Abstract
Mistletoe has been used as treatment of many diseases in traditional and folk medicine. To date, anticancer, immunomodulatory, cardiac, antidiabetic, hepatoprotective, neuropharmacological, antibacterial and antifungal properties of mistletoe extracts have been studied the most. In this review, we summarized in vitro and in vivo studies on the pharmacological activity of Viscum species. Furthermore, we proposed the possible mechanisms of action of this herb, which might include many signalling pathways. Mistletoe could regulate either similar or different targets in various pathways that act on membrane receptors, enzymes, ion channels, transporter proteins and transcriptional targets. Still, pharmacological activities of mistletoe have been investigated mainly for crude extracts. It is a new field for scientists to determined which chemical compounds are responsible for the individual biological activities of mistletoe and how these activities are achieved. As a result, mistletoe might become a source of new complementary therapies supporting the treatment of many diseases.
Keywords: Mistletoe, Viscum, Extracts, Bioactivities, Mechanisms, Pharmacognosy
Introduction
Mistletoe (Viscum L.) belongs to the family of Viscaceae. In Europe, Asia, Africa and Australia, about 100 species of mistletoe can be distinguished, of which the most known are in Santalaceae: Viscum album L. (European mistletoe), Santalaceae: Viscum album subsp. Coloratum Kom. (Viscum coloratum (Kom.) Nakai, Korean mistletoe), Santalaceae: Viscum articulatum Burm. f., Santalaceae: Viscum shimperi Engl., Santalaceae: Viscum capense L.f. and Santalaceae: Viscum cruciatum Sieber ex Boiss. Mistletoe is a semi-parasitic evergreen shrub, which means it depends on having water and some nutrients supplied from another plant (host tree) while it produces carbohydrates in a process of photosynthesis. Viscum species inhabit many types of wooded habitats and parasitize both deciduous and coniferous trees (Bussing 2000). For clinical applications, the most popular species are mistletoe parasitizing fir, maple, almond, birch, hawthorn, ash, apple, pine, poplar, oak, willow, lime and elm (Kienle et al. 2011). Viscum species have been used in the traditional medicine of Europe for centuries. Hippocrates used mistletoe to treat diseases of the spleen and complaints associated with menstruation, while Pliny the Elder used it to treat epilepsy, infertility and ulcers. In the Middle Ages, Paracelsus recommended mistletoe as a treatment for epilepsy. Hildegard von Bingen described mistletoe as a treatment for diseases of the spleen and liver. Mistletoe was also applied for deworming children, to treat labour pains, gout, affections of the lungs and liver, leprosy, mumps, fractures and hepatitis. During the eighteenth century, mistletoe was applied for “weakness of the heart” and oedema (Bussing 2000). By the end of the nineteenth century, mistletoe was rejected by scientists as a folklore remedy. The scientific interest on mistletoe was awakened in the twentieth century, as Gaultier investigated the effect of oral or subcutaneous applications of fresh Viscum album L. extracts on blood pressure in humans and animals (Bussing 2000; Committee on Herbal Medicinal Products 2012). In 1920, Viscum album L. was introduced as a cancer treatment by Rudolf Steiner who recommended a drug extract produced in a complicated manufacturing process combining sap from mistletoe harvested in the winter and summer (Bussing 2000). Mistletoe was also commonly used in other parts of the world. In Japan, mistletoe was used to treat hypertension, spasms of the heart, rheumatic pain, threatened abortion and locally to treat frostbite. In India, a tea prepared from mistletoe leaves was used to treat diabetes, while a preparation of Viscum articulatum Burm. f. was given in fevers with aching limbs. In Africa, Viscum species were a remedy to treat diarrhoea and an enema for stomach troubles in children. In Israel, Viscum cruciatum Sieber ex Boiss. was commonly used to treat constipation in young children and adults. Mistletoe was also used against general pain, backache and arthritis. In the traditional medicine of Egypt, the plant was used for the treatment of epilepsy, arteriosclerosis, and diseases of cardiac arteries, and as a hypotensive (Bussing 2000; Lev et al. 2011; Committee on Herbal Medicinal Products 2012). Such varied pharmaceutical applications result from the rich chemical composition of Viscum species, which largely depend on the host species. The main active compounds are lectins, viscotoxins, flavonoids, phenolic acids, sterols, lignans, terpenoids, phenylpropanoids, alkaloids and fatty acids (Szurpnicka et al. 2019). In this review, we would like to summarize the scientific data on the pharmacological activity of Viscum species and analyse the probable mechanisms of actions of mistletoe.
Anticancer and immunomodulatory activity
In German-speaking countries, mistletoe has been used as complementary anticancer therapy for more than 100 years. Viscum album L. preparations can be divided into phytotherapeutic extracts standardized on a certain lectin level (brand names such as Cefalektin, Eurixor, Lektinol) and anthroposophical/homeopathically produced extracts (brand names such as AbnobaViscum, Helixor, Iscador, Iscucin, Isorel) (Freuding et al. 2019). The main anticancer compounds isolated from Viscum species are lectins (Thies et al. 2005; Eggenschwiler et al. 2007) and viscotoxins (Schaller et al. 1996). Later studies have shown that other compounds, such as phenolic compounds (Melo et al. 2018), triterpene acids (Delebinski et al. 2015) and non-polar compounds (Ćebović et al. 2008), have also shown antitumor properties. Furthermore, it was reported that complete mistletoe extract is more potent at inhibiting tumour cells than isolated compounds (Felenda et al. 2019), and there is synergistic action between different groups of mistletoe compounds (Twardziok et al. 2016; Kleinsimon et al. 2017). Mistletoe shows bi-directional activity in the treatment of cancer. Firstly, it affects the quality of life of cancer patients by the improvement of fatigue, sleep, exhaustion, nausea, vomiting, appetite, depression, anxiety, pain and side effects of traditional treatment (Kienle and Kiene 2010; Brandenberger et al. 2012; Kim et al. 2012). Secondly, it shows antitumor activity by cytotoxicity, induction of apoptosis (Ćebović et al. 2008; Park et al. 2012; Han et al. 2015; Mishra et al. 2018) and inhibition of angiogenesis (Park et al. 2001; Elluru et al. 2009). The mechanism of action is shown in Fig. 1. In vitro studies on anticancer activity of mistletoe have confirmed that it modulates many different pathways, playing key roles in tumour proliferation, including MAPK (mitogen-activated protein kinase) (Park et al. 2012) and PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase B) (Fan et al. 2019). Furthermore, mistletoe can cause cell cycle arrest (Dela Cruz et al. 2015; Kim et al. 2017; Melo et al. 2018), loss of mitochondrial membrane permeability (MMP) (Mishra et al. 2018) and can activate caspases and regulate pro- and anti-apoptotic proteins (Fan et al. 2019) (Table 1). The anticancer activity of Viscum species is linked with their immunomodulatory activity (Oei et al. 2019), such as the increase of maturation and activation of dendritic cells (Elluru et al. 2008; Kim et al. 2014a; Steinborn et al. 2017), abrogation of tumour-induced immunosuppression of dendritic cells (Steinborn et al. 2017), increase of leukocytes, eosinophils, granulocytes (Huber et al. 2005, 2011) and lymphocytes (Semiglasov et al. 2004), increase of cytokines secretion (Hajto et al. 1990; Kovacs 2000; Elluru et al. 2008), increase of activity of natural killer cells (Hajto 1986; Tabiasco et al. 2002; Braedel-Ruoff 2010; Kim et al. 2018), increase of the activities of natural killer cells during surgery (Schink et al. 2007) and enhancement of cellular and humoral immune response (Yoon et al. 2001; Gardin 2009). Clinical studies were done on patients suffering from cancer diseases such as bladder cancer, breast cancer, colorectal cancer, glioma, lung cancer, melanoma and the results of these studies have been published in many articles. Those who are interested in the topic are invited to read review articles focusing on the anti-cancer properties of mistletoe (Ernst et al. 2003; Bar-Sela 2011; Bar-Sela et al. 2013; Steele et al. 2015; Kienle et al. 2016; Schläppi et al. 2017; Freuding et al. 2019). Additionally, it is worth paying attention to studies regarding synergistic interactions of mistletoe preparations with other cancer treatments such as chemotherapy and radiotherapy (Siegle et al. 2001; Hong et al. 2014; Kleinsimon et al. 2017; Schötterl et al. 2019; Menke et al. 2019). Furthermore, we found research that Korean mistletoe lectin affected the self-renewal activity of placenta-derived mesenchymal stem cells (MSCs) (Choi et al. 2012; Kim et al. 2019), however its therapeutic use for cancer is still insufficiently investigated (Hmadcha et al. 2020).
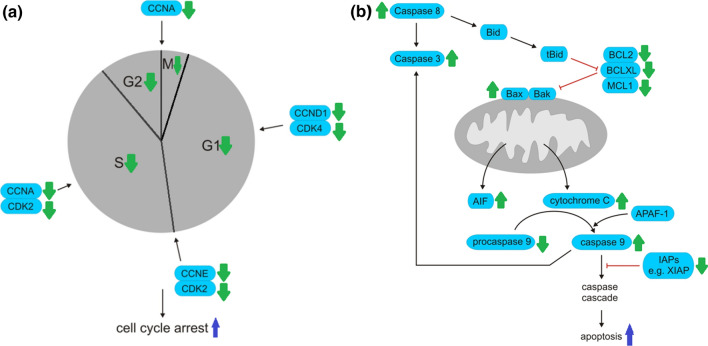
Fig. 1.
Mechanism of anticancer activity of mistletoe. Mistletoe targets two important signalling pathways, PI3K/AKT and MAPK. PI3K/AKT pathway is responsible for growth and survival of cancer cells. Mistletoe induces apoptosis by inhibition of AKT phosphorylation. MAPK pathway is mediated by ERK, p38 and JNK. Mistletoe enhances p38 and JNK1 activation and reduces ERK leading to apoptosis and cell cycle arrest of cancer cells. a Mistletoe downregulates cyclins (CCND1, CCNE, CCNA) and cyclin-dependent protein kinases (CDK4, CDK2) inhibiting cell cycle. b Mistletoe upregulates proapoptotic proteins (Bax) and downregulates inhibitors of apoptosis (IAPs) such as BCL2, BCL2L1, MCL1, XIAP. Furthermore, mistletoe leads to release of cytochrome c and activation of caspases resulting in apoptosis
Table 1.
Antitumor activity of Viscum species—in vitro studies
| Mechanism of action | Preparation/compound (host tree) | Concentration of the extract/compound | Cell line | Observations | References |
|---|---|---|---|---|---|
| Cell cycle | Viscum album L. (apple tree) |
Extract containing 10 ng/mL lectin MLI Extract containing 60 µg/mL oleanolic acid Extract containing 5 ng/mL lectin MLI and 50 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B, Saos-2 and U2OS | G1 arrest in TP53 wild-type (U2OS) and null-mutant (Saos-2) cells, S arrest in TP53 mutant cells (143B), blockage of G1/S transition accompanied by downregulation of CDK4, CCND1, CDK2, CCNE, CCNA, investigations on the transcriptional level revealed secondary TP53 participation, cell cycle arrest was mediated by transcriptionally increased expression of GADD45A and CDKN1A and decreased SKP2 levels | Kleinsimon et al. (2018) |
| Viscum album L | 3% and 5% v/v | Murine melanoma cell line B16F10 | Increased Sub G0 population, probably associated with a consistent decrease in G1, and an increase in S or G2/M populations | Melo et al. (2018) | |
| Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | G2/M cell cycle arrest with a concomitant decrease in some cells at G0/G1 phase | Mishra et al. (2018) | |
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Cell cycle arrest in A549 and NCI- H292 cells at the S and G0/G1 phases, respectively | Fan et al. (2019) | |
| Viscum coloratum (Kom.) Nakai |
Lectin 10–1000 ng/mL Extract 10–1000 µg/mL |
Mouse melanoma cell lines B16BL6 and B16F10 | G0/G1 cell cycle arrest | Han et al. (2015) | |
| MMP | Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | Loss of MMP, which is required for cytochrome c release | Mishra et al. (2018) |
|
Viscum album L (apple tree) |
Extract containing 1.25–7.5 ng/mL lectin MLI Extract containing 30–45 µg/mL oleanolic acid Extract containing 1.25–7.5 ng/mL lectin MLI and 30–45 µg/mL oleanolic acid |
Human alveolar Rhabdomyosarcoma cell line RMS-13 | Stammer et al. (2017) | ||
|
Viscum album L (apple tree) |
Extract containing 1–40 ng/mL lectin MLI Extract containing 10–60 µg/mL oleanolic acid Extract containing 1–40 ng/mL lectin MLI and 10–60 µg/mL oleanolic acid |
Human Ewing sarcoma cell lines TC-71 and MHH-ES-1 | Twardziok et al. (2016) | ||
| Viscum album L. (apple tree) |
Extract containing 2–16 ng/mL lectin MLI Extract containing 20–40 µg/mL oleanolic acid Extract containing 2–16 ng/mL lectin MLI and 20–40 µg/mL oleanolic acid |
Human acute myeloid leukemia cell lines U937 and HL-60 | Delebinski et al. (2015) | ||
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Fan et al. (2019) | ||
| Viscum album L. (apple tree) |
Extract containing 2.5–10 ng/mL lectin MLI Extract containing 40–60 µg/mL oleanolic acid Extract containing 2.5–10 ng/mL lectin MLI and 40–60 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B and Saos-2 | Kleinsimon et al. (2017) | ||
| Cytochrome c and AIF | Viscum album L. (apple tree) |
Extract containing 4–8 ng/mL lectin MLI Extract containing 25–35 µg/mL oleanolic acid Extract containing 4–8 ng/mL lectin MLI and 25–35 µg/mL oleanolic acid |
Human acute myeloid leukemia cell lines U937 and HL-60 | Release of cytochrome c | Delebinski et al. (2015) |
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Release of cytochrome c and AIF | Fan et al. (2019) | |
| MAPK/JNK, ERK and p38 | Lectin II isolated from Viscum coloratum (Kom.) Nakai | 100 ng/mL | Human myeloid leukemia cell line U937 | Increased phosphorylation of the JNK1 substrate, GST-c-Jun N-terminal protein | Park et al. (2000) |
| AbnobaViscum F (ash) | 20 µg/mL | Human myeloid leukemia cell line K562 | Increased phosphorylation of JNK1 and p38, reduced levels of phosphorylated ERK-1/2 | Park et al. (2012) | |
| Viscum album L. (apple tree) |
Extract containing 1–40 ng/mL lectin MLI Extract containing 10–60 µg/mL oleanolic acid Extract containing 1–40 ng/mL lectin MLI and 10–60 µg/mL oleanolic acid |
Human Ewing sarcoma cell lines TC-71 and MHH-ES-1 | Increased phosphorylation of JNK1 and p38 | Twardziok et al. (2017) | |
| Viscum album L. (apple tree) |
Extract containing 10 ng/mL lectin MLI Extract containing 60 µg/mL oleanolic acid Extract containing 5 ng/mL lectin MLI and 50 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B, Saos-2 and U2OS | Activation of JNK1 with simultaneous inactivation of ERK-1/2 | Kleinsimon et al. (2018) | |
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Upregulation of the expression levels of p-ERK1/2 and p-P90RSK | Fan et al. (2019) | |
| PI3K/AKT | 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Downregulation of the phosphorylation of AKT and P70RSK | Fan et al. (2019) |
| Lectin isolated from Viscum coloratum (Kom.) Nakai | 10 ng/mL | Human cancer cell line A253 | Dephosphorylation of AKT | Choi et al. (2004) | |
|
Iscador Qu Spezial (oak) Iscador M (apple tree) |
0.3 mg/mL | Human tongue cancer cell lines SCC9 and SCC25 | Reduced pAKT | Klingbeil et al. (2013) | |
| AbnobaViscum F (ash) | 20 µg/mL | Human myeloid leukemia cell line K562 | Reduced levels of phosphorylated AKT | Park et al. (2012) | |
| COX-2 | VA Qu Spez (oak) | 10–100 µg/mL | Human lung adenocarcinoma cell line A549 | Inhibition of the secretion of IL-1β-induced PGE2 associated with a reduced expression of COX-2 | Hegde et al. (2011) and Saha et al. (2015) |
| Caspases | Viscum album L. (apple tree) |
Extract containing 2–16 ng/mL lectin MLI Extract containing 20–40 µg/mL oleanolic acid Extract containing 2–16 ng/mL lectin MLI and 20–40 µg/mL oleanolic acid |
Human acute myeloid leukemia cell line HL-60 | Activation of caspase-8 and caspase -9 | Delebinski et al. (2015) |
| Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | Activation of caspase-8 and caspase-3 | Mishra et al. (2018) | |
| Viscum album L. (apple tree) |
Extract containing 1.25–7.5 ng/mL lectin MLI Extract containing 30–45 µg/mL oleanolic acid Extract containing 1.25–7.5 ng/mL lectin MLI and 30–45 µg/mL oleanolic acid |
Human alveolar Rhabdomyosarcoma cell line RMS-13 | Activation of caspase-9, caspase-8 and caspase-3 | Stammer et al. (2017) | |
| Viscum album L. (apple tree) |
Extract containing 1–40 ng/mL lectin MLI Extract containing 10–60 µg/mL oleanolic acid Extract containing 1–40 ng/mL lectin MLI and 10–60 µg/mL oleanolic acid |
Human Ewing sarcoma cell lines TC-71 and MHH-ES-1 | Activation of caspase-9, caspase-8 | Twardziok et al. (2016) | |
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Activation of caspase-9 and caspase-3 | Fan et al. (2019) | |
| AbnobaViscum F (ash) | 20 µg/mL | Human myeloid leukemia cell line K562 | Decreased expression of procaspase-9 but increased that of cleaved (active) caspase-9 | Park et al. (2012) | |
| Lectin isolated from Viscum coloratum (Kom.) Nakai | 10 ng/mL | Human cancer cell line A253 | Activation of caspase-3 | Choi et al. (2004) | |
| Viscum album L. (apple tree) |
Extract containing 2.5–10 ng/mL lectin MLI Extract containing 40–60 µg/mL oleanolic acid Extract containing 2.5–10 ng/mL lectin MLI and 40–60 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B and Saos-2 | Activation of caspase-8 and caspase-9 | Kleinsimon et al. (2017) | |
| Viscum Coloratum (Kom.) Nakai |
Lectin 10–1000 ng/mL Extract 10–1000 µg/mL |
Mouse melanoma cell lines B16BL6 and B16F10 | Activation of caspase-1, 3, 4, 5, 6, 7, 8, and 9 | Han et al. (2015) | |
| Antiapoptotic proteins | Viscum album L. (apple tree) |
Extract containing 40 ng/mL lectin MLI Extract containing 40 µg/mL oleanolic acid Extract containing 15 ng/mL lectin MLI and 30 µg/mL oleanolic acid |
Human acute myeloid leukemia cell line HL-60 | Downregulation of BIRC5, XIAP, p53 and claspin | Delebinski et al. (2015) |
| Viscum album L. (apple tree) |
Extract containing 1–40 ng/mL lectin MLI Extract containing 10–60 µg/mL oleanolic acid Extract containing 1–40 ng/mL lectin MLI and 10–60 µg/mL oleanolic acid |
Human Ewing sarcoma cell lines TC-71 and MHH-ES-1 | Downregulation of BIRC5, XIAP, MCL1 and CLSPN, | Twardziok et al. (2016) | |
| Viscum album L. (apple tree) |
