Abstract
Purpose
To clarify the epidemiological, clinical, and therapeutic features of patients with severe COVID-19.
Methods
In this study, we enrolled 681 patients with confirmed cases of severe COVID-19. The epidemiological, demographic, clinical, laboratory, treatment, and outcome data were collected.
Results
The median age of the study participants was 65 years, 53.2% were male, and 104 (15.3%) died. Age, Neutrophil-To-Lymphocyte Ratio (NLR), acute myocardial injury, and levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), and CD3 T cells counts were independently associated with death, while arbidol and ribavirin were protective from death. The combination of NLR and acute myocardial injury on admission (AUC = 0.914) predicted mortality better than NLR, CRP, LDH, and acute myocardial injury. There were 312 (45.8%) patients with cardiovascular disease, of whom 23.4% died. β-blockers, ACEI/ARB, arbidol, and ribavirin might have a beneficial effect for severe COVID-19 patients with cardiovascular disease.
Conclusion
The combination of NLR and acute myocardial injury on admission was highly predictive of mortality and survival. Clinicians should adopt more aggressive strategies for patients with a high NLR (>6.66) combined with myocardial injury. β-blockers and ACEI/ARB, as well as arbidol and ribavirin, were effective in COVID-19 patients with cardiovascular disease.
Keywords: SARS-CoV-2, COVID-19, Clinical characteristics, Treatments, Cardiovascular disease
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CAD, coronary artery disease; CKD, chronic kidney disease; CK-MB, creatine kinase-myocardial isoenzyme; COPD, chronic obstructive pulmonary disease; COVID, coronavirus disease; CRP, C-reactive protein; cTnI, cardiac troponin I; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; IFN-α, Interferon-α; IL-6, Interleukin-6; LDH, lactate dehydrogenase; LDL-C, low density lipoprotein cholesterol; NLR, Neutrophil-To-Lymphocyte Ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride
Highlights
-
•
Combination of NLR and acute myocardial injury could predict mortality.
-
•
Patients combined with higher NLR and myocardial injury need aggressive strategies.
-
•
Arbidol and ribavirin may be benefit to severe COVID-19.
-
•
β-blockers and ACEI/ARB are effective for patients with cardiovascular disease.
1. Introduction
COVID-19, caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), first emerged in Wuhan, China in December of 2019. It has since spread rapidly throughout most of the world and become a global public health crisis. As of April 24, 2020, there have been more than 2.5 million confirmed cases and 160,000 deaths, and these numbers are continuing to rise.
COVID-19 is a highly infectious disease spread via human-to-human transmission, including through droplets, direct contact, and ocular tissues. Each patient can spread the virus to two to three new people [1]. SARS-CoV-2-infected patients are infectious even during the asymptomatic stage, which differs from SARS-CoV [1]. Although the majority of patients infected by SARS-CoV-2 have mild symptoms, the viruses can cause severe lung pneumonia, acute respiratory distress syndrome (ARDS), multiple organ failure, and death [2].
People with mild cases may only require early isolation and close follow-up [3]. For more severe cases, aggressive treatment and intensive care may be urgently needed. To date, the largest case series study enrolled 1591 COVID-19 patients who required ICU admission in Italy [4]. However, the majority (58%) of patients remained in the hospital, and there was no extra description of their medical therapy [4]. In severe cases, the disease progressed quickly while data guiding management remains limited and scarce. In China, Renmin Hospital of Wuhan University was a designated treatment hospital for patients with COVID-19. In this study, we recruited 681 people with confirmed, severe cases of COVID-19 with a clear endpoint (death or discharge) to clarify their epidemiological, clinical, and therapeutic features.
