Abstract
Purpose of review
3D bioprinting technologies hold significant promise for the generation of engineered cardiac tissue and translational applications in medicine. To generate a clinically relevant-sized tissue, the provisioning of a perfusable vascular network that provides nutrients to cells in the tissue is a major challenge. This review summarizes the recent vascularization strategies for engineering 3D cardiac tissues.
Recent findings
Considerable steps towards the generation of macroscopic sizes for engineered cardiac tissue with efficient vascular networks have been made within the past few years. Achieving a compact tissue with enough cardiomyocytes to provide functionality remains a challenging task. Achieving perfusion in engineered constructs with media that contain oxygen and nutrients at a clinically-relevant tissue sizes remains the next frontier in tissue engineering.
Summary
The provisioning of a functional vasculature is necessary for maintaining a high cell viability and functionality in engineered cardiac tissues. Several recent studies have shown the ability to generate tissues up to a centimeter scale with a perfusable vascular network. Future challenges include improving cell density and tissue size. This requires the close collaboration of a multidisciplinary teams of investigators to overcome complex challenges in order to achieve success.
Keywords: 3D printing, cardiac engineered tissue, vascularization, bioprinting, cardiovascular tissue, cardiomyocyte
Introduction
Cardiovascular diseases are the leading causes of death worldwide.(1,2) Beside drug treatment and interventional strategies, specific surgical therapies for heart failure are now available. These surgical strategies include bypass surgery, valve replacement and the implantation of ventricular assist devices. Despite this, the treatment options that are available for patients with end-stage heart failure remain limited. While heart transplantation is available, a critical shortage of available donor organs remains.(3) To alleviate this supply vs demand mismatch, scientists have enthusiastically embraced the use of 3D bioprinting as an alternative approach to generate functional cardiac tissue. (4,5) Furthermore, due to the limited capacity of the heart to regenerate endogenously (6,7), the number of patients with end-stage heart failure has continued to grow. A major research goal for tissue engineers is to generate functional cardiac tissue constructs that would meet the biological and clinical demands for repairing or replace damaged heart tissue.
In recent years, the ability to differentiate human induced pluripotent stem cells (hiPSCs) into cardiomyocytes (CMs) at high purity has enabled investigators to consider using this theoretically unlimited source of CMs to pursue cardiac tissue engineering.(4,5,8,9) For 3D bioprinting of cardiac tissues, multiple properties need to be fulfilled: Cell viability and function, the use of multiple cell types, composition and physical properties mimicking the complex structure of the extracellular matrix.(11,12) The generation of clinically relevant functional tissues necessitates the provisioning of a vasculature with dynamic flow to provide nutrition and oxygen and remove toxic metabolites.(13,14) While a number of pioneering works in 3D bioprinting have been accomplished thus far, the creation of a vascularized, high cell density, fully contractile cardiac tissue has not been achieved.(15–18) In this review, we aim to provide an overview of current technologies for creating 3D bioprinted vascularized cardiac tissue. First, we describe different bioprinting techniques and their advantages and disadvantages. Next, we review the key studies that have made significant advances in this field in recent years. Finally, we discuss the current limitations and future challenges that need to be overcome to advance the field of 3D bioprinting of cardiac tissues.
3D Bioprinting techniques
Current strategies for 3D printing of cardiac tissue includes inkjet, microextrusion, and laser assisted printing.(15–18)(15,16,18,19) Technical differences within these strategies can affect biological factors such as cell viability, number, and feasibility as they have considerably different preparation time, print speed, and costs (Table 1).(11,20)
Table 1:
Advantages and disadvantages of the main 3D bioprinting techniques
| Inkjet based printing | Extrusion based printing | Laser assisted Printing |
|
|---|---|---|---|
| Advantages | low costs fast print high cell viability low viscosity |
high cell density high resolution |
extremely high resolution high cell viability |
| Disadvantages | low resolution low cell density limited materials pallet |
variable cell viability slow print high shear stress noozle clogging |
expensive long preparation thermal damage possible |
| References | (20,25–27) | (11,12,22,23) | (19,28–32) |
Inkjet-based bioprinting
One of the most common printing technique is inkjet bioprinting.(12) This approach gives users the opportunity to transfer controlled volumes of ink to predefined locations.(29) Initially, inkjet printers were modified versions of 2D ink-jet printers where the ink cartridge has been replaced by biological material and an electronically-controlled elevator stage has been added for z-axis movement.(23) After the development of biocompatible printing materials and adjustment of print resolution and speed, this technique was adapted for 3D bioprinting. Inkjet-based bioprinting can be classified in two groups: (1) thermal and (2) piezoelectric forces-based printers. (28,33–38) In thermal printers, a heater produces pressure pulses to eject droplets from the nozzle.(30,31) These printers are known to generate localized temperatures above 200°C. Surprisingly, the impact of the short increase of this localized heating on the cell viability seems to be negligible.(32,33)
Piezoelectric printers generate acoustic waves which create pressure to eject droplets.(34) An advantage of this technique is the avoidance of the high temperature needed to generate droplets, however, the high frequency produced by piezoelectric printers carry the risk of cell damage.(35) Advantages of inkjet based bioprinting are cost efficiency, wide availability, and high speed and resolution.(20,25–27) However, the downsides of this approach include nozzle clogging and low bioink/cell density.(36,37) Inkjet based bioprinting works with a specific viscosity (3.5–12 mPa/s) to enable the printer to generate droplets.(12) Therefore, the bioink has to be a non-viscous liquid which is associated with a weak infrastructural support for encapsulated cells.(38) To overcome these physical limitations, crosslinking after printing using different methods is required.(39,40) However, toxic side effects of cross-linking may lead to decreased cell viability and function.(41) Alternatively, instead of directly inkjet printing the hydrogel precursors, the print-head can be used to pattern a low viscosity crosslinking agent into a bath, such as depositing droplets of calcium chloride into a vat of alginate (27). This method enables the patterning of tougher hydrogels but is limited to working with a single material and requires the use of an elevator platform in the vat to pattern multiple layers.