Extract containing 2.5–10 ng/mL lectin MLI Extract containing 40–60 µg/mL oleanolic acid Extract containing 2.5–10 ng/mL lectin MLI and 40–60 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B and Saos-2 | Downregulation of BIRC5, XIAP, BCL2, and CLSPN | Kleinsimon et al. (2017) | |
| Viscum album L. (apple tree) |
Extract containing 5 ng/mL lectin MLI Extract containing 40 µg/mL oleanolic acid Extract containing 5 ng/mL lectin MLI and 40 µg/mL oleanolic acid |
Human alveolar Rhabdomyosarcoma cell lines RH-30 and RMS-13 | Downregulation of BIRC5, XIAP, BCL2, BCL2L1 and MCL1 | Stammer et al. (2017) | |
| Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | Downregulation of BCL2 | Mishra et al. (2018) | |
| Lectin isolated from Viscum coloratum (Kom.) Nakai | 10 ng/mL | Human hepatocarcinoma cells SK-Hep-1 and Hep3B | Lyu et al. (2002) | ||
| AbnobaViscum F (ash) | 20 µg/mL | Human myeloid leukemia cell line K562 | Downregulation of Mcl-1 | Park et al. (2012) | |
| 1,7-bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Upregulation of Bcl-2 and Bcl-xL | Fan et al. (2019) | |
| Proapoptotic proteins | Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | Upregulation of Bax | Mishra et al. (2018) |
| Lectin isolated from Viscum coloratum (Kom.) Nakai | 10 ng/mL | Human hepatocarcinoma cells SK-Hep-1 and Hep3B | Lyu et al. (2002) | ||
| Viscum album L. (apple tree) |
Extract containing 2.5–10 ng/mL lectin MLI Extract containing 40–60 µg/mL oleanolic acid Extract containing 2.5–10 ng/mL lectin MLI and 40–60 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B and Saos-2 | Kleinsimon et al. (2017) | ||
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 2.5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Downregulation of Bax | Fan et al. (2019) | |
| STAT3 | Viscum album L. (apple tree) |
Extract containing 10 ng/mL lectin MLI Extract containing 60 µg/mL oleanolic acid Extract containing 5 ng/mL lectin MLI and 50 µg/mL oleanolic acid |
Human osteosarcoma cell lines 143B, Saos-2 and U2OS | Dephosphorylation of STAT3 at Tyr705 and Ser727, down-regulation of total STAT3 and its direct downstream targets BIRC5 and C-MYC | Kleinsimon et al. (2018) |
| AbnobaViscum F (ash) | 5–20 µg/mL | Human hepatocellular carcinoma cell line Hep3B | Reduction of C-MYC protein levels which might be mediated by the ubiquitin–proteasome system | Yang et al. (2019) | |
| Telomerase | Lectin isolated from Viscum coloratum (Kom.) Nakai | 10 ng/mL | Human cancer cell line A253 | Inhibition of telomerase activity through downregulation of hTERT | Choi et al. (2004) |
| ROS | Viscum articulatum Burm. f. (Dalbergia latifolia Roxb.) | 0.015–150 µg/mL | Human leukemia cell lines Jurkat E6.1 and THP1 | ROS mediated DNA fragmentation | Mishra et al. (2018) |
| 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one isolated from Viscum coloratum (Kom.) Nakai | 5–20 µM | Human lung cancer cell lines A549 and NCI-H292 | Promotion of ROS generation | Fan et al. (2019) |
Cardiac activity
The cardiac activity of mistletoe has been confirmed in in vitro as well as in vivo studies (Committee on Herbal Medicinal Products 2012; Poruthukaren et al. 2014; Montero et al. 2016) (Table 2). Tenorio et al. (2005) and Tenorio-Lopez et al. (2006) studied the mechanism of vasodilator activity of aqueous extract of Viscum album L. leaves on the Langendorff’s isolated and perfused heart model. They showed that mistletoe induced nitric oxide syntetaze-2 (NOS-2) and nitric oxide syntetaze-3 (NOS-3) overexpression, which was connected with increases in nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) production. Therefore, the vasodilator activity of mistletoe might be mediated by the NO/sGC pathway. Soluble guanylyl cyclase (sGC) is an enzyme catalysing the conversion of GTP to cGMP and mediating several biological functions, such as the inhibition of platelet aggregation, smooth muscle relaxation and vasodilation. NO activates sGC by directly binding to heme to form a ferrous–nitrosyl–heme complex. Once sGC is activated by NO, GTP to cGMP conversion is triggered. Exogenous and endogenous compounds produce vasodilation through increases in cGMP, which in turn, relaxes vascular smooth muscle cells by both desensitising the contractile apparatus to Ca2+ and lowering intracellular Ca2+, with the consequent activation of a protein known as cGMP-dependent protein kinase. NO, synthesised by the enzyme nitric oxide synthase (NOS), maintains a vasodilator tone that is essential for the regulation of blood flow and pressure. NOS is a heme-containing enzyme that has three isoforms, designated as NOS-1, NOS-2 and NOS-3 (Tenorio-Lopez et al. 2006). Furthermore, studies on myocardial ischemia and reperfusion injury in rats as well as isoproterenol-induced heart failure in rats confirmed the cardioprotective effect of Viscum album L. might be mediated by the upregulation of the NO pathway (Karagöz et al. 2016; Suveren et al. 2017). Studies of Nω-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats treated with methanolic extract of Viscum articulatum Burm. f. showed that mistletoe has an antihypertensive effect, which may be attributed to its diuretic, nephroprotective and hypolipidemic action. It was proposed the blood pressure lowering activity of this extract might have been due to the presence of triterpenoids, such as oleanolic acid and betulinic acid (Bachhav et al. 2012). Oleanolic acid isolated from the cuticular wax of Viscum articulatum Burm. f. significantly decreased the systolic blood pressures and cardiac lipid peroxidation levels in glucocorticoid (dexamethasone)-induced hypertensive rats, which might be connected with its antioxidant and nitric oxide releasing action (Bachhav et al. 2011). On the other hand, a study carried out in L-NAME-induced hypertensive rats treated with oleanolic acid showed that this compound did not affect nitric oxide levels, and its antihypertensive effect might be due to diuresis and nephroprotection (Bachhav et al. 2015). Another mechanism of the antihypertension activity of Viscum might be mediated by the calcium channel blockade (CCB). The contractile mechanism in smooth muscle is activated by a rise in the concentration of free intracellural Ca2+ concentration, which activates the contractile elements. The increase in intracellular Ca2+ occurs via either influx from the extracellular fluid through voltage-dependant Ca2+ channels (VDCs) or its release from intracellular stores. Thus, vascular smooth muscle relaxant agents may produce their effects by inhibiting either or both sources of Ca2+ (Mojiminiyi et al. 2008; Khan et al. 2016). A study carried out in rat aortic rings showed that an aqueous extract of leaves of Viscum album L. growing on oil palm trees had vasorelaxant activity, which might be mediated by a non-specific non-competitive inhibition of Ca2+ influx as well as inhibition of Ca2+ mobilization from intracellular stores (Mojiminiyi et al. 2008). Furthermore, a study carried out in rabbit aortic rings showed that vasorelaxant activity of mistletoe is mediated through a voltage-dependent Ca2+ channel blockade (Khan et al. 2016). It was proposed that some of the actions on Ca2+ influx or mobilization from cellular stores observed in the study for Viscum album L. might be partly mediated by NO. This is because NO inhibits Ca2+ influx through ligand gated Ca2+ channels as well as release from cellular stores. Thus, it is probable that Viscum album L. might achieve vasorelaxation through dual mechanisms, the NO/sGC pathway as well as through Ca2+-dependent mechanisms (Mojiminiyi et al. 2008) (Fig. 2). Ofem et al. (2007) suggested that the reduction in blood pressure without any alteration in heart rate by aqueous extract of leaves of Viscum album L. growing on citrus may be due to catecholamine-like blocking agent(s), showing predominantly alpha-1 adrenoceptor antagonist action or agonist-like agents that may be stimulating the beta-2 adrenoceptors to produce the depressor effect. In turn, Radenkovic et al. (2009) proposed that decreases in the blood pressure in rats treated with ethanolic extracts of Viscum album L. steams might be connected with muscarine cholinergic receptors. In rat models of myocardial infarction, flavonoids isolated from Viscum coloratum (Kom.) Nakai reduced ischemic myocardial injuries by blocking the signalling pathway of platelet-activating factor (PAF). A PAF antagonist isolated from mistletoe might be a homoeriodictyol-7-O-β-D-glucoside (Chu et al. 2008). Additionally, Viscum album L. improved haematological parameters in rats. Mistletoe extracts reduced red blood cell count and packed cell volume (Ofem et al. 2009; Ladokun et al. 2015) and brought the elevated total plasma protein levels and reduced erythrocyte sedimentation rate in the high salt-fed rats to near control levels, indicating the ability of the extract to prevent marked changes in the blood viscosity (Ofem et al. 2009).
Table 2.
Pharmacological activity of Viscum species—in vivo studies
| Pharmacological activity | Viscum species/Product | Part | Host tree | Extraction solvent | Compounds | Dose | Route of administration | Study duration | Experimental design | Results | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antihypertensive activity | Viscum album L | Fresh leaves | Citrus | Aqueous | 150 mg/kg, daily | Orally | 6 weeks | Normotensive, renal artery-occluded hypertensive and salt-induced hypertensive rats | Decrease in arterial blood pressure without alteration in heart rate, antihypertensive effect might involve sympathetic mechanism | Ofem et al. (2007) | |
| Viscum album L | Fresh steams | Ethanolic, ether and ethyl acetate | 3.33 × 10−5, 1.00 × 10−4, 3.33 × 10−4, 1.00 × 10−3 mg/kg | Intraperitoneally | Atropine sulfate and hexocycline treated rats | Ethanolic extract exhibited activity even on the lowest dose, the ether and ethyl acetate extracts exhibited activity only by higher doses, antihypertensive effect might involve muscarinic receptors | Radenkovic et al. (2009) | ||||
| Viscum album L | Dried leaves | Pear (Pyrus communis auct. iber.) | Aqueous | 250 mg/kg, daily | Orally by gavage | 24 days | Isoproterenol-induced heart failure in rats | Improvement in all parameters of heart failure including left ventricular diameters, ejection fraction, serum NT-proBNP levels and histopathological changes; decrease in levels of NO, iNOS and hs-CRP | Karagöz et al. (2016) | ||
| Viscum album L | 0.6–2.8 g, daily | Orally | 6 weeks | An open study in 120 patients with light to moderate hypertension (WHO grade I-II) | Decrease in systolic pressure (in rest and during physical exercise) | Committee on Herbal Medicinal Products (2012) | |||||
| Viscum album L | 5 drops of drug every 5 min up to 4 administrations | Sublingually | 264 patients with diagnosis of hypertension | Time of arterial blood pressure reduction was less for the group of patients who received the natural treatment | Montero et al. (2016) | ||||||
| Viscum album L. mother tincture | Ethanolic extract manufactured according to Homoeopathic Pharmacopoeia of India | 10 drops of drug in 30 ml of distilled water, three times a day | Orally | 12 weeks | 37 newly diagnosed hypertensive patients | Decrease in systolic and diastolic pressure, decrease in serum triglyceride | Poruthukaren et al. (2014) | ||||
| Viscum articulatum Burm. f | Dried herb | Cordia macleodii Hook.f. & Thomson | Methanolic | 200 and 400 mg/kg, daily | Orally | 4 weeks | L-NAME-induced hypertensive rats | Antihypertensive effect might be attributed to diuretic, nephroprotective and hypolipidemic actions, and might be due to the presence of triterpenoids | Bachhav et al. (2012) | ||
| Viscum articulatum Burm. f | Cuticular wax | Oleanolic acid | 60 mg/kg, daily | Intraperitoneally | 15 days | Glucocorticoid (dexamethasone)-induced hypertensive rats | Decrease of the systolic blood pressure, which might be connected with its antioxidant and NO releasing action | Bachhav et al. (2011) | |||
| Viscum articulatum Burm. f | Cuticular wax | Oleanolic acid | 60 mg/kg, daily | 4 weeks | L-NAME-induced hypertensive rats | Oleanolic acid did not affected NO level and its antihypertensive effect might be due to diuresis and nephroprotection | Bachhav et al. (2015) | ||||
| Hematological parameters | Viscum album L | Fresh leaves | Citrus | Aqueous | 150 mg/kg, daily | Orally | 6 weeks | High salt-fed rats | Decrease in the red blood cells, packed cell volume, haemoglobin, total plasma protein levels and increase in erythrocyte sedimentation rate | Ofem et al. (2009) | |
| Viscum album L | Dried leaves | Coffee (Coffee arabica), kola (Kola nitida) and cocoa (Theobromae cacao) | Aqueous | 400, 800, 1600 and 3200 mg/kg, daily | Orally | 14 days | Healthy rats | Mistletoe parasitizing on kola significantly, in dose dependent manner, decreased platelets count, mistletoe parasitizing cocoa and coffee reduced haemoglobin concentration, all the extracts reduced packed cell volume, red blood cell and increased white blood cells | Ladokun et al. (2015) | ||
| Antiglycemic, antilipidemic and insulinotropic effect | Viscum album L | Dried leaves | Ethanolic | 250, 500, 750, 1000 mg/kg | 10 h | Normalglycemic and streptozotocin-induced diabetic rats | Reduction in fasting blood glucose level | Nwaegerue et al. (2007) | |||
| Viscum album L | Dried herb | Apricot (Armeniaca vulgaris Lam.), pine (Pinus nigra J.F.Arnold), fir (Abies bornmlleriana Mattf.) | Aqueous and ethanolic | 500 mg/kg | Orally by gastric gavage | 8 days | Streptozotocin-induced diabetic rats | Antidiabetic effect of mistletoe depends on the host tree | Orhan et al. (2005) | ||
| Viscum album L | Fresh leaves | Aqueous | 100 mg/kg | Intravenously | 3 h | Normalglycemic and streptozotocin-induced diabetic rats | No effect on glucose level in normal rats but decrease of the blood glucose level in the diabetic rats, increase of the insulin secretion in normal rats and in the diabetic group | Eno et al. (2008) | |||
| Viscum album L | Dried herb | Kola acuminate | Methanolic | 100 mg/kg, daily | 3 weeks | Streptozotocin-induced diabetic rats | Reduction in fasting blood glucose level, HbA1c, serum triglyceride, urea, lactate dehydrogenase, α-amylase and low density lipoprotein cholesterol, increase of high density lipoprotein cholesterol | Adaramoye et al. (2012) | |||
| Viscum album L | Dried leaves | Ethanolic | 100 mg/kg, daily | Orally by gavage | 10 days | Streptozotocin-induced diabetic rats | No significant difference in glucose level, reduction in oxidative stress | Turkkan et al. (2016) | |||
| Viscum album L | Dried leaves | Citrus | Aqueous | 150 mg/kg, daily | Orally with syringe and orogastric tube | 3 weeks | Streptozotocin-induced diabetic rats | Reduction in fasting blood glucose level | Nna et al. (2013) | ||
| Viscum album L | Dried leaves | Oil been (Pentaclethra macrophylla Benth.) | Aqueous | 200 mg/kg | Intraperitoneally | 4 h | Fasted normalglycemic rats | Decrease in blood glucose level | Ohiri et al. (2003) | ||
| 400 mg/kg | Alloxan-induced diabetic rabbits | ||||||||||
| Viscum album L | Dried leaves | Oak | Aqueous | 500 and 1000 mg/kg, daily | Orally by gavage | 3 days | Alloxan-induced diabetic rats | Decrease in serum glucose concentration and increase in the serum insulin level | Shahaboddin et al. (2011) | ||
| Viscum album L | Dried leaves | Sweet orange (Citrus sinensis (L.) Osbeck), african pear (Dacroydes edulis), guava (Psidium guajava L.) and pepper fruit (Dennettia tripetala Baker f.) | Aqueous | 100 mg/kg, daily | Orally by gavage | 14 days | Alloxan-induced diabetic rats | The strongest activity was exhibited by extracts of mistletoe growing on Citrus sinensis and Pisium guajava | Umoh et al. (2011) | ||
| Viscum album L | Dried leaves | Ethanolic | 2 mg/kg, 16 h | Intraperitoneally | 54 h | Alloxan-induced diabetic rats | Decrease in fasting blood glucose level | Ibegbulem and Chikezie 2013) | |||
| Viscum coloratum (Kom.) Nakai | Dried herb | Oak (Quercus variabilis Blume) | Protein fraction | 50—400 µg/ml | Intraperitoneally | 10 days | Alloxan-induced diabetic mice | Decrease in the blood glucose level and volume of drinking water | Kim et al. (2014b) | ||
| Viscum coloratum (Kom.) Nakai | Dried herb | Oak | Aqueous and ethanolic | Betulin and oleanolic acid | Diet containing 0.2 or 0.6% of extract | Orally | 8 weeks | Partial pancreatectomized rats | Ethanolic extract made β-cell mass greater by increasing β-cell proliferation and decreasing its apoptosis | Ko et al. (2016) | |
| Viscum schimperi Engl | Dried herb | Methanolic | 500 mg/kg, daily | Orally by gavage | 4 weeks | Streptozotocin-induced diabetic rats | Reduction in the fasting blood glucose level; increase of the level of insulin, reduction of total cholesterol, trigliceryde and low density lipoprotein cholesterol and increase of high density lipoprotein cholesterol | Abdel-Sattar et al. (2011) | |||
| Hepatoprotective activity | Viscum album L | Leaves | Ethanolic | 1 g/kg | Orally | Paracetamol-induced hepatotoxity in rats | Reduction of ALT, ALP levels, no influence on the levels of total bilirubin and total protein | Ogbonnanya et al. (2010) | |||
| Viscum album L | Dried leaves | Cocoa (Theobroma cacao L.) and cola (Cola nitida (Vent.) Schott & Endl.) | Methanolic | 1000–5000 mg/kg, daily | Orogastrically | 7 days | Paracetamol-induced hepatotoxity in rats | No significant difference in AST, ALT and ALP for V. album growing on cocoa, significant increase in AST, ALT and ALP for V. album growing on cola at 4000 and 5000 mg/kg doses | Yusuf et al. (2015) | ||
| Viscum album L | Dried leaves | Citrus | Aqueous | 150 mg/kg, daily | Orally (syringe and or gastric tube) | 6 weeks | High salt diet rats | Decrease in serum total bilirubin, serum conjugated bilirubin and serum unconjugated bilirubin | Ofem et al. (2014) | ||
| Viscum album L | Dried leaves | Citrus | Aqueous | 150 mg/kg, daily | Orally (syringe and or ogastric tube) | 3 weeks | Streptozotocin-induced diabetic rats | Decrease in serum total bilirubin, serum conjugated bilirubin and serum unconjugated bilirubin | Nna et al. (2014) | ||