2. Methods
2.1. Study population
This retrospective, cohort study included 681 adult patients (≥18 years old) with COVID-19 who were admitted to the Renmin Hospital of Wuhan University in Wuhan, China, from January 3, 2020, to April 9, 2020. Patients were diagnosed with COVID-19 according to the guidelines issued by the China National Health Commission. All enrolled individuals met at least one of the following criteria: (1) symptoms of respiratory distress, with a respiratory rate ≥30 times/min; (2) a resting state blood oxygen saturation of ≤93%; (3) an oxygenation index (PaO2/FiO2) of ≤300 mmHg (1 mmHg = 0.133 kPa); or (4) lung imaging showing more than 50% of lesions progressing within 24–48 h. The Research Ethics Commission of Qilu Hospital of Shandong University and Renmin Hospital of Wuhan University approved this protocol.
2.2. Data collection
The primary outcomes were all-cause mortality during hospitalization and discharge. The outcomes were used to divide participants into two groups: survivors and non-survivors. Two physicians reviewed the electronic medical records and extracted the epidemiological, demographic, clinical, laboratory, treatment, and outcome data of all patients. All data were confirmed by a third researcher. Neutrophil-To-Lymphocyte Ratio (NLR) was calculated as absolute neutrophil counts divided by absolute lymphocyte counts. Acute myocardial injury was diagnosed if serum levels of cardiac troponin I (cTnI) were above the upper limit of the reference range (>0.04 ng/ml).
2.3. Statistical analysis
All statistical analysis was performed using SPSS Statistics 26.0 (IBM Corp.) and MedCalc (MedCalc Software bvba, Ostend, Belgium). Data are presented as median (IQR) for continuous variables and n (%) for categorical variables. A Mann-Whitney U test, chi-square test, or Fisher's exact test were used to compare the differences between survivors and non-survivors. A P value of <0.05 was considered statistically significant. We used univariable and multivariable logistic regression models to explore the risk factors associated with outcomes. Receiver operating characteristic (ROC) curves were constructed to evaluate the sensitivity and specificity of the parameters. A crossover analysis was performed according to the research of D.W. Hosmer. To minimize bias by confounding, propensity score (PS) matching (1:2 match, caliper 0.2) was used to adjust for imbalances of clinically-relevant parameters that differed between the two groups. Kaplan–Meier curves were used to determine the survival probability.
3. Results
3.1. Characteristics and laboratory findings of patients
The demographic data, clinical characteristics, and laboratory findings of patients were shown in Table 1 . The median age of all subjects was 65.0 years (IQR 54.0–72.0), ranging from 27 to 98 years old. Males accounted for 53.2% of the subjects. Hypertension (43.0%) was the most common comorbidity, followed by diabetes (16.7%) and coronary artery disease (CAD, 11.7%). The main clinical symptoms were fever (85.9%), dry cough (67.8%), and fatigue (51.9%).
Table 1.
Demographic, clinical, and laboratory findings of patients with COVID-19.
| Total patients (n = 681) | Survivors (n = 577) | Non-survivors (n = 104) | P value | |
