S., Birkelund S. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog. Dis. 2013;67(3):206–213. doi: 10.1111/2049-632X.12034. [DOI] [PubMed] [Google Scholar]
- 32.Janeway C.A., Jr., Travers P., Walport M., Shlomchik M.J. The Immune System in Health and Disease. 5th edition. Garland Science; 2001. The distribution and functions of immunoglobulin isotypes, immunobiology. [Google Scholar]
- 33.Cerutti A., Chen K., Chorny A. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte J.H. Functional switching. Nat. Immunol. 2016;17(1):S12. [Google Scholar]
- 35.Herich R. Is the role of IgA in local immunity completely known? Food Agric. Immunol. 2017;28(2):223–237. [Google Scholar]
- 36.Negrao-Correa D. Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal nematode infection: looking at the intestinal mucosae. Rev. Inst. Med. Trop. Sao Paulo. 2001;43:291–299. doi: 10.1590/s0036-46652001000500011. [DOI] [PubMed] [Google Scholar]
- 37.Duarte J., Deshpande P., Guiyedi V., Mécheri S., Fesel C., Cazenave P.-A., Mishra G.C., Kombila M., Pied S. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar. J. 2007;6(1):1. doi: 10.1186/1475-2875-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso A.I., Sixt N., Vallier A., Fayolle J., Buckland R., Wild T.F. Measles virus DNA vaccination: antibody isotype is determined by the method of immunization and by the nature of both the antigen and the coimmunized antigen. J. Virol. 1998;72(3):2516–2518. doi: 10.1128/jvi.72.3.2516-2518.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isa M.a.B.z., Martínez L., Giordano M., Zapata M., Passeggi C., De Wolff M.a.C., Nates S. Measles virus-specific immunoglobulin G isotype immune response in early and late infections. J. Clin. Microbiol. 2001;39(1):170. doi: 10.1128/JCM.39.1.170-174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ethel C.S., Dolores B., Patricia B., Viviana N.S., Victorio P.J. Isotype immune response of IgG antibodies at the persistence and reactivation stages of human herpes virus 6 infection. J. Clin. Virol. 2004;31(4):266–269. doi: 10.1016/j.jcv.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Ferreyra L., Bustos D., Biganzoli P., Isa M.B., Don P.S., Ribechini E., Nates S.V., Pavan J.V. HHV-6 IgG4 isotype response following measles infection. J. Med. Virol. 2010;82(3):396–399. doi: 10.1002/jmv.21702. [DOI] [PubMed] [Google Scholar]
- 42.Hou D., Chen C., Seely E.J., Chen S., Song Y. High-throughput sequencing-based immune repertoire study during infectious disease. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerner R.A. Combinatorial antibody libraries: new advances, new immunological insights. Nat. Rev. Immunol. 2016;16(8):498–508. doi: 10.1038/nri.2016.67. [DOI] [PubMed] [Google Scholar]
- 44.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363(6428):446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 45.Greenberg A.S., Avila D., Hughes M., Hughes A., McKinney E.C., Flajnik M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374(6518):168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 46.Dumoulin M., Conrath K., Van Meirhaeghe A., Meersman F., Heremans K., Frenken L.G.J., Muyldermans S., Wyns L., Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11(3):500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffiths K., Dolezal O., Parisi K., Angerosa J., Dogovski C., Barraclough M., Sanalla A., Casey J.L., González I., Perugini M.A., Nuttall S., Foley M. Shark variable new antigen receptor (VNAR) single domain antibody fragments: stability and diagnostic applications. Antibodies. 2013;2(1) [Google Scholar]
- 48.Desmyter A., Transue T.R., Ghahroudi M.A., Dao Thi M.-H., Poortmans F., Hamers R., Muyldermans S., Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat. Struct. Biol. 1996;3(9):803–811. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen V.K., Hamers R., Wyns L., Muyldermans S. Camel heavy-chain antibodies: diverse germline V(H)H and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 2000;19(5):921–930. doi: 10.1093/emboj/19.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Sapienza G., Rossotti M.A., Tabares-da Rosa S. Single-domain antibodies as versatile affinity reagents for analytical and diagnostic applications. Front. Immunol. 2017;8(977) doi: 10.3389/fimmu.2017.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romão E., Poignavent V., Vincke C., Ritzenthaler C., Muyldermans S., Monsion B. Construction of high-quality camel immune antibody libraries. Methods Mol. Biol. (Clifton, N.J.) 2018;1701:169–187. doi: 10.1007/978-1-4939-7447-4_9. [DOI] [PubMed] [Google Scholar]