| Viscum Fraxini-2 (Viscum album L.) | Ash | Aqueous | 0.1 and 0.2 ml/kg, once weekly | Subcutaneously | 30 days | Carbon tetrachloride-induced hepatotoxity in rats | Decrease in ALT, AST and ALP levels, restoration of the normal architecture of the liver tissue with minimal fibrosis | Abdel-Salam et al. (2010) | |||
| 0.2 ml/kg of mistletoe + 25 mg/kg of sylimarin, once weekly | |||||||||||
| Iscador Qu (Viscum album L.) | Fresh herb | Oak (Quercus robur L. and Quercus petraea (Matt.) Liebl.) | Fermented, aqueous extract | 380 ng/ml of lectins, 14 mg/ml of viscotoxines | Two 5 mg ampules, three times weekly | Subcutaneously | 12 months | 5 patients with chronic hepatitis C | 6–20 fold reduction in viral load (HCV-RNA) and complete remission of elevated AST and ALT in two out of five patients, an increase of HCV RNA in one patient | Tusenius et al. (2001) | |
| Iscador Qu (Viscum album L.) | Fresh herb | Oak (Quercus robur L. and Quercus petraea (Matt.) Liebl.) | Aqueous | 750 ng of lectins | 10 mg, three times weekly | Subcutaneously | 12 months | 21 patients with chronic hepatitis C | Decrease in ALT and AST during the 12 months treatment and slight increase after treatment end | Tusenius et al. (2005) | |
| AbnobaViscum (Viscum album L.) | Fresh herb | Oak | Aqueous | 1000 ng of lectins | 0.15 mg, three times weekly | ||||||
| AbnobaViscum Quercus (Viscum album L.) | Fresh herb | Oak | Aqueous | 65–3610 ng of lectins (mean weekly dose) | Three times a week | Subcutaneously | 9 months | 25 patients with chronic hepatitis C and elevated alanine aminotransferase (ALT) levels | None of the patients had complete or partial normalization of ALT or HCV-RNA levels during treatment period, mean ALT did not change during the study | Huber et al. (2001) | |
| Viscum coloratum (Kom.) Nakai | Dried steams and leaves | Aqueous | Alkaloid fraction | 120 mg/kg, daily | Orally by gastric gavage | 8 weeks | Carbon tetrachloride-induced hepatic fibrosis in rats | Decrease of hepatic fibrosis; reduction in mRNA levels of TGF-β1, procollagen I and TIMPs; increase in TGF-β1, TGF-β1 receptor, phosphorylated Smad 2 and α-SMA proteins in liver tissues; increase in Smad 7 level | Jiang et al. (2014) | ||
| Antiepileptic activity | Viscum album L | Fresh leaves | Citrus | Aqueous | 50 and 150 mg/kg | Orally | Maximum electro shock, isoniazid- and pentylenetetrazole-induced seizures in mice and rats | Reduction in various phases of epileptic seizures, increased latency to the first convulsion, increased convulsion onset and reduction in seizure duration | Gupta et al. (2012) | ||
| Viscum album L | Dried herb | Maple (Acer platanoides L.) | Aqueous and aqueous-ethanolic | 100 mg/kg | Intragastrically | 2 days | Pentylenetetrazole-induced seizures in mice | Effective against pentylenetetrazole-induced seizures | Tsyvunin et al. (2016) | ||
| Willow (Salix alba L.) | Ethanolic | ||||||||||
| Viscum Mali e planta tota (Viscum album L.) | Apple tree |
Initially given in strength D5, 10 granules BID, equivalent to a 1:100,000 dilution of the whole plant extract, later increased to D2, equivalent to a 1:100 dilution, 10 granules twice a day |
12 weeks | 4½-year-old girl suffering from childhood absence epilepsy | The dose increase of Viscum Mali, in addition to an existing combination with valproic acid and clobazam, may have played a key role in achieving seizure freedom for this child | von Schoen-Angerer et al. (2015) | |||||
| Viscum capense L. f | Dried stems | Methanolic | 50 and 100 mg/kg | Intraperitoneally | Pentylenetetrazole-, bicuculline- and N-methyl-DL-aspartic acid- induced seizures in mice | Delayed the onset of pentylenetetrazole—and bicuculline-induced seizures and reduction in the number of convulsing animals; moderate effect against N-methyl-DL-aspartic acid-induced tonic seizures | Amabeoku et al. (1998) | ||||
| Viscum articulatum Burm. f | Dried herb | Methanolic | 100 and 200 mg/kg, daily | Orally | 7 days | Maximum electro shock- and pentylenetetrazole- induced seizures in rats | Reduction in duration of hind limb extensor phase and increase in the latency to convulsions | Geetha et al. (2010) | |||
| Viscum articulatum Burm. f | Fresh herb | Chloroform and methanolic | Syringaresinol | 150 and 300 mg/kg for extracts, 10 and 20 mg/kg for isolated compound | Orally | 7 days | Picrotoxin- induced seizures in rats | Extracts and syringaresinol delayed the onset of tonic convulsions, increase in the brain GABA levels in rats treated with the methanolic extract | Geetha et al. (2018) | ||
| N-methyl-d-aspartic acid—induced seizures in mice | Only the methanolic extract and syringaresinol antagonized the N-methyl-d-aspartic acid -induced turning behaviour | ||||||||||
| Sedative activity | Viscum album L | Fresh leaves | Citrus | Aqueous | 50 and 150 mg/kg | Orally | Mice placed in actophotometer | Reduction in locomotor activity | Gupta et al. (2012) | ||
| Viscum album L | Fresh leaves | Citrus | Aqueous | 50 and 150 mg/kg | Orally | Pentobarbital- induced sleeping time in mice | Increase in duration of sleeping time | Gupta et al. (2012) | |||
| Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 200 and 400 mg/kg for extract, 25 and 50 mg/kg for fractions | Orally | Open field test on mice | Reduction in rearing and crossings | Kumar et al. (2016) | ||||
| Viscum orientale Willd | Dried leaves | Exoecaria agalloch | Methanolic | 300 and 500 mg/kg | Orally | Open field test and hole cross test in mice | Reduction in spontaneous motor activities | Khatun et al. (2016) | |||
| Hypnotic activity | Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 200 and 400 mg/kg for extract, 25 and 50 mg/kg for fractions | Orally | Thiopentone sodium induced-sleeping time assay in mice | Increase in the duration of sleep in mice | Kumar et al. (2016) | |||
| Antipsychotic activity | Viscum album L | Fresh leaves | Citrus | Aqueous | 50 and 150 mg/kg | Orally | Apomorphine-induced stereotypy in mice and rats | Significantly reduction in the stereotyped behaviour | Gupta et al. (2012) | ||
| Viscum album L | Fresh leaves | Citrus | Aqueous | 50 and 150 mg/kg | Orally | Haloperidol-induced catalepsy in mice and rats (bar test) | Enhancement in cataleptic effect of haloperidol | Gupta et al. (2012) | |||
| Antianxiety activity | Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 50 and 100 mg/kg for extract, 5 and 10 mg/kg for fractions | Orally | Elevated plus-maze test on mice (EPM model) | The number of entries and time spent in open arms in the elevated plus-maze test were significantly increased | Kumar et al. (2016) | |||
| Antistress activity | Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 200 and 400 mg/kg for extract, 25 and 50 mg/kg for fractions | Orally | Cold swim test on mice | Reduction in time spent by mice in the immobile state | Kumar et al. (2016) | |||
| Antidepressant activity | Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 200 and 400 mg/kg for extract, 25 and 50 mg/kg for fractions | Orally | Despair swim test on mice | Reduction in the duration of immobility in mice | Kumar et al. (2016) | |||
| Analgesic activity | Viscum album L | Dried herb | Methanolic and its ethyl acetate and 1-butanol fractions | 200 and 400 mg/kg for extract, 25 and 50 mg/kg for fractions | Orally | Tail immersion test was conducted by recording tail withdrawal from heat (flicking response) in mice | Significant analgesic activity | Kumar et al. (2016) | |||
| Viscum album L | Dried leaves and stems | Apricot (Armeniaca vulgaris Lam.) | Ethyl acetate | 2′-Hydroxy-4′,6′-dimethoxy-chalcone-4-O-β-d-glucopyranoside and 5,7-dimethoxy-flavanone-4′-O-[β-D-apiofuranosyl-(1 → 2)]-β-D-glucopyranoside | 125 and 250 mg/kg for extract and 30 mg/kg for isolated compounds | Orally | p-Benzoquinone-induced writhing test in mice and carrageenan-induced hind paw edema model in mice | Ethyl acetate fraction and isolated compounds exhibited antinociceptive and anti-inflammatory activity | Orhan et al. (2006) | ||
| Viscum orientale Willd | Dried leaves | Exoecaria agalloch | Methanolic | 300 and 500 mg/kg | Orally | Acetic acid-induced writhing model in mice and formalin-induced paw licking in mice | Writhing and paw licking inhibition | Khatun et al. (2016) | |||
| Alzheimer’s disease | Viscum album L | Dried leaves | Orange tree | Aqueous | 100 mg/kg, daily | Orally | 21 days | Aluminum chloride-induced Alzheimer’s disease in mice | Increase in the brain-derived neurotrophic factor (BDNF); reduction of aluminum chloride-induced memory impairment and oxidative damage | Ademola et al. (2016) and Ekpenyong et al. (2016) | |
| Viscum coloratum (Kom.) Nacai | Dried herb | Methanolic | 25 and 50 mg/kg, daily | Orally | 7 days | Intracerebroventricular injection of amyloid β protein in mice | Protection from memory impairment induced by intracerebroventricular injection of amyloid β protein | Jang et al. (2015) | |||
| Mood | Eurixor (Viscum album L.) | Fresh herb | Aqueous | Lectin (ML-1) | 1 ng/kg body weight, twice a week | Subcutaneously | 12 weeks | Breast cancer patients (n = 36) | Increased levels of plasma beta-endorphin | Heiny and Beuth 1994) | |
| Eurixor (Viscum album L.) | Fresh herb | Aqueous | Lectin (ML-1) | 0.5–1.0 ng /kg body weight, twice a week | Subcutaneously | 24 weeks | Breast cancer patients (n = 47) | Increased levels of plasma beta-endorphin | Heiny et al. (1998) | ||
| Antiobesity activity | Viscum coloratum (Kom.) Nacai | Dried herb | Oak | Aqueous | 3 g/kg, daily | Orally | 15 weeks | High-fat diet-induced obesity in mice | Reduction in body and epididymal fat pad weights | Jung et al. (2013) | |
| Viscum coloratum (Kom.) Nacai | Dried herb | Oak | Aqueous and ethanolic | Betulin and oleanolic acid | Diet containing 0.2 or 0.6% of extract | Orally | 8 weeks | Partial pancreatectomized rats | Reduction in epididymal fat mass by increasing fat oxidation | Ko et al. (2016) | |
| Endurance capacity | Viscum coloratum (Kom.) Nacai | Dried herb | Oak | Aqueous | 3 g/kg, daily | Orally | 15 weeks | Endurance test with treadmill in high-fat diet- induced obesity mice | Mistletoe treated mice run twice as far as high-fat diet mice | Jung et al. (2013) | |
| Viscum coloratum (Kom.) Nacai | Dried herb | Oak | Aqueous | 400 and 1000 mg/kg, daily | 1 week | Endurance test with treadmill in mice | Mistletoe treated mice run 2.5-times longer than control mice, plasma lactate levels of exhausted mice were significantly lower | Jung et al. (2012) | |||
| 25—400 mg/kg, daily | Forced swim test in mice | The swimming time to exhaustion was prolonged by as much as 212% | |||||||||
| Viscum coloratum (Kom.) Nacai | Leaves | Oak | Aqueous | 500 mg/kg, daily | Orally | 2 weeks | Endurance test with treadmill in mice | Decreases in level of plasma lactate dehydrogenase, increase in the plasma FFA level | Lee et al. (2014) | ||
| Viscum coloratum (Kom.) Nacai | Whole plant | Aqueous | diet Containing 0.3 and 1.5% of extract | Orally | 4 weeks | Treadmill and swimming pool tests in mice | Increased swimming activity and elevated running times on the treadmill | Jeong et al. (2017) | |||
| Activity against muscle decline | Viscum coloratum (Kom.) Nacai | Whole plant | Aqueous | 200 and 500 mg/kg, twice a day | Orally by gavage | 15 days | Denervated mice | Decrease in denervation, decrease in the expression of Atrogin-1, no effect on Murf1 expression | Jeong et al. (2017) | ||
| Diet containing 0.3 and 1.5% of extract | 4 weeks | Mice | Increased whole body weights, a higher weight of quadricepses, increased grip strengths, increased swimming activity and elevated running times on the treadmill, increased skeletal muscle area and diameter | ||||||||
| Viscum coloratum (Kom.) Nacai | Whole plant | Aqueous | 1 and 2 g/d | Orally | 12 weeks | Randomized controlled trial with 67 patients aged 55–75 | Significant differences were found in atrogin-1 mRNA, myogenin mRNA and insulin growth factor 1 receptor phosphorylation | Lim et al. (2017) | |||
| Nephroprotective activity | Helixor M (Viscum album L.) | Fresh herb | Apple tree (Malus domestica Borkh.) | Aqueous | 5 mg/kg | Intraperitoneally | 10 days | Methotrexate-induced acute oxidative stress and nephrotoxicity in rats | Improvement in the glutathione peroxidase and superoxide dismutase activities, decrease in the NO and myeloperoxidase levels was not significant | Sakalli Çetin et al. (2017) | |
| Viscum articulatum Burm. f | Cordia macleodii Hook.f. & Thomson | Oleanolic acid | 40, 60 and 80 mg/kg, daily | Orally | 8 days | Gentamicin-induced nephrotoxicity | Decrease in serum and urine levels of creatinine, albumin and urea | Patil et al. (2010) | |||
| Diuretic activity | Viscum angulatum B.Heyne ex DC | Dried herb | Randia dumetorum (Retz.) Lam | Methanolic | 100, 200 and 400 mg/kg | Orally | 24 h | Rats | Dose-dependent increase in urine excretion volume, significant saluretic and natriuretic activity, the Cl(-)/Na( +) + K( +) ratio, which indicates carbonic anhydrase mediated activity remained unaffected | Jadhav et al. (2010b) | |
| Viscum articulatum Burm. f | Dried herb | Cordia macleodii Hook.f. & Thomson | Methanolic | 100, 200 and 400 mg/kg | Orally | 24 h | Rats | Dose-dependent increase in urine excretion volume, significant saluretic and natriuretic activity | (Jadhav et al. (2010a) | ||
| Wound healing | Viscum album L | Liphohilic extract | Ointment | Topical treatment | 12 patients with 15 BCC lesions | Achievement of hemostasis in bleeding tumor wounds and after a prolonged treatment period a wound epithelialization with a thin epithelial layer | Kunz et al. (2011) and Kuonen et al. (2013) | ||||
| Viscum articulatum Burm. f | Whole plant | Ethanol | 1% extract ointment | Incision, excision and dead space wound model in rats | Reduction in wound area, faster re-epithelization rate | Garg et al. (2012) | |||||
| Antiulcer activity | Viscum articulatum Burm. f | Dried herb | Methanolic | 200 and 400 mg/kg | orally | Ethanol-induced ulcer model and pylorus ligation ulcer model in rats | Inhibition of the gastric lesions | Naganjaneyulu et al. (2011) | |||
| Antibacterial activity | Viscum album L | Dried leaves | Cocoa | Methanolic | 1000 mg/kg, daily | 7 days | Rats with infections of Staphylococcus aureus + Bacillus cereus, Escherichia coli + Pseudomonas aeruginosa, S. aureus + P. aeruginosa and infection of E. coli | Heamatological and histopathological analyses showed therapeutic effects of the extract | Yusuf et al. (2013) |
Fig. 2.
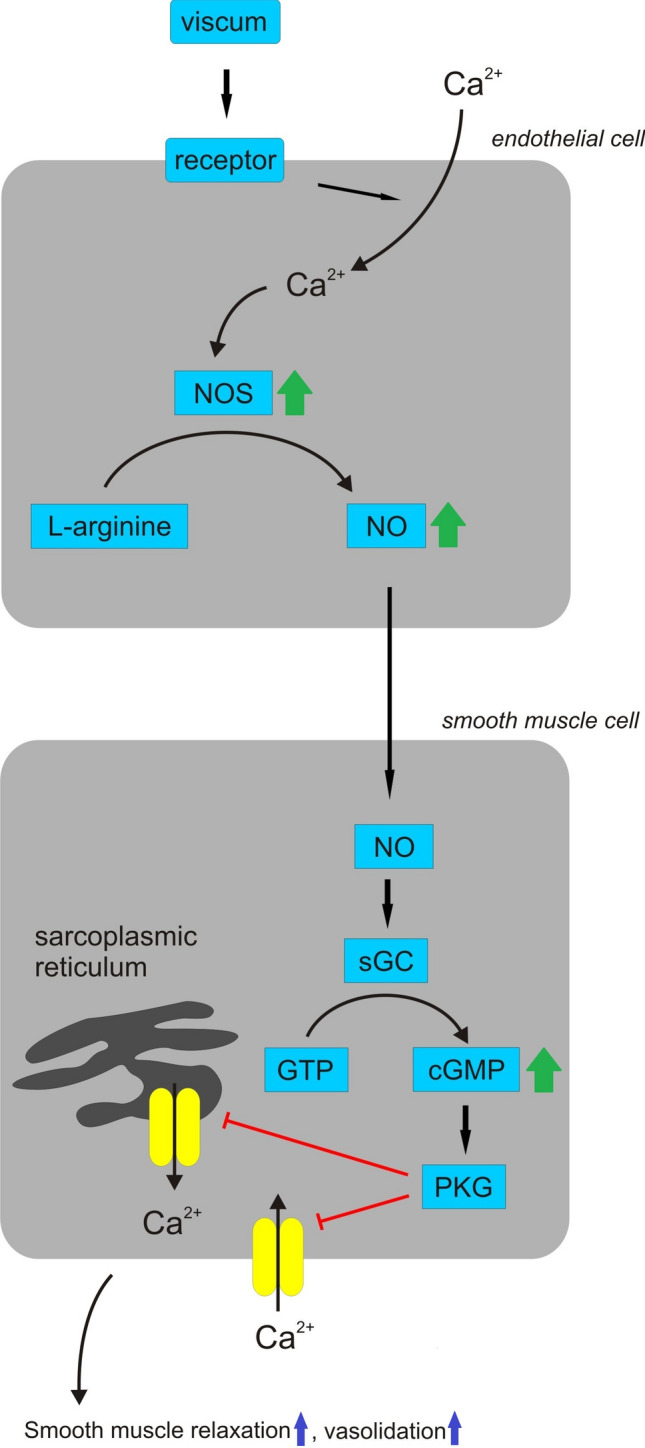
Mechanism of cardiac activity of mistletoe. Mistletoe compounds acting on receptor of endothelial cell might activate influx of Ca2+ ions leading to activation of NOS. NOS catalyzes formation of NO from L-arginine. NO diffuses to smooth muscle cell. Once sGC is activated by NO, GTP to cGMP conversion is triggered. cGMP activates PKG leading to reducing intracellular Ca2+ (by inhibition of Ca2+ influx through ligand gated Ca2+ channels and release from cellular stores). Proposed mechanism is confirmed by the fact that mistletoe induces NOS-2 and NOS-3 overexpression which is connected with increase in NO and cGMP production
Antidiabetic activity
In vivo studies on rats showed that Viscum species exhibit antiglycemic and insulinotropic activity by decreasing blood glucose level and increasing the insulin secretion (Ohiri et al. 2003; Nwaegerue et al. 2007; Eno et al. 2008; Shahaboddin et al. 2011; Abdel-Sattar et al. 2011; Adaramoye et al. 2012; Ibegbulem and Chikezie 2013; Kim et al. 2014b; Turkkan et al. 2016) (Table 2). Furthermore, the effects of mistletoe have been shown to be dependent on host trees (Orhan et al. 2005; Umoh et al. 2011). The antilipidemic activity of mistletoe was shown in the reduction in low density lipoprotein cholesterol (LDL) and the increase in high density lipoprotein cholesterol (HDL) (Abdel-Sattar et al. 2011; Adaramoye et al. 2012; Kim et al. 2015) as well as improvement of HOMA-IR (Homeostatic Model Assessment of Insulin Resistance), which is an indicator of insulin resistance (Kim et al. 2015). Gray and Flatt (1999) showed that aqueous extract of Viscum album L. exhibited dose-dependent activity to stimulate insulin secretion by rat clonal pancreatic β-cells, and the effect was not mediated by lectins. Furthermore, Kim et al. (2014b) showed that Korean mistletoe growing on oak increased the insulin secretion from the rat pancreatic β-cells (RINm5F cells) without any effects of cytotoxicity. The lectin-free protein fraction induced insulin secretion was similar to the Korean mistletoe extract. It was also reported that the protein fraction upregulated pancreatic and duodenal homeobox 1 (PDX-1) and beta2 (neuroD), which are transcription factors regulating the expression of insulin gene. An ethanolic extract of Korean mistletoe growing on oak also made β-cell mass greater by increasing β-cell proliferation and decreasing its apoptosis. An in vitro study showed that betulin potentiated insulin-stimulated glucose uptake by increasing PPAR-γ (peroxisome proliferator-activated receptor γ) activity and insulin signalling in 3T3-L1 adipocytes, whereas oleanolic acid enhanced glucose-stimulated insulin secretion and cell proliferation in insulinoma cells (Ko et al. 2016). Aqueous Viscum coloratum (Kom.) Nakai extract significantly increased the secretion of insulin and an insulin precursor, C-peptide, by RINm5F cells. In differentiated C2C12 cells, the extract enhanced the expression of glucose transporter type 4 (GLUT-4), insulin receptor substrate 1 (IRS-1), and protein kinase B (AKT), which are involved in the glucose uptake signalling pathway. Viscothionin, a polypeptide isolated from mistletoe, increased the level of insulin secretion by more than 20-fold compared to that induced by the extract (Park et al. 2019). Furthermore, it was reported that mistletoe extracts inhibited α-glucosidase activity, an enzyme catalysing the cleavage of glucose from disaccharide, impeding the digestion and adsorption of glucose, eliciting attenuated postprandial plasma glucose levels (Önal et al. 2005; Park et al. 2019). The mechanism of action is shown on Fig. 3.
