Abstract
Purpose of Review
Neurovascular compression in the upper extremity is rare but can affect even those participating in high-level competitive athletics. To assess optimal approaches to treatment, in this review, we evaluate the current literature on neurovascular compressive syndromes affecting the upper extremity, with a special focus on the thoracic outlet syndrome (TOS).
Recent Findings
Neurovascular compression at the thoracic outlet can involve the brachial plexus, subclavian artery, or subclavian vein, each with distinct clinical manifestations. Neurogenic TOS is best treated with surgical decompression, if physical therapy has not improved symptoms. Venous TOS results in acute thrombosis superimposed on chronic venous compression. Treatment is best directed at early anticoagulation, catheter-directed thrombolysis, and surgical decompression, with most patients able to discontinue anticoagulation and return to high-level athletic activity. Arterial TOS is related to aneurysmal degeneration of the subclavian artery with distal embolization, leading to limb-threatening ischemia. This should be aggressively treated with surgery. Similar degenerative changes can occur in the axillary artery and its branches, leading to distal embolization. Prompt recognition of these potential sources of limb-threatening ischemia is critical to limb preservation.
Summary
TOS includes rare but important conditions in the overhead athlete. Recent advances in physical therapy and image-guided diagnostic techniques have facilitated more accurate diagnosis. Surgical treatment remains the gold standard to maximize function or for limb preservation, and future research is needed to clarify optimal pain and physiotherapy regimens, as well as to examine novel approaches to neurovascular decompression.
Keywords: Thoracic outlet syndrome, Paget-Schroetter syndrome, First rib, Neurovascular compression, Subclavian artery aneurysm, Subclavian vein thrombosis
Introduction
Neurovascular compressive conditions of the upper extremity are rare and often overlooked as an explanation for progressive limitations and high-level athletic performance in overhead athletes [1]. Some of the most common, and significant, upper extremity neurovascular compressive conditions involve the brachial plexus and the subclavian artery and vein, therefore grouped together under the banner of thoracic outlet syndrome (TOS) [2, 3•]. However, compression of neurovascular structures at other sites is also gaining more thorough understanding, including at the quadrilateral space [4] and axillary artery levels [5]. Some of these conditions can even put the limb at risk, and in this chapter, we will review the current strategies for diagnosis and treatment of these conditions. The aim is to maximize successful treatment outcomes, as well as to minimize serious complications. In all cases, prompt recognition, early treatment, and thorough surgical decompression can allow for an effective return to high-level athletic performance.
Neurogenic Thoracic Outlet Syndrome
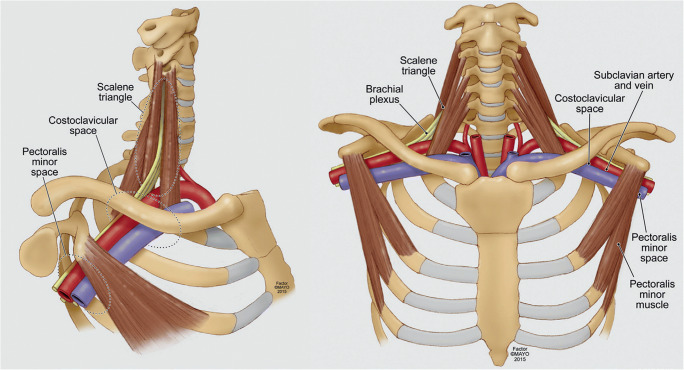
Neurogenic thoracic outlet syndrome is caused by compression and subsequent irritation of the brachial plexus nerves as they pass through the scalene triangle at the base of the neck, between the clavicle and first rib, or in the infraclavicular pectoralis minor space (Fig. 1) [3•, 6]. The underlying mechanism is an ongoing process of repetitive injury leading to fibrosis and hypertrophy of the scalene or pectoralis minor muscles, followed by scar deposition onto the brachial plexus nerves themselves. This may be exacerbated by predisposing anatomical factors such as musculotendinous abnormalities or cervical ribs.
Fig. 1.
Overview of the structures of the thoracic outlet. There several important features in the thoracic outlet at the base of the neck: the scalene triangle, the costoclavicular space, and the subcoracoid (pectoralis minor) space. The scalene triangle is bounded by the anterior middle scalene muscles, as well as the 1st rib. Through this space passes the brachial plexus, which is composed of five nerve roots (C5, C6, C7, C8, and T1), as well as the brachial artery. The subclavian vein passes through the costoclavicular space, anterior to the anterior scalene muscle. Modified with permission from Illig KA, Donahue D, Duncan A, Freischlag J, Gelabert H, Johansen K, Jordan S, Sanders R, and Thompson R. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg 2016;64(3):e23-e35
Clinical Recognition
The classic symptoms of neurogenic TOS involve pain, numbness, paresthesia, and weakness radiating from the neck and shoulder and extending into the arm and hand. It does not follow a single peripheral nerve or cervical nerve root distribution, and the symptoms may fluctuate depending upon the duration or intensity of upper extremity activity. A key finding is the ability to provoke symptoms with overhead arm use, as well as by direct palpation over the brachial plexus at the supraclavicular or infraclavicular space, depending upon the principal area of nerve compression. The latter is a key component of the physical diagnosis. Neurogenic TOS is considered a “diagnosis of exclusion” in that imaging and/or electrophysiology studies are usually negative and serve more importantly to help exclude other diagnoses as opposed to confirming a diagnosis of neurogenic TOS [7]. A recent publication of standardized clinical diagnostic criteria for neurogenic thoracic outlet syndrome (Table 1) has brought more uniformity and recognition to the diagnosis of this condition [8••, 9•]. The elevated arm stress test (EAST) is performed with the patient seated and with the arms at 90 degrees of abduction and full external rotation (the “surrender” position) while opening and closing the hands for up to 3 min. Patients are asked to describe all changes in the nature and distribution of symptoms in the neck and upper extremity and are instructed to lower the arms if unable to continue because of pain. Most patients with neurogenic TOS report the rapid onset of typical upper extremity symptoms within 20 to 30 s of initiating the EAST and are often unable to continue the exercise beyond 60 s. The upper limb tension test (ULTT) is performed with the patient placing both arms at 90 degrees of abduction from the trunk, with the elbows extended and the palms flat. Reproduction of arm or hand symptoms during wrist extension, with some degree of relief during wrist flexion, is a frequent finding in those with neurogenic TOS.
Table 1.
CORE-TOS clinical diagnostic criteria for neurogenic TOS
| Upper extremity symptoms extending beyond the distribution of a single cervical nerve root or peripheral nerve, present for at least 12 weeks, not satisfactorily explained by another condition, AND meeting at least 1 criterion in at least 4 of the following 5 categories: | |
| Principal symptoms | |
|
1A: Pain in the neck, upper back, shoulder, arm and/or hand 1B: Numbness, paresthesia, and/or weakness in the arm, hand, or digits | |
| Symptom characteristics | |
|
2A: Pain/paresthesia/weakness exacerbated by elevated arm positions 2B: Pain/paresthesia/weakness exacerbated by prolonged or repetitive arm/hand use, including prolonged work on a keyboard or other repetitive strain tasks 2C: Pain/paresthesia radiate down the arm from the supraclavicular or infraclavicular spaces | |
| Clinical history | |
|
3A: Symptoms began after occupational, recreational, or accidental injury of the head, neck, or upper extremity, including repetitive upper extremity strain or overuse 3B: Previous ipsilateral clavicle or first rib fracture, or known cervical rib 3C: Previous cervical spine or ipsilateral peripheral nerve surgery without sustained improvement in symptoms 3D: Previous conservative or surgical treatment for ipsilateral TOS | |
| Physical examination | |
|
4A: Local tenderness on palpation over the scalene triangle and/or subcoracoid space 4B: Arm/hand/digit paresthesia on palpation over the scalene triangle and/or subcoracoid space 4C: Objectively weak handgrip, intrinsic muscles, or digit 5, or thenar/hypothenar atrophy | |
| Provocative maneuvers | |
|
5A: Positive upper limb tension test (ULTT) 5B: Positive 3-min elevated arm stress test (EAST) |
Diagnostic criteria developed by the Consortium for Research and Education on Thoracic Outlet Syndrome (CORE-TOS). With permission from Balderman J, Holzem K, Field BJ, Bottros MM, Abuirqeba AA, Vemuri C, and Thompson RW. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg 2017;66(2):533–544
In the high-level athlete, the fluctuation of symptoms can often lead to a long interval from symptom onset to clinical diagnosis. Periods of minimal symptoms corresponding to periods of rest or even normal day-to-day activities should not be used to rule out neurogenic TOS, especially when there is significant arm fatigue, heaviness, or other symptoms when throwing [1, 10••, 11]. Diminished pitch count and inning longevity are semi-objective metrics that may be useful in uncovering neurogenic TOS [10••]. Finally, the diagnosis of neurogenic TOS can be facilitated by exercise-enhanced, ultrasound-guided, local anesthetic anterior scalene and/or pectoralis minor muscle blocks [12•]. There are no clear indications for noninvasive Doppler ultrasound tests in the evaluation of the patients with suspected neurogenic TOS. Electromyography and nerve conduction studies are of limited utility for evaluating neurogenic TOS because they are usually negative or nonspecific due to limitations in obtaining accurate proximal measurements for the brachial plexus, as well as the intermittent nature of the nerve compression.