- 52.Kurosaki T., Kometani K., Ise W. Memory B cells. Nat. Rev. Immunol. 2015;15:149. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 53.Persson M., Caothien R.H., Burton D.R. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc. Natl. Acad. Sci. 1991;88(6):2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amersdorfer P., Wong C., Smith T., Chen S., Deshpande S., Sheridan R., Marks J.D. Genetic and immunological comparison of anti-botulinum type A antibodies from immune and non-immune human phage libraries. Vaccine. 2002;20(11):1640–1648. doi: 10.1016/s0264-410x(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 55.Miethe S., Rasetti-Escargueil C., Liu Y., Chahboun S., Pelat T., Avril A., Frenzel A., Schirrmann T., Thullier P., Sesardic D., Hust M. Development of neutralizing scFv-Fc against botulinum neurotoxin A light chain from a macaque immune library. mAbs. 2014;6(2):446–459. doi: 10.4161/mabs.27773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DiGiandomenico A., Warrener P., Hamilton M., Guillard S., Ravn P., Minter R., Camara M.M., Venkatraman V., MacGill R.S., Lin J., Wang Q., Keller A.E., Bonnell J.C., Tomich M., Jermutus L., McCarthy M.P., Melnick D.A., Suzich J.A., Stover C.K. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012;209(7):1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenzel E.V., Bosnak M., Tierney R., Schubert M., Brown J., Dübel S., Efstratiou A., Sesardic D., Stickings P., Hust M. Human antibodies neutralizing diphtheria toxin in vitro and in vivo. Sci. Rep. 2020;10(1):571. doi: 10.1038/s41598-019-57103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang-Lin S.X., Balthasar J.P. Pharmacokinetic and pharmacodynamic considerations for the use of monoclonal antibodies in the treatment of bacterial infections. Antibodies (Basel) 2018;7(1):5. doi: 10.3390/antib7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McConnell M.J. Where are we with monoclonal antibodies for multidrug-resistant infections? Drug Discov. Today. 2019;24(5):1132–1138. doi: 10.1016/j.drudis.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Men R., Yamashiro T., Goncalvez A.P., Wernly C., Schofield D.J., Emerson S.U., Purcell R.H., Lai C.-J. Identification of chimpanzee Fab fragments by repertoire cloning and production of a full-length humanized immunoglobulin G1 antibody that is highly efficient for neutralization of dengue type 4 virus. J. Virol. 2004;78(9):4665–4674. doi: 10.1128/JVI.78.9.4665-4674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruyama T., Rodriguez L.L., Jahrling P.B., Sanchez A., Khan A.S., Nichol S.T., Peters C.J., Parren P.W.H.I., Burton D.R. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 1999;73(7):6024. doi: 10.1128/jvi.73.7.6024-6030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodchild S.A., Dooley H., Schoepp R.J., Flajnik M., Lonsdale S.G. Isolation and characterisation of Ebolavirus-specific recombinant antibody fragments from murine and shark immune libraries. Mol. Immunol. 2011;48(15):2027–2037. doi: 10.1016/j.molimm.2011.06.437. [DOI] [PubMed] [Google Scholar]
- 63.Zebedee S.L., Barbas C.F., Hom Y.L., Caothien R.H., Graff R., DeGraw J., Pyati J., LaPolla R., Burton D.R., Lerner R.A. Human combinatorial antibody libraries to hepatitis B surface antigen. Proc. Natl. Acad. Sci. 1992;89(8):3175. doi: 10.1073/pnas.89.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen W.-H., Qin W.-J., Gao H., Zhao J., Jia L.-T., Liao Q.-H., Meng Y.-L., Jin B.-Q., Yao L.-B., Chen S.-Y., Yang A.-G. An hepatitis B virus surface antigen specific single chain of variable fragment derived from a natural immune antigen binding fragment phage display library is specifically internalized by HepG2.2.15 cells. J. Viral Hepat. 2007;14(7):512–519. doi: 10.1111/j.1365-2893.2007.00843.x. [DOI] [PubMed] [Google Scholar]