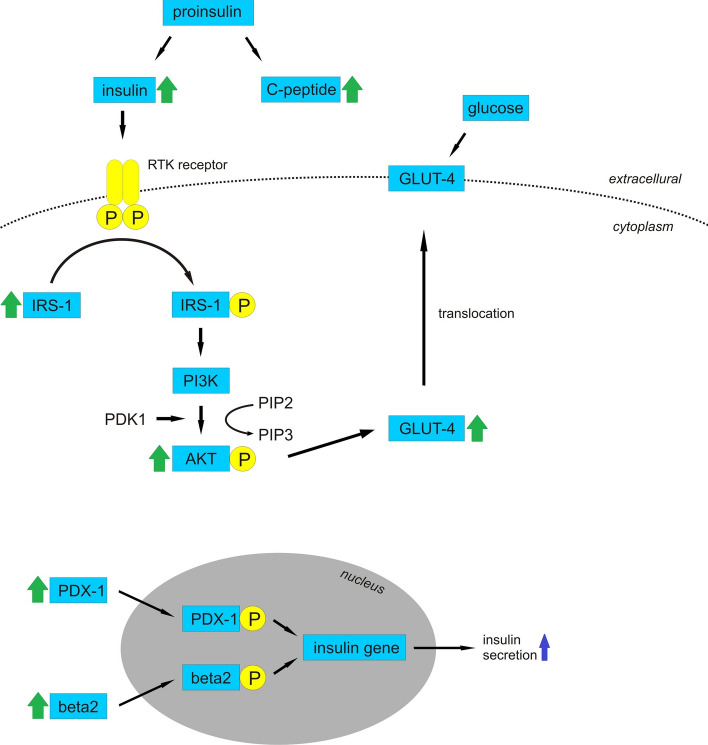
Fig. 3.
Mechanism of antidiabetic activity of mistletoe. Mistletoe increases the secretion of insulin and insulin precursor, C-peptide. Insulin binds to tyrosine kinase receptor (RTK). The activated receptor phosphorylates the IRS-1 protein leading to activation of PI3K which catalyzes the addition of phosphate group to PIP2, converting it to PIP3. PIP3 activates PDK1 leading to AKT phosphorylation, recruitment of the glucose transporter GLUT-4 to the membrane and glucose inflow. Mistletoe enhances the expression of GLUT-4, IRS-1 and AKT. Furthermore, protein fraction of mistletoe upregulates transcription factors PDX-1 and beta2 (neuroD). PDX-1 and beta2 become phosphorylated (this process might be mediated by PI3K and ERK1/2 pathways) and regulate insulin gene transcription
Hepatoprotective activity
Indicators of liver cell injury are increased levels of serum aminotransferases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as well as alkaline phosphatase (ALP). Studies on rats with hepatic damage showed that Viscum species decreased levels of ALT, AST and ALP (Abdel-Salam et al. 2010; Ogbonnanya et al. 2010; Yusuf et al. 2015) (Table 2). Furthermore, aqueous extracts of leaves of Viscum album L. growing on citrus decreased levels of serum bilirubin in high-salt-fed rats and in streptozotocin-induced diabetic rats (Nna et al. 2014; Ofem et al. 2014). The results of studies on patients with chronic hepatitis C were ambiguous. Treatment with either Iscador or AbnobaViscum caused significant improvement for AST and ALT (Tusenius et al. 2005). Furthermore, in two out of five patients treated with Iscodar, a 6–20 fold viral load reduction (HCV-RNA) and improvements for AST and ALT were observed (Tusenius et al. 2001). On the other hand, none of the patients with chronic hepatitis C and elevated ALT levels had complete or partial normalization of ALT or HCV-RNA levels during treatment with AbnobaViscum Quercus (Huber et al. 2001). The mechanism of hepatoprotection by mistletoe is not clear, but it might be mediated by the TGF-β/Smad pathway (Fig. 4). It is accepted that hepatic fibrosis is characterized by an excessive accumulation of extracellular matrix (ECM) proteins. Transforming growth factor-β1 (TGF-β1) is a cytokine leading to the activation of hepatic stellate cells (HSCs), and it stimulates ECM production while inhibiting its degradation. Once activated, TGF-β1 binds its cognate receptors and functions in autocrine and paracrine manners to exert its activities via Smad-dependent and -independent pathways. Smads are signal transduction molecules transmitting signals directly from cell surface receptors to the nucleus. Smad signal transduction pathways are thought to mediate TGF-β1-induced collagen synthesis and to play a crucial role in the process of liver damage. Nine Smads have been reported and classified into three groups. When TGF-β1 binds to its receptor, Smad 2/3 is phosphorylated and binds with Smad 4, and they move together into the nucleus for translation and expression of the target gene. Smad 7 is an inhibitory Smad that negatively regulates Smad 2/3 activation and functions by targeting the TGF-β1 receptor. An in vivo study on rats with carbon tetrachloride-induced hepatotoxicity showed that TGF-β1, TGF-β1 receptor and phosphorylated Smad 2 protein levels were reduced and Smad 7 level was increased after treatment with mistletoe alkaloid fractions. The mRNA levels of collagen I and tissue inhibitors of metalloproteinases (TIMP-1) were also downregulated. Collagen I is the prototype constituent of the fibril-formatting matrix in fibrotic liver, whereas TIMP-1 is an endogenous inhibitor of the matrix metalloproteinase (MMP) degradation of ECM. Furthermore, the mistletoe alkaloid fractions blocked α-SMA (α smooth muscle actin), the marker of activated HSC. An in vitro study on HSC-T6 cells showed that treatment with mistletoe alkaloid fractions induced Smad 7 expression and inhibited the expression of α-SMA, TGFβ1, TGF-β1 receptors, Smad 2 and TIMP-1 (Jiang et al. 2014).
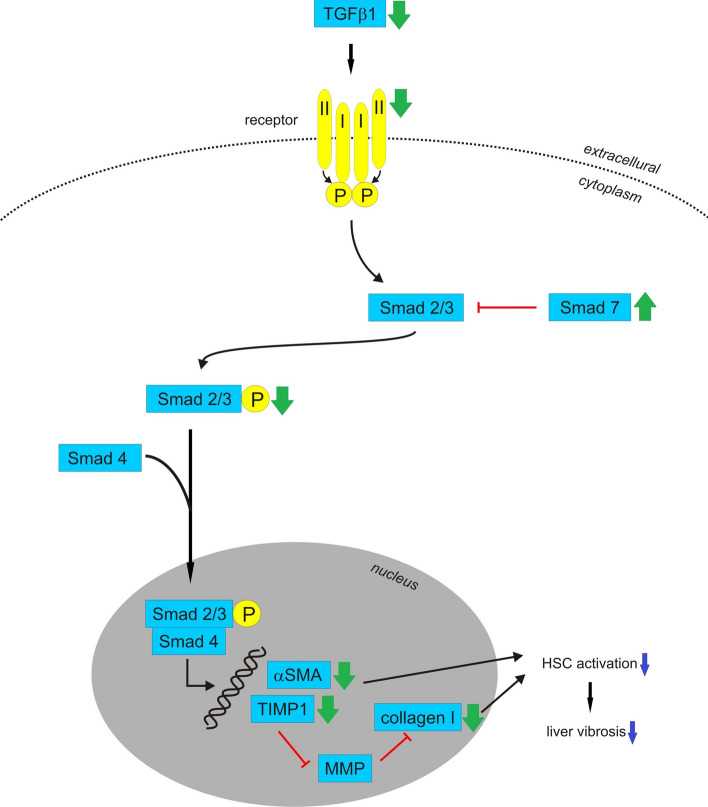
Fig. 4.
Mechanism of hepatoprotective activity of mistletoe, TGFβ/Smad pathway. TGFβ1 binds to its receptor, which consists of two type I and two type II subunits. Type II subunit phosphorylates type I subunit, which then phosphorylates Smad 2 and Smad 3. Phosphorylated Smad 2 and Smad 3 bind with Smad 4 and together they move into the nucleus to regulate expression of target genes. Smad 7 is an inhibitory Smad that negatively regulates Smad 2/3 activation. In vivo study showed that mistletoe alkaloid fractions downregulate TGF-β1, TGF-β1 receptor, phosphorylated Smad 2 and α-SMA proteins as well as downregulate the mRNA levels of TGF-β1, collagen I and TIMP-1. In contrast, Smad 7 level is upregulated. In vitro study showed that mistletoe alkaloid fractions induce Smad 7 expression and inhibit the expression of α-SMA, TGFβ1, TGF-β1 receptor, Smad 2 and TIMP-1
Neuropharmacological activity
The influence of Viscum species on the central nervous system (CNS) is differential, and it was reviewed by Szurpnicka et al. (2019) In vivo studies on mice and rats showed that Viscum species exhibited antiepileptic (Amabeoku et al. 1998; Geetha et al. 2010, 2018; Gupta et al. 2012; Tsyvunin et al. 2016), sedative (Gupta et al. 2012; Khatun et al. 2016; Kumar et al. 2016), analgesic (Orhan et al. 2006; Khatun et al. 2016), antianxiety, antidepressant, hypnotic, anti-stress (Kumar et al. 2016) and antipsychotic activity (Gupta et al. 2012) (Table 2). Several studies have proposed that the CNS activity of mistletoe is mediated by GABA (γ-aminobutyric acid) receptors. GABA is the most important inhibitory neurotransmitter in the human central nervous system. GABA is involved in epilepsy, sedation and anxiolysis, and it works by binding to GABAA receptors. GABAA receptors are heteromeric GABA-gated chloride channels. The transmembrane ion channel is opened by a stimulus generated by GABA, which allows an influx of chloride ions. This results in a decrease of the depolarizing effects of an excitatory input, thereby depressing excitability. As a result, the cell is inhibited and an anticonvulsant, sedative or anxiolytic activity is achieved. The type of activity obtained depends on the subtype of the receptor. The GABAA receptor consists of five subunits, made up of two α, two β and one γ or δ subunit. Several isoforms exist (α1–α6, β1-β3, γ1–γ3, δ), potentially giving a vast number of combinatorial mixes. However, only ten subunit combinations make up the physiologically relevant GABAA receptors in the brain (Jäger and Saaby 2011). In addition to GABA binding sites, the GABAA receptor possesses binding sites for compounds that allosterically modify the chloride channel gating of GABA, such as benzodiazepines and barbiturates. Benzodiazepine site agonists increase the GABA-induced chloride channel opening frequency and have established efficacy in the treatment of anxiety, insomnia and epilepsy as well as muscle relaxant, sedative, hypnotic, and cognition impairing effects (Diniz et al. 2015) (Fig. 5). Furthermore, it has been reported that mistletoe extract standardized for galactoside-specific lectin (ML-1) increases the beta-endorphin plasma levels in breast cancer patients (Heiny and Beuth 1994; Heiny et al. 1998). Endorphins act through opiate receptors. Three major type of opioid receptors have been identified, mu (μ), delta (δ) and kappa (κ). Beta-endorphin has a relatively high affinity at mu and delta receptors. Mu (μ) (agonist morphine) receptors are responsible for supraspinal analgesia, respiratory depression, euphoria, sedation, decreased gastrointestinal motility and physical dependence (Sharma et al. 2015). Treatment with an aqueous extract of Viscum album L. might also increase brain-derived neurotrophic factor (BDNF) (Ademola et al. 2016; Ekpenyong et al. 2016). BDNF plays a prominent role in modulating cognition and memory. BDNF is a neurotrophin that belongs to a family of proteins that promote the survival, functions and development of neurons. BDNF enhances neurogenesis and neurotransmission across the synapses, promotes synaptic growth and modulates synaptic plasticity (Fig. 6). BDNF also induces hippocampal long-term potentiation, which is important for memory formation. It was found that higher peripheral BDNF levels protect the older adults against Alzheimer’s disease (Ng et al. 2019).
Fig. 5.
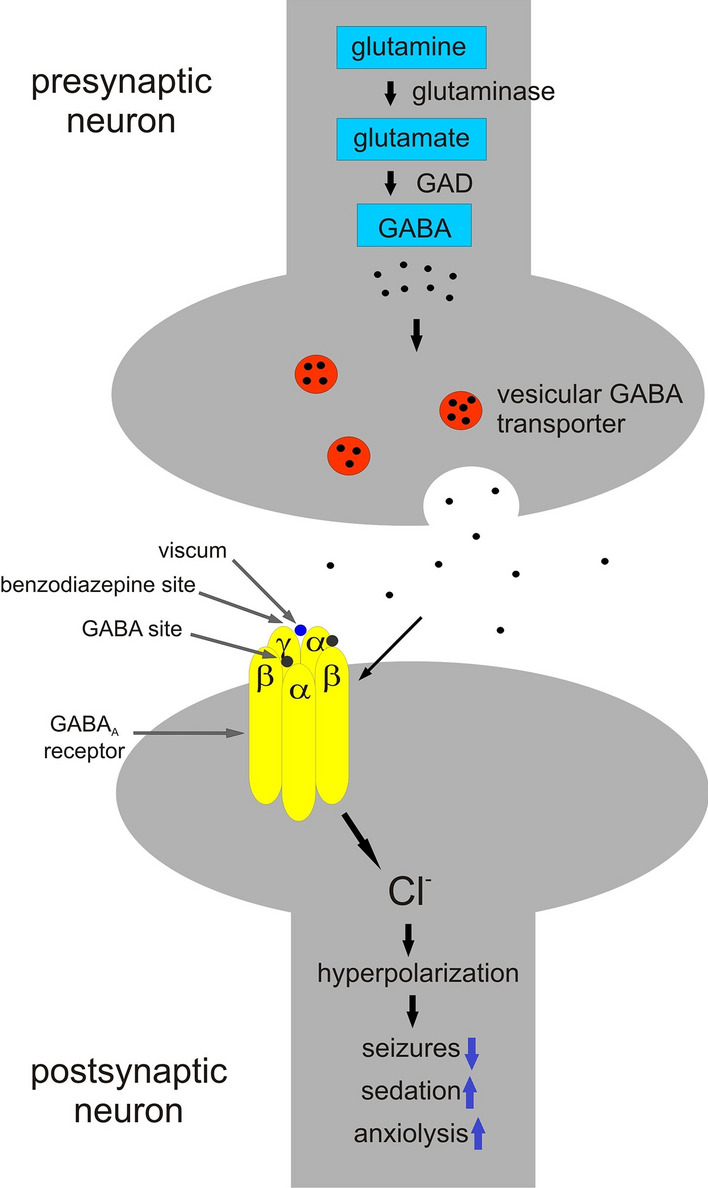
Mechanism of neuropharmacological activity of mistletoe, GABAergic signalling. Mistletoe compounds might be positive allosteric modulators of GABAA receptor. They might bind to benzodiazepine site increasing the binding affinity of the receptor for GABA. This results in increased frequency of chloride ion channel opening, increased influx of chloride ions and hyperpolarization leading to anticonvulsant, sedative and anxiolytic activity
Fig. 6.