|---|---|---|---|---|
| Age (years) | 65.0 (54.0–72.0) | 63.0 (52.0–70.0) | 72.5 (65.0–80.8) | 0.000 |
| <40 years old | 46 (6.8%) | 43 (7.5%) | 3 (2.9%) | 0.088 |
| 40–65 years old | 314 (46.1%) | 289 (50.1%) | 25 (24.0%) | 0.000 |
| >65 years old | 321 (47.1%) | 245 (42.5%) | 76 (73.1%) | 0.000 |
| Gender | ||||
| Male | 362 (53.2%) | 297 (51.5%) | 65 (62.5%) | 0.038 |
| Female | 319 (46.8%) | 280 (48.5%) | 39 (37.5%) | |
| Smoking | 29 (4.3%) | 26 (4.5%) | 3 (3.1%) | 0.706 |
| Signs and symptoms | ||||
| Fever | 584 (85.9%) | 494 (85.8%) | 90 (86.5%) | 0.835 |
| Dry cough | 462 (67.8%) | 397 (68.8%) | 65 (62.5%) | 0.205 |
| Fatigue | 352 (51.9%) | 299 (52.1%) | 53 (51.0%) | 0.832 |
| Dyspnea | 123 (18.1%) | 95 (16.5%) | 28 (26.9%) | 0.011 |
| Diarrhea | 119 (17.5%) | 104 (18.1%) | 15 (14.4%) | 0.366 |
| Palpitation | 17 (2.5%) | 15 (2.6%) | 2 (1.9%) | 0.944 |
| Comorbidity | ||||
| Hypertension | 293 (43.0%) | 227 (39.3%) | 66 (63.5%) | 0.000 |
| Diabetes | 114 (16.7%) | 96 (16.6%) | 18 (17.3%) | 0.866 |
| CAD | 80 (11.7%) | 55 (9.5%) | 25 (24.0%) | 0.000 |
| Cerebral infarction | 33 (4.8%) | 20 (3.5%) | 13 (12.5%) | 0.000 |
| CKD | 27 (4.0%) | 20 (3.5%) | 7 (6.7%) | 0.194 |
| COPD | 15 (2.2%) | 13 (2.3%) | 2 (1.9%) | 1.000 |
| Laboratory findings | ||||
| White blood cells (109/L) | 5.90 (4.39–7.94) | 5.66 (4.27–7.39) | 8.25 (5.60–12.45) | 0.000 |
| Neutrophils (109/L) | 4.17 (2.81–6.26) | 3.83 (2.70–5.50) | 7.36 (4.77–11.41) | 0.000 |
| Lymphocytes (109/L) | 0.97 (0.68–1.42) | 1.05 (0.73–1.49) | 0.56 (0.35–0.87) | 0.000 |
| NLR | 3.84 (2.29–8.24) | 3.38 (2.14–6.33) | 12.27 (7.73–24.50) | 0.000 |
| Hemoglobin (g/L) | 123.0 (112.0–134.0) | 123.0 (112.5–134.0) | 127.0 (108.5–138.0) | 0.491 |
| Platelet (109/L) | 214.0 (161.5–279.5) | 222.0 (168.5–290.0) | 173.0 (118.0–227.5) | 0.000 |
| CRP (mg/L) | 40.1 (7.4–82.3) | 29.4 (5.0–68.6) | 101.0 (59.1–180.7) | 0.000 |
| IL-6 (pg/mL) | 6.08 (1.89–20.00) | 4.43 (1.50–15.33) | 78.90 (26.47–118.28) | 0.000 |
| ALT (U/L) | 25.0 (17.0–45.5) | 26.0 (16.0–46.0) | 24.0 (18.0–42.0) | 0.747 |
| AST (U/L) | 30.0 (20.0–43.5) | 28.0 (20.0–40.0) | 41.0 (25.5–62.5) | 0.000 |
| Creatinine (μmol/L) | 61.0 (50.0–74.5) | 60.0 (50.0–72.0) | 73.0 (54.0–112.0) | 0.000 |
| LDH (U/L) | 286.0 (217.0–398.5) | 273.0 (212.0–349.0) | 477.0 (352.0–652.0) | 0.000 |
| CK-MB (ng/mL) | 1.09 (0.70–2.00) | 0.99 (0.66–1.51) | 3.27 (1.72–5.95) | 0.000 |
| cTnI (ng/mL) | 0.00 (0.00–0.03) | 0.00 (0.00–0.01) | 0.15 (0.03–0.78) | 0.000 |
| Acute myocardial injury | 139 (20.4%) | 64 (11.1%) | 75 (72.1%) | 0.000 |
| BNP elevation | 78 (13.6%) | 38 (8.0%) | 40 (42.1%) | 0.000 |
| CD3 count (/μL) | 558.0 (356.0–858.8) | 611.0 (409.5–905.5) | 287.0 (177.0–433.0) | 0.000 |
| CD4 count (/μL) | 349.5 (205.0–542.8) | 387.0 (237.5–576.5) | 172.0 (106.0–283.0) | 0.000 |
| CD8 count (/μL) | 200.0 (107.3–311.5) | 209.0 (123.0–325.0) | 77.0 (48.0–147.0) | 0.000 |
Data were presented as n (%) or median (IQR).
P values are comparing Survivor and Non-survivor. CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; IL-6, Interleukin-6; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK-MB, creatine kinase-myocardial isoenzyme.; cTnI, cardiac troponin I; BNP, brain natriuretic peptide.