- 65.Li C.-Z., Liang Z.-K., Chen Z.-R., Lou H.-B., Zhou Y., Zhang Z.-H., Yu F., Liu S., Zhou Y., Wu S., Zheng W., Tan W., Jiang S., Zhou C. Identification of HBsAg-specific antibodies from a mammalian cell displayed full-length human antibody library of healthy immunized donor. Cell. Mol. Immunol. 2011;9:184. doi: 10.1038/cmi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burton D.R., Barbas C.F., 3rd, Persson M.A., Koenig S., Chanock R.M., Lerner R.A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. U. S. A. 1991;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Björling E., Garrelts E. von, Mörner A., Ehnlund M., Persson M.A. Human neutralizing human immunodeficiency virustype 2-specific Fab molecules generated by phage display. J. Gen. Virol. 1999;80(8):1987–1993. doi: 10.1099/0022-1317-80-8-1987. [DOI] [PubMed] [Google Scholar]
- 68.Bowley D.R., Labrijn A.F., Zwick M.B., Burton D.R. Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage. Protein Eng. Des. Sel. 2007;20(2):81–90. doi: 10.1093/protein/gzl057. [DOI] [PubMed] [Google Scholar]
- 69.Choudhry V., Zhang M.-Y., Sidorov I.A., Louis J.M., Harris I., Dimitrov A.S., Bouma P., Cham F., Choudhary A., Rybak S.M. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007;363(1):79–90. doi: 10.1016/j.virol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strokappe N., Szynol A., Aasa-Chapman M., Gorlani A., Quigley A.F., Hulsik D.L., Chen L., Weiss R., de Haard H., Verrips T. Llama antibody fragments recognizing various epitopes of the CD4bs neutralize a broad range of HIV-1 subtypes A, B and C. PLoS One. 2012;7(3):e33298. doi: 10.1371/journal.pone.0033298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Throsby M., van den Brink E., Jongeneelen M., Poon L.L.M., Alard P., Cornelissen L., Bakker A., Cox F., van Deventer E., Guan Y., Cinatl J., Meulen J.t., Lasters I., Carsetti R., Peiris M., de Kruif J., Goudsmit J. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3(12) doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Carvalho Nicacio C., Williamson R.A., Parren P.W.H.I., Lundkvist A., Burton D.R., Björling E. Neutralizing human Fab fragments against measles virus recovered by phage display. J. Virol. 2002;76(1):251–258. doi: 10.1128/JVI.76.1.251-258.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer R.A., Marissen W.E., Goudsmit J., Visser T.J., Clijsters-Van der Horst M., Bakker A.Q., de Jong M., Jongeneelen M., Thijsse S., Backus H.H., Rice A.B., Weldon W.C., Rupprecht C.E., Dietzschold B., Bakker A.B., de Kruif J. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur. J. Immunol. 2005;35(7):2131–2145. doi: 10.1002/eji.200526134. [DOI] [PubMed] [Google Scholar]
- 74.Barbas C.F., Crowe J.E., Cababa D., Jones T.M., Zebedee S.L., Murphy B.R., Chanock R.M., Burton D.R. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc. Natl. Acad. Sci. 1992;89(21) doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marasco W.A., Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007;25(12):1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kashyap A.K., Steel J., Oner A.F., Dillon M.A., Swale R.E., Wall K.M., Perry K.J., Faynboym A., Ilhan M., Horowitz M., Horowitz L., Palese P., Bhatt R.R., Lerner R.A. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl. Acad. Sci. U. S. A. 2008;105(16):5986–5991. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwick M.B., Labrijn A.F., Wang M., Spenlehauer C., Saphire E.O., Binley J.M., Moore J.P., Stiegler G., Katinger H., Burton D.R., Parren P.W.H.I. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75(22):10892. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.U.B.B.K. Arbeitskreis Blut Malaria. Transfus. Med. Hemother. 2009;36(1):48–60. doi: 10.1159/000197327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roeffen W.F., Raats J.M., Teelen K., Hoet R.M., Eling W.M., van Venrooij W.J., Sauerwein R.W. Recombinant human antibodies specific for the Pfs48/45 protein of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2001;276(23):19807–19811. doi: 10.1074/jbc.M100562200. [DOI] [PubMed] [Google Scholar]