Treatment
The hallmark of the initial stages of treatment for neurogenic TOS involves rest of the affected extremity; focused physical therapy to relax the scalene muscles and decompress the scalene triangle and subcoracoid spaces; as well as the use of muscle relaxants and anti-inflammatory medications [13–17]. The use of longer-acting muscle injections, such as botulinum toxin, has fallen out of favor due to a lack of durable benefit [18]. The use of “highly selective” algorithms for surgical treatment [19, 20] has also fallen out of favor, and surgical treatment is recommended for both patients who have not achieved sufficient improvement with conservative therapy alone and remain with substantial disability [21•, 22•, 23]. Surgical decompression for neurogenic TOS can be achieved through either a supraclavicular or transaxillary incision, with subsequent anterior and middle scalene resection and removal of the first rib, as well as brachial plexus neurolysis. A separate incision is often used for release of the pectoralis minor tendon for patients with evidence of compression at that level [24]. Minimally invasive approaches, utilizing an extrapleural approach with video assistance (thorascopic), have been popularized, but this still remains experimental without any clear advantages over standard surgical approaches [25]. Of the standard surgical approaches, there is some evidence to suggest that the recurrence rate with the supraclavicular approach is lower than with the transaxillary approach, particularly in long-term follow-up [26]. The supraclavicular approach is associated with a more complete removal of the anterior and middle scalene muscles, ease of identifying and managing anatomic variants, and the unfettered ability to perform a full exploration of the brachial plexus for neurolysis [27].
Rehabilitation
Immediate postoperative care after thoracic outlet decompression is focused around pain control, maintenance of full range of motion at the shoulder and the neck, optimization of wound healing, and avoidance of muscle spasm through initiation of light physical therapy and multimodal pain control (Table 2) [6]. The course of physical therapy is expanded over the next 3–4 weeks and is focused on passive and assisted exercises based on shoulder range of motion, avoidance of strength training, “nerve glides “for neural mobilization, maintenance and improvement of posture, minimizing scapular winging with appropriate muscle mechanics, conditioning the diaphragm for breathing patterns, and maintaining general conditioning. Strenuous activity as well as submersion in water should be avoided due to wound healing for several weeks after surgery. At the 8-week mark, resistive strength training for the mid and lower trapezius, serratus anterior, and rotator cuff muscles begins, as well as continued efforts to maintain full range of motion and natural movement patterns, as well as reintroduction of gentle throwing to preserve the overall throwing motion. Between 8 and 12 weeks, the care is transitioned from the physical therapist to the athletic trainer, and at the 12-week mark, a more formal graduated throwing program may begin and progress as tolerated. It is critical that all of these steps work in concert during the rehabilitation process, as attempts to rush the process may lead to the development of excessive muscle spasm with subsequent perineural scar tissue deposition and increased risk of recurrent neurogenic TOS symptoms. Full rehabilitation and return to high-level athletic competition often take 9–12 months after surgical decompression [27].
Table 2.
Overview of postoperative rehabilitation for neurogenic TOS
| Stage I: Inpatient | |
| •Hospital length of stay 3–5 days | |
| •Patient self-directed exercises (cervical and shoulder ROM) | |
| •Surgeon office follow-up visit postoperative day 5–7, drain removal | |
| Stage II: First postoperative month | |
| •Protection of surgical tissues to promote incisional healing and minimize muscle spasm (propping arm with pillows while sitting and sleeping, ice, medications) | |
| •Maintain cervical and glenohumeral range of motion (hospital self-exercises) | |
| •Light conditioning activity (walking, bicycle) | |
| Stage III: Second postoperative month | |
| •Physical therapist visits near home, 1–2 per week | |
| •Pain management (ice or heat to surgical area) | |
| •Scar hypersensitivity and local tissue edema | |
| •Posture (head, shoulders, and scapulae), monitor for scapular winging | |
| •Light neural mobilizations | |
| •Gentle stretching of levator, upper trapezius, and pectoral muscles | |
| •Movement of scapula into upward rotation and elevation | |
| •Breathing pattern (chest versus diaphragm) | |
| •Conditioning activity (walking, bicycle, elliptical, treadmill) but avoid vigorous use of involved upper limb | |
| •Activities of daily living, ergonomics, work environment | |
| •Cautions: No strengthening including use of weights or bands, avoid manual therapies that may irritate sensitive healing tissue, no immersion in water until incisions fully healed | |
| Stage IV: Third and fourth postoperative months | |
| •Continue physical therapist visits near home, 1–2 per week | |
| •Symptom management, may introduce manual therapies | |
| •Continue conditioning activity (bicycling, walking, elliptical, treadmill) | |
| •Strengthening mid and lower trapezius, serratus anterior, and rotator cuff muscles | |
| •Increasing range of motion of upper limb, throwing motion, optimize movement patterns | |
| •Introduce gentle throwing and progress as tolerated | |
| Stage V: Fourth to sixth postoperative months | |
| •Supervised throwing program, progressing as tolerated: | |
| •Step 1: 1 × 25 throws at 60 ft | |
| •Step 2: 2 × 25 throws at 60 ft | |
| •Step 3: 1 × 25 throws at 60 ft, 1 × 25 throws at 90 ft | |
| •Step 4: 1 × 30 throws at 60 ft, 1 × 25 throws at 90 ft | |
| •Step 5: 1 × 30 throws at 60 ft, 1 × 25 throws at 90 ft | |
| •Step 6: 1 × 30 throws at 90 ft, 1 × 25 throws at 120 ft | |
| •Step 7: 2 × 20 throws at 120 ft | |
| •Step 8: 1 × 20 throws at 120 ft, 1 × 20 ft at 150 ft | |
| •Step 9: 1 × 20 throws at 150 ft, 10 pitches from mound | |
| •Step 10: Long toss, 35-pitch bullpen session | |
| •Gradually increase activity toward unrestricted return to competition at 6 to 9 months |
Outcomes
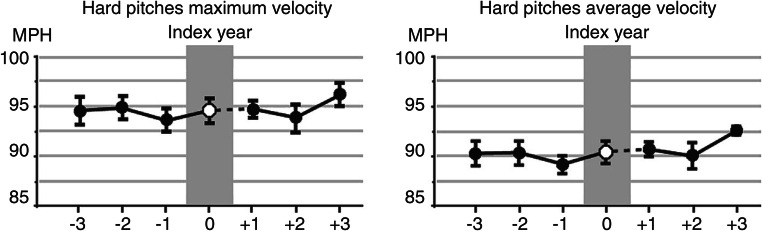
Surgical outcomes for neurogenic thoracic outlet decompression are well-established, with 85–90% of the general population reporting significant symptom improvement, and recent studies have shown that this is near the same in the high-level overhead athlete population [10, 20]. Given the increased awareness of this condition, particularly among baseball athletes of all levels, as well as refinement in the surgical treatment, has allowed many athletes to resume careers that previously would have been derailed. The most robust study used objective sabermetric data to follow the performance of Major League Baseball (MLB) pitchers before and after surgical decompression [10••]. This involved 10 pitchers that underwent surgery between July 2001 and July 2014, who achieved a sustained return to major league pitching. The mean age was 30.2 ± 1.4 years with a mean postoperative rehabilitation period of 10.8 ± 1.8 months prior to return. Using 15 traditional pitching metrics (including earned run average [ERA], fielding independent pitching [FIP], strikeouts to walks ratio [SO/BB], walks per 9 innings [BB/9], and walks plus hits per innings pitched [WHIP]) and 72 advanced metrics for 3 years before and after the date of surgery identified no significant differences. In fact, maximal and average hard pitch velocities (Fig. 2) were similar between the pre- and postoperative periods. This was the first study looking at MLB pitchers returning after surgical decompression and demonstrated that they had capabilities equivalent or better than their historical baselines—even prior to the onset of symptoms.
