Abstract
Purpose of Review
Advances in technology, implant design, and surgical technique have lowered the dislocation rate in primary total hip arthroplasty (THA). Despite these advances, there remain a large number of instability episodes without a known etiology. Recent research suggests that the pelvic and lumbar spine interrelationship may be the explanation in prosthetic dislocations without a known cause. In this review, we describe the biomechanics, measurements, diagnoses, classification, management, and outcomes of total hip and revision total hip instability as it relates to spinopelvic alignment.
Recent Findings
As a person goes from standing to sitting, lumbar lordosis decreases, and the sacrum and entire pelvis tilts posteriorly with sacrum and coccyx rotating posterior-inferiorly, resulting in increased acetabular cup anteversion to accommodate femoral flexion. A fused spine and associated fixed acetabulum can result in abnormal pelvic femoral motion, impingement, and dislocation. Classifying the spinopelvic mechanics by sacral motion based on sitting and standing lateral radiographs provides an understanding of how the acetabulum behaves in space. This information helps appropriate cup positioning, reducing the risk of femoral side impingement and subsequent dislocation.
Summary
Surgical techniques to consider in the spinopelvic at-risk patient are positioning considerations in acetabular cup inclination and anteversion, high offset femoral stems, high offset acetabular liners, dual mobility articulations, and removal of impinging structures. Future research is needed to define the safest order of operation in concomitant hip and spine pathology, the effects on pelvic femoral biomechanics in spine surgery, and whether preoperative and intraoperative management strategies have a long-term beneficial effect on the dislocation rate.
Keywords: Spinopelvic motion, total hip arthroplasty instability, Ante-inclination, Sacro-acetabular angle, Spinopelvic mobility
THA Instability
Total hip arthroplasty has been described as one of the most successful operations of the past century due to decreased pain, increased functional outcomes, and improved quality of life. Even with modern implant technology, contemporary surgical techniques, perioperative pain management, and rapid recovery protocols, complications from total hip arthroplasty (THA) surgery still exist. The most common reason for early revision surgery is prosthetic instability [1].
Traditional risk factors have included those related to surgical technique as well as those related to the individual patient. Surgical approach, appropriate component selection and position, capsular repair, length, and offset restoration are examples of surgery-related factors important for stability. Age, cerebral dysfunction, neuromuscular disease, gender, body habitus, and compliance are examples of patient factors that affect prosthetic stability.
Recently, Wera et al. classified 6 causes for THA instability requiring revision that were identified at the time of the surgery. Acetabular and femoral component malpositioning together were the leading causes with 41% (33% and 8% respectively), abductor deficiency was second at 36%, impingement was 9% of the etiologies, late polyethylene wear was 7%, and unidentifiable factors were another 7% [2]. Other authors have also listed unidentifiable causes of dislocation varying from 7 to 18% [3–5].
The historical Lewinnek safe zone, which had been considered the desired acetabular position for over 30 years, has been shown to be insufficient [6, 7••, 8–10]. Recent evidence has identified an additional etiology of THA instability, especially in late instability development and in people with previous or subsequent spinal fusions [11–15, 16••]. We now know that spinal motion is linked to pelvic motion within a defined biomechanical relationship [17–23, 24••]. Understanding how the spinopelvic interaction works and how to identify, manage, and treat people with hip arthritis and spine pathology will be the goal of this article.