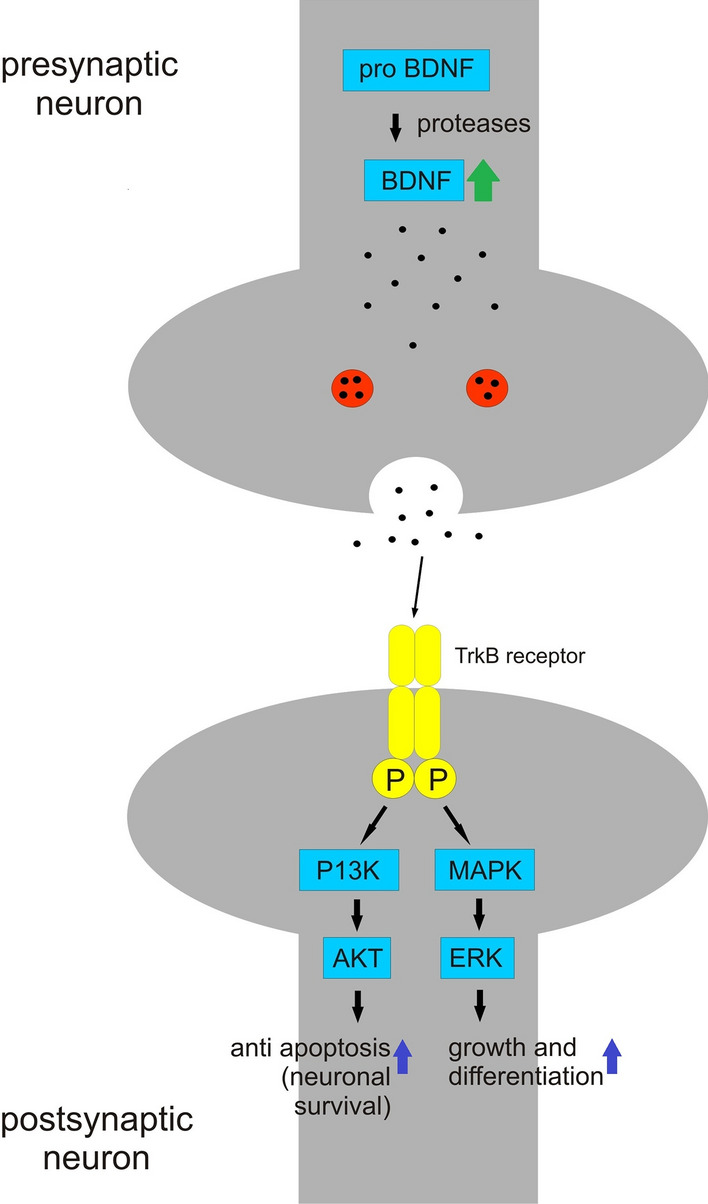
Mechanism of neuropharmacological activity of mistletoe, BDNF signalling. Mistletoe has been reported to increase brain-derived neurotrophic factor (BDNF) level. BDNF binds to tyrosine receptor kinase B leading to its phosphorylation and activation of signaling pathways. The P13K pathway activates AKT leading to neuronal survival whereas MAPK/ERK pathway leads to neuronal growth and differentiation
Antiobesity activity
Treatment with mistletoe parasitizing oak might influence body and epididymal fat pad weights in vivo and inhibit adipogenic factors in vitro (Table 2). It is known that obesity is related with adipocyte differentiation and the extent of subsequent fat accumulation. Adipogenesis can be induced through the action of enzymes, such as fatty acid synthase (FAS), acyl-CoA synthase (ACC) and acyl-CoA synthetase (ACS). The expressions of these genes are regulated by transcription factors, including peroxisome proliferator-activated receptor γ (PPAR-γ), CCAAT/enhancer-binding protein-α (C/EBP-α) and sterol regulatory element binding element protein-1c (SREBP-1c), which are known to be crucial activators for adipogenesis and show early changes in gene expression during adipocyte differentiation (Jung et al. 2013). It has been shown that mistletoe treatment significantly decreased SREBP-1c, C/EBP-α, and PPAR-γ mRNA expression in cultured 3T3-L1 adipocytes and inhibited expression of adipocyte-specific proteins—FAS, ACC, ACS, and LPL (lipoprotein lipase) (Jung et al. 2013). Furthermore, in ovariectomized rats fed a high-fat diet, Korean mistletoe decreased FAS and SREBP-1c expression as well as increased carnitine palmitoyltransferase-1 (CPT-1) expression, a key regulator of fatty acid oxidation (Kim et al. 2015) (Fig. 7).
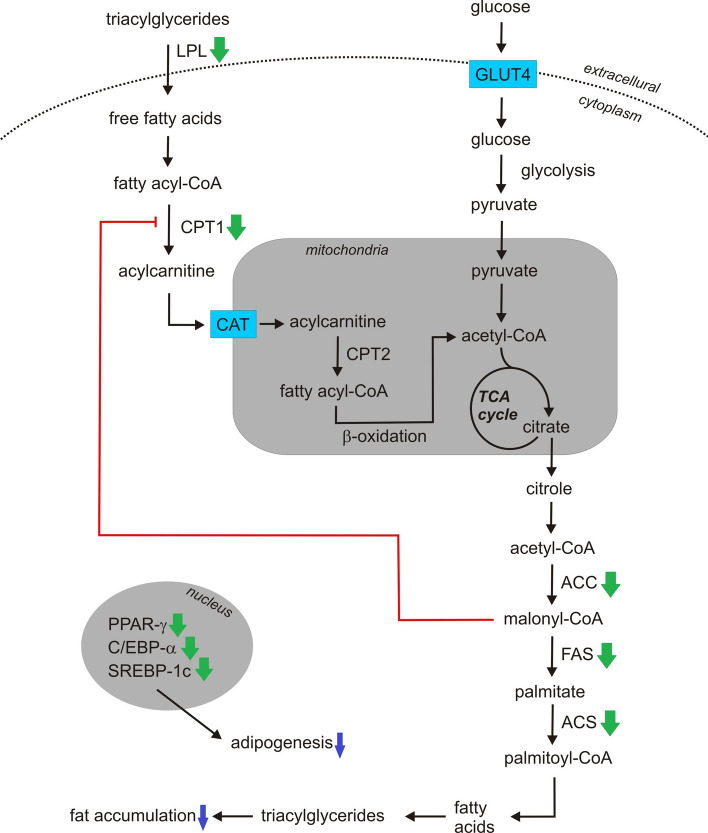
Fig. 7.
Probable mechanism of antiobesity activity of mistletoe. LPL converts triacylglycerides into free fatty acids. Free fatty acids are moved into the cell and activated to acyl-CoA. CPT1 converts acyl-CoA to acylcarnitine, which is transported into the mitochondria by CAT. CPT2 converts acylcarnitine back to acyl-CoA, and then acyl-CoA enters β-oxidation pathway. Acetyl-CoA goes into TCA cycle. Citrate exits mitochondria and is converted to acetyl-CoA, which is carboxylated to malonyl-CoA by ACC. FAS undergoes the reductive synthesis of palmitate which is converted to palmitiyl-CoA leading to formation of triacylogliceryes. Additionally, malonyl-CoA inhibits CPT-1. Mistletoe decreases expression of FAS, ACC, ACS and LPL and decreases SREBP-1c, C/EBP-α, and PPAR-γ mRNA expression
Muscle mitochondrial activity
It was determined that the administration of an aqueous extract of Korean mistletoe might enhance exercise performance in mice (Jung et al. 2012, 2013; Lee et al. 2014) (Table 2). Jung et al. (2012) showed that an increase in endurance capacity might be mediated by improvement of mitochondrial biogenesis (Fig. 8). Korean mistletoe treatment significantly increased the mitochondrial oxygen consumption rate (OCR) in L6 cells (rat myoblast cell line) as well as increased the mRNA expression of peroxisome proliferator-activated receptor γ coactivator (PGC-1α) and silent mating type information regulation 2 homolog 1 (SIRT-1), two major genes related to mitochondrial biogenesis and function in C2C12 cells (mouse myoblast cell line). Korean mistletoe treatment increased the expression of PGC-1α transcriptional targets, such as PGC-1β, NRF-1 (nuclear respiratory factor-1), ERRα (estrogen-related receptor α), Tfam (mitochondrial transcription factor A), PPARβ/δ (peroxisome proliferator–activated receptor β/δ), MB (myoglobin) and TNNI2 (troponin I) in C2C12 cells. Additionally, Korean mistletoe decreased levels of plasma lactate and lactate dehydrogenase, parameters of tissue damage and muscle fatigue in exhausted mice (Jung et al. 2012; Lee et al. 2014). Furthermore, exercise training increases the muscular glycogen and plasma free fatty acid (FFA) level, and Korean mistletoe administration increased the plasma FFA level, indicating that Korean mistletoe administration alters the energy resources in muscle (Lee et al. 2014).
Fig. 8.
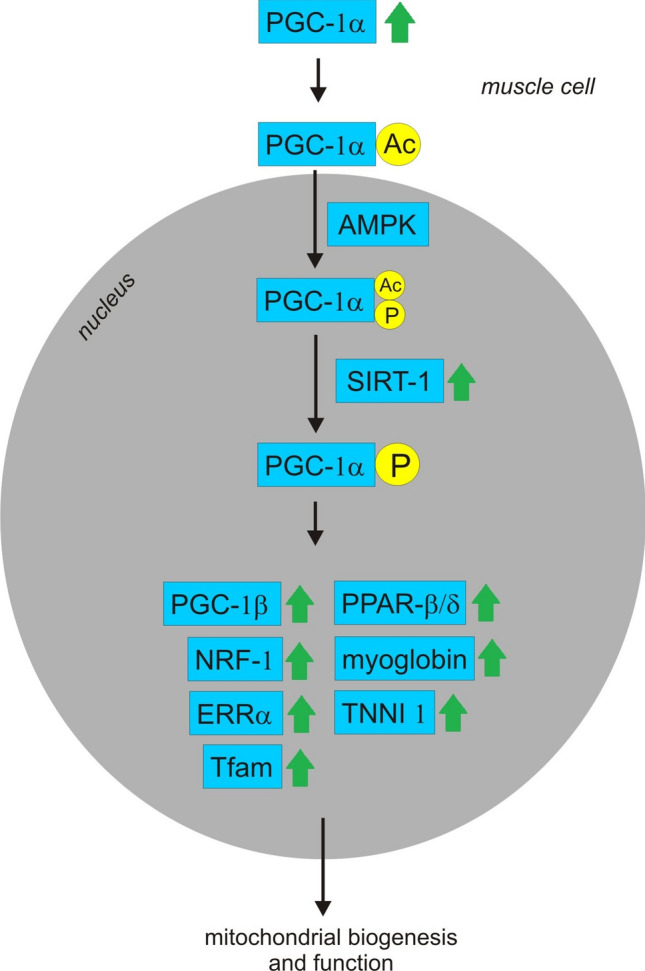
Probable effect of mistletoe on muscle mitochondrial activity. Two major genes related to mitochondrial biogenesis and function are SIRT-1 and PGC-1α. PGC-1α translocates into the nucleus where it is phosphorylated by AMPK and deacetylated by SIRT-1. Once phosphorylated and deacetylated, PGC-1α activity is increased, leading to increased transcription of mitochondrial genes. Korean mistletoe increases the mRNA expression of PGC-1α and SIRT-1 and increases the expression of PGC-1α transcriptional targets such as PGC-1β, NRF-1, ERRα, Tfam, PPARβ/δ, myoglobin and TNNI2
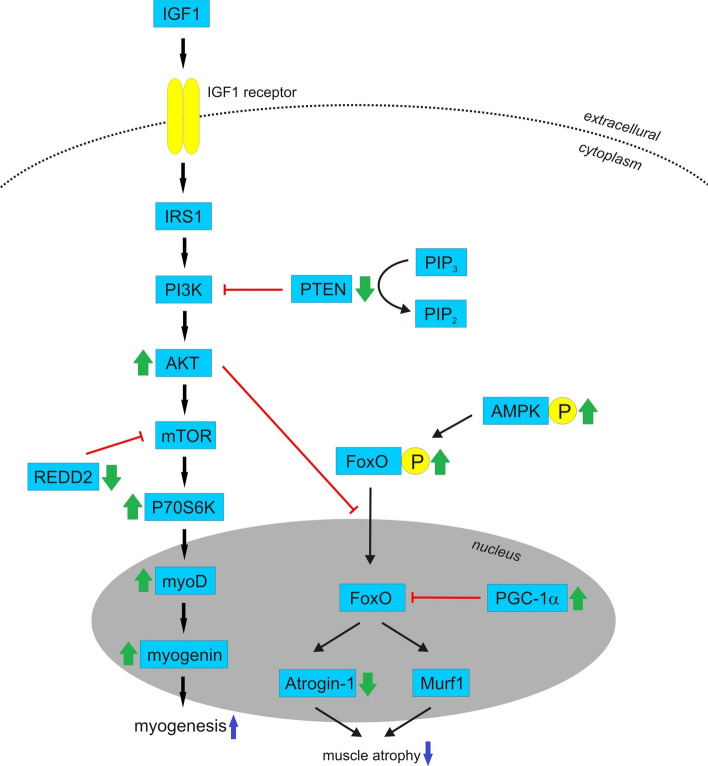
Activity against muscle decline
Supplementation with mistletoe might be effective against age-related decline in muscle mass (Table 2). An in vitro study showed that an aqueous extract of Korean mistletoe caused higher phosphorylation of AKT in C2C12 cells (mouse myoblast cell line), suggesting that mistletoe has an effect on the regulation of the muscle mass through the activation of the AKT/mTOR (protein kinase B/ mammalian target of rapamycin) signalling pathway (Jeong et al. 2017) (Fig. 9). Furthermore, mistletoe showed increased phosphorylation of FoxO (forkhead box transcription factors of the class O) supporting the observation that mistletoe could induce the phosphorylation of AMPK (AMP-activated protein kinase), which is a repressor of FoxO. FoxO is a key molecule inducing muscle atrophy by stimulating the E3 ubiquitin ligases Murf1 and Atrogin-1. In C2C12 cells, as well as in denervated mice, mistletoe decreased gene expression of Atrogin-1. On the contrary, in C2C12 cells, mistletoe increased mRNA expression of PGC-1α, GLUT-4, and SREBP-1c genes related to the inhibition of muscle atrophy and related to the induction of muscle hypertrophy by regulating the expression of Atrogin-1and Murf1 (Jeong et al. 2017). Lim et al. (2017) conducted randomized controlled trial confirming that supplementation with Korean mistletoe extract and exercise affects muscle mass and functional capabilities. Supplementation with tablets containing aqueous extracts of Viscum coloratum (Kom.) Nakai was effective for suppressing intracellular pathways related to muscle protein degradation, but stimulated those related to myogenesis. The mRNA expressions levels related to muscle protein degradation (REDD2, TSC2, FoxO1, and atrogin-1) and myogenesis (mTOR, S6K1Rheb, c-Myc, myogenin, and MyoD) as well as the phosphorylation of proteins related to muscle protein degradation (GSK3β, GSK3α, TSC2, and PTEN) and myogenesis (IGF1R, IR, IRS-1, AKT, mTOR, P70S6K, RPS6 and ERK) were studied. Significant differences were found in atrogin-1 mRNA, myogenin mRNA and insulin growth factor 1 receptor phosphorylation. A single administration of mistletoe induced decreases in atrogin-1 gene expression and PTEN (phosphatase and tension homolog) phosphorylation and an increase in myogenin gene expression. A 12-week treatment induced consistent changes in atrogin-1 and myogenin gene expression. Furthermore, the increase of REDD2 gene expression and a decrease of IGF1R phosphorylation shown by the placebo group were retarded in the mistletoe treated group at a 12-week administration. In patients treated with mistletoe, along with an endurance exercise program, the body composition was significantly changed, and knee strength and the dynamic balance ability were improved (Lim et al. 2017).
Fig. 9.
Probable effect of mistletoe against muscle decline. IGF1 binds to IGF1 receptor leading to activation of PI3K/AKT/mTOR pathway. Mistletoe leads to higher phosphotylation of AKT resulting in activation of P70S6K, upregulation of myoD and myogenin expression and myogenesis. Mistletoe decreases phosphorylation of PTEN which dephosphorylates PIP3, increasing PIP2 level and resulting in a decreased AKT activity. Furthermore, mistletoe decreases expression of REDD2 which inhibits mTOR pathway. Mistletoe increases phosphorylation of FoxO supporting the observation that mistletoe could induce the phosphorylation of AMPK, which is a repressor of FoxO. AKT also causes phosphorylation and nuclear exclusion of FoxO which is key molecule inducing muscle atrophy by stimulating Murf1 and Atrogin-1. Additionally, FoxO-dependent activation of muscle atrophy is inhibited by PGC-1α
Antioxidative activity
Oxidative stress, defined as an imbalance between oxidants and antioxidants in favour of oxidants, leads to many biochemical changes in organisms and is an important contributing factor in several human chronic diseases, such as atherosclerosis and cardiovascular diseases, mutagenesis and cancer, several neurodegenerative disorders, and aging process (Frei 1999). It is suggested that increasing intake of dietary antioxidant may help to maintain a tolerable antioxidant status and help in the disease prevention (Nimse and Pal 2015). Numerous mistletoe extracts and isolated lectin showed radical-scaveging activity and protective effects against oxidative stress induced by free radicals, nitric oxide and superoxide anion (O2−) (Sengul et al. 2009; Papuc et al. 2010; Kim et al. 2010, 2016; Kusi et al. 2015). It was studied that the activity of the more polar extracts was higher in different antioxidant mechanism and might result from high level of phenolic and flavonoid compounds (Orhan et al. 2014; Khatun et al. 2016; Pietrzak et al. 2017). Furthermore, antioxidant activity of Viscum species depends on host tree (Vicas and Prokisch, 2009; Vicas et al. 2011; Orhan et al. 2014; Pietrzak et al. 2017) and the time of harvest (ÖnayUçar et al. 2006).
Nephroprotective and antidiuretic activity
Helixor M, extract from Viscum album L. growing on apple tree, showed activity against methotrexate (MTX)-induced acute oxidative stress and nephrotoxicity in rats. The mechanism included antioxidant and anti-inflammatory properties, as evident from significant increase in the activities of the antioxidative enzymes, superoxide dismutase (SOD) and glutathione peroxidise (GSH-Px) (Sakalli Çetin et al. 2017). Oleanolic acid isolated from Viscum articulatum Burm. f. showed protective effects on gentamicin-induced renal damage in rats. Oleanolic acid decreased creatinine, albumin and urea levels in the serum and urine. It protected the rat kidneys from histological alterations induced by gentamicin and improved the glomerular filtration rate. The mechanism might be due to antioxidant and diuretic activity (Patil et al. 2010). Diuretic activity was tested for Viscum angulatum B. Heyne ex DC. and Viscum articulatum Burm. f. It was showed that mistletoe had a significant effect on the urine excretion volume. The higher natriuretic activity (Na+/K+) observed suggested a potassium-sparing diuretic effect. Furthermore, the extract showed less influence on the ion quotient (Cl−/Na+ + K+) which suggested no inhibition of carbonic anhydrase (Jadhav et al. 2010a, b).
Wound healing
Viscum articulatum Burm. f., extract showed reduction in wound area in an excision wound model in rats (Table 2). Furthermore, the re-epithelization rate was found to be faster and granuloma breaking strength as well as dry granulation tissue were significantly increased in extract-treated rats (Garg et al. 2012). Kunz et al. (2011) showed in a prospective case series study, wound healing promoting and anti-tumour effects by the topical treatment of basal cell carcinoma with ointment containing Viscum album L. lipophilic extract. More specifically, an achievement of haemostasis in bleeding tumour wounds, and after a prolonged treatment period, a wound epithelialization with a thin epithelial layer (Kuonen et al. 2013). It is known that, in wound healing processes, many different cell types are involved, including fibroblasts and keratinocytes. As fibroblasts are responsible for initiating angiogenesis, epithelialization, collagen formation and synthesis of extracellular matrix proteins an important step of the proliferative phase of wound healing is the activation of fibroblast migration into the wounded area. An in vitro study showed that Viscum album L. liphophilic extract and its predominant triterpene–oleanolic acid significantly and dose-dependently promoted the migration of NIH/3T3 fibroblasts, thereby leading to an enhanced wound closure (Kuonen et al. 2013).