Compared with the survivors, non-survivors were older, more male, and had a higher heart rate and systolic blood pressure (SBP) (P < .05). Non-survivors had higher hypertension, CAD, and cerebral infarction comorbidity rates (P < .001), as well as significantly higher white blood cell and neutrophil counts and CRP, interleukin-6 (IL-6), NLR, aspartate aminotransferase (AST), creatinine, blood glucose, triglycerides (TG), LDH, creatine kinase-myocardial isoenzyme (CK-MB), and cTnI levels. Lymphocyte, platelet, CD3, CD4, CD8 counts and total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were lower (P < .05) in non-survivors. There were also more cases of acute myocardial injury (72.1% vs. 11.1%) and BNP elevation (42.1% vs. 8.0%) among non-survivors as compared to the survivors. There was no significant difference in diastolic blood pressure (DBP), aspartate aminotransferase (ALT), potassium, sodium, and hemoglobin between the two groups (Table 1, Supplementary Table1).
3.2. Interventions and clinical outcomes
Oxygen support was administered to patients according to hypoxemia severity. Non-survivors received more noninvasive and invasive ventilation (P < .01). Among the total 681 patients, 666 (97.8%) received antiviral treatment, including arbidol, ribavirin, ganciclovir, or oseltamivir, etc. The proportion of patients who received antibiotic therapy or immunoglobulin and glucocorticoid treatment was 83.8%, 54.6%, and 48.8%, respectively. Compared with the survivors, non-survivors used more antibiotics, glucocorticoids, immunoglobulin, and antifungal drugs, while less arbidol was administered (P < .05) (Table 2 ).
Table 2.
Interventions of patients with COVID-19.
| Total patients (n = 681) | Survivors (n = 577) | Non-survivors (n = 104) | P value | |
|---|---|---|---|---|
| Oxygen support | ||||
| Nasal cannula | 476 (70.1%) | 442 (76.9%) | 34 (32.7%) | 0.000 |
| Non-invasive ventilation | 164 (24.2%) | 125 (21.8%) | 39 (37.5%) | 0.001 |
| Invasive mechanical ventilation | 38 (5.6%) | 7 (1.2%) | 31 (29.8%) | 0.000 |
| Treatment | ||||
| Antiviral therapy | 666 (97.8%) | 566 (98.1%) | 100 (96.2%) | 0.380 |
| Arbidol | 571 (83.8%) | 500 (86.7%) | 71 (68.3%) | 0.000 |
| Ribavirin | 279 (41.0%) | 245 (42.5%) | 34 (32.7%) | 0.060 |
| Ganciclovir | 92 (13.5%) | 72 (12.5%) | 20 (19.2%) | 0.064 |
| Oseltamivir | 261 (38.4%) | 218 (37.8%) | 43(41.7%) | 0.446 |
| IFN-α | 119 (17.5%) | 96 (16.6%) | 23 (22.1%) | 0.176 |
| Antibiotic therapy | 571 (83.8%) | 470 (81.5%) | 101 (97.1%) | 0.000 |
| Glucocorticoids | 332 (48.8%) | 266 (46.2%) | 66 (63.5%) | 0.001 |
| Immunoglobulin | 372 (54.6%) | 305 (52.9%) | 67 (64.4%) | 0.029 |
| Vitamin C | 214 (31.4%) | 175 (30.3%) | 39 (37.5%) | 0.147 |
| Antifungal drugs | 19 (2.8%) | 10 (1.7%) | 9 (8.7%) | 0.000 |
IFN-α, Interferon-α;
3.3. Independent predictors of death
We performed a multivariable logistic regression to determine the parameters associated with death in patients with severe cases of COVID-19. We found that NLR (odds ratio (95% CI), 1.057 (1.010–1.107); P = .018) and acute myocardial injury (7.716 (3.812–15.619); P = .000) were independently and negatively associated with death in patients with severe COVID-19. The risk of death increases by 5.7% for every one-unit increase in NLR. In addition, age, CRP, LDH, CD3 counts, arbidol, and ribavirin were independently associated with adverse outcomes (Table 3 ).
