Abstract
Purpose of Review
The complexity of the human extremity, particularly the upper extremity and the hand, allows us to interact with the world. Prosthetists have struggled to recreate the intuitive motor control, light touch sensation, and proprioception of the innate limb in a manner that reflects the complexity of its native form and function. Nevertheless, recent advances in prosthesis technology, surgical innovations, and enhanced rehabilitation appear promising for patients with limb loss who hope to return to their pre-injury level of function. The purpose of this review is to illustrate recent technological advances that are moving us one step closer to the goal of multi-functional, self-identifiable, durable, and intuitive prostheses.
Recent Findings
Surgical advances such as targeted muscle reinnervation, regenerative peripheral nerve interfaces, agonist-antagonist myoneural interfaces, and targeted sensory reinnervation; development of technology designed to restore sensation, such as implanted sensors and haptic devices; and evolution of osseointegrated (bone-anchored) prostheses show great promise. Augmented and virtual reality platforms have the potential to enhance prosthesis design, pre-prosthetic training, incorporation, and use.
Summary
Emerging technologies move surgeons, rehabilitation physicians, therapists, and prosthetists closer to the goal of creating highly functional prostheses with elevated sensory and motor control. Collaboration between medical teams, scientists, and industry stakeholders will be required to keep pace with patients who require durable, high-functioning prostheses.
Keywords: Amputation, Rehabilitation, Adaptive technology, Prosthetics, Limb loss, Myoelectric prostheses
Introduction
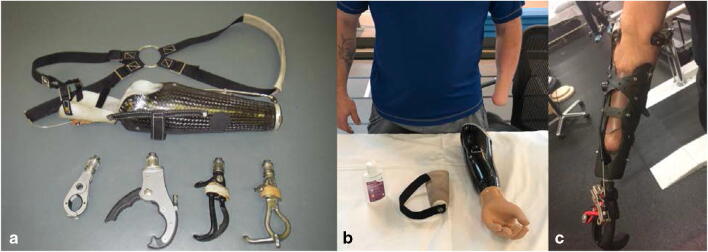
An estimated 1.6 million Americans were living with limb loss in 2005, with a projected increase to 3.6 million by 2050 [1]. Upper extremity limb loss accounts for approximately one-third of that group, though major amputations (those proximal to the digits) represent only 8% of upper extremity amputations [1]. Trauma is the second leading cause of amputation, occurring at approximately one-eighth the rate as amputation secondary to vascular disease [2]. The Congressional Research Service reported 1645 patients with major limb amputations from 2001 to 2015 related to battle injuries incurred during US military operations [3]. Importantly, the demographic affected by upper extremity amputations, and particularly those related to trauma, is younger than those affected by lower extremity amputations [4–6]. These patients desire durable, high-functioning prostheses that allow them to pursue active, independent lifestyles [7]. The number of young amputees is expected to grow as mortality following high-energy trauma and war injuries continues to decline. Specifically, recent US combat operations have demonstrated an increasing need for prostheses capable of returning service members to duty and amputees to the workforce (Fig. 1).
Fig. 1.
Common designs of upper extremity prostheses for the transradial amputation. a Body-powered design. This figure-of-eight harness anchors the prosthesis to the contralateral axilla. A combination of scapular abduction and/or glenohumeral flexion pulls the cable to open the terminal device, while relaxation allows it to passively close. The quick-disconnect wrist unit allows the user to easily interchange terminal devices for varying activities. b Myoelectric design. The particular limb is designed for suspension with a silicone liner and lanyard and has electrodes embedded in the prosthesis for EMG control. No external harness is needed. Note the cosmetic “skin” over the hand which gives the prosthesis a more aesthetic appearance. c Modular socket design. This prefabricated design can be used when a simple and durable prosthesis is needed such as in dirty or wet environments. The socket fitting is user-adjustable and the terminal device can be interchanged
Prosthesis abandonment is surprisingly common among patients living with limb loss. A 2007 review of the literature demonstrated that 26% and 23% of adults abandoned their body-powered and electric devices, respectively. Rejection was even higher in the pediatric population, with a rate of 45% for body-powered and 35% for electric prostheses [8]. A separate review of 172 traumatic upper extremity amputees revealed that 29% of patients elected not to use their prosthesis [9]. Additionally, prosthesis rejection is higher at levels that involve the elbow and above. Despite modern myoelectric technology, passive (body-powered or cosmetic) prostheses are still used by one-third of upper extremity amputees [10].
Prosthesis abandonment has been linked to discomfort, residual limb pain, and functional superfluity (Fig. 2) [9]. Fitting complications may be related to sweating, limb atrophy, and weight gain or loss. Sweating in particular causes issues with socket and liner position by altering contact between the prosthesis and the skin. Myoelectric prosthesis users report that sweating alters the location of the electrodes and interferes with signal transmission, thus limiting the efficacy of the myoelectric device. When they are not rejected entirely, myoelectric prosthetics are often repurposed as passive devices by the amputee [11].
Fig. 2.