Fig. 2.
Performance metrics in MLB pitchers before and after surgical treatment for neurogenic TOS. Pitch velocity metrics in the 3 years before and after undergoing surgical treatment for neurogenic TOS, as represented by the mean ± SEM. Note there is no significant difference before or after surgical decompression. With permission from Thompson RW, Dawkins C, Vemuri C, Mulholland MW, Hadzinsky TD, McLaughlin LN, and Pearl GJ. Performance metrics in professional baseball pitchers before and after surgical treatment for neurogenic thoracic outlet syndrome. Ann Vasc Surg 2017;39(1):216–227
Schutze et al. reported similar satisfaction with outcomes in a survey of competitive athlete who had undergone surgical treatment for neurogenic TOS [28]. They surveyed 67 athletes ranging from 14 to 48 years old, at an average of 3.9 years post procedure. There was an improvement in pain medication use in 96% and resolution of symptoms in 82%, with 75% reporting that they would undergo contralateral decompression if necessary and 94% stating that they were unlimited in performing standard day-to-day activities. Finally, 70% of these individuals had returned to the same or better subjective levels of activity, and half reached that goal 1 year after surgery.
Venous Thoracic Outlet Syndrome
Venous TOS, otherwise known as axillary-subclavian vein effort thrombosis or Paget-Schroetter syndrome, is the most frequently encountered vascular disorder in competitive athletes [29, 30]. Unfortunately, delayed diagnosis or incomplete treatment is common and can reduce athletic tolerance and ability and prevent further participation. Thus, it is critical that sports medicine physicians have a sound understanding of this condition and its management. As with neurogenic TOS, the prognosis for patients with venous TOS is quite good following surgical decompression, and most athletes are able to return to previous high levels of functional performance within several months of treatment [30].
Pathophysiology
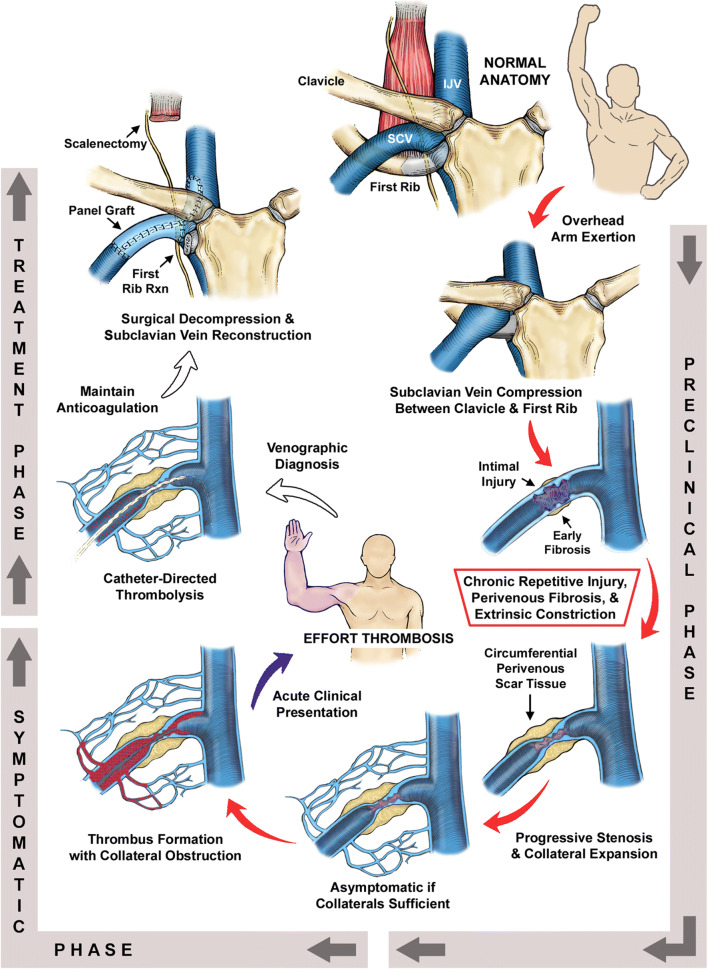
Historically, venous TOS was thought of as an acute thrombosis of the subclavian vein between the clavicle and the 1st rib, occurring as a result of unusual overhead or lifting effort or as a complication of a primary hypercoagulable state. A more modern understanding of this disease process recognizes that this is a chronic disease process, underpinned by repetitive venous injury during arm use and elevation in the setting of normal anatomic arrangements in the costoclavicular space. This space is the area between the clavicle, the first rib, the anterior scalene muscle, and the subclavius muscle (Fig. 3) [30, 31]. In the chronic state, this is reflected as a cycle of injury and tissue repair, with progressive deposition of perivenous constricting scar tissue. This condition may remain asymptomatic for many years, as the body is able to develop collateral draining pathways, until the formation of an acute thrombus in the main subclavian vein develops and is propagated, and a significant amount of collateral circulation is thereby obstructed in addition to the main venous drainage. This acute-on-chronic venous obstruction results in sudden spontaneous swelling of the entire arm, with cyanosis, heaviness, early fatigue, and pain with use (Fig. 4). Pulmonary embolism may occur; however, this is much less frequent than in iliofemoral deep vein thrombosis, and it is usually hemodynamically insignificant and even clinically silent, often being an incidental finding on imaging studies.
Fig. 3.
Underlying pathophysiology and surgical management of subclavian vein effort thrombosis. Normal anatomy of the thoracic outlet (top center) illustrating relationships between the subclavian vein (SCV), internal jugular vein (IJV), clavicle, and first rib. In the preclinical phase, vigorous activities requiring overhead positions of the arm are associated with the development of SCV effort thrombosis. SCV compression between the clavicle and first rib results in focal vein wall injury. Chronic repetitive compression injury of the SCV leads to formation of circumferential perivenous scar tissue, which can severely constrict the lumen. Thrombus formation within the lumen of the constricted SCV causes complete obstruction of the SCV, with extension of thrombus to the axillary vein causing obstruction of collateral veins and symptomatic presentation of acute SCV effort thrombosis. Catheter-directed venography and thrombolytic infusion remove the thrombus and demonstrate the underlying injury to the subclavian vein. Definitive treatment is achieved by thoracic outlet decompression with first rib resection and subclavian vein reconstruction. Reproduced with permission from Melby SJ, Vedantham S, Narra VR, et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome). J Vasc Surg 2008;47:809–820
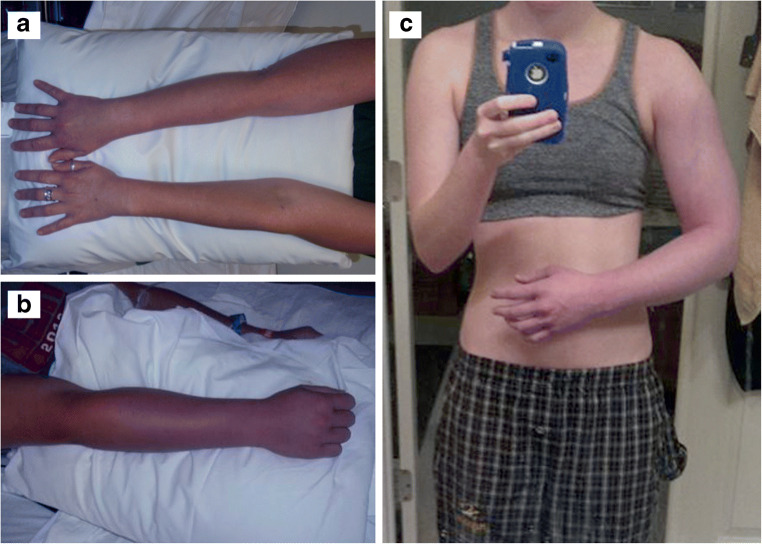
Fig. 4.