Microextrusion-based bioprinting
Microextrusion-based bioprinters extrude continuous lines of biomaterial onto a defined substrate.(31,39,42) These computer-controlled devices most commonly use either pneumatic or mechanical (piston or screw) systems to dispense the biomaterial and allows for a layer-by-layer deposition of biomaterial. The adjustment of printhead or stage height allows the printer to print in the z-axis.(43,44) Microextrusion-based bioprinting (MBB) allows one to use different bioinks/cell types in the same print run via multiple syringes/nozzles.(20) The rheological properties of the biomaterial used for MBB affects cell viability, cell density, and integrity of the scaffold in which the cells are embedded.(31,42) Biomaterials with a higher viscosity provide structural support for encapsulated cells while a very high cell density carries the risk of nozzle clogging.(39,45) Furthermore, while MBB bioinks with a higher shear yield stress and storage modulus can generate more complex 3D structures with higher aspect ratios, the increased shear stress during printing can result in a lower cell viability.(46,47) To overcome this tradeoff, several temperature-sensitive biomaterials have been used as bioinks such that their viscosity can be tuned during printing.(48) Due to the increased cell viability and function is lower (40–80%) compared to inkjet based bioprinting.(34,43,44,49) The development of shear-thinning biomaterials in recent years has demonstrated significant promise to reduce the shear stress and improve cell viability during printing.
Laser-assisted bioprinting
Laser-based bioprinters, particularly Laser-Induced Forward Transfer (LIFT), use a laser pulse which is transmitted on a glass-based ribbon.(25) This ribbon contains an energy absorbing (metallic) layer and a suspension of biological material (e.g. including viable cells).(27,50) The interaction of the laser volatizes the biomaterial and thereby creates a vapor pocket which induces droplet formation targeting the receiving substrate. Advantages of this technique are high cell density and avoidance of nozzle clogging.(28,30,32,43,56) Disadvantages include metallic residues on the receiving substrate and high costs.(11,16,21) The generation of the ribbon requires advanced skills regarding cell distribution and is time consuming.(12)
Bioinks for 3D bioprinted vascularized cardiac tissue
Success or failure of 3D bioprinting is highly dependent on having the appropriate biomaterials for the specific printing application desired. Having a correct match between the biomaterial properties and the parameters of printing are required to provide optimal function of cells within the printed construct.(51,52) Materials used for printing need to be biocompatible/non-toxic.(53) At the same time, mechanical properties such as stiffness and degradation rate influence structural integrity and cell viability/function.(22,54,55) Bioinks used for 3D printing of cardiac tissues need to mimic the stiffness of the extracellular matrix of heart tissue and should contain appropriate chemical cues for cell survival.(36) To meet these requirements, both natural and synthetic bioinks have been explored.
Natural bioinks that have been used in cardiac tissue generation include collagen, gelatin, fibrin and hyalonuronic acid.(56–59) Within natural bioinks, decellularized extracellular matrix (dECM), particularly from heart tissue, may be the most ideal since it can recapitulate most of the chemical cues of the native heart tissue to preserve cell survival, differentiation, and function. Decellularized ECM are obtained from the organ of interest by detergent treatment to remove cells. This then leaves the ECM behind while keeping and architecture of the cell-cell interaction intact. The improved viability and function achieved with this material need to be balanced by the high costs and effort necessary for carrying out the decellularization process.(45,60,61) Natural bioinks facilitate cell viability greatly due to their biomimicry and biodegradability and can promote cell-matrix communication leading to matrix driven neo tissue formation. However, natural bioinks provide poor mechanical support and microstructure for embedded cells.(62,63)
Synthetic bioinks such as PEG offer the necessary mechanical support, yet they lack active binding sites for cells which leads to inhibition of cell adhesion and death. (22,52,64) To overcome the disadvantages of natural and synthetic bioinks, chemical conjugations of natural or synthetic bioinks have been tried (e.g. gelatin-methacrylate).(46,65) Gelatin provides cell adhesion as a natural ink with improve printability through high viscosity. Methacrylate crosslinks under UV conditions and the construct stiffness can be adjusted by UV intensity and exposure time. Pluronic F127 is a synthetic block polymer mainly used as sacrificial material to print scaffolds within generated cardiac tissue.(66,67)
To print a vasculature mimetic that can be perfused, Kolesky et al. used a thrombin-containing fugitive ink (Pluronic F127) to print channels for a timed-dependent release of thrombin to induced matrix cross-linking. A gelatin and fibrinogen combination ink was used to embed the cells surrounding the channels and this matrix is then crosslinked by thrombin diffusion and transglutaminase-driven crosslinking. After removing the fugitive ink, exposed channels were manually endothelialized to create the vasculature mimetic.(46) Alternatively, Maiullari et al. created constructs containing HUVECs and hiPSC-CMs by using chemically conjugated inks: Alginate and PEG- diacrylate. Printability and cell viability was provided by using alginate and fibrinogen, while PEG-diacrylate provides the necessary mechanical support. Stiffness was adjusted by UV-crosslinking which carries the risk of having a negative impact on cell viability due to cell damage.
Vascularization in 3D printed cardiac tissue
The progress that has been made in the field of tissue engineering and 3D bioprinting raises the potential that one day soon we may be able to generate tissues that are of clinically-relevant size. However, for an engineered tissue to be considered as successful, physiologic levels of CM function has to be achieved. The ability to provide electrical coupling and macroscopic beating remains challenge thus far.(5,68,69) Several groups reported the engineering of cardiac tissue with sizes near 1 cm thick.(46,70–72) The generation of large cardiac constructs can be advantageous in numerous different applications including drug screening, cardiac disease modeling, and even transplantation of functional tissue for the treatment of end-stage heart failure. For this purpose, iPSC-derived CMs are a great cell source for individualized heart failure cell therapy.(8,70) Compact tissue such as myocardium requires adequate supply of nutrients which limits feasibility and long-term survival of these constructs. Therefore, vascularization of 3d printed tissue has to address following challenges:
Cell density and nutrition/diffusion distance
Resolution of printed channels
Proper types of cells used for large vessel construction as well as small capillary structures
Fragility of cardiomyocytes – a delicate cell type requiring specific biomaterial stiffness and density for proper function (i.e. beating). Table 2 provides a summary of recent studies with different approaches used to create vascularized tissues.