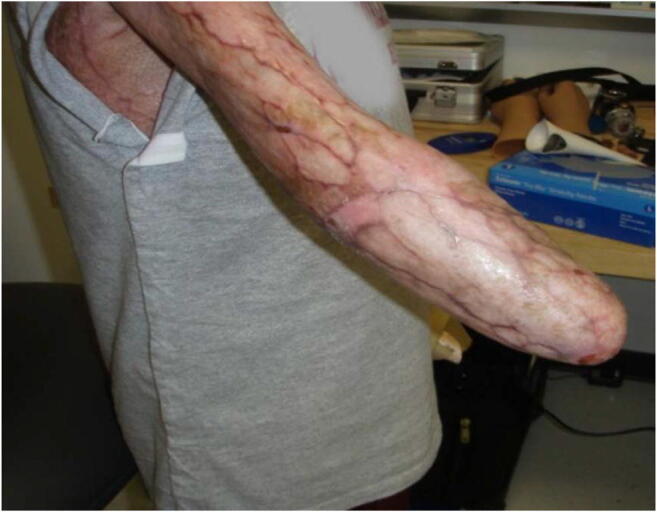
Burns, skin grafts, underlying heterotopic ossification, chronic ulcerations, and sweating can compromise the skin-socket interface. Prosthesis discomfort, residual limb pain, and functional superfluity (functional adaptation where the residual limb is used as a “helper hand” in the absence of a prosthesis) have been linked to prosthesis abandonment. Image courtesy of Ryan Blanck, CPO
Recent technological and surgical advances have begun to shape prosthetic design and the lives of those who wear them. However, despite centuries of work towards the creation of artificial limbs, the development of a device whose function closely approaches that of a biological extremity is far from complete. Advances in areas such as myoelectric sensors, osseointegration, augmented reality, and targeted muscle reinnervation (TMR) may hold the key to powered prosthetics that are multi-functional, self-identifiable, durable, and intuitive.
New Technologies for Myoelectric Prosthetics
Powered prostheses have been in existence since the early twentieth century, with electronic prostheses first emerging near the end of World War II [12–15]. Body-powered prostheses use cables or harnesses that capture the motion of more proximal joints to control the device. In contrast, myoelectric prostheses translate electromyographic (EMG) signals from an activated muscle or muscle group into a specific preprogrammed function. In their most basic form, myoelectric devices rely on antagonistic muscle groups to provide opposing functions. For example, one muscle or muscle group provides the “hand open” signal while its antagonist muscle or muscle group provides the “hand close” signal [12, 16]. These devices provide direct control—sequential one-dimensional functions where the user switches modes (usually through muscle co-contractions) before initiating the next action. More recently, pattern recognition technology has allowed the user to correlate a specific EMG pattern (often the EMG signals from several synergistic muscles as opposed to the EMG signal from a single muscle) with a corresponding pre-programmed action. The benefits of pattern recognition include simultaneous control of multiple degrees of freedom; more intuitive and adaptable control of the prosthesis; and elimination of the need for mode switching. Surgical innovations such as targeted muscle reinnervation (see the “Surgical Innovations” section below) complement pattern recognition technology [17•]. Nevertheless, in our experience, many patients still prefer direct control prostheses with sequential limb function because it does not require extensive training and is viewed as predictable and reliable.
Traditionally, EMG signals have been recorded by surface electrodes, which have the advantage of being non-invasive. However, several factors may degrade EMG signals interpreted by surface electrodes and consequently interfere with limb function. These include anatomic factors such as excessive adipose tissue or a compromised soft tissue envelope; physical factors that compromise the muscle-prosthesis interface such as sweat pooling under the device, a change in limb position, or motion; and “cross talk” between adjacent muscles [18]. As a result, there has been substantial research into devices that address communication challenges between the patient and their prosthetic.
One novel implant aims to provide a more consistent signal by anchoring an implant within the skin. Hahne et al. [19••] introduced a percutaneous titanium electrode for myoelectric control in 2016. The investigators implanted four electrodes in the forearm of an adult volunteer using a 10 mm skin incision. With this approach, the implants are constructed of two disks connected by a stem and are designed to provide more reliable muscle signal detection than traditional surface-based electrodes. One disk is located on the skin surface while the other rests deep to the dermis; the stem traverses the skin. The implants have superior electrical properties and less mechanical interference than current surface-based electrodes. The authors concluded these novel electrodes could serve as an interface for myoelectric control in prostheses while surpassing many drawbacks of traditional surface electrodes.
Implanted sensors within muscles or nerves may also improve the accuracy of signal transduction and prosthetic function. One new device detects muscle movement instead of muscle activation. Tarantino et al. [20, 21] reported promising possibilities using small magnets implanted in residual muscle tissues. Instead of tracking myoelectrical signals, this approach uses sensors to track the movement of implanted magnetic markers during muscle contraction. The investigators tracked muscle contractions in a forearm mockup using this device and highlighted the potential for direct and simultaneous control over individual digits of a prosthetic hand. They note that this technology could be implemented in both upper and lower extremity prostheses.
Devices that attach directly to nerves and that can detect both efferent and afferent signals are also under investigation. One of these devices is the Utah Slanted Electrode Array (USEA). Davis et al. [22••] implanted the USEA, containing 96 recording/stimulating electrodes, into peripheral nerves of amputees to restore motor control and sensory feedback. Two subjects, one whose device was placed in the median nerve and one whose device was placed in the ulnar nerve, were studied for 30 days. Over the experimental period, the patients were able to regain control of individual fingers of a virtual robotic hand. Wendelken et al. [23] performed a similar study by placing two 100-channel USEAs for 4 to 5 weeks in the median and ulnar nerves of two patients. In addition to generating sensory feedback, the implant enabled multi-degree-of-freedom control of virtual hand movement. While these studies demonstrate promising results, albeit in small clinical cohorts, this technology is not yet available commercially.