Clinical presentations of subclavian vein thrombosis in healthy adults. Photographs depicting: a A 32-year-old woman with right SCV thrombosis; b a 24-year-old male with right SCV thrombosis; and c a 17-year-old woman with right SCV thrombosis. Note how all of them have demonstrated the classic symptoms of upper extremity swelling and cyanotic discoloration, extending from the shoulder to involve the entire arm. With permission from Vemuri C, Salehi P, Benarroch-Gampel J, McLaughlin LN, and Thompson RW. Diagnosis and treatment of effort-induced thrombosis of the axillary-subclavian vein due to venous thoracic outlet syndrome. J Vasc Surg Venous Lymphat Disord 2016;4(4):485–500
Clinical Recognition
The index of suspicion for venous TOS should be high in any young, healthy, active individual who presents with pulmonary embolism or the sudden onset of arm swelling and cyanosis, particularly in those involved in repetitive overhead activities or weightlifting [29–31]. Additional history will often include a sense of heaviness in the arm that is worse with elevation, with occasional numbness and tingling in the arm and hand. In contrast to neurogenic TOS, in venous TOS, there is no significant tenderness along the path of the brachial plexus. However, physical examination reveals substantial swelling of the entire arm, from the shoulder extending into the hand, with cyanosis, as well as engorgement of subcutaneous veins draining from the arm around the shoulder and across the upper chest and back.
Duplex ultrasound imaging of the upper extremity may be used in the initial evaluation of patients suspected to have venous TOS; however, there are several technical limitations imposed on imaging this area of the body, including acoustic shadowing from the bony structures and difficulty compressing the subclavian vein. One retrospective review identified a 21% false-negative rate for diagnosis of subclavian vein thrombus, which was associated with a delay in definitive imaging and thrombolysis and associated with an increase in need for major subclavian vein reconstruction at the time of surgical decompression [32••]. Because of this substantial false-negative rate, duplex ultrasound should not be relied upon to rule out a diagnosis of venous TOS. Effort thrombosis may also be evaluated by contrast-enhanced CT or MR venography, often with the arm in resting and overhead positions. This type of study may readily establish the diagnosis and may also be relied upon to exclude venous TOS when negative [7]. The most definitive diagnostic test for venous TOS remains catheter-directed venography. This provides the most complete information regarding flow through the axillary-subclavian vein as well as the collateral pathways and allows for potential for single-session treatment with thrombolysis using a variety of techniques. Catheter-based venography should therefore be considered the initial diagnostic test of choice, whenever available.
Anticoagulation and Thrombolysis
The initial treatment of patients suspected to have venous TOS is anticoagulation with intravenous heparin to stabilize the clot and prevent extension of the thrombus. Catheter-directed venography should also be used, as well as attempts at clearing the clot and restoring in-line flow through the subclavian vein (Fig. 5). There are a variety of devices and pharmaceutical agents used in these procedures that are beyond the scope of this chapter. Balloon angioplasty may be attempted to improve any residual stenosis; however, this is often unsuccessful given fibrous scarring within the subclavian vein wall and persistent external compression. It is essential to realize that thrombolysis does not represent definitive long-term treatment. Additionally, it is important to emphasize that stenting in this area is contraindicated and should be strongly avoided, given the well-documented high rate of mechanical stent failure in the absence of surgical decompression [33].
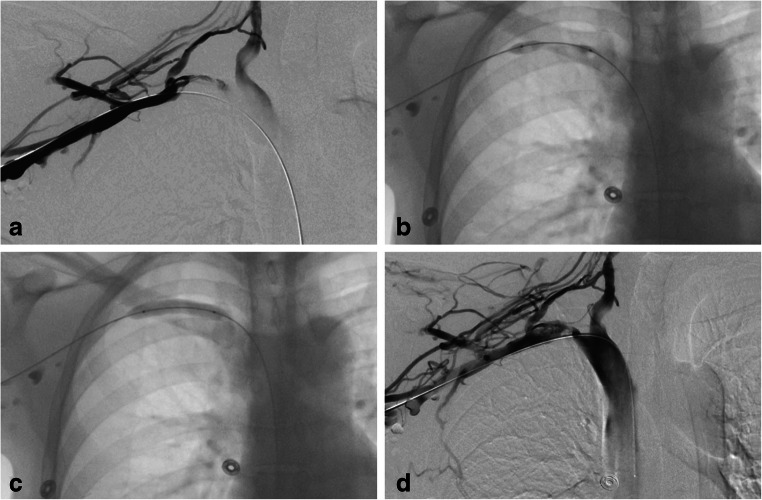
Fig. 5.
Venography and initial management of subclavian vein thrombosis. Initial management of a young woman with right-sided SCV effort thrombosis is best achieved with catheter-directed venography. a Initial venogram demonstrates extensive collateral veins, as well as underlying axillary-subclavian vein occlusion. b After catheter-directed pharmacomechanical therapy, a flow channel is cleared, and residual narrowing is observed and the proximal SCV. c Balloon angioplasty at the area of the residual stenosis demonstrates the external compression of the bony structures. d Completion venogram demonstrating improved flow of the SCV common decompression of the collaterals. With permission from Vemuri C, Salehi P, Benarroch-Gampel J, McLaughlin LN, and Thompson RW. Diagnosis and treatment of effort-induced thrombosis of the axillary-subclavian vein due to venous thoracic outlet syndrome. J Vasc Surg Venous Lymphat Disord 2016;4(4):485–500
Fortunately, most patients experience rapid improvement in symptoms following thrombolysis and are then maintained on systemic anticoagulation. In the past, conservative treatment with anticoagulation alone has been attempted, but this is associated with a substantial incidence of recurrent thrombosis and persistence of symptoms with limitations in arm activity. It should be recognized that axillary-subclavian vein effort thrombosis is not a primary thrombotic process like deep venous thrombosis of the lower extremities, as there is a distinct cause due to external compression and injury to the vein. Thus, it is unknown at what time interval anticoagulation can be discontinued in the absence of thoracic outlet decompression, and it may need to be continued on an indefinite basis. While these limitations may be acceptable to elderly sedentary individuals, for young healthy athletes, the use of long-term anticoagulation and restrictions on arm activity represent an undue burden that is unacceptable, making surgical treatment the best recommendation both for long-term reduction in symptoms and to allow discontinuation of anticoagulation. Therefore, following thrombolysis, the standard of care for venous TOS is referral for surgical treatment.
Surgical Management
The goals of surgical decompression for venous TOS are focused around decompressing the axillary-subclavian vein and to restore normal venous blood flow in a durable manner [29]. In all the surgical per protocols to date, thoracic outlet decompression is accomplished by first rib resection along with removal or division of the anterior scalene, as well as removal of the subclavius muscle and perivenous scar. The most common approaches involve a transaxillary, paraclavicular, or infraclavicular approach. The transaxillary approach typically involves partial resection of the anterior portion of the first rib and division of the scalene muscle attachments. Given the limitations on exposure or control of the subclavian vein, this approach is often done in conjunction with a plan for intraoperative or postoperative venography and balloon angioplasty. The infraclavicular approach involves resection of the anterior portion of the first rib to its junction with the sternum and as far back as is reasonably viewed. The anterior scalene muscle is also divided, and the subclavius muscle is removed. A limited external venolysis of the subclavian vein can be performed from this approach. In contrast, the paraclavicular approach allows for full resection of the entire first rib and complete removal of the anterior scalene, middle scalene, and subclavius muscles. It allows a full exposure of the axillary-subclavian vein by which to perform a thorough external venolysis, as well as sufficient exposure for potential patch angioplasty repair or interposition bypass reconstruction, as indicated by the findings of intraoperative venography (Fig. 6). Following surgical decompression, the gradual initiation of anticoagulation and physical therapy are important for achieving a steady return to full function. Anticoagulation is generally continued for 3 months after surgery and then discontinued.