Table 2:
Vascularization approaches for 3D bioprinted engineered tissue
| Cell source | Cell source (vasculature) | Sacrificial Bioink | Perfusion | Main outcome | Reference |
|---|---|---|---|---|---|
| hepatocytes (rat) | HUVECs | Carbohydrate glass | Passive | Construct size limited to 1mm Short cell viability | 2012 Miller(73) |
| hMScs hNDFs | HUVECs | Pluronic F127 Thrombin | more than 6 weeks | Perfusable chip with a size of > 1 cm | 2016 Kolesky(46) |
| - | HUVECs | Collagen | Microfluidic chip with hydrostatic driven flow | 2016 Skylar-Scott(74) | |
| hCPCs | MSC-VEGF | Decellularized ECM | In vivo | Cell maturation of hCPCs Capillary formation EF improvement |
2016 Jang(75) |
| iPS-CM (murine) | HUVECs | Alginate and PEG-Fibrinogen | In vivo and in vitro | Vascularization, CM mature but no functionality (beating) | 2018 Maiullari(70) |
| hESC-CM | hESC-ECs | Collagen | In vivo and in vitro | Neovascularization and anastomosis under flow conditions | 2019 Redd(71) |
Miller et al. created small (1 mm diameter) vascular channel-containing constructs by printing sacrificial water-soluble carbohydrate glass rods and then encapsulate them with cell-ladden hydrogels.(73) With this approach, endothelial cell (HUVECs)-line lumen were generated but the construct was incubated in media and not directly perfused. However, the provisioning of these vascular channels was able to improve cell survival and some function, as demonstrated by urea and albumin production in rat hepatocyte-containing constructs. However, due to the small size of these constructs and the lack of controlled inlet and outlet, these constructs were unable to be directly perfused, thus limiting the number of applications for this approach to tissue engineering.
Kolesky et al. in 2016 then followed this with preformed perfusable channels in a 3D bioprinted construct using sacrificial material.(46) To create an osteogenic lineage, human mesenchymal stem cells (HMCs) and human neonatal dermal fibroblasts (hNDFs) were embedded in an engineered extracellular matrix. This cell-laden ink was then cast over 3D printed channels within a perfusion chip. (Figure 1) Subsequently, the sacrificial Pluronic F127 channels was washed out leaving the vascular network behind. HUVECs were then injected into the remaining channels and pump-driven perfusion was initated and maintained for more than six weeks.
Figure 1:
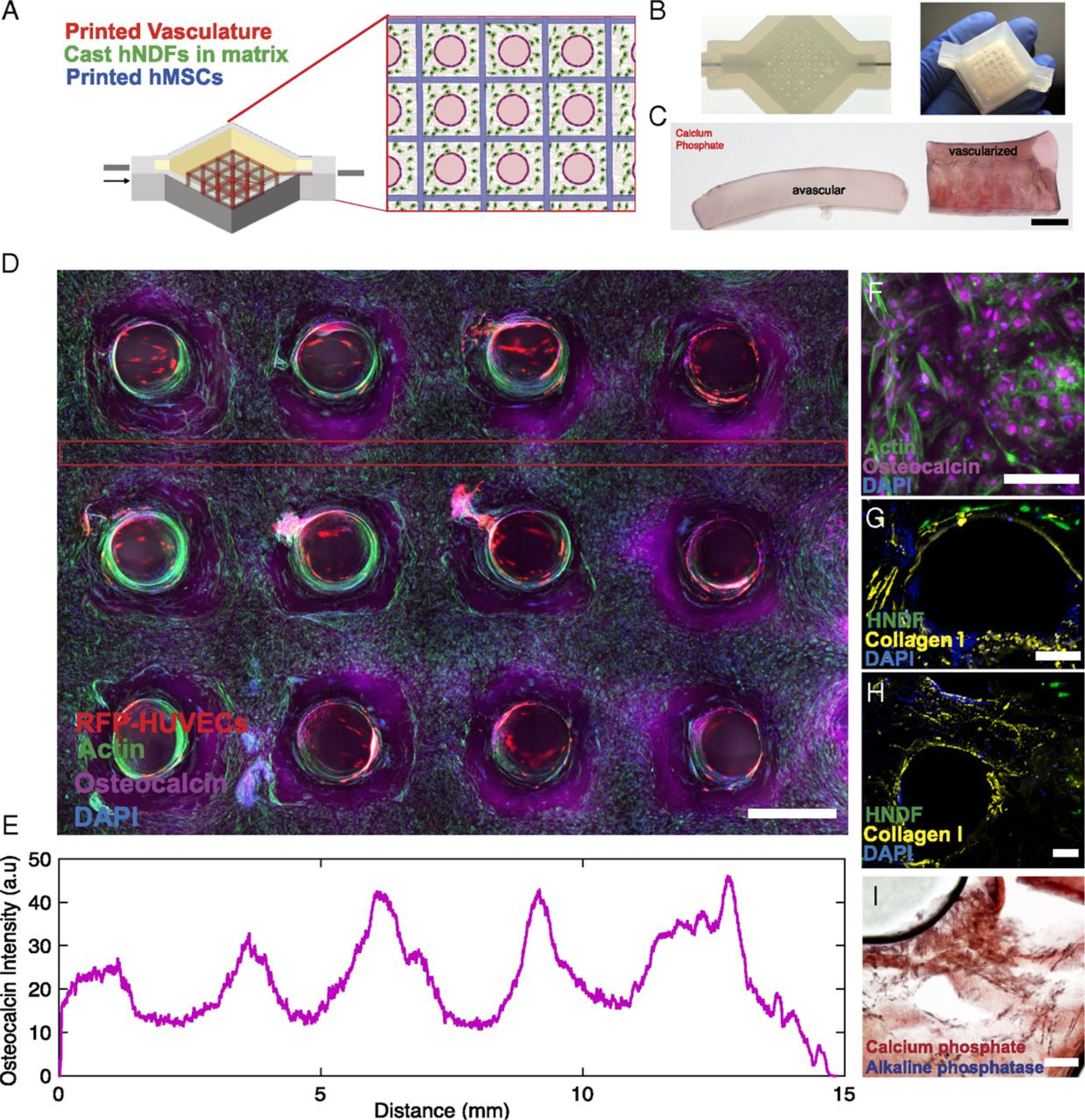
Three-dimensional vascularized tissues remain stable during long-term perfusion. (A)Schematic depicting a single HUVEC-lined vascular channel supporting a fibroblast cell-laden matrix and housed within a 3D perfusion chip. (B and C) Confocal microscopy image of the vascular network after 42 d, CD-31 (red), vWF (blue), and VE-Cadherin (magenta). (Scale bars: 100 μm.) (D) Long-term perfusion of HUVEC-lined (red) vascular network supporting HNDF- laden (green) matrix shown by top-down (Left) and cross-sectional confocal microscopy at 45 d (Right). (Scale bar: 100 μm.) (E) Quantification of barrier properties imparted by endothelial lining of channels, demonstrated by reduced diffusional permeability of FITC-dextran. (F) GFP-HNDF distribution within the 3D matrix shown by fluorescent intensity as a function of distance from vasculature. Reproduced with permission from Kolesky et al. PNAS 2016
Skylar-Scott et al. used 3D multi-photon photolithography to generate arbitrarily shaped 3D microchannels down to single micron resolutions exhibited by the smallest capillaries.(74) HUVECs were seeded into the channels adhering to the collagen walls building a confluent monolayer of cells. The lumen generated had a diameter of 20–50 μm mimicking capillaries. In this work, a uniform single cell type was used. Brandenberg and Lutolf demonstrated that laser ablation of 3D channels into in a cell laden gel could generate capillary-scale channels that could be lined with endothelium, thus enabling multicellular microvascularized constructs.(76) The application of these high resolution pulsed-laser approaches to a therapeutic scale scaffold will require multiplexing printheads for faster printing due to the extremely high resolution and therefore large number of voxels per construct.