Surgical Innovations
Several recent surgical innovations have improved the functionality of myoelectric prostheses.
Targeted muscle reinnervation (TMR) is a surgical procedure that gives traumatically or surgically transected nerves a new motor target. The procedure has dual advantages: preventing or treating painful neuromas and improving myoelectric prosthesis functionality [17•]. By giving the transected nerve “somewhere to go and something to do,” it regenerates in an organized fashion and is less likely to develop a painful neuroma [24, 25]. After TMR, muscle reinnervated by the transected nerve produces EMG signals and becomes a myosite, a signal for a myoelectric prosthesis. In this way, a patient can intuitively control the prosthesis through activation of muscular targets whose identity and function have been “reassigned” to that of its new motor nerves [26, 27]. TMR was first developed among patients with shoulder disarticulations and above-elbow amputations (Fig. 3) [17•, 28–30]. However, this powerful procedure is now being used among patients with transradial, transfemoral, and transtibial amputations to optimize myoelectric prosthesis functionality (in the upper extremity) and to prevent and treat painful neuromas (in the upper and lower extremities) [18, 30, 31, 32•, 33, 34••, 35].
Fig. 3.
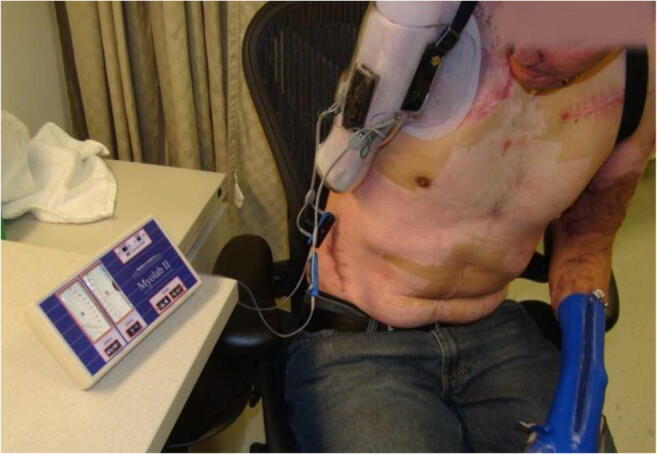
A patient uses the Myolab II (Fillauer, Chattanooga, TN) to train in preparation for his myoelectric prosthesis following shoulder disarticulation and targeted muscle reinnervation. Image courtesy of Ryan Blanck, CPO
TMR can be augmented with haptics and other sensory devices to connect stimulated sensory nerves to the sensory cortex and can be integrated with pattern recognition algorithms for prosthetic control [30, 36–38]. Plasticity of the human brain has potential to allow for greater control and feedback from new prosthetic devices. Sensory feedback provided by targeted reinnervation has been demonstrated to cause sensory cortical remapping, resulting in reorganization that may be advantageous for sensory feedback devices [37, 39].
Like targeted muscle reinnervation, the regenerated peripheral nerve interface (RPNI) is a surgical technique that matches amputated peripheral nerves with denervated muscle grafts. Unlike TMR, the muscle targets used for RPNI are small (less than 40 mm × 20 mm × 5 mm) devascularized muscle grafts taken from either the amputated limb or elsewhere in the body and transposed to the residual limb. The transected nerves are sutured to the center of the graft and the graft is then tubularized [40]. Over time, axonal regeneration occurs and the graft is reinnervated and revascularized [41]. While RPNI was initially developed to provide peripheral nerve signals for myoelectric prosthesis control [42•, 43], it has been successfully used to prevent and treat symptomatic neuromas and phantom pain [44, 45]. An algorithm for translating EMG signals from RPNI constructs have been validated in an animal model for reliable proportional prosthetic control [42•]. Like TMR, this technique has promising implications for augmenting prosthesis function, managing residual limb pain, and perhaps even restoring sensation following upper extremity limb loss [46].
Restoring Sensation
Sensory feedback allows individuals to interact with their environment. Not surprisingly, a lack of sensory feedback has been cited as a cause of prosthesis rejection [47, 48]. When direct sensory feedback is not provided by a prosthetic device, the patient must rely on other sensory inputs to direct the prosthesis and manipulate objects [36, 38, 49–51]. Environment feedback is obtained by prosthetic users visually (77%), acoustically (67%), and using residual limb sensation (57%) [38, 50, 51]. When using a body-powered limb, tactile feedback is generated by activation of the harness when opening the terminal device or when flexing the elbow. When it is available, tactile feedback by the prosthesis increases the amputee’s sense of sense of self-identification with the prosthesis [36, 49].
Many sensory implants are undergoing research and development. These range from implants placed on or within peripheral nerves to those that directly stimulate the somatosensory cortex. Cuff electrodes (surgically implanted electrodes that are wrapped around a peripheral nerve) preserve nerve integrity, have been used on human subjects in multiple studies, and have shown long-term stability but lack the high selectivity of more invasive electrodes [49, 52, 53, 54•, 55]. In contrast, implants containing thin filaments, which are surgically inserted into a nerve, provide more selectivity but may result in intraneural scarring, which may reduce their utility and longevity [49, 54•, 56, 57]. New implantation techniques including syringe injection (as opposed to surgical implantation) are under development as new materials and smaller devices are refined [58]. Some implants, including the USEA, have been able to simultaneously provide both sensation (light touch and proprioception) and motor control of prostheses [22••, 23]. Despite these promising advances, implanted sensory technology is still under investigation and will require further study prior to routine clinical use [54•].