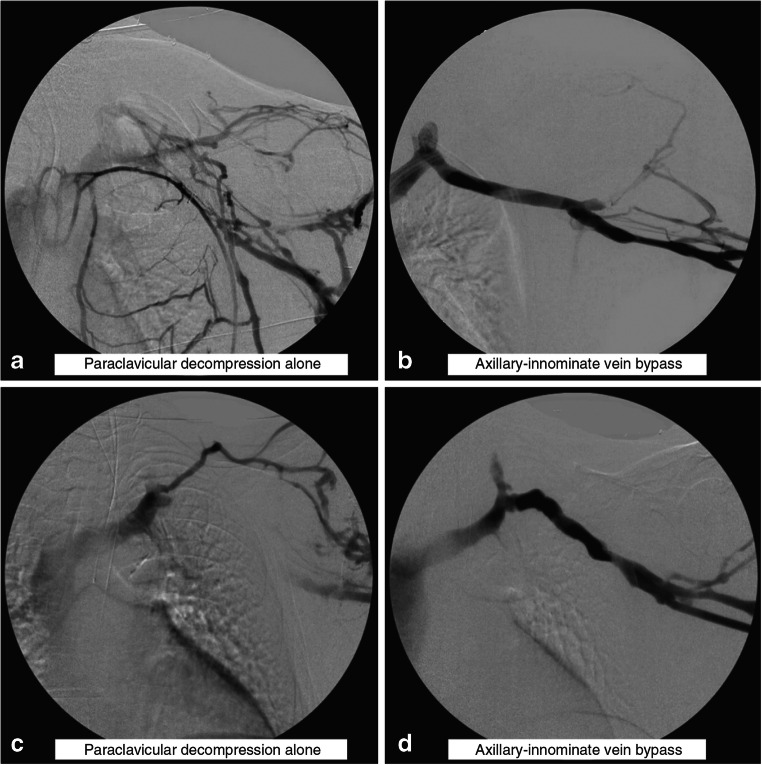
Fig. 6.
Subclavian vein bypass graft reconstruction. Intraoperative left upper extremity venograms in two different patients depicting residual obstruction of the SCV immediately following paraclavicular decompression and external venolysis (a and c); both patients required reconstruction with cryopreserved femoral vein conduits (b and d). With permission from Vemuri C, Salehi P, Benarroch-Gampel J, McLaughlin LN, and Thompson RW. Diagnosis and treatment of effort-induced thrombosis of the axillary-subclavian vein due to venous thoracic outlet syndrome. J Vasc Surg Venous Lymphat Disord 2016;4(4):485–500
Outcomes
Our results for venous thoracic outlet decompression using the paraclavicular approach have been previously reviewed in this series of 32 high-performing overhead athletes [30]. With a mean age of 20.3 years and a mean interval of symptom onset to definitive venographic diagnosis of 20.2 ± 5.6 days (range 1 to 120), catheter-directed thrombolysis was performed in 26 (81%) with adjuvant balloon angioplasty required in 12. During surgical decompression, none of the patients experienced any disruption of the bony landmarks at the sternoclavicular joint. Patency of the axillary and subclavian vein was restored by external venolysis and decompression alone in 56%, while the remaining 44% required axillary-subclavian vein reconstruction. All patients resumed unrestricted use of the upper extremity as well as participation and high-level athletics, with a median interval of 3.5 months (range 2 to 10 months) between operation and the return to competitive athletics. Similar to earlier reports, the overall duration from onset of symptoms to a full return to activity was correlated with the time interval from venographic diagnosis to surgical decompression and was lengthened in patients with persistent symptoms or rethrombosis prior to definitive surgical referral.
In a review of the three major surgical decompression protocols, those based on the transaxillary and infraclavicular approaches resulted in successful outcomes in approximately 75% of patients, whereas over 95% of those who underwent paraclavicular decompression had a successful outcome [31]. These experiences demonstrate that successful outcomes for venous TOS can be most predictably achieved using an aggressive approach, centered around early diagnosis using venography, prompt thrombolysis, and referral for surgical decompression using paraclavicular thoracic outlet decompression with possible reconstruction, followed by short-course postoperative anticoagulation. Optimal outcomes for this condition can therefore be achieved by early recognition and prompt referral to a center where comprehensive endovascular and surgical management are available.
Arterial Thoracic Outlet Syndrome
Arterial TOS is characterized by pathology of the subclavian artery as it passes through the scalene triangle in the setting of precipitating anatomical factors, most commonly bony abnormalities such as a cervical rib, hypoplastic first rib, or clavicle fracture. It is the least frequent form of TOS, accounting for 1–3% of patients in the largest clinical series [1, 2]. It is important to emphasize that arterial TOS is distinct from positional compression of the subclavian artery (reduction of the pulses during arm elevation), which is not an infrequent finding in normal patients on physical exam and has no potential for occlusion or distal embolization. In contrast, positional compression of the subclavian artery may be present in patients with symptoms more characteristic of neurogenic TOS, in which case it should be apparent that the cause of symptoms is not related to the reduction of pulses but rather to simultaneous brachial plexus nerve compression.
Clinical Recognition
Unilateral hand or digital ischemia is a common presenting finding in patients with arterial TOS. This may be accompanied by symptoms of numbness, tingling, altered temperature sensation, and delayed capillary refill. It is important to note that these findings may be seen even in the presence of palpable radial and ulnar pulses, although this is not universal. There may be loss of the ulnar, radial, or brachial pulses with diminished blood pressure measurements on the affected extremity. The underlying mechanism is proximal embolization from the subclavian artery, most commonly manifest as an aneurysm.
The presence of digital ischemia is not universally due to subclavian artery pathology. More proximal and distal sources of embolization should be evaluated and differentiated between localized digital artery occlusion and primary vasospasm (such as in the setting of Raynaud’s syndrome) (Table 3). The workup for a source of embolization should include a transthoracic echocardiogram, to evaluate for cardiac source of embolus, as well as contrast-enhanced angiography, whether by CT or MR. Similar to venous TOS, catheter-directed angiography remains the most accurate and definitive means of diagnosis relating to the level of disease, as well as to accurately evaluate the distal vasculature and the level of embolization for treatment planning purposes.
Table 3.
Differential diagnosis of digital ischemia
| Thromboembolism from a cardiac source | |
| Arrhythmia, valvular disease, paradoxical (patent septal defect) | |
| Thromboembolism from a proximal arterial source | |
| Aorta: endothelial ulceration, dissection, or penetrating ulcer | |
| Subclavian or axillary arteries: aneurysm, occlusion, stenosis or ulceration | |
| Thromboembolism from a distal arterial source | |
| Brachial, radial or ulnar arteries: local trauma | |
| Palmar arteries: hypothenar hammer syndrome | |
| Systemic diseases associated with vasculitis | |
| Scleroderma, rheumatoid arthritis, polyarteritis nodosa, Takayasu’s, Buerger’s disease | |
| Local vascular diseases | |
| Hemangioma, arteriovenous malformation, glomus tumor, synovitis | |
| Primary digital artery thrombosis | |
| Local repetitive trauma | |
| Primary vasospasm | |
| Raynaud’s disease, cold exposure, tobacco use, cocaine |
Pathophysiology and Surgical Therapy
Subclavian artery aneurysms form as a result of poststenotic dilatation in the setting of prolonged and sustained compression of the subclavian artery. This is most commonly seen in the setting of bony abnormalities, such as a congenital cervical rib or hypoplastic first rib. The poststenotic dilatation of the artery leads to aneurysmal wall degeneration, with intimal ulceration and thrombus formation that is prone to distal embolization. When the diagnosis is made, whether on axial imaging or by catheter-based angiography (Fig. 7), antiplatelet agents and anticoagulation should be initiated to help stabilize the mural thrombus and prevent further embolization. Intra-arterial thrombolytics may have a role for the management of distal embolic disease but should be used with caution and in the setting of planned surgical decompression, as it may exacerbate the potential for distal embolization.