Jang et al. developed a 3D pre-vascularized stem cell patch through spatial organization of c-kit+ cardiac progenitor cells (hCPCs).(75) dECM from porcine origin was used to promote rapid vascularization and maturation of cardiac progenitor cells within the patch. Each patch had a diameter of 8 mm and a height of 0.5 mm and the most promising results were achieved with multicellular patterning of both cell types. Mesenchymal stem cells derived endothelial cells showed CD31 expression and capillary formation with a diameter of 50 μm as well as maturation of hCPCs with an increase of troponin I and alpha sarcomeric actin expression. For in vivo testing, a myocardial infarction model in rats was generated. These investigators reported a slight increase in ejection fraction and reduced scar formation after one week of construct implantation.
Maiullari et al 3D bioprinted a HUVECs and iPSC-CM construct using an extrusion-based 3D bioprinter and multiple bioinks (PEG-Fibrinogen and alginate).(70) The murine iPSC-CMs and HUVECs were arrayed orthogonally within a cubic stack called the “Janus” construct that alternated one layer of HUVEC containing hydrogel with another hydrogel layer containing iPSC-CM.(Figure 2) UV and Ca2+ crosslinking lead to polymerization of PEG monoacrylate-fibrinogen for structural support. This construct was deem to be the best where it promoted the homogeneity of HUVEC distribution compared to other construct shapes. The encapsulated CM also showed signs of maturation (cardiac TNN I and alpha-myosin-heavy chain expression) at the histological level but the construct did not beat at the macroscopic level. Furthermore, other functionalities of CMs were not reported. One of the limitations of this construct is the long time it takes to differentiate and culture sufficient number of iPSC-CMs (40 × 10⁶/ml). Another is the limited number of days (14 days) that the incorporated CM could be maintained alive which is likely due to the lack of active perfusion of nutrients within the construct.
Figure 2:
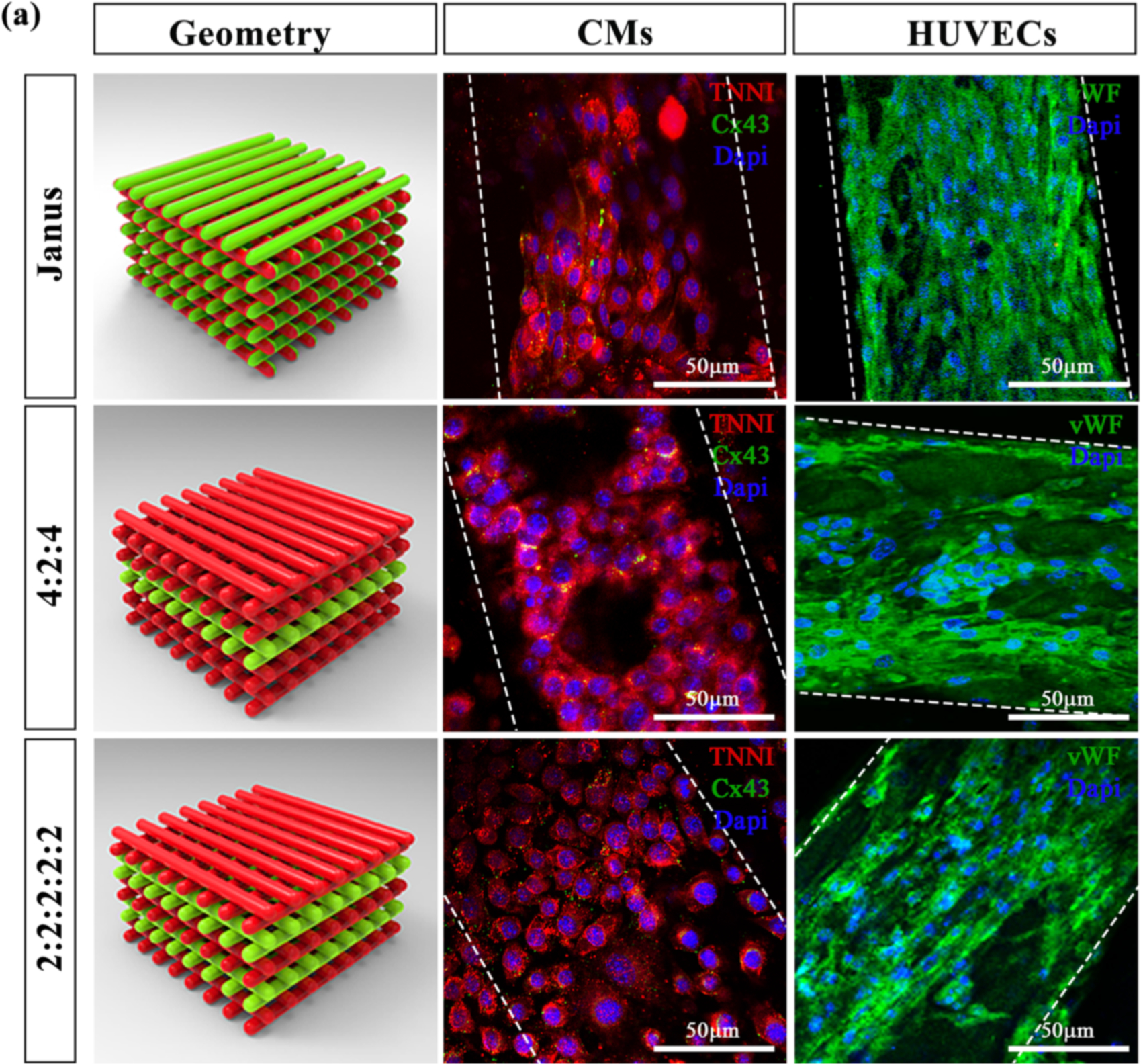
Multi-cellular 3D bioprinted cardiac tissue constructs. Representative images showing TNNI (red) and Cx43 (green) expressions in CMs and vWF (green) labelling in HUVEC, after 7 days of culture, printed in three different spatial geometries. Janus constructs contained the two different cell lineages within the each laid fiber; 4:2:4 and 2:2:2:2:2 structures were printed altering two layers of HUVEC with two or four layers of CM. Scale bars represent 50 μm. Reproduced with permission from Maiullari et al. Scientific Reports 2018.