Stimulation of the somatosensory cortex with intracortical microstimulation (ICMS) is also under study. ICMS has the potential to convey multiple types of somatosensory information and may hold the key to overcoming limitations of peripheral nerve stimulation [59, 60].
In contrast to implanted sensors, surface-level sensors can be integrated into prosthetic skin and represent promising advances for light touch, proprioception, and temperature detection. Sim et al. [61••] recently describe the use of metal oxide semiconductor nanomembranes and their potential integration into prostheses’ cosmetic “skin” or glove. These ultrathin devices, which can endure about 30% of mechanical deformation, could offer intelligent feedback and a closed-loop human-machine interface. Whether this level of deformation is adequate for functional pinch or for grip strength for higher activity tasks remains to be seen. Temperature sensors placed on a robotic hand that applies voltage to a soft thermal stimulator on human skin may be used to detect temperature of the external environment or of specific objects.
Haptics is another method of communicating information from the environment to the patient through a prosthesis. Haptic devices use various sensory modalities such as vibration, stretch, and pressure to provide the user with sensory feedback. Use of tactile feedback to convey pressure and vibration sensed by a prosthesis has previously been established and continues to evolve as smaller and more powerful sensors, actuators, and processors are developed [50, 62]. The use of vibrating devices placed on sensory-intact areas can aid in visual feedback and can promote the prosthetic as the part of the patient’s self-identification [36]. The combination of haptics with targeted sensory reinnervation (“reinnervating” intact proximal skin with sensory nerves devoid of their targets following amputation) is particularly promising, as it provides the patient with sensation that seems to come from the more distal (amputated) site [38, 63, 64]. This technique may help restore input to areas of the brain used to receiving input from the missing limb [38, 50, 51].
Osseointegration
Osseointegration, or the stable integration of implants into bone, was originally investigated by Swedish dentist Per-Ingvar Brånemark and was further developed by his son, Rickard Brånemark [65–67]. Osseointegrated prostheses such as Brånemark’s Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) device (Integrum, Sweden), the Compress Transcutaneous Implant (CTI; Zimmer Biomet, USA), and the Osseointegrated Prosthetic Limb (OPL; OrthoDynamics, Australia) have an intramedullary component connected to a percutaneous fixture to which the prosthetic limb is attached (Figs. 4 and 5). Osseointegration addresses or circumvents many of the complications of and restrictions inherent to socket-based prostheses, particularly among patients with compromised soft tissue envelopes (e.g., decreased tissue compliance, history of skin grafting, persistent ulcerations or wounds, underlying heterotopic bone, painful neuromas) or a short residual limb. It also eliminates issues related to socket fit and signal transduction between the myosites and the electrodes, improving the efficiency of these devices. Osseointegration improves functionality, durability, and freedom of motion in the prosthesis as well vibrotactile and pressure feedback secondary to osseoperception [68–72]. A 2-year survival rate of 92% in 51 transfemoral amputees has been observed [73].
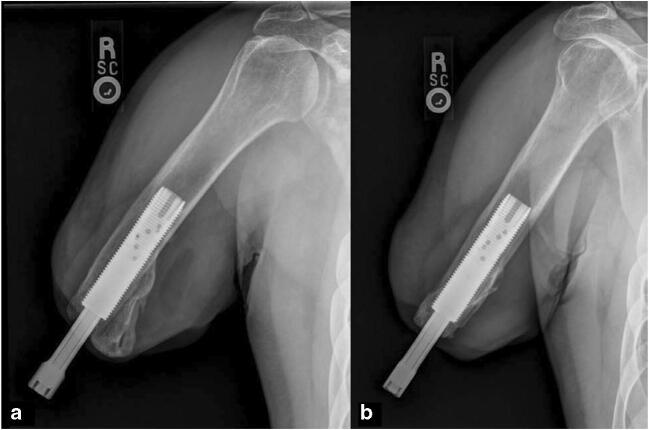
Fig. 4.
Orthogonal radiographs showing the Osseoanchored Prosthesis for the Rehabilitation of Amputees (OPRA; Integrum, Sweden) fixture (intramedullary component) and abutment (extradermal component) following ingrowth. Note the presence of heterotopic bone medial on the medial aspect of the distal residual humerus which, in the absence of a socket, will not adversely impact prosthesis wear. a AP humerus radiograph. b Lateral humerus radiograph. Images courtesy of the Department of Defense Osseointegration Program
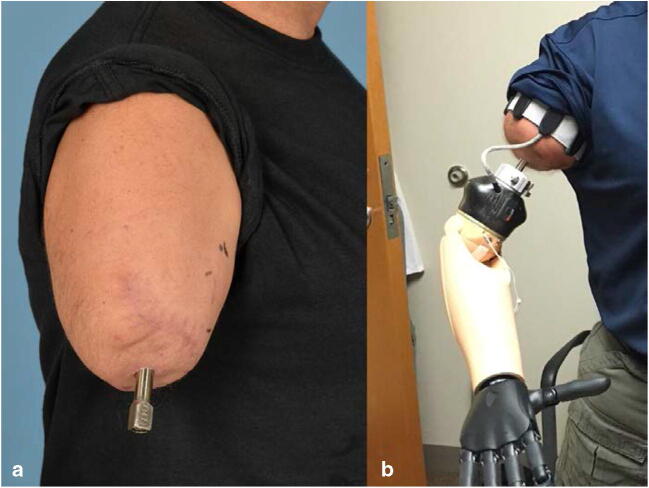
Fig. 5.