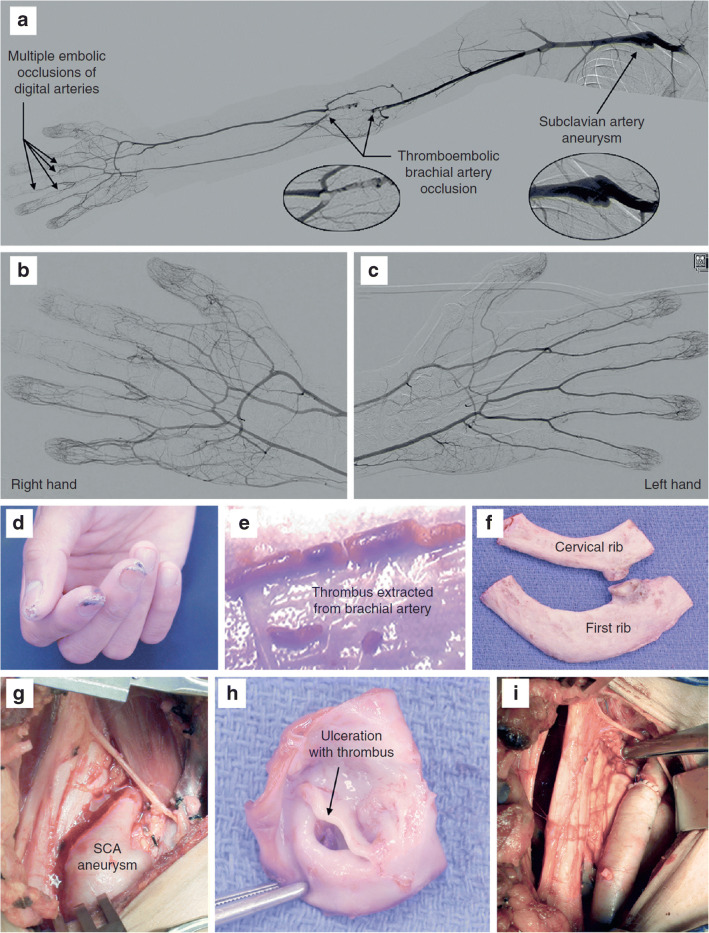
Fig. 7.
Subclavian Artery Aneurysm Causing Digital Ischemia. Photographs depicting a otherwise healthy 28-year-old right-handed man who presented with right digital ischemia. a A right upper extremity arteriogram demonstrates multilevel disease, beginning with the subclavian artery aneurysm, as well as distal occlusion of the brachial artery, as well as distal digital artery occlusion. b and c Angiographic views of the right hand (b) and left hand (c) illustrate the ipsilateral difference in perfusion between the hands. d Ischemic digital lesions in the right hand. e Brachial artery thromboembolectomy and patch angioplasty repair was initially performed due to the acute limb-threatening nature. f Several days later, the patient went for removal of the 1st and cervical ribs. g Subclavian artery aneurysm viewed from right supraclavicular exposure. h There is intimal disruption with ulceration and thrombus formation in the subclavian artery. i Interposition bypass grafting was necessary for subclavian artery reconstruction. With permission from Thompson RW. Management of digital emboli, vasospasm, and ischemia in ATOS. In: Illig KA, Thompson RW, Freischlag JA, Donahue DM, Jordan SE, Edgelow PI, eds. Thoracic Outlet Syndrome. London:Springer; 2013:p.557–563
The definitive surgical approach for subclavian artery aneurysms in the setting of arterial TOS is dependent upon the location and extent of the aneurysm. In all cases, it begins with the supraclavicular incision and proceeds with scalenectomy and rib resection similar to operations for neurogenic TOS. In cases where the subclavian artery aneurysm extends underneath the level of the clavicle, a separate infraclavicular incision may be necessary to obtain distal axillary artery control for the performance of an arterial bypass. The choice of bypass conduit is largely determined by the extent of the aneurysm. In those that do not cross under the clavicle, it is reasonable to consider repair with short autologous or cryopreserved vessel conduits. However, if the lesion crosses underneath the clavicle and an infraclavicular distal anastomosis is required, greater consideration may be given to use of externally supported ringed polytetrafluoroethylene (PTFE) or Dacron grafts. In patients who presented acutely, the emphasis should be on reestablishing flow to the distal extremity, often through a more distal brachial artery exposure in the arm. Depending upon the clinical picture, it is not unreasonable to stage surgical management of the subclavian artery aneurysm, until several weeks after the acute limb-threatening event has passed.
Outcomes
The senior author’s experience with arterial TOS over an 8-year period has been previously reported [34]. This single institution-based series encompassed 40 patients with a mean age of 40.3 ± 2.2 years (range, 13–68 years), with over half presenting with upper extremity ischemia (N = 21) or posterior cerebral circulation strokes (N = 2), 8 of whom required separate brachial artery thromboembolectomy for limb-threatening ischemia. The remaining 17 presented with a nonvascular etiology, including superimposed neurogenic TOS or asymptomatic neck mass. All of the patients underwent surgical decompression, and 75% had a cervical rib (6 partial, 24 complete), 5 had a first rib abnormality, and four had a clavicular fracture.
Subclavian artery reconstruction for significant dilation was performed in 28 (70%). Patency of the reconstruction in a follow-up of 4.5 ± 0.4 years was 72%, and no patients (including those who did not undergo reconstruction) reported further embolization or had noted dilatation at follow-up. Chronic symptoms remained in 6 patients, four of which were post-ischemic and 2 of which had neurogenic symptoms. Based on this experience, it was concluded that for limb-threatening events, it is safe to stage the brachial embolectomy from the proximal subclavian aneurysm repair, and secondly, that prompt surgical decompression and repair of the subclavian aneurysm can lead to excellent results and a full return to high level of function within months. However, the residual symptom rate is not insignificant (15%), and such patients should be counseled on this possibility at the time of surgical therapy.
Compressive Axillary Artery Lesions
There is an even more rare vascular condition related to the thoracic outlet, seen almost exclusively in volleyball and baseball pitchers, that is marked by injuries to the axillary artery or its branches at the level of the head of the humerus (Fig. 8) [4, 5, 35, 36]. This may be seen in the main axillary artery in its third portion or, more frequently, in the proximal branch vessels (anterior and posterior circumflex humeral arteries or subscapular artery). Given the location and fixation of the axillary artery just beyond the pectoralis minor muscle and the relatively fixed positions of the anterior and posterior circumflex humeral and subscapular branch vessels, these lesions are believed to be provoked by recurrent trauma against the humeral head as the axillary artery is pulled tight during the overhead throwing position. A variant of this lesion, termed “vascular quadrilateral space syndrome,” is due to repetitive trauma to the posterior circumflex humeral artery (PCHA) as it courses through the quadrilateral space in the upper arm [4]. This space is bounded by the long head of the triceps medially, medial edge of the humerus laterally, the teres major inferiorly, and the teres minor superiorly [37]. As a result of this trauma, there is repetitive injury at the origin of the branch points, with intimal disruption and dissection, leading to both aneurysmal degeneration and thrombotic occlusion with formation of mural thrombus and distal thromboembolization. Primary dissection of the axillary artery itself is possible and may extend proximally or distally with subsequent ischemic complications.
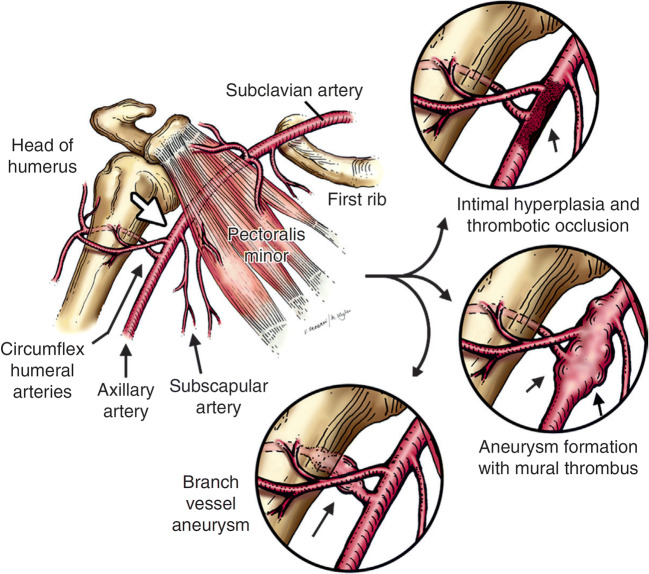
Fig. 8.