Antiulcer activity
We found a research regarding the antiulcer activity of mistletoe. Methanolic extract of Viscum articulatum Burm. f was tested in Pyrolus ligation ulcer and ethanol induced ulcer models in rats. The extract showed significant inhibition of the gastric lesions in both models. Significant reduction in gastric volume, free acidity and ulcer index was observed compared to control. The authors proposed that antiulcerogenic and ulcer healing properties might be due to antisecretory activity of mistletoe (Naganjaneyulu et al. 2011).
Antibacterial activity
In the need to find new potent antibacterial compounds, the activity of Viscum species was examined in vitro using wide selection of bacterial strains such as Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Bacillus atrophaeus, Enterococcus faecium, Escherichia coli, Bordetella bronchisiptica, Salmonella typhi, Pseudomonas aeruginosa, Pseudomonas syringe, Enterobacter cloacae, Proteus vulgaris, Proteus mirabilis, Klebsiella pneumoniae, Klebsiellaaerogenes, Serratiamarcescens, Streptococcus pyogenes, Mycobacterium tuberculosis, Erwinia carotovora, Agrobacterium tumefaciens, Propionibacterium acnes and Xanthomonas campestris (Satish et al. 1999; Deliorman et al. 2001; Erturk et al. 2003; Oguntoye et al. 2008; Sengul et al. 2009; Hussain et al. 2011; Turker et al. 2012; Assaf et al. 2013; Abualhasan et al. 2014; Kusi et al. 2015; Shah et al. 2017). The results obtained are difficult to compare, because researchers used different solvents and various extortion methods to obtain extracts. In addition, some extracts were obtained from the entire plant, and others were obtained from its individual parts such as fruits, leaves or steams (Hussain et al. 2011; Shah et al. 2017). Mistletoe was also obtained from various types of host trees (Deliorman et al. 2001; Turker et al. 2012). Those interested are invited to read the quoted articles. We found only one in vivo study carried out on rats infected by Staphylococcus aureus + Bacillus cereus, Escherichia coli + Pseudomonas aeruginosa, Staphylococcus aureus + Pseudomonas aeruginosa and Escherichia coli. Haematological and histopathological studies, after 7 days of treatment with methanolic extract of Viscum album L. parasitizing cocoa trees, exhibited its therapeutic effect (Yusuf et al. 2013) (Table 2). Researchers have noticed that antibacterial activity of the extracts was more effective against Gram-negative bacteria than against Gram-positive bacteria (Erturk et al. 2003; Hussain et al. 2011) and postulated that the antibacterial effects were through anti-biofilm activity (Kenar et al. 2016).
Antifungal activity
Antifungal activity was most often tested on Candida species, which are important pathogens causing substantial morbidity and mortality in hospitalized critically ill patients (Jahagirdar et al. 2018). Nacsa-Farkas et al. (2014) tested activity of ethanolic extract of Viscum album L. against twelve Candida species, of which the most sensitive was Candida inconspicua (MIC 5.65 mg/mL). A methanolic extract of Viscum cruciatum Sieber ex Boiss. leaves exhibited activity against Candida albicans with MIC 1.25 mg/mL (Assaf et al. 2013). Furthermore, an n-hexane extract of Viscum album subsp. abietis (Wiesb.) Abrom. and its two fractions after flash column preparation were tested against Candida albicans. The first fraction was active at a concentration of 1 mg/mL (ZI (zone inhibition) 11 mm), whereas the second fraction showed activity at a concentration of 10 mg/mL (ZI 10 mm) (Erturk et al. 2003). Shah et al. (2017) compared the activity of different parts of Viscum album L. against Candida albicans. For steam distillations, the most active was the butanol extract, which showed 25.66 and 30.00 mm ZI at 1 and 2 mg/disc, respectively. For leaves, the methanol extract was the most active, reducing the growth of Candida albicans as 25.00 and 30.00 mm ZI at 1 and 2 mg/disc, respectively. For fruits, the most potent was the n-hexane extract, which reduced the growth of Candida albicans by 22.00 mm ZI at both concentrations (1 and 2 mg/disc). On the other hand, methanolic, dichloromethane and aqueous extracts of Viscum capense L. f. stems had no effect on the growth of Candida albicans (Amabeoku et al. 1998). Furthermore, Hussain et al. (2011) studied various extracts of Viscum album L. leaves and twigs and found that they were not effective against Saccharomyces cerevisiae and Aspergillus flavus. Methanolic extracts of leaves of Viscum album L. growing on cocoa and cola trees inhibited the growth of Fusarium oxysporium, Penicillium oxalium and Microsporum canis, but the highest activity was obtained against Aspergillus niger (ZI 10.66 ± 1.45 mm) (Yusuf et al. 2014). The petroleum ether extract of Viscum album L. leaves was found to be more effective against Aspergillus niger, Fusarium oxysporium, Botryodiplodia theobromae and Geotrichum candidum than methanolic extract. The highest activity was exhibited by the petroleum ether extract against Botryodiplodia theobromae with ZI of 15.85 mm (Akalazu et al. 2016). It was proposed that two compounds, caprylamide and linolelaidic acid methyl ester, might be the major antifungal compounds in the leaves of mistletoe (***Akalazu et al. 2016). Other compounds active against fungi might be viscotoxines. Viscotoxin A3 and viscotoxin B, at a concentration of 10 µM, blocked the germination of spores, and at lower concentration than 10 µM, inhibited the mycelial growth of Fusarium solani, Sclerotinia sclerotiorum and Phytophthora infestans. It was postulated that viscotoxin A3 might bind to membranes and form ion channels, leading to destabilization and disruption of the plasma membrane (Giudici et al. 2003, 2004, 2006).
Antiviral activity
So far, the antiviral activity of mistletoe has not been extensively investigated. The aqueous extract of leaves of Viscum album L. growing on lime trees was found to be effective against human parainfluenza virus type 2 (HPIV-2) growth in Vero cells. The extract, at a dose of 1 µg/mL, inhibited HPIV-2 replication and suppressed virus production by 99.7% (ED50 0.53 µg/mL) with no toxic effect on host cells (Karagöz et al. 2003). The methanolic extract of Viscum album L. leaves was active against measles virus growth in Vero cells at 0.063 μg/μL (IC50 0.031 μg/μL) and 0.031 μg/μL (IC50 0.039 μg/μL). On the other hand, the polio, yellow fever and simplex virus-1 (HSV-1) viruses were resistant to the same extract (Obi and Shenge 2018). Due to the strong impact of mistletoe on the immune system, it was proposed that it might be useful as a complementary treatment of patients with human immunodeficiency virus (HIV). Studies to confirm the induction of immunomodulation and possibility of the inhibition of the progression of HIV disease are needed (Gorter et al. 1996, 1999; Stoss and Gorter 1998; Stoss et al. 1999). An in vitro study revealed that a phenolic glycoside, homoeriodictyol-7-O-β-D-glucopyranoside-4′-O-β-D-apiofuranoside, isolated from Viscum articulatum Burm. f. growing on Fagaceae: Lithocarpus variolosus (Franch.) Chunex showed weak anti-HIV-1 activity with CC50 > 200 μg/mL and EC50 = 18.09 μg/mL. This compound exerted its weak protection of HIV-1ШB-induced MT-4 host cell lytic effects with a TI > 11.06 (Li et al. 2008).
Conclusion
Traditionally mistletoe has been used in a treatment of many diseases. To date, the anticancer and immunomodulatory activities of Viscum species were the most studied. In Europe, mainly in German-speaking countries, this resulted in the launch of extracts for subcutaneous or intravenous administration to improve the quality of life and survival of cancer patients. Lately, an extensive number of in vitro and in vivo studies have been conducted to investigate the other pharmaceutical activities of mistletoe. The results of these studies (Table2) showed that mistletoe might be a potential source of new drugs and complementary therapies to treat hypertension, diabetes, liver diseases, epilepsy and Alzheimer’s disease. Furthermore, it might be used to improve endurance and muscle strength, enhance wound healing and as antibacterial and antifungal agent. Such a wide variety of pharmaceutical properties is due to the content of many biologically active compounds from various chemical groups. The chemical composition of mistletoe depends on part of the plant (stem, leaves, fruits) and host species as well as the place and time of harvest. To date, the active compounds responsible for the individual pharmacological activities of mistletoe have not been identified. Further studies on fractionation and isolation of main active compounds and the development of methods of standardization of the extracts are required. Such studies should be conducted for extracts prepared from mistletoe parasitizing various host trees, different harvesting periods and different parts of the plant. In the next step of research, the mechanisms of action need to be tested, not only for individual isolated active compounds, but also for the whole extracts. This is because the therapeutic effect of mistletoe might be a result of the synergistic interactions of various secondary metabolites. Those interactions might include both low-molecular weight compounds (such as phenolic acids, flavonoids and fatty acids) and high-molecular weight substances (such as viscotoxines and lectins). Because of the diverse synergistic interactions, the mechanism of action of mistletoe might include many signalling pathways. Mistletoe might regulate either the same or different targets in various pathways, while acting on membrane receptors, enzymes, ion channels, transporter proteins and transcriptional targets. In this review, we summarized the existing studies on pharmacological activities of Viscum species and proposed possible mechanisms of action. Still, this is a new field for scientific research, and further studies on compound isolation and identification, synergistic interactions, metabolism, mechanisms of action and toxicity are required. We believe that due to this research, mistletoe will become a source of new complementary therapies supporting the treatment of many diseases.
Acknowledgements
The authors received no financial support for publication of this article.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Salam OM, Sleem AA, Shaffie NM. Effect of Viscum album on acute hepatic damage caused by carbon tetrachloride in rats. Turk J Med Sci. 2010;40:421–426. doi: 10.3906/sag-0803-12. [DOI] [Google Scholar]
- Abdel-Sattar EA, Elberry AA, Harraz FM, Ghareib SA, Nagy AA, Gabr SA. Antihyperglycemic and hypolipidaemic effects of the methanolic extract of Saudi mistletoe (Viscum schimperi Engl.) J Adv Res. 2011;2:171–177. doi: 10.1016/J.JARE.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Abualhasan M, Jaradat N, Abu-Hasan N, Almasri M, Taha AA, Rabbaa A, Natsheh N, Shalalfeh S, Najib M. Bioactivity of Viscum album extracts from olive and almond host plants in Palestine. Pharmacogn J. 2014;6:117–123. doi: 10.5530/pj.2014.2.7. [DOI] [Google Scholar]
- Adaramoye O, Amanlou M, Habibi-Rezaei M, Pasalar P, Ali M-M, et al. Methanolic extract of African mistletoe (Viscum album) improves carbohydrate metabolism and hyperlipidemia in streptozotocin-induced diabetic rats. Asian Pac J Trop Med. 2012;5:427–433. doi: 10.1016/S1995-7645(12)60073-X. [DOI] [PubMed] [Google Scholar]
- Ademola O, Edem E, Olufunke D, Oladunni K. Cognitive-enhancing and neurotherapeutic prospects of Viscum album in experimental model of Alzheimer’s disease. African J Cell Pathol. 2016;7:11–16. [Google Scholar]
- Akalazu JN, Ohazurike NC, Onuoha CI. Antifungal activity of Viscum album (African mistletoe) leaf extract on Dioscorea rotundata (poir) (white yam) tuber rot disease. Int J Adv Sci Eng Technol. 2016;4:189–191. doi: 10.13140/RG.2.2.29874.66248. [DOI] [Google Scholar]
- Amabeoku GJ, Leng MJ, Syce JA. Antimicrobial and anticonvulsant activities of Viscum capense. J Ethnopharmacol. 1998;61:237–241. doi: 10.1016/s0378-8741(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Assaf AM, Haddadin RN, Aldouri NA, Alabbassi R, Mashallah S, Mohammed M, Bustanji Y. Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medicine of Jordan. J Ethnopharmacol. 2013;145:728–736. doi: 10.1016/j.jep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- Bachhav SS, Patil SD, Bhutada MS, Surana SJ. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phyther Res. 2011;25:1435–1439. doi: 10.1002/ptr.3431. [DOI] [PubMed] [Google Scholar]
- Bachhav SS, Bhutada MS, Patil SD, Baser B, Chaudhari KB. Effect of Viscum articulatum Burm. (Loranthaceae) in Nω-nitro-l-arginine methyl ester induced hypertension and renal dysfunction. J Ethnopharmacol. 2012;142:467–473. doi: 10.1016/j.jep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Bachhav SS, Bhutada MS, Patil SP, Sharma KS, Patil SD. Oleanolic acid prevents increase in blood pressure and nephrotoxicity in nitric oxide dependent type of hypertension in rats. Pharmacognosy Res. 2015;7:385–392. doi: 10.4103/0974-8490.159575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sela G. White-Berry Mistletoe (Viscum album L.) as complementary treatment in cancer: does it help? Eur J Integr Med. 2011;3:e55–e62. doi: 10.1016/J.EUJIM.2011.03.002. [DOI] [Google Scholar]
- Bar-Sela G, Wollner M, Hammer L, Agbarya A, Dudnik E, Haim N. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: a randomised phase II study. Eur J Cancer. 2013;49:1058–1064. doi: 10.1016/J.EJCA.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Braedel-Ruoff S. Immunomodulatory effects of Viscum album Extracts on natural killer cells: review of clinical trials. Res Complement Med. 2010;17:63–73. doi: 10.1159/000288702. [DOI] [PubMed] [Google Scholar]
- Brandenberger M, Simões-Wüst AP, Rostock M, Rist L, Saller R. An exploratory study on the quality of life and individual coping of cancer patients during mistletoe therapy. Integr Cancer Ther. 2012;11:90–100. doi: 10.1177/1534735411413267. [DOI] [PubMed] [Google Scholar]
- Bussing A. Mistletoe. The genus Viscum. Amsterdam: Hardwood Academic Publishers; 2000. [Google Scholar]
- Ćebović T, Spasić S, Popović M. Cytotoxic effects of the Viscum album L. extract on Ehrlich tumour cells in vivo. Phyther Res. 2008;22:1097–1103. doi: 10.1002/ptr.2464. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lyu SY, Lee HJ, Jung J, Park WB, Kim GJ. Korean mistletoe lectin regulates self-renewal of placenta-derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif. 2012;45:420–429. doi: 10.1111/j.1365-2184.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lyu SY, Park WB. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res. 2004;27:68–76. doi: 10.1007/BF02980049. [DOI] [PubMed] [Google Scholar]
- Chu W, Qiao G, Bai Y, Pan Z, Li G, Piao X, Wu L, Lu Y, Yang B. Flavonoids from Chinese Viscum coloratum produce cytoprotective effects against ischemic myocardial injuries: inhibitory effect of flavonoids on PAF-Induced Ca2+ overload. Phyther Res. 2008;22:134–137. doi: 10.1002/ptr.2267. [DOI] [PubMed] [Google Scholar]
- Committee on Herbal Medicinal Products (2012) Assessment report on Viscum album L., herba. Committee on Herbal Medicinal Product, London
- Dela Cruz JF, Kim YS, Lumbera WM, Hwang SG. Viscum album var hot water extract mediates anti-cancer effects through G1 phase cell cycle arrest in SK-Hep1 human hepatocarcinoma cells. Asian Pac J Cancer Prev. 2015;16:6417–6421. doi: 10.7314/APJCP.2015.16.15.6417. [DOI] [PubMed] [Google Scholar]
- Delebinski CI, Twardziok M, Kleinsimon S, Hoff F, Mulsow K, Rolff J, Jager S, Eggert A, Seifert G. A natural combination extract of Viscum album L. containing both triterpene acids and lectins is highly effective against AML in vivo. PLoS ONE. 2015;10:e0133892. doi: 10.1371/journal.pone.0133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliorman D, Ergun F, Sener B, Palittapongarnpim P. Evaluation of antimycobacterial activity of Viscum album subspecies. Pharm Biol. 2001;39:381–383. doi: 10.1076/phbi.39.5.381.5900. [DOI] [Google Scholar]
- Diniz TC, Silva JC, de Lima-Saraiva SR, Ribeiro FP, de Freitas RM, Quintans-Júnior LJ, Quintans Jde S, Mendes RL, Almeida JR. The role of flavonoids on oxidative stress in epilepsy. Oxid Med Cell Longev. 2015;2015:9. doi: 10.1155/2015/171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler J, von Balthazar L, Stritt B, Pruntsch D, Ramos M, Urech K, Rist L, Simoes-Wust P, Viviani A. Mistletoe lectin is not the only cytotoxic component in fermented preparations of Viscum album from white fir (Abies pectinata) BMC Complement Altern Med. 2007;7:1–7. doi: 10.1186/1472-6882-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpenyong EE, Ayodele OA, Linus E. Viscum album enhanced blood serum brain-derived neurotrophic factor (BDNF) level in experimental model of Alzheimer’s disease. World J Pharm Pharm Sci. 2016;5:157–171. [Google Scholar]
- Elluru SR, Duong Van Huyen JP, Delignat S, Prost F, Heudes D, Kazatchkine MD, Friboulet A, Kaveri SV. Antiangiogenic properties of Viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res. 2009;29:2945–2950. [PubMed] [Google Scholar]
- Elluru SR, van Huyen JPD, Delignat S, Kazatchkine MD, Friboulet A, Kaveri SV, Bayry J. Induction of maturation and activation of human dendritic cells: a mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer. 2008;8:161. doi: 10.1186/1471-2407-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eno AE, Ofem OE, Nku CO, Ani EJ, Itam EH. Stimulation of insulin secretion by Viscum album (mistletoe) leaf extract in streptozotocin-induced diabetic rats. Afr J Med Med Sci. 2008;37:141–147. [PubMed] [Google Scholar]