Right transhumeral amputation following targeted muscle reinnervation and osseointegration with the Osseoanchored Prosthesis for the Rehabilitation of Amputees (OPRA) device (Integrum, Sweden). a The abutment penetrates through the skin at the end of the residual limb and is secured to the fixture (intramedullary component) with an abutment screw. b The myoelectric prosthesis attaches to the abutment with a connection device (white ring). In the absence of a socket, the electrodes sensing muscle contraction and controlling the device are contained within the soft arm strap. Images courtesy of the Department of Defense Osseointegration Program
An exciting development in osseointegration is the incorporation of motosensory devices into the implant itself. The e-OPRA device (Integrum, Sweden), for example, contains a bidirectional interface using electrodes integrated with the device to improve control of the osseointegrated prosthesis. The e-OPRA device is currently undergoing clinical trials in the USA with an estimated study completion date of January 2023 [74]. Early clinical trials have displayed promising results, with more precision and reliability of implanted electrodes than surface electrodes found among transhumeral amputees [52, 75••]. Mastinu et al. [75••] studied grip control and motor coordination in three transhumeral amputees with the e-OPRA. While the e-OPRA demonstrated superior controllability over conventional surface electrodes during the Pick and Lift and the Virtual Eggs tests, the authors acknowledged that patients remained highly dependent on visual feedback despite incidental sensory feedback (auditory and osseoperceptive). Nevertheless, the synergism of bone-anchored devices, advanced neuromuscular interfaces, and targeted muscle reinnervation could significantly improve the sensory and motor function of advanced prostheses while avoiding the complications associated with socket wear.
Advanced Rehabilitation: Augmented and Virtual Reality
Rehabilitation physicians, therapists, and prosthetists have begun to employ augmented and virtual reality platforms to optimize prosthesis design, incorporation, and use. On the design side, the development of a virtual limb prosthesis has been used to test devices prior to their construction in a virtual reality environment [76]. Putrino et al. [76] trained two non-human primates (Macaca mulatta) to use the virtual prosthetic and observed that they performed reaching and grasping tasks. The authors describe virtual prostheses as highly versatile and applicable to multiple rehabilitative situations, to include simulation of prosthetics prior to production.
Perry et al. [77] have demonstrated that a virtual reality (VR) training platform can be used to efficiently train upper extremity amputees. In this study, thirteen active-duty military personnel with 14 upper extremity amputations learned to control a virtual avatar over 1 to 2 months. Residual limb muscle contraction patterns could be accurately interpreted by computer software as distinct active motion commands (Fig. 6).
Fig. 6.
Client doing virtual training with a pattern recognition system (Coapt, Chicago, Illinois) prior to prosthetic fitting. The client practices bilateral hand movements while the EMG signals are tracked and prosthetic hand movement is simulated. In this figure, the system is learning her EMG pattern for opening the hand. She will continue to train for hand closing and could progress to wrist rotation, various grip patterns, and individual finger use. Photo courtesy of Coapt, LLC
Game-based training has been shown to improve myoelectric prosthesis use (such as muscle control) and could potentially improve rehabilitation success through enhanced training outside the clinic [78]. Melero et al. [79] recently reported on Upbeat, the augmented reality-guided dancing program for upper limb amputees. The authors suggest that improved and tailored rehabilitation will increase patient satisfaction, with further clinical studies to evaluate its impact still pending.
Conclusion
A significant amount of research and funding has been dedicated to the development of advanced prosthesis for individuals living with upper extremity limb loss. The goal of these emerging technologies is to create multi-functional, self-identifiable, durable, and intuitive prostheses. Collaboration between surgeons, rehabilitation specialists, therapists, prosthetists, engineers, and industry stakeholders will be necessary if investigatory protocols and surgical techniques are going to keep pace with active patients’ needs and expectations.
Funding Information
No funding was received for the production of this review article.
Compliance with Ethical Standards
Conflict of Interest
Taylor Bates, John Fergason, and Sarah Pierrie declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimers
The opinions and assertions contained herein are the private views of the authors and are not to be constructed as official or reflecting the views of the Departments of the Air Force, Army, or Defense.
Footnotes
This article is part of the Topical Collection on The Use of Technology in Orthopaedic Surgery—Intraoperative and Post-Operative Management
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014;25:1–8. doi: 10.1016/j.pmr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer, Hannah (2015) A Guide to U.S. Military Casualty Statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom.
- 4.Kejlaa GH The social and economic outcome after upper limb amputation. 7. [DOI] [PubMed]
- 5.Postema SG, Bongers RM, Brouwers MA, Burger H, Norling-Hermansson LM, Reneman MF, Dijkstra PU, van der Sluis CK. Upper limb absence: predictors of work participation and work productivity. Arch Phys Med Rehabil. 2016;97:892–899. doi: 10.1016/j.apmr.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Tintle SM, Keeling JJ, Shawn SB, Forsberg JA, Potter BK. Traumatic and trauma-related amputations: part I: general principles and lower-extremity amputations. J Bone Jt Surg Am. 2010;92:2852–68. [DOI] [PubMed]
- 7.Pierce RO, Kernek CB, Ambrose TA. The plight of the traumatic amputee. Orthopedics. 1993;16:793–797. doi: 10.3928/0147-7447-19930701-08. [DOI] [PubMed] [Google Scholar]
- 8.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthetics Orthot Int. 2007;31:236–257. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 9.Otto IA, Kon M, Schuurman AH, van Minnen LP. Replantation versus prosthetic fitting in traumatic arm amputations: a systematic review. PLoS One. 2015;10:e0137729. doi: 10.1371/journal.pone.0137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maat B, Smit G, Plettenburg D, Breedveld P. Passive prosthetic hands and tools: a literature review. Prosthetics Orthot Int. 2018;42:66–74. doi: 10.1177/0309364617691622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chadwell A, Kenney L, Thies S, Galpin A, Head J. The reality of myoelectric prostheses: understanding what makes these devices difficult for some users to control. Front Neurorobot. 2016;10. 10.3389/fnbot.2016.00007. [DOI] [PMC free article] [PubMed]
- 12.Roche AD, Hubertus Rehbaum DF, Aszmann OC. Prosthetic myoelectric control strategies: a clinical perspective. Curr Surg Rep. 2014.