Pathogenesis of compressive axillary artery lesions. The arrow annotated is the area of compression of the axillary artery against the proximal portion of the humeral head, posterior to the level of the pectoralis minor tendon. This is most commonly seen in the overhead position. Repetitive injury can lead to a variety of sequelae eyes, including intimal hyperplasia and thrombotic occlusion, aneurysm formation with mural thrombus, or event vessel aneurysm and dissection. With permission from Duwayri YM, Emery VB, Driskill MR, Earley JA, Wright RW, Paletta GA Jr., and Thompson RW. Positional compression of the axillary artery causing upper extremity thrombosis and embolism in the elite overhead throwing athlete. J Vasc Surg 2011;53(5):1329–1340
Diagnosis and Management
Similar to arterial TOS, this condition is often manifested as hand or digital ischemia as well as exertional fatigue in the arm. Similar to the management of arterial TOS, initial treatment involves antiplatelet medication and anticoagulation, as well as angiographic diagnosis. It is important during imaging assessment to assess for dynamic positioning, as dynamic arterial obstruction may be best visualized with catheter-based imaging techniques.
As with arterial TOS, antiplatelet and anticoagulation management alone is not sufficient, nor is angioplasty or stent placement at the time of catheter-directed angiography, given the potential for exacerbating any underlying dissection or causing distal embolization. Surgical treatment is optimal management for compressive lesions of the axillary artery and its branches. The preferred operative approach involves an upper arm incision overlying the course of the upper brachial and distal axillary artery, across the level of the pectoralis minor space. The pectoralis minor tendon may be divided if necessary for exposure, and the entire axillary artery should be fully dissected free of surrounding tissues and attachments, taking care not to injure the adjacent brachial plexus nerves. For lesions affecting the axillary artery branches alone, it is sufficient to ligate and transect the branch vessel off of the main axillary artery. However, for those lesions involving the main axillary artery, the affected segment should be completely excised. Primary repair is ill-advised due to tethering of the axillary artery; thus, patch angioplasty or interposition bypass grafting is preferable, with saphenous vein being the preference for the young athlete (Fig. 9). All attempts possible should be made to preserve or reimplant at least one of the axillary branches onto the bypass graft or the distal anastomosis, to maintain adequate perfusion and optimal performance of the humeral head and the muscles of the shoulder girdle.
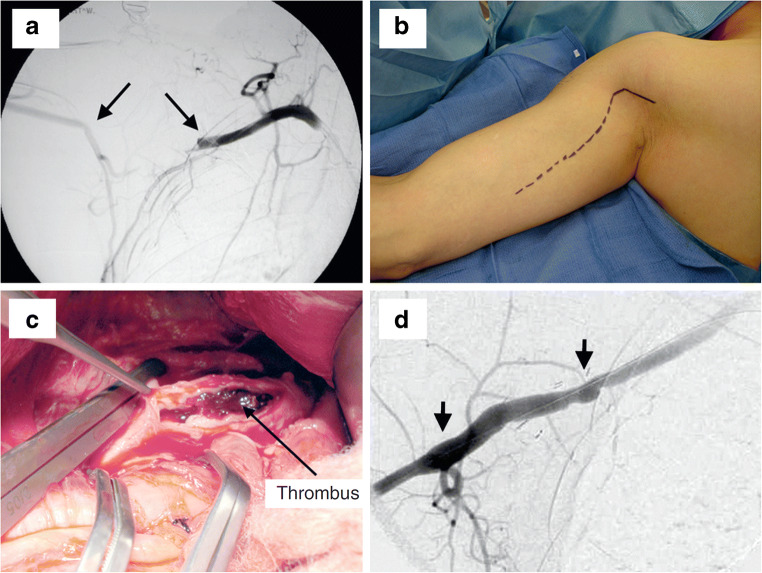
Fig. 9.
Axillary artery thrombosis. a Intraoperative angiogram demonstrates occlusion of the right axillary artery id a 29-year-old baseball pitcher. b The incision shown for the upper arm exploration is demonstrated, with noted of the cutoff at the level of the pectoralis minor. c The arteries controlled and then opened longitudinally to identify thickened and disrupted intima, as well as thrombus formation of both acute and chronic nature. d This area was excised and replaced with an interposition vein graft, shown on the intraoperative angiogram. Note the preserved large circumflex humeral artery at the 2nd arrow. With permission from Duwayri YM, Emery VB, Driskill MR, Earley JA, Wright RW, Paletta GA Jr., and Thompson RW. Positional compression of the axillary artery causing upper extremity thrombosis and embolism in the elite overhead throwing athlete. J Vasc Surg 2011;53(5):1329–1340
Prognosis
Postoperative recovery is typically complete by the 12-week mark, and in our reported series of compressive axillary artery lesions, all patients returned to high-level professional baseball [35]. Of these 9 patients, the mean age was 30.9 ± 2.9 years, and they underwent angiography and surgical repair at a mean of 2.5 ± 0.8 weeks after the onset of symptoms (range 1–8 weeks). The majority (6 patients) had occlusion of the distal axillary artery at the level of the humeral head, 1 had axillary artery dissection with positional occlusion, and 2 had thrombosis of posterior circumflex humeral artery aneurysms. Surgical treatment consisted of distal artery thrombectomy and thrombolysis, with ligation and excision of circumflex humeral artery aneurysms in 2, patch angioplasty of the axillary artery alone in 2, and interposition bypass graft using saphenous vein in 5. The hospital stay was just under 4 days (3.8 ± 0.5 days), unrestricted overhead throwing was begun at 10.8 ± 2.7 weeks, and all were able to return to professional baseball. Primary patency of the arterial repair was 89%, and secondary patency was 100%, at a median follow-up of 15 months. This study leads us to advocate for prompt surgical management and exploration of these patients, to facilitate a return to high-level athletic activities.
Conclusions
Upper extremity pain, numbness, tingling, and fatigue are hallmarks of neurogenic TOS, and in the high-performance athlete, these symptoms may not be present at rest. Surgical decompression for neurogenic TOS is effective and can lead to a full return to athletic activities if the condition is identified and treated early. Median recovery time to competitive athletic activity is 9–10 months, with targeted physical therapy needed during recovery and rehabilitation. Subclavian vein thrombosis due to venous TOS should be aggressively evaluated with catheter-based venography, and anticoagulation is initial management strategy. Surgical decompression is essential to allow discontinuation of anticoagulation and a return to high-level athletic activity. Unilateral digital ischemia, most commonly in the throwing arm, may arise from multiple sources of emboli including the subclavian artery in arterial TOS, as well as the axillary artery or its branches. This finding should prompt urgent evaluation with contrast-enhanced angiography. Arterial TOS is caused by bony abnormalities, most often a cervical rib, with chronic compression and injury to the subclavian artery resulting in with aneurysm formation. Arterial obstruction may be largely positional, and this should be taken into consideration during imaging evaluation. There is increasing awareness and understanding of neurovascular compressive symptoms affecting the upper extremities, and these should be promptly referred to specialists and surgical centers of excellence upon recognition.
Compliance with Ethical Standards
Conflict of Interest
J Westley Ohman and Robert W Thompson declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. All external articles referenced by the authors of this article were approved by the local Institutional Review Board.