Table 3.
Multivariate analysis for predicting the risk of death in COVID-19 patients.
| Characteristics | Multivariate |
|
|---|---|---|
| OR (95%CI) | P | |
| Age (years) | 1.049 (1.019–1.080) | 0.001 |
| Acute myocardial injury | 7.716 (3.812–15.619) | 0.000 |
| NLR | 1.057 (1.010–1.107) | 0.018 |
| CRP (mg/L) | 1.007 (1.001–1.013) | 0.017 |
| LDH (U/L) | 1.003 (1.001–1.005) | 0.002 |
| CD3 count (/μL) | 0.998 (0.996–0.999) | 0.007 |
| Arbidol | 0.280 (0.126–0.625) | 0.002 |
| Ribavirin | 0.477 (0.232–0.982) | 0.044 |
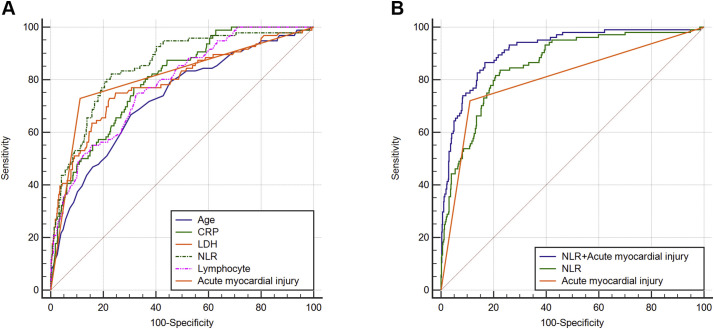
ROC analysis identified an NLR of 6.66 as the optimal cutoff to discriminate between discharge and death outcomes (area under the curve [AUC] [95% CI], 0.860 [0.821–0.898]; P < .001; Youden's index is 0.611). The area under the ROC curve was the largest in NLR, followed by acute myocardial injury (0.805), CRP (0.800), LDH (0.797), lymphocyte counts (0.788), and age (0.726). NLR predicted mortality better than age, LDH, CRP, and lymphocyte levels, suggesting it may be the best predictive parameter (P < .05). Importantly, we found that the predictive effect of a combination of NLR and acute myocardial injury on death was better than that of NLR or acute myocardial injury alone (0.914 [0.884–0.944]; P < .001; Youden's index = 0.697) (P < .001, combined prediction vs. acute myocardial injury, NLR) (Fig. 1 ).
Fig. 1.
ROC analysis of the association between NLR, Acute myocardial injury, and their combination effect in relation to death. A. The ROC curves of age, CRP, LDH, NLR, lymphocyte counts, and acute myocardial injury. B. The ROC curves of NLR, acute myocardial injury, and their combination.
We further analyzed the relationship between survival probability, NLR, and acute myocardial injury. Increased NLR levels and acute myocardial injury reduced the survival probability. There were significant differences between the survival probability when NLR ≤6.66 and NLR >6.66, with or without acute myocardial injury (P < .001, Supplementary Fig. 1). A decision tree was built to predict death using the NLR cutoff threshold and acute myocardial injury as the predictor variable. Patients were split into two groups according to acute myocardial injury and then stratified into two subgroups according to NLR (≤6.66 or >6.66). A total of 91 patients who had an acute myocardial injury and an NLR greater than 6.66 were in the first group, in which the mortality rate was 70.3%. A total of 416 patients with an NLR ≤6.66 who did not have acute myocardial injury were enrolled in the second group, in which only six (1.4%) patients died. The sensitivity of the diagnostic protocol was 0.84, the specificity was 0.77, the positive predictive value was 0.46, and the negative predictive value was 0.96 (Supplementary Fig. 2). The results suggested that a combination of NLR (>6.66) and acute myocardial injury have a better predictive value of death in patients with COVID-19.