- 13.Childress DS. Historical aspects of powered limb prostheses. Prosthet Orthot. 1985;9:2–13. [Google Scholar]
- 14.Zecca M, Micera S, Carrozza MC, Dario P. Control of multifunctional prosthetic hands by processing the electromyographic signal. Crit Rev Biomed Eng. 2017;45:383–410. doi: 10.1615/CritRevBiomedEng.v45.i1-6.150. [DOI] [PubMed] [Google Scholar]
- 15.Zecca M, Micera S, Carrozza MC, Dario P. Control of multifunctional prosthetic hands by processing the electromyographic signal. Crit Rev Biomed Eng. 2002;30:459–485. doi: 10.1615/critrevbiomedeng.v30.i456.80. [DOI] [PubMed] [Google Scholar]
- 16.Peerdeman B, Boere D, Witteveen H, in ‘t Veld RH, Hermens H, Stramigioli S, Rietman H, Veltink P, Misra S. Myoelectric forearm prostheses: state of the art from a user-centered perspective. J Rehabil Res Dev. 2011;48:719–737. doi: 10.1682/jrrd.2010.08.0161. [DOI] [PubMed] [Google Scholar]
- 17.• Pierrie SN, Gaston RG, Loeffler BJ. Current concepts in upper-extremity amputation. J Hand Surg. 2018;43:657–67 Summary of current strategies for the care of patients with amputations proximal to the wrist with emphasis on recent advancements in surgical techniques and prosthetics. [DOI] [PubMed]
- 18.Hahne JM, Farina D, Jiang N, Liebetanz D. A novel percutaneous electrode implant for improving robustness in advanced myoelectric control. Front Neurosci. 2016;10:114. doi: 10.3389/fnins.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•• Tarantino S, Clemente F, Barone D, Controzzi M, Cipriani C. The myokinetic control interface: tracking implanted magnets as a means for prosthetic control. Sci Rep. 2017;7:17149 Proposes a new approach using implanted magnetic markers for direct control of multiple degrees-of-freedom in a prosthesis. [DOI] [PMC free article] [PubMed]
- 20.Tarantino S, Clemente F, De Simone A, Cipriani C. Feasibility of tracking multiple implanted magnets with a myokinetic control interface: simulation and experimental evidence based on the point dipole model. IEEE Trans Biomed Eng. 2019;67:1282–1292. doi: 10.1109/TBME.2019.2935229. [DOI] [PubMed] [Google Scholar]
- 21.Davis TS, Wark HAC, Hutchinson DT, Warren DJ, O’Neill K, Scheinblum T, Clark GA, Normann RA, Greger B. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J Neural Eng. 2016;13:036001. doi: 10.1088/1741-2560/13/3/036001. [DOI] [PubMed] [Google Scholar]
- 22.Wendelken S, Page DM, Davis T, Wark HAC, Kluger DT, Duncan C, Warren DJ, Hutchinson DT, Clark GA. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J Neuro Eng Rehabil. 2017;14:121. doi: 10.1186/s12984-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetics Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 24.Mioton LM, Dumanian GA. Targeted muscle reinnervation and prosthetic rehabilitation after limb loss: MIOTON and DUMANIAN. J Surg Oncol. 2018;118:807–814. doi: 10.1002/jso.25256. [DOI] [PubMed] [Google Scholar]
- 25.Kim PS, Ko JH, O’Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg. 2012;37:1609–1616. doi: 10.1016/j.jhsa.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 26.Tintle SM, Baechler MF, Nanos GP, Forsberg JA, Potter BK. Traumatic and trauma-related amputations: part II: upper extremity and future directions. J Bone Jt Surg Am. 2010;92:2934–45. [DOI] [PubMed]
- 27.Ovadia S, Askari M. Upper extremity amputations and prosthetics. Semin Plast Surg. 2015;29:055–061. doi: 10.1055/s-0035-1544171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 29.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 30.Cheesborough JE, Smith LH, Kuiken TA, Dumanian GA. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg. 2015;29:62–72. doi: 10.1055/s-0035-1544166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan EN, Kyle Potter B, Souza JM, Tintle SM, Nanos GP. Targeted muscle reinnervation for transradial amputation: description of operative technique. Tech Hand Up Extrem Surg. 2016;20:166–171. doi: 10.1097/BTH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 32.• Pierrie SN, Gaston RG, Loeffler BJ. Targeted muscle reinnervation for prosthesis optimization and neuroma management in the setting of transradial amputation. J Hand Surg. 2019;44:525.e1–8 Description of a new technique for forearm targeted muscle reinnervation following transradial amputation with a discussion of selected nerve transfer, sensory nerve management, and pattern recognition technology. [DOI] [PubMed]