Recently, Redd et al. created perfusable microvascular constructs using human embryonic stem-cell derived endothelial cells (hESC-ECs).(71) The generated constructs that contain 150k ECs each and were 8 mm in x-y dimensions with a thickness of 1 mm. The microfluidic channel networks in collagen gel matrices were lithographically fabricated with and without seeding of GFP-hESC-ECs and some of the constructs were additionally seeded with mTm-hESC-ECs in their channels (Figure 3). The purpose of the having two different hESC-ECs having two distinct colors was to see if an integration/anastomosis of both EC populations occurs. After 4–7 days of gravity-driven flow mTm-hESC-ECs spread into the bulk matrix showing direct connections and anastomosis between both EC types. In vitro studies showed vascular remodeling by increasing the total perfusable area within the constructs over time (using fluorescent beads). To assess potential integration of engineered microchannel networks with vasculature from the host myocardium, the authors implanted the engineered constructs in infarcted rat models over the epicardial surface of the left ventricle. Following engraftment, these hearts were excised and real time ex vivo imaging using optical microangiography (OMAG) was performed. While it was exciting to see that hESC-EC-lined channels represent up to 10% of the total perfused vessels, indicating that host vascular ingrowth into the construct and connection to engineered channels have taken place, the perfusion rate was significantly lower than in non-infarcted healthy regions of the same heart. Additional studies from this work showed the incorporation of hESC-CMs in the constructs and their implantation onto the epicardial surface of infarcted rat hearts. Encouragingly, these implanted constructs show high cell viability after a relatively brief period (5 days) post-implantation.
Figure 3:
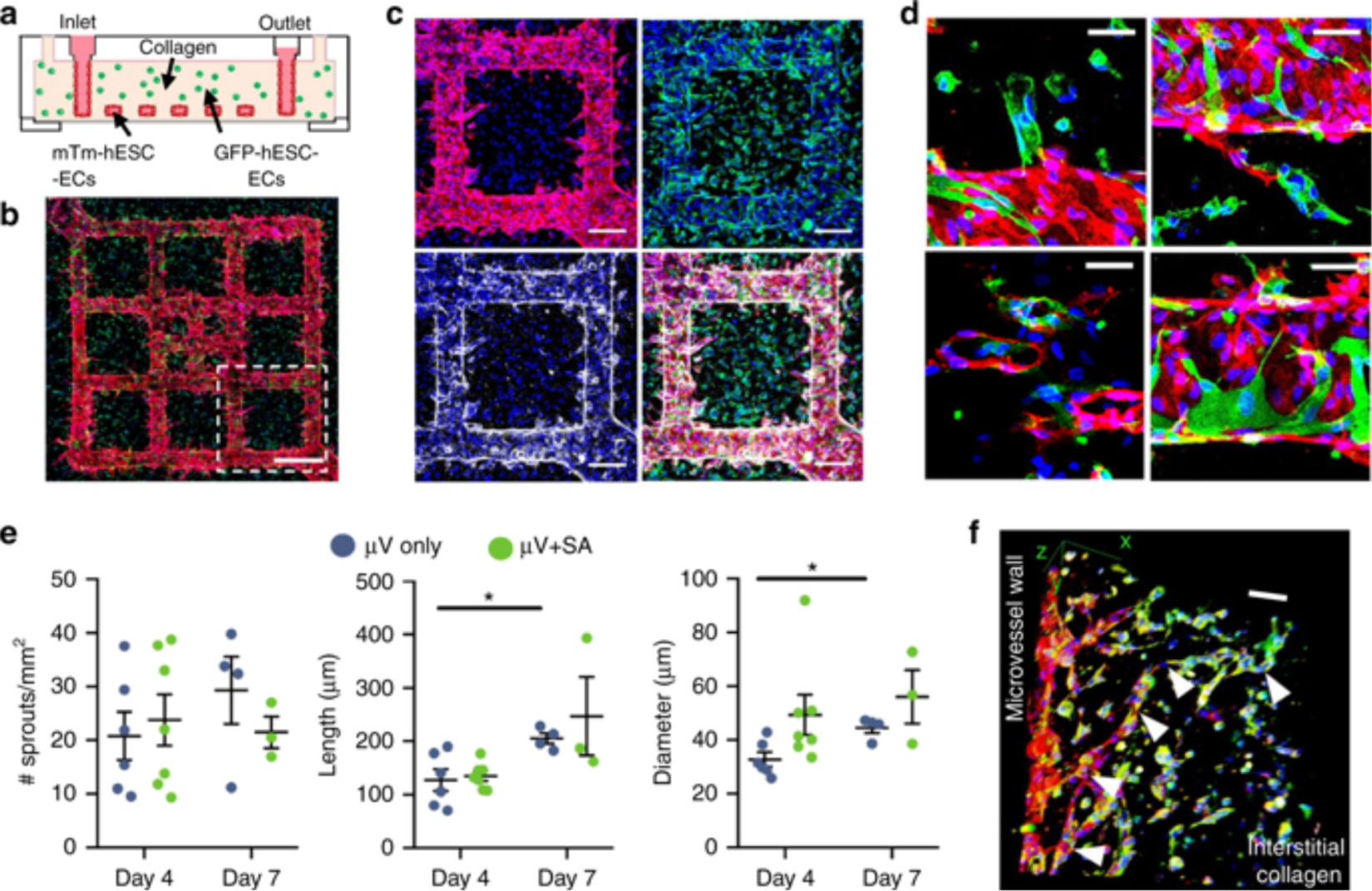
In vitro anastomosis of human embryonic stem cell-derived endothelial cells (hESC-ECs) in engineered microvessels (μVs). a Schematic of in vitro culture device for μV+SA constructs: mTm-hESC-EC μVs formed via perfusion and attachment with bulk-seeded GFP-hESC-ECs in the surrounding collagen gel. b Maximum intensity projection of stitched large image confocal z-stack of μV+SA construct cultured for 4 days and stained for DsRed (red) and GFP (green) to detect mTm- and GFP-expressing hESC-ECs, respectively. Scale bar, 500 μm. c Outlined region (white box) in b stained for DsRed (red, top left), GFP (green, top right), and VE-cadherin (white, bottom left). Merged image, bottom right. Scale bar, 200 μm. d High magnification images of GFP-hESC-ECs (green) integrated with mTm-hESC-EC (red) patterned vessel in μV+SA constructs. Scale bar, 50 μm. e Quantitation of sprouts from patterned μVs by sprout density (no. of sprouts per vessel surface area), sprout length, and sprout diameter in μV only (blue circles) and μV+SA (green circles) constructs after 4 days and 7 days of culture. N = 6, 7, 4, and 3 biologically independent samples for D4 μV only, D4 μV+SA, D7 μV only, and D7 μV+SA, respectively. p = 0.011 for length and p = 0.007 for diameter for D4 μV only and D7 μV only, p > 0.05 for all others (two-tailed t test). f 3D view of GFP+ de novo lumen integrated with mTm+ microvascular sprout (white arrowheads) stained for CD31 (red) and GFP (green). Scale bar, 100 μm. Representative images for b–d, f from seven biologically independent samples of D4 μV+SA, with similar results. Hoechst-stained nuclei, blue. Error bars, mean ± SEM. *p < 0.05 determined using two-tailed t test. D4 after 4 days of culture, D7 after 7 days of culture. Reproduced with permission from Redd et al. Nature communications 2019
Cell source for vascularization of engineered cardiac tissues
One important consideration for the generation of functional engineered constructs containing blood vessel system and CMs is the source of cell used to generate these constructs. CMs are highly metabolically active so have a high energy demand. This is supported by the observation that in adult hearts in vivo every CM neighbors a capillary that ensures the availability of adequate nutrient supply for that CM.(77,78) Another consideration for engineered tissue construct is the need to recreate two types of vessels - arteries and veins - so that there is directional flow of oxygen into the tissue construct and carbon dioxide out. Engineering of arterial vessels requires that these vessels be made to resist higher flow rate, pressure, and shear stress within the system. On the other hand, veins are low resistance and high capacitance circuit made to deliver blood flow out of the tissue construct. For both artery and vein, there are three cell layers needed to recapitulate the normal architecture: the intima, consisting of endothelial cells and pericytes, the media (in arteries) with layers of smooth muscle cells and elastic fibers, and the externa consisting of connective tissues. In addition, a wide range of vessels sizes from terminal capillaries that are 5 μm in diameter (79) to multiple centimeter-sized arteries and veins require different composition of cell types for their assembly. Since the diffusion limit of oxygen is around 150 μm, capillaries must also be sufficiently dense to be able to provide nutrients for each cell on a microscopic scale.(77–79) Within diffusion dependent engineered tissue, this limits the volume of constructs to 2–3 mm³.