Footnotes
This article is part of the Topical Collection on Injuries in Overhead Athletes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Thompson RW. Thoracic outlet syndrome and neurovascular conditions of the shoulder in baseball players. In: Ahmad, editor. Baseball Sports Medicine. Philadelphia: Wolters Kluwer Health; 2019. pp. 269–283. [Google Scholar]
- 2.de Mooij T, Duncan AA, Kakar S. Vascular injuries in the upper extremity in athletes. Hand Clin. 2015;31(1):39–52. doi: 10.1016/j.hcl.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Illig KA, Thompson RW, Freischlag JA, Donahue DM, Jordan SE, Edgelow PI. Thoracic Outlet Syndrome (TOS) 1. London: Springer-Verlag; 2013. [Google Scholar]
- 4.Rollo J, Rigberg D, Gelabert H. Vascular Quadrilateral Space Syndrome in 3 Overhead Throwing Athletes: An Underdiagnosed Cause of Digital Ischemia. Ann Vasc Surg. 2017;42:63.e1–63.e6. doi: 10.1016/j.avsg.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 5.Zajac JM, Angeline ME, Bohon TM, Loftus M, Potter HG, Weiland AJ, Thompson RW, Coleman SH, Altchek DW. Axillary artery thrombosis in a major league baseball pitcher: a case report and rehabilitation guide. Sports Health. 2013;5(5):402–406. doi: 10.1177/1941738113495647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RW, Bartoli MA. Neurogenic thoracic outlet syndrome. In: Rutherford RB, editor. Vascular Surgery. 6. Philadelphia: Elsevier Saunders; 2005. pp. 1347–1365. [Google Scholar]
- 7.Raptis CA, Sridhar S, Thompson RW, Fowler K, Bhalla S. Imaging of the patient with thoracic outlet syndrome. Radiographics. 2016;36(4):984–1000. doi: 10.1148/rg.2016150221. [DOI] [PubMed] [Google Scholar]
- 8.Illig KA, Donahue D, Duncan A, Freischlag J, Gelabert H, Johansen K, Jordan S, Sanders R, Thompson R. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg. 2016;64(3):e23–e35. doi: 10.1016/j.jvs.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Balderman J, Holzem K, Field BJ, Bottros MM, Abuirqeba AA, Vemuri C, Thompson RW. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2017;66(2):533–544. doi: 10.1016/j.jvs.2017.03.419. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RW, Dawkins C, Vemuri C, Mulholland MW, Hadzinsky TD, McLaughlin LN, Pearl GJ. Performance metrics in professional baseball pitchers before and after surgical treatment for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2017;39(1):216–227. doi: 10.1016/j.avsg.2016.05.103. [DOI] [PubMed] [Google Scholar]
- 11.Twaij H, Rolls A, Sinisi M, Weiler R. Thoracic outlet syndromes in sport: a practical review in the face of limited evidence--unusual pain presentation in an athlete. Br J Sports Med. 2013;47(17):1080–1084. doi: 10.1136/bjsports-2013-093002. [DOI] [PubMed] [Google Scholar]
- 12.Bottros MM, AuBuchon JD, McLaughlin LN, Altchek DW, Illig KA, Thompson RW. Exercise-enhanced, ultrasound-guided, local anesthetic anterior scalene/pectoralis minor muscle blocks can facilitate diagnosis of neurogenic thoracic outlet syndrome in the high-performance overhead athlete. Am J Sports Med. 2017;45(1):189–194. doi: 10.1177/0363546516665801. [DOI] [PubMed] [Google Scholar]
- 13.Totten PA, Hunter JM. Therapeutic techniques to enhance nerve gliding in thoracic outlet syndrome and carpal tunnel syndrome. Hand Clin. 1991;7(3):505–520. [PubMed] [Google Scholar]
- 14.Aligne C, Barral X. Rehabilitation of patients with thoracic outlet syndrome. Ann Vasc Surg. 1992;6(4):381–389. doi: 10.1007/BF02008798. [DOI] [PubMed] [Google Scholar]
- 15.Walsh MT. Therapist management of thoracic outlet syndrome. J Hand Ther. 1994;7(2):131–144. doi: 10.1016/s0894-1130(12)80083-4. [DOI] [PubMed] [Google Scholar]
- 16.Wehbe MA, Schlegel JM. Nerve gliding exercises for thoracic outlet syndrome. Hand Clin. 2004;20(1):51–55. doi: 10.1016/s0749-0712(03)00090-8. [DOI] [PubMed] [Google Scholar]
- 17.Watson LA, Pizzari T, Balster S. Thoracic outlet syndrome part 2: conservative management of thoracic outlet. Man Ther. 2010;15(4):305–314. doi: 10.1016/j.math.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Finlayson HC, O’Connor RJ, Brasher PM, Travlos A. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152(9):2023–2028. doi: 10.1016/j.pain.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Chandra V, Olcott C, 4th, Lee JT. Early results of a highly selective algorithm for surgery on patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2011;54(6):1698–1705. doi: 10.1016/j.jvs.2011.05.105. [DOI] [PubMed] [Google Scholar]
- 20.Chandra V, Little C, Lee JT. Thoracic outlet syndrome in high-performance athletes. J Vasc Surg. 2014;60(4):1012–1017. doi: 10.1016/j.jvs.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Caputo FJ, Wittenberg AM, Vemuri C, Driskill MR, Earley JA, Rastogi R, Emery VB, Thompson RW. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg. 2013;57(1):149–157. doi: 10.1016/j.jvs.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Peek J, Vos CG, Unlu C, van de Pavoordt HDWM, van den Akker PJ, de Vries JPM. Outcome of surgical treatment for thoracic outlet syndrome: systematic review and meta-analysis. Ann Vasc Surg. 2017;40:303–326. doi: 10.1016/j.avsg.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 23.Rinehardt EK, Scarborough JE, Bennett KM. Current practice of thoracic outlet decompression surgery in the United States. J Vasc Surg. 2017;66(3):858–865. doi: 10.1016/j.jvs.2017.03.436. [DOI] [PubMed] [Google Scholar]
- 24.Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg. 2014;27(2):86–117. doi: 10.1053/j.semvascsurg.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Hwang J, Min BJ, Jo WM, Shin JS. Video-assisted thoracoscopic surgery for intrathoracic first rib resection in thoracic outlet syndrome. J Thorac Dis. 2017;9(7):2022–2028. doi: 10.21037/jtd.2017.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altobelli GG, Kudo T, Haas BT, Chandra FA, Moy JL, Ahn SS. Thoracic outlet syndrome: pattern of clinical success after operative decompression. J Vasc Surg. 2005;42(1):122–128. doi: 10.1016/j.jvs.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RW, Vemuri C. Neurogenic thoracic outlet syndrome exposure and decompression: supraclavicular (chapter) In: Mulholland MW, Hawn MT, Hughes SJ, Albo D, Sabel MS, Dalman RL, editors. Operative techniques in surgery. Philadelphia: Wolters Kluwer Health; 2015. pp. 1848–1861. [Google Scholar]
- 28.Shutze W, Richardson B, Shutze R, Tran K, Dao A, Ogola GO, Young A, Pearl G. Midterm and long-term follow-up in competitive athletes undergoing thoracic outlet decompression for neurogenic thoracic outlet syndrome. J Vasc Surg. 2017;66(6):1798–1805. doi: 10.1016/j.jvs.2017.06.108. [DOI] [PubMed] [Google Scholar]
- 29.Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg. 2010;51(6):1538–1547. doi: 10.1016/j.jvs.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Melby SJ, Vedantham S, Narra VR, Paletta GA, Jr, Khoo-Summers L, Driskill M, Thompson RW. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome) J Vasc Surg. 2008;47(4):809–820. doi: 10.1016/j.jvs.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Vemuri C, Salehi P, Benarroch-Gampel J, McLaughlin LN, Thompson RW. Diagnosis and treatment of effort-induced thrombosis of the axillary subclavian vein due to venous thoracic outlet syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(4):485–500. doi: 10.1016/j.jvsv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Brownie ER, Abuirqeba AA, Ohman JW, Rubin BG, Thompson RW. False-negative upper extremity ultrasound in the initial evaluation of patients with suspected subclavian vein thrombosis due to thoracic outlet syndrome (Paget-Schroetter syndrome) J Vasc Surg Venous Lymphat Disord. 2020;8(1):118–126. doi: 10.1016/j.jvsv.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Urschel HC, Jr, Patel AN. Paget-Schroetter syndrome therapy: failure of intravenous stents. Ann Thorac Surg. 2003;75(6):1693–1696. doi: 10.1016/s0003-4975(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 34.Vemuri C, McLaughlin LN, Abuirqeba AA, Thompson RW. Clinical presentation and management of arterial thoracic outlet syndrome. J Vasc Surg. 2017;65(5):1429–1439. doi: 10.1016/j.jvs.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Duwayri YM, Emery VB, Driskill MR, Earley JA, Wright RW, Paletta GA, Jr, Thompson RW. Positional compression of the axillary artery causing upper extremity thrombosis and embolism in the elite overhead throwing athlete. J Vasc Surg. 2011;53(5):1329–1340. doi: 10.1016/j.jvs.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 36.van de Pol D, Kuijer PPFM, Langenhorst T, Maas M. High prevalence of self-reported symptoms of digital ischemia in elite male volleyball players in the Netherlands: a cross-sectional national survey. Am J Sports Med. 2012;40(10):2296–2302. doi: 10.1177/0363546512456973. [DOI] [PubMed] [Google Scholar]
- 37.McClelland D, Paxinos A. The anatomy of the quadrilateral space with reference to quadrilateral space syndrome. J Shoulder Elb Surg. 2008;17:162–164. doi: 10.1016/j.jse.2007.05.013. [DOI] [PubMed] [Google Scholar]