- 80.Sowa K.M., Cavanagh D.R., Creasey A.M., Raats J., McBride J., Sauerwein R., Roeffen W.F., Arnot D.E. Isolation of a monoclonal antibody from a malaria patient-derived phage display library recognising the Block 2 region of Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 2001;112(1):143–148. doi: 10.1016/s0166-6851(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 81.Deckers N., Saerens D., Kanobana K., Conrath K., Victor B., Wernery U., Vercruysse J., Muyldermans S., Dorny P. Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int. J. Parasitol. 2009;39(5):625–633. doi: 10.1016/j.ijpara.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Hoe L.-N., Wan K.-L., Nathan S. Construction and characterization of recombinant single-chain variable fragment antibodies against Toxoplasma gondii MIC2 protein. Parasitology. 2005;131(6):759–768. doi: 10.1017/S0031182005008450. [DOI] [PubMed] [Google Scholar]
- 83.Rahumatullah A., Abdul Karim I.Z., Noordin R., Lim T.S. Antibody-based protective immunity against helminth infections: antibody phage display derived antibodies against BmR1 antigen. Int. J. Mol. Sci. 2017;18(11):2376. doi: 10.3390/ijms18112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahumatullah A., Ahmad A., Noordin R., Lim T.S. Delineation of BmSXP antibody V-gene usage from a lymphatic filariasis based immune scFv antibody library. Mol. Immunol. 2015;67(2):512–523. doi: 10.1016/j.molimm.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 85.Mazumdar S. Raxibacumab. mAbs. 2009;1(6):531–538. doi: 10.4161/mabs.1.6.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franka R., Carson W.C., Ellison J.A., Taylor S.T., Smith T.G., Kuzmina N.A., Kuzmin I.V., Marissen W.E., Rupprecht C.E. In vivo efficacy of a cocktail of human monoclonal antibodies (CL184) against diverse north American bat rabies virus variants. Trop. Med. Infect. Dis. 2017;2(3):48. doi: 10.3390/tropicalmed2030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saylor C., Dadachova E., Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine. 2009;27(Suppl. 6):G38–G46. doi: 10.1016/j.vaccine.2009.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker L.M., Burton D.R. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018;18(5):297–308. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mechaly A., Zahavy E., Fisher M. Development and implementation of a single-chain Fv antibody for specific detection of <em>Bacillus anthracis</em> spores. Appl. Environ. Microbiol. 2008;74(3):818. doi: 10.1128/AEM.01244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walper S.A., Anderson G.P., Brozozog Lee P.A., Glaven R.H., Liu J.L., Bernstein R.D., Zabetakis D., Johnson L., Czarnecki J.M., Goldman E.R. Rugged single domain antibody detection elements for bacillus anthracis spores and vegetative cells. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0032801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pelat T., Hust M., Laffly E., Condemine F., Bottex C., Vidal D., Lefranc M.P., Dubel S., Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob. Agents Chemother. 2007;51(8):2758–2764. doi: 10.1128/AAC.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Z., Moayeri M., Zhao H., Crown D., Leppla S.H., Purcell R.H. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl. Acad. Sci. 2009;106(32) doi: 10.1073/pnas.0906581106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayhurst A., Happe S., Mabry R., Koch Z., Iverson B.L., Georgiou G. Isolation and expression of recombinant antibody fragments to the biological warfare pathogen Brucella melitensis. J. Immunol. Methods. 2003;276(1):185–196. doi: 10.1016/s0022-1759(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 94.Hussack G., Arbabi-Ghahroudi M., van Faassen H., Songer J.G., Ng K.K.-S., MacKenzie R., Tanha J. Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J. Biol. Chem. 2011;286(11):8961–8976. doi: 10.1074/jbc.M110.198754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H., Yu R., Fang T., Yu T., Chi X., Zhang X., Liu S., Fu L., Yu C., Chen W. Tetanus neurotoxin neutralizing antibodies screened from a human immune scFv antibody phage display library. Toxins. 