To create a clinically-relevant sized cardiac tissue construct, a vast capillary network has to be engineered. To meet the myriad of challenges that need to be overcome in order to be successful, one must ensure that the ECs used is appropriately functional for the intended purpose.(80,81) While HUVECs appears to be most frequently used for engineering of vascularized tissues (Table 2), other sources of ECs may be more ideal.(82,83) Within vessels, ECs not only support the delivery of oxygen supply, but they also have crucial functions such as metabolization, secretion, and reaction to hormonal substances. Pro-and antithrombotic reactions are also part of their functions. However, ECs are quite heterogenous so even within one organ, different ECs can be found and each of them show different angiogenic response, molecular permeability, hemostasis, and immune tolerance.(84) Since HUVECs are extracted from umbilical cords, they do not typically resemble ECs from the heart. However, because of their ability to expand, they have been a favorite endothelial cell type to use among tissue engineers. However, HUVEC attachment to the 3D construct cannot always be guaranteed. This cell type also tends to dedifferentiate quickly within a few passages which limits their use. For in vivo applications, immune compatibility of the ECs used will need to be addressed.
Another source for endothelial cells is mature ECs derived from autologous vascular tissue. The use of this source is limited by the low proliferation of mature ECs and the invasive extraction (biopsy).(85) Human ESC is a difficult source of cells to use for EC-line constructs due to ethical limitation.(80) Several methods for the generation of ECs from iPSCs have recently been reported. Although improvement has been made, the efficiency for generating CD31+ ECs using growth factors varies from 5–57 %.(86) Not only does the high variability of iPSC differentiation reduce their feasibility, but once made, the differentiated ECs also show rapid dedifferentiation in culture.(87) As an alternative to media-directed differentiation, over-expression of the ETS variant 2 transcription factor (ETV2) can derive endothelial cells from pluripotent stem cells in as short as five days with an efficiency of CD31+ cells at 46%.(88) The consequences of coculturing ECs with other cell types to improve vessel formation have been reported in several studies.(89–97) Regarding promotion of angiogenesis and anastomosis of capillaries, the co-culturing of ECs and fibroblasts has shown promising results.(89–91) The presence of fibroblasts seems to provide a stable surrounding for the creation of endothelialized tubes both by infrastructural support and angiogenesis promotion via secretion of mediators.(88–90) Within this context, vascular smooth muscle cells, which are part of the media of vessels, seem to have a stabilizing function for the formation of vessel like networks.(95–97) Beyond the issue of endothelial and vascular smooth muscle cell sources, future tissue engineering considerations will need to address the complex functions of ECs such as resistance to shear stress, release of hormones and paracrine factors, and response to chemical and other small molecule cues. Furthermore, the ability of tissue engineered vessel network to undergo angiogenic sprouting and generate neovasculature is crucial for proper cell functioning. Once implanted in patients, immunogenicity of the construct will need to be addressed since it is unlikely that the fabrication of an entirely autologous construct will be practical for in vivo application.
Conclusion and Future Perspectives
In the past few years, 3D bioprinting has gained substantial interests from investigators in tissue engineering and regenerative medicine and continues to be a rapidly growing field. This is reflected by the estimated market size for 3D printing to go from $2.2B in 2012 to $10.8B by 2021.(98) Although tremendous progress has been made thus far, the generation of engineered cardiac tissue on a macroscopic scale is still challenging due to various reasons as discussed above. In particular, the technologies needed to vascularize and perfuse a large, clinically-relevant sized construct are still limited. Important early steps towards the creation of functional vascular networks have been demonstrated thus far.(46,71) Yet, important challenges remain regarding the incorporation of CMs within these constructs. These challenges include: the number of cardiomyocytes needed in each construct to achieve a final cell density of 100–1000M cells/ml.(20) This will require a tremendous cell culturing effort to maintain and differentiate a massive number of iPSCs. Even when the relevant-sized engineered cardiac tissue construct has been made with a sufficiently dense vasculature, other challenges such as the presence of directional flow with optimized flow conditions that mimic in vivo tissue will need to be ensured. Shear stress, rheology of whole blood and high flow rate will bring additional challenges to tissue engineering. A highly specialized multidisciplinary teams will need to be assembled in order to meet these challenges in the future.