We performed a crossover analysis to further clarify whether there was an interaction between NLR and acute myocardial injury and their impact on adverse outcomes. We found that the synergy index (S) was 4.79 (P < .05), suggesting that there was a positive interaction between NLR and acute myocardial injury on death (Supplementary Table 2).
3.4. Primary outcomes in the PS-matched cohort
From the unadjusted analysis, we determined that the clinical status of patients with an NLR >6.66 was worse at admission. To account for the confusion bias between patients with an NLR ≤ 6.66 vs. those with an NLR >6.66, we performed PS matching and eventually had two balanced cohorts. The results indicated that the mortality of patients with an NLR >6.66 was higher compared to those patients with an NLR ≤6.66. The NLR also increased significantly (P < .001, Supplementary Table 3).
3.5. The treatments and outcomes of patients with cardiovascular disease
Among all study subjects, 312 (45.8%) had cardiovascular disease (hypertension or CAD). Of these patients, 73 (23.4%) died. The deceased patients received less antiplatelet drugs, statins, β-blockers, and ACEI/ARB, but used more diuretics. We conducted a multivariable logistic regression in patients combined with COVID-19 and cardiovascular disease to evaluate the efficiency of the intervention. The results showed that age, acute myocardial injury, NLR, CRP, and LDH were still independent risk factors for death in this patient population. Moreover, arbidol and ribavirin were also effective in these patients. Surprisingly, β-blockers and ACEI/ARB were independently and positively associated with decreased mortality and might have a beneficial effect for COVID-19 patients with cardiovascular disease (Table 4 , Supplementary Table 4).
Table 4.
Multivariate analysis of parameters associated with death in patients with cardiovascular disease.
| Characteristics | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age | 1.077 (1.031–1.125) | 0.001 | 1.081 (1.032–1.131) | 0.001 |
| Acute myocardial injury | 5.781 (2.368–14.116) | 0.000 | 7.708 (2.988–19.887) | 0.000 |
| NLR | 1.129 (1.059–1.204) | 0.000 | 1.132 (1.060–1.210) | 0.000 |
| CRP (mg/L) | 1.015 (1.007–1.023) | 0.000 | 1.016 (1.008–1.024) | 0.000 |
| LDH (U/L) | 1.004 (1.001–1.006) | 0.008 | 1.004 (1.001–1.006) | 0.005 |
| β-blockers | 0.255 (0.076–0.853) | 0.026 | ||
| ACEI/ARB | 0.136 (0.035–0.532) | 0.004 | ||
| Arbidol | 0.246 (0.084–0.723) | 0.011 | 0.205 (0.067–0.631) | 0.006 |
| Ribavirin | 0.238 (0.082–0.685) | 0.008 | 0.208 (0.070–0.618) | 0.005 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
4. Discussion
COVID-19 is a serious threat to public health. Those with severe cases may wonder: “Will I live or die?” Therefore, to clarify their clinic characteristics and seek an efficient therapy to improve their survival and decrease the mortality is urgently needed. In this descriptive study, 681 patients with severe, confirmed cases of COVID-19 were enrolled and all of them reached a clear endpoint (death or hospital discharge). To our best knowledge, this is the largest cohort study of severe COVID-19 cases with a definite endpoint in China. First, we found that age, CRP and LDH levels, CD3 counts, NLR, and acute myocardial injury were independently associated with death in severe COVID-19 patients. The combination of NLR and acute myocardial injury was highly predictive of mortality and survival exclusion diagnosis, more so than NLR or acute myocardial injury alone. Second, arbidol and ribavirin may be a beneficial therapy for COVID-19 and may be associated with improved survival rates. Third, β-receptor blocking agents, ACEI/ARB, arbidol, and ribavirin were independently and positively associated with death in patients who had COVID-19 and cardiovascular disease. These treatments might be effective in this patient population.
SARS-CoV-2 infection preferentially afflicts the elderly and those with chronic comorbidities [5]. Compared to survivors, non-survivors were typically older - 73.1% of them were over the age of 65 years, while 2.9% were younger than 40 years old - and had more comorbid conditions. Multivariable logistic regression revealed that older age was independently and negatively associated with death in COVID-19 patients. Meanwhile, 375 patients had underlying comorbidities, with hypertension being the most common, followed by diabetes, and CAD. However, a retrospective study involving 191 patients did not find that comorbidities can independently predict an increased risk of death [6]. Our multivariable regression had similar findings and did not establish an independent predictive value between underlying diseases and mortality.