2016;8(9):266. doi: 10.3390/toxins8090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tremblay J.M., Mukherjee J., Leysath C.E., Debatis M., Ofori K., Baldwin K., Boucher C., Peters R., Beamer G., Sheoran A., Bedenice D., Tzipori S., Shoemaker C.B. A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect. Immun. 2013;81(12):4592–4603. doi: 10.1128/IAI.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reason D.C., Wagner T.C., Lucas A.H. Human Fab fragments specific for the Haemophilus influenzae b polysaccharide isolated from a bacteriophage combinatorial library use variable region gene combinations and express an idiotype that mirrors in vivo expression. Infect. Immun. 1997;65(1):261–266. doi: 10.1128/iai.65.1.261-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reiche N., Jung A., Brabletz T., Vater T., Kirchner T., Faller G. Generation and characterization of human monoclonal scFv antibodies against Helicobacter pylori antigens. Infect. Immun. 2002;70(8):4158–4164. doi: 10.1128/IAI.70.8.4158-4164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berger S., Hinz D., Bannantine J.P., Griffin J.F.T. Isolation of high-affinity single-chain antibodies against Mycobacterium avium subsp. paratuberculosis surface proteins from sheep with Johne's disease. Clin. Vaccine Immunol. 2006;13(9):1022–1029. doi: 10.1128/CVI.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamidon N.H., Suraiya S., Sarmiento M.E., Acosta A., Norazmi M.N., Lim T.S. Immune TB antibody phage display library as a tool to study B cell immunity in TB infections. Appl. Biochem. Biotechnol. 2018;184(3):852–868. doi: 10.1007/s12010-017-2582-5. [DOI] [PubMed] [Google Scholar]
- 101.Singh P.K., Agrawal R., Kamboj D.V., Gupta G., Boopathi M., Goel A.K., Singh L. Construction of a single-chain variable-fragment antibody against the superantigen staphylococcal enterotoxin B. Appl. Environ. Microbiol. 2010;76(24):8184–8191. doi: 10.1128/AEM.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foord A.J., Muller J.D., Yu M., Wang L.-F., Heine H.G. Production and application of recombinant antibodies to foot-and-mouth disease virus non-structural protein 3ABC. J. Immunol. Methods. 2007;321(1–2):142–151. doi: 10.1016/j.jim.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 103.Pereira S.S., Moreira-Dill L.S., Morais M.S., Prado N.D., Barros M.L., Koishi A.C., Mazarrotto G.A., Gonçalves G.M., Zuliani J.P., Calderon L.A. Novel camelid antibody fragments targeting recombinant nucleoprotein of Araucaria hantavirus: a prototype for an early diagnosis of hantavirus pulmonary syndrome. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koch J., Liang M., Queitsch I., Kraus A.A., Bautz E.K.F. Human recombinant neutralizing antibodies against hantaan virus G2 protein. Virology. 2003;308(1):64–73. doi: 10.1016/s0042-6822(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 105.Schofield D., Satterfield W., Emerson S., Purcell R. Four chimpanzee monoclonal antibodies isolated by phage display neutralize hepatitis A virus. Virology. 2002;292(1):127–136. doi: 10.1006/viro.2001.1252. [DOI] [PubMed] [Google Scholar]
- 106.Chan S.-W., Bye J.M., Jackson P., Allain J.-P. Human recombinant antibodies specific for hepatitis C virus core and envelope E2 peptides from an immune phage display library. J. Gen. Virol. 1996;77(10):2531–2539. doi: 10.1099/0022-1317-77-10-2531. [DOI] [PubMed] [Google Scholar]
- 107.Barban V., Fraysse-Corgier S., Paranhos-Baccala G., Petit M., Manin C., Berard Y., Prince A., Mandrand B., Meulien P. Identification of a human epitope in hepatitis C virus (HCV) core protein using a molecularly cloned antibody repertoire from a non-symptomatic, anti-HCV-positive patient. J. Gen. Virol. 2000;81(2):461–469. doi: 10.1099/0022-1317-81-2-461. [DOI] [PubMed] [Google Scholar]