Acknowledgments
We thank Mark Skylar-Scott, PhD (Wyss Institute for Biologically Inspired Engineering, Harvard University) for his comments and edits on the manuscript.
Financial & conflict of interest disclosure
Funding for this research was provided by the German Research Foundation/ DFG (PU 690/1-1) (N.P.), the NIH Office of Director’s Pioneer Award LM012179-03, the American Heart Association Established Investigator Award 17EIA33410923, the Stanford Cardiovascular Institute, the Hoffmann and Schroepfer Foundation, and the Stanford Division of Cardiovascular Medicine, Department of Medicine (S.M.W). The authors declare no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Footnotes
Conflict of Interest
Nazan Puluca, Soah Lee, Stephanie Doppler, Andrea Münsterer, Martina Dreßen, Markus Krane and Sean M. Wu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet Lond Engl. 2006. May 27;367(9524):1747–57. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019. January 31;CIR0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Chambers DC, Cherikh WS, Goldfarb SB, Hayes D, Kucheryavaya AY, Toll AE, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2018. October;37(10):1169–83. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, et al. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed Mater Bristol Engl. 2015. June 11;10(3):034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010. April;16(2):169–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009. April 3;324(5923):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte Turnover in the Aging Human Heart. Circ Res. 2010. November 26;107(11):1374–86. [DOI] [PubMed] [Google Scholar]
- 8.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014. August;11(8):855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009. February 27;104(4):e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011. May 19;473(7347):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact Mater. 2018. June;3(2):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014. August;32(8):773–85. [DOI] [PubMed] [Google Scholar]
- 13.Ubil E, Duan J, Pillai ICL, Rosa-Garrido M, Wu Y, Bargiacchi F, et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014. October 30;514(7524):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Altalhi W, Nunes SS. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv Drug Deliv Rev. 2016. January 15;96:183–94. [DOI] [PubMed] [Google Scholar]
- 15.Ali M, Pages E, Ducom A, Fontaine A, Guillemot F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication. 2014. September 12;6(4):045001. [DOI] [PubMed] [Google Scholar]
- 16.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012. September;33(26):6020–41. [DOI] [PubMed] [Google Scholar]
- 17.Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, et al. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett. 2015. November;37(11):2349–55. [DOI] [PubMed] [Google Scholar]
- 18.Ning L, Chen X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol J. 2017. August;12(8). [DOI] [PubMed] [Google Scholar]
- 19.Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, et al. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat Commun. 2014. May 8;5:3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM. Bioengineering cardiac constructs using 3D printing. J 3D Print Med. 2017. April;1(2):123–39. [Google Scholar]
- 21.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013. March;60(3):691–9. [DOI] [PubMed] [Google Scholar]
- 22.Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016. 23;8(3):032002. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Baicu C, Aho M, Zile M, Boland T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication. 2009. September;1(3):035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopp B, Smausz T, Kresz N, Barna N, Bor Z, Kolozsvári L, et al. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005. December;11(11–12):1817–23. [DOI] [PubMed] [Google Scholar]
- 25.Hopp B Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt Eng. 2012. January 31;51(1):014302. [Google Scholar]
- 26.Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010. October;31(28):7250–6. [DOI] [PubMed] [Google Scholar]
- 27.Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005. October 20;92(2):129–36. [DOI] [PubMed] [Google Scholar]
- 28.Gruene M, Deiwick A, Koch L, Schlie S, Unger C, Hofmann N, et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods. 2011. January;17(1):79–87. [DOI] [PubMed] [Google Scholar]
- 29.Calvert P MATERIALS SCIENCE: Printing Cells. Science. 2007. October 12;318(5848):208–9. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009. October;30(31):6221–7. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011. July;98(1):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto T, Suzuki T, Yamamoto N. Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nat Biotechnol. 2000. April;18(4):438–41. [DOI] [PubMed] [Google Scholar]
- 33.Goldmann T, Gonzalez JS. DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J Biochem Biophys Methods. 2000. March 16;42(3):105–10. [DOI] [PubMed] [Google Scholar]
- 34.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008. January;29(2):193–203. [DOI] [PubMed] [Google Scholar]
- 35.Cui X, Boland T, D’Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. 2012. August;6(2):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pati F, Jang J, Ha D-H, Won Kim S, Rhie J-W, Shim J-H, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014. June 2;5:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009. April;30(12):2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JD, Choi JS, Kim BS, Chan Choi Y, Cho YW. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer. 2010. May;51(10):2147–54. [Google Scholar]
- 39.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013. January;101(1):272–84. [DOI] [PubMed] [Google Scholar]
- 40.Khalil S, Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater Sci Eng C. 2007. April;27(3):469–78. [Google Scholar]
- 41.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002. January 17;54(1):13–36. [DOI] [PubMed] [Google Scholar]
- 42.Turksen K, editor. Bioprinting in regenerative medicine. Cham Heidelberg New York: Springer; 2015. 140p. (Stem cell biology and regenerative medicine). [Google Scholar]
- 43.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016. January;76:321–43. [DOI] [PubMed] [Google Scholar]
- 44.Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A. 2008. January;14(1):41–8. [DOI] [PubMed] [Google Scholar]
- 45.Jones N Science in three dimensions: the print revolution. Nature. 2012. July 4;487(7405):22–3. [DOI] [PubMed] [Google Scholar]
- 46.••.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016. March 22;113(12):3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript shows pioneering work creating thick perfusable tissue.