Although most SARS-CoV-2-infected patients are asymptomatic and have mild cases, severe cases can lead to acute respiratory distress syndrome (ARDS), multiple organ failure, and even death [2]. As of April 20, 2020, the total worldwide case-fatality rate of COVID-19 was 5.2%. However, the mortality was higher in severe and critical patients. One study of 41 patients indicated that 15% of patients died, while the mortality rate increased by 38% in patients requiring ICU admission [5]. In one report in China of 44,672 confirmed cases, including 2087 (5%) critical cases, the overall case-fatality rate was 2.3%, while 49% were critical cases [7]. In another study in Italy of 1591 patients confirmed to have COVID-19, 405 (26%) patients died in ICU [4]. Our mortality rate was 15.3%, and we found that 80% of cases died within 14 days of admission. This heterogeneity may be associated with differences in illness severity.
Viral invasion and rapid replication cause the change of white blood cells and the production of proinflammatory cytokines, resulting in a cytokine storm and pulmonary tissue damage [8]. Elevated inflammatory indicators are predictive of a fatal outcome [9]. Consistent with previous research [10], we found that traditional infection markers, including the number of white blood cells and serum levels of CRP and IL-6, were significantly higher in non-survivors than in survivors. As a specific marker of systemic inflammation and infection, NLR has prognostic value in predicting the 30-day mortality rate in community-acquired pneumonia [11]. In our study, NLR was significantly higher in non-survivors as compared with survivors. Moreover, higher NLR at admission was associated with an increased risk of death.
SARS-CoV-2 can cause acute myocardial injury, which was present in 7.2%–30% of hospitalized patients [9,12]. Acute myocardial injury might be an important cause of severe clinical phenotypes or adverse endpoint events and is considered a predictor of mortality in patients with COVID-19 [13]. In this study, levels of LDH and CK-MB were significantly increased in non-survivors, and 72.1% of non-survivors had elevated serum cTnI levels while 11.1% of survivors did. We found that NLR and acute myocardial injury on admission were the two best predictors of mortality in patients with severe cases of COVID-19. Moreover, the combined diagnostic value of these two indicators was significantly better than either alone. The AUC of NLR and acute myocardial injury combined was significantly higher than that of traditional infection markers, such as CRP, and myocardial injury markers, such as LDH and CK-MB. We built a decision tree that showed a combination of NLR and acute myocardial injury was highly predictive of mortality and survival exclusion diagnosis. Therefore, patients with a high NLR (>6.66) and myocardial injury require more aggressive treatment strategies, including appropriate respiratory support or admission to the ICU.
Impaired immune homeostasis and function are characteristic of severe SARS-CoV-2 infections [20] . Patients with weakened immune systems are more likely to become severely ill [8]. We found both lymphocytes and T cell subset counts (including CD3, CD4, and CD8 T cells) were lower in non-survivors, suggestive of immune function damage. A multivariable logistic regression showed CD3 counts were independently and positively associated with mortality in severe cases of COVID-19. Therefore, a promising treatment strategy for COVID-19 may be to increase the number of immune cells and to recover immune function.
Although physicians want efficient treatments for SARS-CoV-2 infections, there is currently no specific therapeutic drug or vaccine. Antiviral treatment may be promising, antiviral drugs (including arbidol, oseltamivir, lopinavir, and ritonavir, etc.) have been used to treat COVID-19. However, clinical efficacy data is limited. Recently, in one study of 199 patients hospitalized with severe COVID-19, lopinavir-ritonavir treatment showed no benefit beyond standard care, and some patients developed adverse gastrointestinal events during therapy [14]. In an in vitro experiment, arbidol effectively suppressed SARS reproduction [15]. Ribavirin was widely used in Hong Kong during the SARS outbreak [8]. A multivariable regression analysis showed that arbidol and ribavirin may be associated with improved chances of survival and may be beneficial in COVID-19 therapy. IFN-α was recommended in the guidelines issued by the National Health Commission of China, based on the beneficial effect seen during SARS [16]. However, we did not see this efficiency in COVID-19 cases. Therefore, every drug regimen for severe cases should include arbidol or ribavirin. Considering the side effects, it is not recommended to use three or more antiviral drugs at the same time [8]. Many patients, particularly severely ill ones, develop a bacterial infection. As such, antibiotic therapy should be considered.