- Ernst E, Schmidt K, Steuer-Vogt MK. Mistletoe for cancer? A systematic review of randomised clinical trials. Int J Cancer. 2003;107:262–267. doi: 10.1002/ijc.11386. [DOI] [PubMed] [Google Scholar]
- Erturk Ö, Kati H, Yayli N, Demirbag Z. Antimicrobial activity of Viscum album L. subsp. abietis (Wiesb) Turk J Biol. 2003;27:255–258. [Google Scholar]
- Fan J, Wu M, Wang J, Ren D, Zhao J, Yang G. 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one induces lung cancer cell apoptosis via the PI3K/Akt and ERK1/2 pathways. J Cell Physiol. 2019;234:6336–6349. doi: 10.1002/jcp.27364. [DOI] [PubMed] [Google Scholar]
- Felenda JE, Turek C, Stintzing FC. Antiproliferative potential from aqueous Viscum album L. preparations and their main constituents in comparison with ricin and purothionin on human cancer cells. J Ethnopharmacol. 2019;236:100–107. doi: 10.1016/j.jep.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Frei B. Molecular and biological mechanisms of antioxidant action. FASEB J. 1999;13:963–964. doi: 10.1096/fasebj.13.9.963. [DOI] [PubMed] [Google Scholar]
- Freuding M, Keinki C, Micke O, Buentzel J, Huebner J. Mistletoe in oncological treatment: a systematic review: part 1: survival and safety. J Cancer Res Clin Oncol. 2019;145:695–707. doi: 10.1007/s00432-018-02837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardin NE. Immunological response to mistletoe (Viscum album L.) in cancer patients: a four-case series. Phyther Res. 2009;23:407–411. doi: 10.1002/ptr.2643. [DOI] [PubMed] [Google Scholar]
- Garg S, Patil UK, Shrivastava TP. Wound healing potential of Viscum Articulum Brm., an ethnomedical plant of Sikkim on rat. Int J Res Phytochem Pharmacol. 2012;2:138–142. doi: 10.1186/s13002-017-0148-9. [DOI] [Google Scholar]
- Geetha KM, Bhaskara Gopal PVVS, Murugan V. Antiepileptic activity of aerial parts of Viscum articulatum (Viscaceae) in rats. J Pharm Res. 2010;3:2886–2887. [Google Scholar]
- Geetha KM, Murugan V, Pavan Kumar P, Wilson B. Antiepileptic activity of Viscum articulatum Burm and its isolated bioactive compound in experimentally induced convulsions in rats and mice. Eur J Biomed Pharm Sci. 2018;5:311–318. [Google Scholar]
- Giudici AM, Regente MC, Villalain J, Pfuller K, Pfuller U, De La Canal L. Mistletoe viscotoxins induce membrane permeabilization and spore death in phytopathogenic fungi. Physiol Plant. 2004;121:2–7. doi: 10.1111/j.0031-9317.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- Giudici M, Poveda JA, Molina ML, De La Canal L, Gonzales-Ros JM, Pfuller K, Pfuller U, Villalain J. Antifungal effects and mechanism of action of viscotoxin A3. FEBS J. 2006;273:72–83. doi: 10.1111/j.1742-4658.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- Giudici M, Pascual R, De La Canal L, Pfuller K, Pfuller U, Villalain J. Interaction of viscotoxins A 3 and B with membrane model systems: implications to their mechanism of action. Biophys J. 2003;85:971–981. doi: 10.1016/S0006-3495(03)74536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter RW, Stein J, Stoss M, Linder M. Prospektive, longitudinale, Dosis-eskalierende, randomisierte Phase-L/Ll-Studie mit Iscador® QuFrF und Iscador® Qu Spezial mit HIV-Positiven, Krebspatienten und gesunden, nichtrauchenden Probanden. Complement Med Res. 1996;3:169–175. doi: 10.1159/000210221. [DOI] [Google Scholar]
- Gorter RW, van Wely M, Reif M, Stoss M. Tolerability of an extract of European mistletoe among immunocompromised and healthy individuals. Altern Ther Health Med. 1999;5(37–44):47–48. [PubMed] [Google Scholar]
- Gray AM, Flatt PR. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe) J Endocrinol. 1999;160:409–414. doi: 10.1677/joe.0.1600409. [DOI] [PubMed] [Google Scholar]
- Gupta G, Kazmi I, Afzal M, Rahman M, Saleem S, Ashraf S, Khusroo MJ, Nazeer K, Ahmed S, Mujeeb M, Ahmed Z, Anwar F. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J Ethnopharmacol. 2012;141:810–816. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Hajto T. Immunomodulatory effects of Iscador: a Viscum album preparation. Oncology. 1986;43:51–65. doi: 10.1159/000226420. [DOI] [PubMed] [Google Scholar]
- Hajto T, Hostanska K, Frei K, Rordorf C, Gabius HJ. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990;50:3322–3326. [PubMed] [Google Scholar]
- Han SY, Hong CE, Kim HG, Lyu SY. Anti-cancer effects of enteric-coated polymers containing mistletoe lectin in murine melanoma cells in vitro and in vivo. Mol Cell Biochem. 2015;408:73–87. doi: 10.1007/s11010-015-2484-1. [DOI] [PubMed] [Google Scholar]
- Hegde P, Maddur MS, Friboulet A, Bayry J, Kaveri SV. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE. 2011;6:e26312. doi: 10.1371/journal.pone.0026312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiny BM, Beuth J. Mistletoe extract standardized for the galactoside-specific lectin (ML-1) induces beta-endorphin release and immunopotentiation in breast cancer patients. Anticancer Res. 1994;14:1339–1342. [PubMed] [Google Scholar]
- Heiny BM, Albrecht V, Beuth J. Correlation of immune cell activities and beta-endorphin release in breast carcinoma patients treated with galactose-specific lectin standardized mistletoe extract. Anticancer Res. 1998;18:583–586. [PubMed] [Google Scholar]
- Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CE, Park AK, Lyu SY. Synergistic anticancer effects of lectin and doxorubicin in breast cancer cells. Mol Cell Biochem. 2014;394:225–235. doi: 10.1007/s11010-014-2099-y. [DOI] [PubMed] [Google Scholar]
- Huber R, Lüdtke H, Wieber J, Beckmann C. Safety and effects of two mistletoe preparations on production of Interleukin-6 and other immune parameters—a placebo controlled clinical trial in healthy subjects. BMC Complement Altern Med. 2011;11:116. doi: 10.1186/1472-6882-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Lüdtke R, Klassen M, Muller-Buscher G, Wolff-Vorbeck G, Scheer R. Effects of a mistletoe preparation with defined lectin content on chronic hepatitis C: an individually controlled cohort study. Eur J Med Res. 2001;6:399–405. [PubMed] [Google Scholar]
- Huber R, Rostock M, Goedl R, Ludtke R, Urech K, Buck S, Klein R. Mistletoe treatment induces GM-CSF- and IL-5 production by PBMC and increases blood granulocyte- and eosinophil counts: a placebo controlled randomized study in healthy subjects. Eur J Med Res. 2005;10:411–418. [PubMed] [Google Scholar]
- Hussain MA, Khan MQ, Hussain N, Habib T. Antibacterial and antifungal potential of leaves and twigs of Viscum album L. J Med Plants Res. 2011;5:5545–5549. [Google Scholar]
- Ibegbulem CO, Chikezie PC. Hypoglycemic properties of ethanolic extracts of Gongronema latifolium, Aloe perryi, Viscum album and Allium sativum administered to alloxan-induced diabetic albino rats (Rattus norvegicus) Pharmacogn Commun. 2013;3:12–16. doi: 10.5530/pc.2013.2.4. [DOI] [Google Scholar]
- Jadhav N, Patil CR, Chaudhari KB, Wagh JP, Surana SJ, Jadhav RB. Diuretic and natriuretic activity of two mistletoe species in rats. Pharmacognosy Res. 2010;2:50–57. doi: 10.4103/0974-8490.60576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav RB, Bhatnagar SP, Surana SJ. Diuretic activity of squamate mistletoe, Viscum angulatum. Pharm Biol. 2010;48:417–421. doi: 10.3109/13880200903150427. [DOI] [PubMed] [Google Scholar]
- Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahagirdar V, Davane M, Aradye S, Nagoba B. Candida species as potential nosocomial pathogens—a review. Eur J Gen Med. 2018;15:71–75. doi: 10.29333/ejgm/82346. [DOI] [Google Scholar]
- Jang JY, Kim SY, Song KS, Seong YH. Korean mistletoe (Viscum album var. coloratum) inhibits amyloid beta protein (25–35)-induced cultured neuronal cell damage and memory impairment. Nat Prod Sci. 2015;21:134–140. [Google Scholar]
- Jeong J, Park CH, Kim I, Kim YH, Yoon JM, Kim KS, Kim JB. Korean mistletoe (Viscum album coloratum) extract regulates gene expression related to muscle atrophy and muscle hypertrophy. BMC Complement Altern Med. 2017;17:68. doi: 10.1186/s12906-017-1575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang C, Li YY, Wang XC, An JD, Wang YJ, Wang XJ. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-β/Smad interference. J Ethnopharmacol. 2014;158:230–238. doi: 10.1016/J.JEP.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Jung HY, Kim YH, Kim IB, Jeong JS, Lee JH, Do MS, Jung SP, Kim KS, Kim KT, Kim JB. The Korean mistletoe (Viscum album coloratum) extract has an antiobesity effect and protects against hepatic steatosis in mice with high-fat diet-induced obesity. Evid Based Complement Altern Med. 2013;2013:1–9. doi: 10.1155/2013/168207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Lee AN, Song TJ, An HS, Kim YH, Kim KD, Kim IB, Kim KS, Han BS, Kim CH, Kim KS, Kim JB. Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. J Med Food. 2012;15:621–628. doi: 10.1089/jmf.2010.1469. [DOI] [PubMed] [Google Scholar]
- Karagöz A, Kesici S, Vural A, Usta M, Tezcan B, Semerci T, Teker E. Cardioprotective effects of Viscum album L. ssp. album (Loranthaceae) on isoproterenol-induced heart failure via regulation of the nitric oxide pathway in rats. Anatol J Cardiol. 2016;16:923–930. doi: 10.14744/AnatolJCardiol.2016.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagöz A, Önay E, Arda N, Kuru A. Antiviral potency of mistletoe (Viscum album ssp. album) extracts against human parainfluenza virus type 2 in Vero cells. Phyther Res. 2003;17:560–562. doi: 10.1002/ptr.1163. [DOI] [PubMed] [Google Scholar]
- Kenar N, Erdonmez D, Turkmen KE. Anti-quorum sensing and anti-biofilm activity of Viscum album L. on different pathogenic bacteria. J Biotechnol. 2016;1000:1000. doi: 10.1016/J.JBIOTEC.2016.05.138. [DOI] [Google Scholar]
- Khan T, Ali S, Qayyum R, Hussain I, Wahid F, Shah AJ. Intestinal and vascular smooth muscle relaxant effect of Viscum album explains its medicinal use in hyperactive gut disorders and hypertension. BMC Complement Aternative Med. 2016;16:251. doi: 10.1186/s12906-016-1229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun A, Rahman M, Rahman MM, Hossain H, Jahan IA, Nesa L. Antioxidant, antinociceptive and CNS activities of Viscum orientale and high sensitive quantification of bioactive polyphenols by UPLC. Front Pharmacol. 2016;7:176. doi: 10.3389/fphar.2016.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L. in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011 doi: 10.1186/1472-6882-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienle GS, Kiene H. Influence of Viscum album L (European Mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142–157. doi: 10.1177/1534735410369673. [DOI] [PubMed] [Google Scholar]
- Kienle GS, Mussler M, Fuchs D, Kiene H. Intravenous mistletoe treatment in integrative cancer care: a qualitative study exploring the procedures, concepts, and observations of expert doctors. Evid Based Complement Altern Med. 2016;2016:1–16. doi: 10.1155/2016/4628287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Choi MJ, Park KY, Cho EJ. Protective effects of Korean mistletoe lectin on radical-induced oxidative stress. Biol Pharm Bull. 2010;33:1152–1158. doi: 10.1248/bpb.33.1152. [DOI] [PubMed] [Google Scholar]
- Kim GD, Choi JH, Lim SM, Jun JH, Moon JW, Kim GJ. Alterations in IL-6/STAT3 signaling by Korean mistletoe lectin regulate the self-renewal activity of placenta-derived mesenchymal stem cells. Nutrients. 2019;11:2604. doi: 10.3390/nu11112604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Hwang YH, Kang K-Y, Kim I, Kim JB, Park JH, Yoo YC, Yee ST. Enhanced dendritic cell maturation by the B-chain of Korean mistletoe lectin (KML-B), a novel TLR4 agonist. Int Immunopharmacol. 2014;21:309–319. doi: 10.1016/J.INTIMP.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Kim KC, Yook JH, Eisenbraun J, Kim BS, Huber R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—a randomized, controlled pilot study. BMC Complement Altern Med. 2012;12:172. doi: 10.1186/1472-6882-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Yang SH, Kim JB. Protein fractions from Korean mistletoe (Viscum album coloratum) extract induce insulin secretion from pancreatic beta cells. Evid Based Complement Altern Med. 2014;703624:8. doi: 10.1155/2014/703624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Park JH, Kwon DY, Yang HJ, Kim DS, Kang S, Shin BK, Moon NR, Song BS, Kim JH, Park S. The supplementation of Korean mistletoe water extracts reduces hot flushes, dyslipidemia, hepatic steatosis, and muscle loss in ovariectomized rats. Exp Biol Med. 2015;240:477–487. doi: 10.1177/1535370214551693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Yang EJ, Son YK, Yeo JH, Song KS. Enhanced anti-oxidative effect of fermented Korean mistletoe is originated from an increase in the contents of caffeic acid and lyoniresinol. Food Funct. 2016;7:2270–2277. doi: 10.1039/c6fo00138f. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim KC, Lee C. Mistletoe (Viscum album) extract targets Axl to suppress cell proliferation and overcome cisplatin- and erlotinib-resistance in non-small cell lung cancer cells. Phytomedicine. 2017;36:183–193. doi: 10.1016/J.PHYMED.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim I, Park CH, Kim JB. Korean mistletoe lectin enhances natural killer cell cytotoxicity via upregulation of perforin expression. Asian Pac J Allergy Immunol. 2018;36:175–183. doi: 10.12932/AP-030417-0067. [DOI] [PubMed] [Google Scholar]
- Kleinsimon S, Kauczor G, Jaeger S, Eggert A, Seifert G, Delebinski C. ViscumTT induces apoptosis and alters IAP expression in osteosarcoma in vitro and has synergistic action when combined with different chemotherapeutic drugs. BMC Complement Altern Med. 2017;17:26. doi: 10.1186/s12906-016-1545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsimon S, Longmuss E, Rolff J, Jager S, Eggert A, Delebinski C, Seifert G. GADD45A and CDKN1A are involved in apoptosis and cell cycle modulatory effects of viscumTT with further inactivation of the STAT3 pathway. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-24075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil MG, Xavier FCA, Sardinha LR, Severino P, Mathor MB, Rodrigues RV, Pinto DS. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol Rep. 2013;30:2316–2322. doi: 10.3892/or.2013.2732. [DOI] [PubMed] [Google Scholar]
- Ko BS, Kang S, Moon BR, Ryuk JA, Park S. A 70% ethanol extract of mistletoe rich in betulin, betulinic acid, and oleanolic acid potentiated β-cell function and mass and enhanced hepatic insulin sensitivity. Evid Based Complement Altern Med. 2016;7836823:13. doi: 10.1155/2016/7836823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. Serum levels of IL-12 and the production of IFN-gamma, IL-2 and IL-4 by peripheral blood mononuclear cells (PBMC) in cancer patients treated with Viscum album extract. Biomed Pharmacother. 2000;54:305–310. doi: 10.1016/S0753-3322(00)80052-9. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar D, Kaushik D, Kumar S. Screening of neuropharmacological activities of Viscum album and estimation of major flavonoid constituents using TLC densitometry. Int J Toxicol Pharmacol Res. 2016;8:179–186. [Google Scholar]
- Kunz C, Heiligtag HR, Hintze A, Urech K. Treatment of basal cell carcinoma with Viscum album lipophilic extract—a case series study. Phytomedicine. 2011;18:S25. doi: 10.1016/j.phymed.2011.09.060. [DOI] [Google Scholar]
- Kuonen R, Weissenstein U, Urech K, Kunz M, Hostanska K, Estko M, Heusser P, Baumgartner S. Effects of lipophilic extract of Viscum album L. and oleanolic acid on migratory activity of NIH/3T3 fibroblasts and on HaCat keratinocytes. Evid Based Complement Alternat Med. 2013;2013:718105. doi: 10.1155/2013/718105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusi M, Shrestha K, Malla R. Study on phytochemical, antibacterial, antioxidant and toxicity profile of Viscum album Linn associated with Acacia catechu. Nepal J Biotechnol. 2015;3:60–65. doi: 10.3126/njb.v3i1.14234. [DOI] [Google Scholar]
- Ladokun O, Ojezele M, Arojojoye O. Comparative study on the effects of aqueous extracts of Viscum album (mistletoe) from three host plants on hematological parameters in albino rats. Afr Health Sci. 2015;15:606–612. doi: 10.4314/ahs.v15i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim IB, Kim JB, Park DH, Min KJ. The effects of Korean mistletoe extract on endurance during exercise in mice. Anim Cells Syst (Seoul) 2014;18:34–40. doi: 10.1080/19768354.2014.881917. [DOI] [Google Scholar]
- Lev E, Ephraim M, Ben-Arye E. European and Oriental mistletoe: from mythology to contemporary integrative cancer care. Eur J Integr Med. 2011;3:e133–e137. doi: 10.1016/J.EUJIM.2011.05.052. [DOI] [Google Scholar]
- Li Y, Zhao YL, Huang N, Zheng YT, Yang YP, Li XL. Two new phenolic glycosides from Viscum articulatum. Molecules. 2008;13:2500–2508. doi: 10.3390/molecules13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim NJ, Shin JH, Kim HJ, Lim Y, Kim JY, Lee WJ, Han SJ, Kwon O. A combination of Korean mistletoe extract and resistance exercise retarded the decline in muscle mass and strength in the elderly: a randomized controlled trial. Exp Gerontol. 2017;87:48–56. doi: 10.1016/j.exger.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Lyu SY, Choi SH, Park WB. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch Pharm Res. 2002;25:93–101. doi: 10.1007/BF02975269. [DOI] [PubMed] [Google Scholar]
- Melo MNO, Oliveira AP, Wiecikowski AF, Carvalho RS, Castro JL, de Oliveira FAG, Pereira HMG, da Veiga VF, Capello MMA, Rocha L, Holandino C. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm J. 2018;26:311–322. doi: 10.1016/j.jsps.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke K, Schwermer M, Falke K, Eisenbraun J, Schramm A, Zuzak TJ. Preclinical investigation of interaction of mistletoe extract (Viscum album L.) with radio- and chemotherapy in pediatric tumor cell lines. Phytomedicine. 2019;61:4. doi: 10.1016/j.phymed.2019.09.123. [DOI] [Google Scholar]