- 47.Irvine SA, Agrawal A, Lee BH, Chua HY, Low KY, Lau BC, et al. Printing cell-laden gelatin constructs by free-form fabrication and enzymatic protein crosslinking. Biomed Microdevices. 2015. February;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017. 16;8:15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015. October;10(10):1568–77. [DOI] [PubMed] [Google Scholar]
- 50.Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication. 2010. September;2(3):032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panwar A, Tan LP. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Mol Basel Switz. 2016. May 25;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chimene D, Lennox KK, Kaunas RR, Gaharwar AK. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann Biomed Eng. 2016;44(6):2090–102. [DOI] [PubMed] [Google Scholar]
- 53.Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res [Internet]. 2018. December [cited 2019 Feb 28];22(1). Available from: https://biomaterialsres.biomedcentral.com/articles/10.1186/s40824-018-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tirella A, Orsini A, Vozzi G, Ahluwalia A. A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication. 2009. December;1(4):045002. [DOI] [PubMed] [Google Scholar]
- 55.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials. 2016;102:20–42. [DOI] [PubMed] [Google Scholar]
- 56.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013. May;101(5):1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Shengjie, Xiong Zhuo, Wang Xiaohong, Yan Yongnian, Liu Haixia, Zhang Renji. Direct Fabrication of a Hybrid Cell/Hydrogel Construct by a Double-nozzle Assembling Technology. J Bioact Compat Polym. 2009. May;24(3):249–65. [Google Scholar]
- 58.Gaetani R, Feyen DAM, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015. August;61:339–48. [DOI] [PubMed] [Google Scholar]
- 59.Duan B, Kapetanovic E, Hockaday LA, Butcher JT. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014. May;10(5):1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Censi R, van Putten S, Vermonden T, di Martino P, van Nostrum CF, Harmsen MC, et al. The tissue response to photopolymerized PEG-p(HPMAm-lactate)-based hydrogels. J Biomed Mater Res A. 2011. June 1;97(3):219–29. [DOI] [PubMed] [Google Scholar]
- 61.Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJA, Hutmacher DW, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013. May;13(5):551–61. [DOI] [PubMed] [Google Scholar]
- 62.Stanton MM, Samitier J, Sánchez S. Bioprinting of 3D hydrogels. Lab Chip. 2015. August 7;15(15):3111–5. [DOI] [PubMed] [Google Scholar]
- 63.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002. January 17;54(1):3–12. [DOI] [PubMed] [Google Scholar]
- 64.Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater Sci Eng. 2016. October 10;2(10):1662–78. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2015. December 22;7(4):045009. [DOI] [PubMed] [Google Scholar]
- 66.Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015. May;112(5):1047–55. [DOI] [PubMed] [Google Scholar]
- 67.Müller M, Becher J, Schnabelrauch M, Zenobi-Wong M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication. 2015. August 11;7(3):035006. [DOI] [PubMed] [Google Scholar]
- 68.Ruan J-L, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, et al. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation. 2016. November 15;134(20):1557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004. December 28;101(52):18129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maiullari F, Costantini M, Milan M, Pace V, Chirivì M, Maiullari S, et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep [Internet]. 2018. December [cited 2019 Feb 27];8(1). Available from: http://www.nature.com/articles/s41598-018-31848-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.••.Redd MA, Zeinstra N, Qin W, Wei W, Martinson A, Wang Y, et al. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat Commun. 2019. February 4;10(1):584. [DOI] [PMC free article] [PubMed] [Google Scholar]; Current state-of-the-art showing vascular remodeling and integration of engineered microchannel networks.
- 72.Zhang YS, Arneri A, Bersini S, Shin S-R, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012. September;11(9):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skylar-Scott MA, Gunasekaran S, Lewis JA. Laser-assisted direct ink writing of planar and 3D metal architectures. Proc Natl Acad Sci. 2016. May 31;113(22):6137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang J, Park H-J, Kim S-W, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–74. [DOI] [PubMed] [Google Scholar]
- 76.Brandenberg N, Lutolf MP. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv Mater Deerfield Beach Fla. 2016. September;28(34):7450–6. [DOI] [PubMed] [Google Scholar]
- 77.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003. January;83(1):59–115. [DOI] [PubMed] [Google Scholar]
- 78.Montgomery M, Zhang B, Radisic M. Cardiac Tissue Vascularization: From Angiogenesis to Microfluidic Blood Vessels. J Cardiovasc Pharmacol Ther. 2014. July;19(4):382–93. [DOI] [PubMed] [Google Scholar]
- 79.Potter RF, Groom AC. Capillary diameter and geometry in cardiac and skeletal muscle studied by means of corrosion casts. Microvasc Res. 1983. January;25(1):68–84. [DOI] [PubMed] [Google Scholar]
- 80.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005. July;23(7):879–84. [DOI] [PubMed] [Google Scholar]
- 81.Tremblay P-L, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2005. May;5(5):1002–10. [DOI] [PubMed] [Google Scholar]
- 82.Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, et al. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998. November 6;273(45):29786–93. [DOI] [PubMed] [Google Scholar]
- 83.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol Berl Ger. 1993. February;7(1):96–104. [DOI] [PubMed] [Google Scholar]
- 84.Baiguera S, Ribatti D. Endothelialization approaches for viable engineered tissues. Angiogenesis. 2013. January;16(1):1–14. [DOI] [PubMed] [Google Scholar]
- 85.Perry L, Flugelman MY, Levenberg S. Elderly Patient-Derived Endothelial Cells for Vascularization of Engineered Muscle. Mol Ther J Am Soc Gene Ther. 2017. 05;25(4):935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurokawa YK, Yin RT, Shang MR, Shirure VS, Moya ML, George SC. Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for Three-Dimensional Microphysiological Systems. Tissue Eng Part C Methods. 2017. August;23(8):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurisaki A, Ito Y, Onuma Y, Intoh A, Asashima M. In vitro organogenesis using multipotent cells. Hum Cell. 2010. February 1;23(1):1–14. [DOI] [PubMed] [Google Scholar]
- 88.Elcheva I, Brok-Volchanskaya V, Kumar A, Liu P, Lee J-H, Tong L, et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014. July 14;5:4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009. June;15(6):1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes CCW. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008. May;15(3):204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu S, Zhang H, Zhang X, Lu W, Huang X, Xie H, et al. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng Part A. 2011. March;17(5–6):725–39. [DOI] [PubMed] [Google Scholar]
- 92.D’Amore PA. Capillary growth: a two-cell system. Semin Cancer Biol. 1992. April;3(2):49–56. [PubMed] [Google Scholar]
- 93.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CCW, Jeon NL, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008. March 1;94(5):1930–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Velazquez OC, Snyder R, Liu Z-J, Fairman RM, Herlyn M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J Off Publ Fed Am Soc Exp Biol. 2002. August;16(10):1316–8. [DOI] [PubMed] [Google Scholar]
- 95.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996. December 27;87(7):1153–5. [DOI] [PubMed] [Google Scholar]
- 96.Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999. January;103(2):157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darland DC, D’Amore PA. Cell-cell interactions in vascular development. Curr Top Dev Biol. 2001;52:107–49. [DOI] [PubMed] [Google Scholar]
- 98.Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication. 2016. March 1;8(1):014103. [DOI] [PubMed] [Google Scholar]