Coexisting conditions, particularly cardiovascular disease, were common in patients with COVID-19, which might increase the risk of death [17]. A meta-analysis of 1576 patients with COVID-19 indicated that the prevalence of hypertension and CAD was approximately 21.1% and 8.4%, respectively [18]. Patients with cardiovascular disease are more susceptible to severe and fatal SARS-CoV-2 infection, which accounts for a large proportion of COVID-19 deaths [17]. In this study, 45.8% of patients had preexisting cardiovascular disease, of which 23.4% died. During hospitalization, increased cTnI and BNP were observed in 31.1% and 20.5% of patients with cardiovascular disease, respectively. Therefore, medically managing coexisting conditions and preventing cardiac complications should garner more attention. Antiplatelet drugs, statins, β-blockers, and ACEI/ARB have been wildly used to treat cardiovascular diseases. In this study, we assessed the safety and efficiency of these drugs, and we found both β-blockers and ACEI/ARB, as well as arbidol and ribavirin, were independently and positively associated with death in patients with COVID-19 and cardiovascular disease, and might be effective in these patients. Recently, Vaduganathan M et al. indicated that patients with COVID-19 and myocardial injury might have higher early risks after ACEI/ARB withdraw, suggesting ACRI/ARB may play a key role in COVID-19 [19].
There are several limitations to our study. First, as this is a retrospective study, a number of confounding factors may influence the clinical outcomes. Second, the sample size of our study population was not large enough. Further, multicenter studies are required.
5. Conclusion
The combination of NLR and acute myocardial injury on admission was highly predictive of mortality and survival exclusion diagnosis. Patients with a high NLR (>6.66) and myocardial injury require a more aggressive treatment strategy. Arbidol and ribavirin may be beneficial in severe cases of COVID-19. To the patients combined with COVID-19 and cardiovascular disease, β-blockers and ACEI/ARB as well as arbidol and ribavirin were also effective. We are anxiously awaiting the results of randomized, controlled clinical trials currently underway, which may provide therapy recommendations for patients with COVID-19.
Funding
This work was supported by the Program of Introducing Talents of Discipline to Universities [BP 0719033], the State Key Program of National Natural Science of China [81530014] and the International Collaboration and Exchange program of China [81920108003]. These funding had no involvement in study design, collection, analysis and interpretation of data, writing of the report and the decision to submit the article for publication.
Ethics approval and consent to participate
The ethical approval or individual consent was not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Xiao Meng and Yun Zhang participated in study design and study conception. Fangfang Chen and Ming Zhong performed data analysis. Yi Zhang, Kai Zhang and Dezhen Su recruited patients and collected data. Ya Liu checked the data. Fangfang Chen and Xiao Meng drafted the manuscript. All authors provided critical review of the manuscript and approved the final draft for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2020.07.003.
Appendix A. Supplementary data
Supplementary material
References
- 1.Kakodkar P., Kaka N., Baig M. A comprehensive literature review on the clinical presentation, and management of the Pandemic Coronavirus Disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspective. Int J Antimicrob Agents. 2020;2019:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cataudella E., Giraffa C.M., Di Marca S., Pulvirenti A., Alaimo S., Pisano M. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J Am Geriatr Soc. 2017;65:1796–1801. doi: 10.1111/jgs.14894. [DOI] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khamitov R.A., Loginova S.I., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 16.Ströher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F. Severe acute respiratory syndrome–related coronavirus is inhibited by interferon-α. J Infect Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/nejmsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material