- 33.Bowen JB, Ruter D, Wee C, West J, Valerio IL. Targeted Muscle Reinnervation Technique in Below-Knee Amputation. Plast Reconstr Surg. 2019;143:309–312. doi: 10.1097/PRS.0000000000005133. [DOI] [PubMed] [Google Scholar]
- 34.•• Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270:238–46 A prospective, randomized multicenter clinical trial demonstrating the effectiveness of targeted muscle reinnervation (TMR) in treating phantom limb pain and painful neuromas after major limb amputation. [DOI] [PubMed]
- 35.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop. 2014;472:2984–2990. doi: 10.1007/s11999-014-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marasco PD, Kim K, Colgate JE, Peshkin MA, Kuiken TA. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain. 2011;134:747–758. doi: 10.1093/brain/awq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J, Chen A, Kuiken T, Carmona C, Dewald J. Sensory cortical re-mapping following upper-limb amputation and subsequent targeted reinnervation: a case report. NeuroImage Clin. 2015;8:329–336. doi: 10.1016/j.nicl.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tintle SM, LeBrun C, Ficke JR, Potter BK. What Is New in Trauma-Related Amputations. J Orthop Trauma. 2016;30:S16–S20. doi: 10.1097/BOT.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 39.Serino A, Akselrod M, Salomon R, Martuzzi R, Blefari ML, Canzoneri E, Rognini G, van der Zwaag W, Iakova M, Luthi F, Amoresano A, Kuiken T, Blanke O. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain. 2017;140:2993–3011. doi: 10.1093/brain/awx242. [DOI] [PubMed] [Google Scholar]
- 40.Kubiak CA, Kemp SWP, Cederna PS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153:681. doi: 10.1001/jamasurg.2018.0864. [DOI] [PubMed] [Google Scholar]
- 41.Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative Peripheral Nerve Interface Viability and Signal Transduction with an Implanted Electrode. Plast Reconstr Surg. 2014;133:1380–1394. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 42.• Frost CM, Ursu DC, Flattery SM, et al. Regenerative peripheral nerve interfaces for real-time, proportional control of a Neuroprosthetic hand. J Neuro Eng Rehabil. 2018;15:108 Animal study validating an algorithm for translating EMG signals from regenerative peripheral nerve interfaces (RPNI) constructs for reliable proportional prosthetic hand control in an animal model. [DOI] [PMC free article] [PubMed]
- 43.Vu PP, Vaskov AK, Irwin ZT, et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci Transl Med. 2020;12:eaay2857. doi: 10.1126/scitranslmed.aay2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS (2016) Regenerative Peripheral Nerve Interfaces for the Treatment of Postamputation Neuroma Pain: A Pilot Study 8. [DOI] [PMC free article] [PubMed]
- 45.Kubiak CA, Kemp SWP, Cederna PS, Kung TA. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg. 2019;144:421e–430e. doi: 10.1097/PRS.0000000000005922. [DOI] [PubMed] [Google Scholar]
- 46.Nghiem BT, Sando IC, Gillespie RB, McLaughlin BL, Gerling GJ, Langhals NB, Urbanchek MG, Cederna PS. Providing a Sense of Touch to Prosthetic Hands. Plast Reconstr Surg. 2015;135:1652–1663. doi: 10.1097/PRS.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 47.Farina D, Aszmann O. Bionic limbs: clinical reality and academic promises. Sci Transl Med. 2014;6:257ps12-257ps12. doi: 10.1126/scitranslmed.3010453. [DOI] [PubMed] [Google Scholar]
- 48.Shull PB, Damian DD. Haptic wearables as sensory replacement, sensory augmentation and trainer - a review. J Neuroeng Rehabil. 2015;12:59. doi: 10.1186/s12984-015-0055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis S, Russold M, Dietl H (2012) User demands for sensory feedback in upper extremity prostheses. In: 2012 IEEE Int. Symp Med. Meas Appl MeMeA. IEEE, pp 1–4.