- 108.Schofield D., Glamann J., Emerson S., Purcell R. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000;74(12):5548–5555. doi: 10.1128/jvi.74.12.5548-5555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burioni R., Williamson R.A., Sanna P.P., Bloom F.E., Burton D.R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc. Natl. Acad. Sci. 1994;91(1):355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cattani P., Rossolini G.M., Cresti S., Santangelo R., Burton D.R., Williamson R.A., Sanna P.P., Fadda G. Detection and typing of herpes simplex viruses by using recombinant immunoglobulin fragments produced in bacteria. J. Clin. Microbiol. 1997;35(6):1504–1509. doi: 10.1128/jcm.35.6.1504-1509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trott M., Weiß S., Antoni S., Koch J., von Briesen H., Hust M., Dietrich U. Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: a neutralizing V3- and a trimer-specific gp41 antibody. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ekiert D.C., Kashyap A.K., Steel J., Rubrum A., Bhabha G., Khayat R., Lee J.H., Dillon M.A., O'Neil R.E., Faynboym A.M., Horowitz M., Horowitz L., Ward A.B., Palese P., Webby R., Lerner R.A., Bhatt R.R., Wilson I.A. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489:526. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arakawa M., Yamashiro T., Uechi G.i., Tadano M., Nishizono A. Construction of human fab (y1/k) library and identification of human monoclonal fab possessing neutralizing potency against Japanese encephalitis virus. Microbiol. Immunol. 2007;51(6):617–625. doi: 10.1111/j.1348-0421.2007.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 114.Froude J.W., Pelat T., Miethe S., Zak S.E., Wec A.Z., Chandran K., Brannan J.M., Bakken R.R., Hust M., Thullier P., Dye J.M. Generation and characterization of protective antibodies to Marburg virus. mAbs. 2017;9(4):696–703. doi: 10.1080/19420862.2017.1299848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Z., Chumakov K., Dragunsky E., Kouiavskaia D., Makiya M., Neverov A., Rezapkin G., Sebrell A., Purcell R. Chimpanzee-human monoclonal antibodies for treatment of chronic poliovirus excretors and emergency postexposure prophylaxis. J. Virol. 2011;85(9):4354–4362. doi: 10.1128/JVI.02553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee Y.-C., Leu S.-J.C., Hu C.-J., Shih N.-Y., Huang I.-J., Wu H.-H., Hsieh W.-S., Chiang B.-L., Chiu W.-T., Yang Y.-Y. Chicken single-chain variable fragments against the SARS-CoV spike protein. J. Virol. Methods. 2007;146(1–2):104–111. doi: 10.1016/j.jviromet.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duan J., Ji X., Feng J., Han W., Zhang P., Cao W., Guo X., Qi C., Yang D., Jin G., Gao G., Yan X. A human neutralizing antibody against a conformational epitope shared by oligomeric SARS S1 protein. Antivir. Ther. 2006;11(1):117–123. [PubMed] [Google Scholar]
- 118.Liang M.-f., Du R.-l., Liu J.-z., Li C., Zhang Q.-f., Han L.-l., Yu J.-s., Duan S.-m., Wang X.-f., Wu K.-x. SARS patients-derived human recombinant antibodies to S and M proteins efficiently neutralize SARS-coronavirus infectivity. Biomed. Environ. Sci. 2005;18(6):363. [PubMed] [Google Scholar]
- 119.Duggan J.M., Coates D.M., Ulaeto D.O. Isolation of single-chain antibody fragments against Venezuelan equine encephalomyelitis virus from two different immune sources. Viral Immunol. 2001;14(3):263–273. doi: 10.1089/088282401753266774. [DOI] [PubMed] [Google Scholar]
- 120.Throsby M., Geuijen C., Goudsmit J., Bakker A.Q., Korimbocus J., Kramer R.A., Clijsters-van der Horst M., de Jong M., Jongeneelen M., Thijsse S., Smit R., Visser T.J., Bijl N., Marissen W.E., Loeb M., Kelvin D.J., Preiser W., ter Meulen J., de Kruif J. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J. Virol. 2006;80(14):6982. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duan T., Ferguson M., Yuan L., Xu F., Li G. Human monoclonal Fab antibodies against West Nile virus and its neutralizing activity analyzed in vitro and in vivo. J. Antivir. Antiretrovir. 2009;1(1):036. doi: 10.4172/jaa.1000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S.-H., Hwang S.-Y., Lee Y.-S., Choi I.-H., Park S.-G., Kho W.-G. Single-chain antibody fragment specific for Plasmodium vivax Duffy binding protein. Clin. Vaccine Immunol. 2007;14(6):726. doi: 10.1128/CVI.00456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]