- Mishra R, Sharma S, Sharma RS, Singh S, Sardesai MM, Sharma S, Mishra V. Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J Ethnopharmacol. 2018;219:91–102. doi: 10.1016/J.JEP.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Mojiminiyi FBO, Owolabi ME, Igbokwe UV, Ajagbonna OP. The vasorelaxant effect of Viscum album leaf extract is mediated by calcium-dependent mechanism. Niger J Physiol Sci. 2008;23:115–120. doi: 10.4314/njps.v23i1-2.54947. [DOI] [PubMed] [Google Scholar]
- Montero GD, Valladares MB, Tornes CYLF, Agramonte REA, Calderon JB, Fundora HR (2016) Tratamiento homeopático y convencional de la hipertensión arterial. Rev Méd Homeopat 9:53–58. 10.1016/J.HOMEO.2016.07.005
- Nacsa-Farkas E, Kerekes E, Kerekes EB, Krisch J, Roxana P, Vlad DC, Ivan P, Vagvolgyi C. Antifungal effect of selected European herbs against Candida albicans and emerging pathogenic non-albicans Candida species. Acta Biol Szeged. 2014;58:61–64. [Google Scholar]
- Naganjaneyulu R, Kumar CKA, Kumar GA, Dalith MD, Basha DJ. Antiulcer activity of Viscum articulatum Burm f. (viscaceae) Int J Innov Pharm Res. 2011;2:139–143. [Google Scholar]
- Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RC. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): a systematic review and meta-analysis. Int J Mol Sci. 2019;20:1–26. doi: 10.3390/ijms20020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/c4ra13315c. [DOI] [Google Scholar]
- Nna VU, Ofem OE, Oka VO, Aluko EO, Ofutet EO. Comparative effects of Aloe vera gel and aqueous leaf extract of Viscum album on bilirubin excretion in streptozotocin-induced diabetic rats. Int J Biochem Res Rev. 2014;4:99–115. doi: 10.9734/ijbcrr/2014/7512. [DOI] [Google Scholar]
- Nna VU, Oka VO, Aluko EO, Helen OT. Comparative effects of aqueous leaf extract of Viscum album (Mistletoe ) and Aloe vera gel in the management of streptozotocin-induced diabetes mellitus. Int J Diabetes Res. 2013;2:84–89. doi: 10.5923/j.diabetes.20130205.01. [DOI] [Google Scholar]
- Nwaegerue E, Nweke IN, Ezeala CC, Unekwe PC. Glucose lowering effect of leaf extracts of Viscum album in normal and diabetic rats. J Res Med Sci. 2007;12:235–240. [Google Scholar]
- Obi RK, Shenge JA. In vitro antiviral activities of Bryophyllum pinnatum (Odaa opuo) and Viscum album (Awuruse) Res J Microbiol. 2018;13:138–146. doi: 10.3923/jm.2018.138.146. [DOI] [Google Scholar]
- Oei SL, Thronicke A, Schad F. Mistletoe and immunomodulation: insights and implications for anticancer therapies. Evid Based Complement Altern Med. 2019;2019:1–6. doi: 10.1155/2019/5893017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofem OE, Eno AE, Imoru J, Nkanu E, Unoh F, Ibu JO. Effect of crude aqueous leaf extract of Viscum album (mistletoe) in hypertensive rats. Indian J Pharmacol. 2007;39:15–19. doi: 10.4103/0253-7613.30756. [DOI] [Google Scholar]
- Ofem OE, Eno AE, Nku CO, Antai AB. Viscum album (mistletoe) extract prevents changes in levels of red blood cells, PCV, Hb, serum proteins and ESR in high salt-fed rats. J Ethnopharmacol. 2009;126:421–426. doi: 10.1016/j.jep.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Ofem OE, Nna VU, Essien NM. Reduction in serum bilirubin concentration following administration of crude leaf extract of Viscum album (mistletoe) in high salt fed rats. Br J Pharm Res. 2014;4:352–361. doi: 10.9734/BJPR/2014/7359. [DOI] [Google Scholar]
- Ogbonnanya AE, Mounmbegna EP, Monago CC. Effect of ethanolic extract of mistletoe (Viscum album L.) leaves on paracetamol-induced hepatotoxicity in rats. J Pharm Res. 2010;3:1888–1891. [Google Scholar]
- Oguntoye SO, Olatunji GA, Kolawole OM, Enonbun KI. Phytochemical screening and antibacterial activity of Viscum album (mistletoe) extracts. Plant Sci Res. 2008;1:44–46. [Google Scholar]
- Ohiri FC, Esimone CO, Nwafor SV, Okoli CO, Ndu OO. Hypoglycemic properties of Viscum album (mistletoe) in alloxan-induced diabetic animals. Pharm Biol. 2003;41:184–187. doi: 10.1076/phbi.41.3.184.15098. [DOI] [Google Scholar]
- Önal S, Timur S, Okutucu B, Zihnioğlu F. Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol. 2005;35:29–36. doi: 10.1081/PB-200041438. [DOI] [PubMed] [Google Scholar]
- Önay-Uçar E, Karagöz A, Arda N. Antioxidant activity of Viscum album ssp. album. Fitoterapia. 2006;77:556–560. doi: 10.1016/j.fitote.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Orhan DD, Aslan M, Sendogdu N, Ergun F, Yesilada E. Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J Ethnopharmacol. 2005;98:95–102. doi: 10.1016/J.JEP.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Orhan DD, Küpeli E, Yesilada E, Ergun F. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z Naturforsch. 2006;61c:26–30. doi: 10.1515/znc-2006-1-205. [DOI] [PubMed] [Google Scholar]
- Orhan DD, Senol FS, Hosbas S, Orhan IE. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant properties of Viscum album L. samples collected from different host plants and its two principal substances. Ind Crops Prod. 2014;62:341–349. doi: 10.1016/J.INDCROP.2014.08.044. [DOI] [Google Scholar]
- Papuc C, Crivineanu M, Goran G, Nicorescu V, Durdun N. Free radicals scavenging and antioxidant activity of European mistletoe (Viscum album) and European Birthwort (Aristolochia clematitis) Rev Chim. 2010;61:619–622. [Google Scholar]
- Park JH, Kim YN, Kim JK, Park HY, Song BS. Viscothionin purified from mistletoe (Viscum album var. coloratum Ohwi) induces insulin secretion from pancreatic beta cells. J Ethnopharmacol. 2019;234:172–179. doi: 10.1016/j.jep.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Park R, Kim MS, So HS, Jung BH, Moon SR, Chung SY, Ko CB, Kim BR, Chung HT. Activation of c-Jun N-terminal kinase 1 (JNK1) in mistletoe lectin II-induced apoptosis of human myeloleukemic U937 cells. Biochem Pharmacol. 2000;60:1685–1691. doi: 10.1016/S0006-2952(00)00482-2. [DOI] [PubMed] [Google Scholar]
- Park WB, Lyu SY, Kim JH, Choi SH, Chung HK, Ahn SH, Hong SY, Yoon TJ, Choi MJ. Inhibition of tumor growth and metastasis by Korean mistletoe lectin is associated with apoptosis and antiangiogenesis. Cancer Biother Radiopharm. 2001;16:439–447. doi: 10.1089/108497801753354348. [DOI] [PubMed] [Google Scholar]
- Park YK, Do YR, Jang BC. Apoptosis of K562 leukemia cells by Abnobaviscum F®, a European mistletoe extract. Oncol Rep. 2012;28:2227–2232. doi: 10.3892/or.2012.2026. [DOI] [PubMed] [Google Scholar]
- Patil CR, Jadhav RB, Singh PK, Mundada S, Patil PR. Protective effect of oleanolic acid on gentamicin induced nephrotoxicity in rats. Phyther Res. 2010;24:33–37. doi: 10.1002/ptr.2861. [DOI] [PubMed] [Google Scholar]
- Pietrzak W, Nowak R, Gawlik-Dziki U, Lemieszek MK, Rzeski W. LC-ESI-MS/MS identification of biologically active phenolic compounds in mistletoe berry extracts from different host trees. Molecules. 2017;22:1–15. doi: 10.3390/molecules22040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poruthukaren KJ, Palatty PL, Baliga MS, Suresh S. Clinical evaluation of Viscum album mother tincture as an antihypertensive: a plot study. J Evid Based Complement Altern Med. 2014;19:31–35. doi: 10.1177/2156587213507726. [DOI] [PubMed] [Google Scholar]
- Radenkovic M, Ivetic V, Popovic M, Brankovic S, Gvozdenovic L. Effects of mistletoe (Viscum album L., Loranthaceae) extracts on arterial blood pressure in rats treated with atropine sulfate and hexocycline. Clin Exp Hypertens. 2009;31:11–19. doi: 10.1080/10641960802409820. [DOI] [PubMed] [Google Scholar]
- Saha C, Hegde P, Friboulet A, Bayry J, Kaveri SV. Viscum album-mediated COX-2 inhibition implicates destabilization of COX-2 mRNA. PLoS ONE. 2015;10:e0114965. doi: 10.1371/journal.pone.0114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakalli Çetin E, Tetiker H, İlhan Çelik Ö, Yilmaz N, Hakki Cigerci I. Methotrexate-induced nephrotoxicity in rats: protective effect of mistletoe Viscum album L.) extract. Complement Med Res. 2017;24:364–370. doi: 10.1159/000468984. [DOI] [PubMed] [Google Scholar]
- Satish S, Raveesha KA, Janardhana GR. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett Appl Microbiol. 1999;28:145–147. doi: 10.1046/j.1365-2672.1999.00479.x. [DOI] [Google Scholar]
- Schaller G, Urech K, Giannattasio M. Cytotoxicity of different viscotoxins and extracts from the European subspecies of Viscum album L. Phyther Res. 1996;10:473–477. doi: 10.1002/(SICI)1099-1573(199609)10:6<473::AID-PTR879>3.0.CO;2-Q. [DOI] [Google Scholar]
- Schink M, Tröger W, Dabidian A, Goyert A, Scheuerecker H, Meyer J, Fischer IU, Glaser F. Mistletoe extract reduces the surgical suppression of natural killer cell activity in cancer patients. A randomized phase III trial. Complement Med Res. 2007;14:9–17. doi: 10.1159/000098135. [DOI] [PubMed] [Google Scholar]
- Schläppi M, Ewald C, Kuehn JJ, Weinert T, Huber R. Fever therapy with intravenously applied mistletoe extracts for cancer patients: a retrospective study. Integr Cancer Ther. 2017;16:479–484. doi: 10.1177/1534735416658121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schötterl S, Miemietz JT, Ilina EI, Wirsik NM, Ehrlich I, Gall A, Huber SM, Lentzen H, Mittelbronn M, Naumann U. Mistletoe-based drugs work in synergy with radio-chemotherapy in the treatment of glioma in vitro and in vivo in glioblastoma bearing mice. Evid Based Complement Alternat Med. 2019;2019:1376140. doi: 10.1155/2019/1376140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiglasov VF, Stepula VV, Dudov A, Lehmacher W, Mengs U. The standardised mistletoe extract PS76A2 improves QoL in patients with breast cancer receiving adjuvant CMF chemotherapy: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2004;24:1293–1302. [PubMed] [Google Scholar]
- Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci. 2009;22:102–106. [PubMed] [Google Scholar]
- Shah SS, Rehman YU, Iqbal A, Rahman ZU, Zhou B, Peng M, Li Z. Phytochemical screening and antimicrobial activities of stem, leaves and fruit extracts of Viscum album L. J Pure Appl Microbiol. 2017;11:1337–1349. doi: 10.22207/JPAM.11.3.14. [DOI] [Google Scholar]
- Shahaboddin ME, Pouramir M, Moghadamnia AA, Lakzaei M, Mirhashemi SM, Motallebi M. Antihyperglycemic and antioxidant activity of Viscum album extract. Afr J Pharm Pharmacol. 2011;5:432–436. doi: 10.5897/AJPP11.136. [DOI] [Google Scholar]
- Sharma V, Dutt S, Kumar R, Sharma P, Javed A, Guleria R. OPIOID Pharmacology: a review. IJSRST. 2015;1:1–11. [Google Scholar]
- Siegle I, Fritz P, McClellan M, Gutzeit S, Mürdter TE. Combined cytotoxic action of Viscum album agglutinin-1 and anticancer agents against human A549 lung cancer cells. Anticancer Res. 2001;21:2687–2691. [PubMed] [Google Scholar]
- Stammer RM, Kleinsimon S, Rolff J, Jager S, Eggert A, Seifert G, Delebinski CI. Synergistic antitumour properties of viscumTT in alveolar rhabdomyosarcoma. J Immunol Res. 2017;2017:1–13. doi: 10.1155/2017/4874280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Use and safety of intratumoral application of European mistletoe (Viscum album L.) preparations in oncology. Integr Cancer Ther. 2015;14:140–148. doi: 10.1177/1534735414563977. [DOI] [PubMed] [Google Scholar]
- Steinborn C, Klemd AM, Sanchez-Campillo AS, Rieger S, Scheffen M, Sauer B, Garcia-Kaufer M, Urech K, Follo M, Ucker A, Kienle GS, Huber R, Grundemann C. Viscum album neutralizes tumor-induced immunosuppression in a human in vitro cell model. PLoS ONE. 2017;12:e0181553. doi: 10.1371/journal.pone.0181553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoss M, Gorter RW. No evidence of IFN-γ increase in the serum of HIV-positive and healthy subjects after subcutaneous injection of a non-fermented Viscum album L. extract. Nat Immun. 1998;16:157–164. doi: 10.1159/000069440. [DOI] [PubMed] [Google Scholar]
- Stoss M, Van Wely M, Musielsky H, Gorter RW. Study on local inflammatory reactions and other parameters during subcutaneous mistletoe application in HIV-positive patients and HIV-negative subjects over a period of 18 weeks. Arzneimittelforschung. 1999;49:366–373. doi: 10.1055/s-0031-1300428. [DOI] [PubMed] [Google Scholar]
- Suveren E, Baxter GF, Iskit AB, Turker AU. Cardioprotective effects of Viscum album L. subsp. album (European misletoe) leaf extracts in myocardial ischemia and reperfusion. J Ethnopharmacol. 2017;209:203–209. doi: 10.1016/J.JEP.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Szurpnicka A, Zjawiony JK, Szterk A. Therapeutic potential of mistletoe in CNS-related neurological disorders and the chemical composition of Viscum species. J Ethnopharmacol. 2019;231:241–252. doi: 10.1016/j.jep.2018.11.025. [DOI] [PubMed] [Google Scholar]
- Tabiasco J, Pont F, Fournié J-J, Vercellone A. Mistletoe viscotoxins increase natural killer cell-mediated cytotoxicity. Eur J Biochem. 2002;269:2591–2600. doi: 10.1046/j.1432-1033.2002.02932.x. [DOI] [PubMed] [Google Scholar]
- Tenorio-Lopez FA, Del Valle ML, Olvera GZ, Narvaez JCT, Pastelin G. Viscum album aqueous extract induces NOS-2 and NOS-3 overexpression in Guinea pig hearts. Nat Prod Res. 2006;20:1176–1182. doi: 10.1080/14786410600898979. [DOI] [PubMed] [Google Scholar]
- Tenorio FA, del Valle L, González A, Pastelín G. Vasodilator activity of the aqueous extract of Viscum album. Fitoterapia. 2005;76:204–209. doi: 10.1016/J.FITOTE.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Thies A, Nugel D, Pfüller U, Schumacher U. Influence of mistletoe lectins and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology. 2005;207:105–116. doi: 10.1016/j.tox.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Tsyvunin V, Shtrygol S, Prokopenko Y, Georgiyants V, Blyznyuk N. Influence of dry herbal extracts on pentylenetetrazole-induced seizures in mice: screening results and relationship “chemical composition-pharmacological effect”. Sci Pharm Sci. 2016;1:18–28. doi: 10.15587/2519-4852.2016.71518. [DOI] [Google Scholar]
- Turker AU, Yildirim AB, Karakas FP. Antitumor and antibacterial activities of Viscum album L. grown on different host trees. Spat DD. 2012;2:229–236. doi: 10.5455/spatula.20121224052924. [DOI] [Google Scholar]
- Turkkan A, Savas HB, Yavuz B, Yigit A, Uz E, Bayram NA, Kale B. The prophylactic effect of Viscum album in streptozotocin-induced diabetic rats. North Clin Istanbul. 2016;3:83–89. doi: 10.14744/nci.2016.22932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusenius KJ, Spoek AM, van Hattum J. Exploratory study on the effects of treatment with two mistletoe preparations on chronic hepatitis C. Arzneimittelforschung. 2005;55:749–753. doi: 10.1055/s-0031-1296925. [DOI] [PubMed] [Google Scholar]
- Tusenius KJ, Spoek JM, Kramers CW. Iscador Qu for chronic hepatitis C: an exploratory study. Complement Ther Med. 2001;9:12–16. doi: 10.1054/ctim.2000.0408. [DOI] [PubMed] [Google Scholar]
- Twardziok M, Kleinsimon S, Rolff J, Jager S, Eggert A, Seifert G, Delebinski CI. Multiple active compounds from Viscum album L. synergistically converge to promote apoptosis in ewing sarcoma. PLoS ONE. 2016;11:e0159749. doi: 10.1371/journal.pone.0159749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardziok M, Meierhofer D, Börno S, Timmermann B, Jager S, Boral S, Eggert A, Delebinski CI, Seifert G. Transcriptomic and proteomic insight into the effects of a defined European mistletoe extract in Ewing sarcoma cells reveals cellular stress responses. BMC Complement Altern Med. 2017;17:237. doi: 10.1186/s12906-017-1715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umoh UF, Ekpo BAJ, Bala DN, Udobang JA, Cocobassey M, Etim EI. Phytochemical and comparative antidiabetic studies of leaf extracts of Viscum album from different plant hosts. Int J Biol Chem Sci. 2011;5:1448–1454. doi: 10.4314/ijbcs.v5i4.11. [DOI] [Google Scholar]
- Vicas S, Prokisch J, Rugina D, Socaciu C. Hydrophilic and lipophilic antioxidant activities of mistletoe (Viscum album) as determined by FRAP method. Not Bot Hortic Agrobot Cluj-Napoca. 2009;37:112–116. doi: 10.15835/nbha3723244. [DOI] [Google Scholar]
- Vicas SI, Rugina D, Leopold L, Pintea A, Socaciu C. HPLC fingerprint of bioactive compounds and antioxidant activities of Viscum album from different host trees. Not Bot Hortic Agrobot Cluj-Napoca. 2011;39:48–57. doi: 10.15835/nbha3913455. [DOI] [Google Scholar]
- von Schoen-Angerer T, Madeleyn R, Kienle G, Kiene H, Vagedes J. Viscum album in the treatment of a girl with refractory childhood absence epilepsy. J Child Neurol. 2015;30:1048–1052. doi: 10.1177/0883073814541473. [DOI] [PubMed] [Google Scholar]
- Yang P, Jiang Y, Pan Y, Ding X, Rhea P, Ding J, Hawke DH, Felsher D, Narla G, Lu Z, Lee RT. Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression. Sci Rep. 2019;9:6428. doi: 10.1038/s41598-019-41444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon TJ, Yoo YC, Kang TB, Her E, Kim SH, Kim K, Azuma I, Kim JB. Cellular and humoral adjuvant activity of lectins isolated from Korean mistletoe (Viscum album colaratum) Int Immunopharmacol. 2001;1:881–889. doi: 10.1016/s1567-5769(01)00024-8. [DOI] [PubMed] [Google Scholar]
- Yusuf L, Oladunmoye MK, Ogundare AO. Hepatoprotective effect of methanolic leave extracts of V. album on paracetamol-induced laboratory animals. Int J Pharmacol Phytochem Ethnomedicine. 2015;1:46–54. doi: 10.18052/www.scipress.com/ijppe.1.46. [DOI] [Google Scholar]
- Yusuf L, Oladunmoye MK, Ogundare AO. In-vivo antibacterial activities of mistletoe (Viscum album) leaves extract growing on cocoa tree in Akure North, Nigeria. Eur J Biotechnol Biosci. 2013;1:37–42. [Google Scholar]
- Yusuf L, Oladunmoye MK, Ogundare AO, Mohmo AO, Daudu OAY. Comparative antifungal and toxicological effects of the extract of mistletoes growing on two different host plants in Akure North, Nigeria. Int J Biotechnol Food Sci. 2014;2:31–34. [Google Scholar]