- 50.Wright TW, Hagen AD, Wood MB. Prosthetic usage in major upper extremity amputations. J Hand Surg. 1995;20:619–622. doi: 10.1016/S0363-5023(05)80278-3. [DOI] [PubMed] [Google Scholar]
- 51.Lundborg G, Rosén B. Sensory substitution in prosthetics. Hand Clin. 2001;17:481–488. [PubMed] [Google Scholar]
- 52.Ortiz-Catalan M, Hakansson B, Branemark R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci Transl Med. 2014;6:257re6-257re6. doi: 10.1126/scitranslmed.3008933. [DOI] [PubMed] [Google Scholar]
- 53.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014;6:257ra138-257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.• Rijnbeek EH, Eleveld N, Olthuis W. Update on peripheral nerve electrodes for closed-loop neuroprosthetics. Front Neurosci. 2018;12:350 Review of recent advances of various types of electrodes for stimulation and recording activity of peripheral nerves for the control of neuroprosthetic limbs. [DOI] [PMC free article] [PubMed]
- 55.Christie BP, Freeberg M, Memberg WD, Pinault GJC, Hoyen HA, Tyler DJ, Triolo RJ. Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J Neuroeng Rehabil. 2017;14:70. doi: 10.1186/s12984-017-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raspopovic S, Capogrosso M, Petrini FM, et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med. 2014;6:222ra19-222ra19. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 57.Di Pino G, Denaro L, Vadalà G, et al. Invasive neural interfaces: the perspective of the surgeon. J Surg Res. 2014;188:77–87. doi: 10.1016/j.jss.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Fu T-M, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol. 2015;10:629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vidal GWV, Rynes ML, Kelliher Z, Goodwin SJ. Review of brain-machine interfaces used in neural prosthetics with new perspective on somatosensory feedback through method of signal breakdown. Scientifica. 2016;2016:1–10. doi: 10.1155/2016/8956432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249–266. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.•• Sim K, Rao Z, Zou Z, Ershad F, Lei J, Thukral A, et al. Metal oxide semiconductor nanomembrane-based soft unnoticeable multifunctional electronics for wearable human-machine interfaces. Sci Adv. 2019;5:eaav9653 Describes the use of ultrathin stretchable nanomembranes and their potential integration into prostheses’ cosmetic “skin” or glove. [DOI] [PMC free article] [PubMed]
- 62.Antfolk C, D’Alonzo M, Rosén B, Lundborg G, Sebelius F, Cipriani C. Sensory feedback in upper limb prosthetics. Expert Rev Med Devices. 2013;10:45–54. doi: 10.1586/erd.12.68. [DOI] [PubMed] [Google Scholar]
- 63.Hebert JS, Olson JL, Morhart MJ, Dawson MR, Marasco PD, Kuiken TA, Chan KM. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2014;22:765–773. doi: 10.1109/TNSRE.2013.2294907. [DOI] [PubMed] [Google Scholar]
- 64.Hebert JS, Chan KM, Dawson MR. Cutaneous sensory outcomes from three transhumeral targeted reinnervation cases. Prosthetics Orthot Int. 2016;40:303–310. doi: 10.1177/0309364616633919. [DOI] [PubMed] [Google Scholar]
- 65.Zuo KJ, Olson JL. The evolution of functional hand replacement: From iron prostheses to hand transplantation. 2014;22:8. [PMC free article] [PubMed]
- 66.Brånemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: an experimental in vivo study in the rat. Biomaterials. 1997;18:969–978. doi: 10.1016/s0142-9612(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 67.Tsikandylakis G, Berlin Ö, Brånemark R. Implant survival, adverse events, and bone remodeling of osseointegrated percutaneous implants for transhumeral amputees. Clin Orthop. 2014;472:2947–2956. doi: 10.1007/s11999-014-3695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jönsson S, Caine-Winterberger K, Brånemark R. Osseointegration amputation prostheses on the upper limbs: methods, prosthetics and rehabilitation. Prosthetics Orthot Int. 2011;35:190–200. doi: 10.1177/0309364611409003. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs R, Brånemark R, Olmarker K, Rydevik B, Van Steenberghe D, Brånemark PI. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthetics Orthot Int. 2000;24:133–142. doi: 10.1080/03093640008726536. [DOI] [PubMed] [Google Scholar]
- 70.Hagberg K, Brånemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthetics Orthot Int. 2008;32:29–41. doi: 10.1080/03093640701553922. [DOI] [PubMed] [Google Scholar]
- 71.Lundborg G, Brnemark P-I, Rosén B. Osseointegrated thumb prostheses: a concept for fixation of digit prosthetic devices. J Hand Surg. 1996;21:216–221. doi: 10.1016/s0363-5023(96)80103-1. [DOI] [PubMed] [Google Scholar]
- 72.Lundborg G, Waites A, Björkman A, Rosén B, Larsson E-M. Functional magnetic resonance imaging shows cortical activation on sensory stimulation of an osseointegrated prosthetic thumb. Scand J Plast Reconstr Surg Hand Surg. 2006;40:234–239. doi: 10.1080/02844310600787005. [DOI] [PubMed] [Google Scholar]
- 73.Brånemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. Bone Jt J. 2014;96-B:106–113. doi: 10.1302/0301-620X.96B1.31905. [DOI] [PubMed] [Google Scholar]
- 74.(2018) e-OPRA Implant System for Lower Limb Amputees. In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03720171. Accessed 4 Nov 2019.
- 75.Mastinu E, Clemente F, Sassu P, Aszmann O, Brånemark R, Håkansson B, Controzzi M, Cipriani C, Ortiz-Catalan M. Grip control and motor coordination with implanted and surface electrodes while grasping with an osseointegrated prosthetic hand. J Neuroeng Rehabil. 2019;16:49. doi: 10.1186/s12984-019-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Putrino D, Wong YT, Weiss A, Pesaran B. A training platform for many-dimensional prosthetic devices using a virtual reality environment. J Neurosci Methods. 2015;244:68–77. doi: 10.1016/j.jneumeth.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry BN, Armiger RS, Yu KE, Alattar AA, Moran CW, Wolde M, McFarland K, Pasquina PF, Tsao JW. Virtual integration environment as an advanced prosthetic limb training platform. Front Neurol. 2018;9:785. doi: 10.3389/fneur.2018.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winslow BD, Ruble M, Huber Z. Mobile, game-based training for myoelectric prosthesis control. Front Bioeng Biotechnol. 2018;6:94. doi: 10.3389/fbioe.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melero M, Hou A, Cheng E, Tayade A, Lee SC, Unberath M, Navab N. Upbeat: augmented reality-guided dancing for prosthetic rehabilitation of upper limb amputees. J Healthcare Eng. 2019;2019:1–9. doi: 10.1155/2019/2163705. [DOI] [PMC free article] [PubMed] [Google Scholar]