Definitions
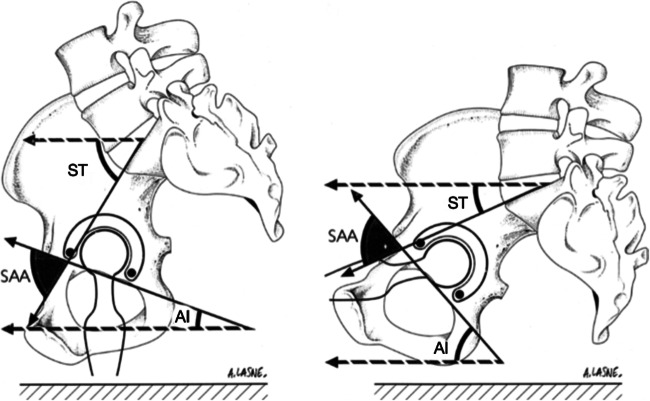
Several spinopelvic parameters have been developed to help understand the biomechanics of this motion (Table 1; Fig. 1). Well established by Legaye et al., pelvic incidence (PI) is a fixed angle measuring from the center of the S1 end plate with one arm perpendicular to the slope of the S1 endplate and the other towards the center of the femoral head on a lateral view of the pelvis showing the lower lumbar spine from L3 down, sacrum, and hip joint and proximal femur. Pelvic tilt (PT) angle uses the same arm from the center of the S1 endplate to the center of the femoral head and then projects vertically [25]. Sacral slope (SS) also known as sacral tilt is the angle between the S1 endplate and a horizontal line drawn perpendicular to the vertical lines used to determine the PI and the PT. These three angles are balanced in the equation: PI = PT + SS. Similarly, Lazennec and Dorr reported a fixed angle called the sacro-acetabular angle (SAA) and its relationship with ante-inclination (AI) also known as sagittal tilt or acetabular tilt (AT) and sacral tilt also known as sacral slope (SS), cumulating in the equation: SAA = AI + SS [17, 19, 26]. Ante-inclination is the horizontal line and a line between the edges of the walls of the acetabulum. What is confusing for readers is that Lazennec originally named this angle acetabular tilt, or sagittal acetabular tilt. Dorr named this angle ante-inclination. We will name this angle ante-inclination to prevent confusion. SS or sacral tilt was previously described, and SAA is the angle formed from the crossing arms of AI and SS. The field of spine surgery usually refers to sacral tilt as sacral slope (SS). Additionally, they typically describe pelvic motion in respect to the pelvis and not the acetabulum. Pelvic anteversion means the pelvis rotates counterclockwise on a lateral radiograph, with the ASIS rotating anteriorly and inferiorly and the coccyx moving posteriorly superiorly.
Table 1.
Spinopelvic radiographic parameters, performed on lateral pelvic radiographs
| Angle | How to measure | Normal values (Lazennec) | Normal values (Dorr) | |
|---|---|---|---|---|
| Pelvic tilt (PT) | Angle from center of femoral head; one arm goes vertical and the other arm goes towards center of S1 sacral endplate | |||
| Sacral slope (SS) or sacral tilt (ST) | Angle from anterior S1 end plate; one arm travels along the tangent of the end plate and the other arm goes horizontal | 40° standing, 20° sitting | 40° ± 10° standing, 20° ± 9° sitting | |
| Pelvic incidence (PI) | Fixed angle from middle of S1 endplate; one arm goes towards center of femoral head and the other arm goes perpendicular to slope of S1 end plate | 56–58° | 53° ± 11° standing or sitting | |
| Ante-inclination (AI) or sagittal tilt | Angle from posterior inferior edge of acetabulum; one arm goes horizontal and the other arm travels to anterior edge. This measures the cup opening angle. | 36–47° standing, 51–58° sitting | 35° ± 10° standing, 52° ± 11° sitting | |
| Sagittal acetabular angle (SAA) | Fixed angle created from slope of S1 end plate and line from posterior inferior acetabulum to anterior edge of acetabulum | 69° | 75° ± 15° | |
| Anterior pelvic plane (APP) | Angle from pubic tubercle; one arm goes towards ASIS and the other arm travels directly vertically. Surrogate for pelvic tilt using computer navigation | |||
| Lumbar lordosis (LL) | Angle between superior L1 endplate and inferior L5 endplate | |||
| Pelvic femoral angle (PFA) | Angle centered over femoral head; one arm goes towards middle of S1 endplate and the other arm travels parallel to proximal femur. | 180° ± 15° standing, 132° ± 12° sitting |
Values from Lazennec JY, Brusson A, Rousseau MA. Hip-spine relations and sagittal balance clinical consequences Eur Spine J. 2011;20 Suppl 5(Suppl 5):686–98 and Ike H, Dorr LD, Trasolini N, Stefl M, McKnight B, Heckmann N. Spine-pelvis-hip relationship in the functioning of a total hip replacement. J Bone Joint Surg Am. 2018 Sep 19;100(18):1606–1615
Fig. 1.
Sitting and standing radiographs. ST: sacral tilt (aka sacral slope), SA: sacro-acetabular angle, AT: acetabular tilt (aka ante-inclination)
Recently, pelvic femoral angle (PFA) has been suggested as an additional measurement representative of motion between these two angles. It may be helpful as impingement and dislocation in spinopelvic motion occurs between the femur and the pelvis, not between the spine and the pelvis. Dorr’s research group has investigated their averages in sitting and standing, suggesting numbers outside of this range may be at risk for impingement and subsequent dislocation (Table 1) [10, 26, 27•]. Lower angles in sitting suggest the femur has to undergo more flexion, with a possibility of anterior impingement and posterior dislocation if ante-inclination is low in sitting. High angles in standing suggest the femur may hyperextend, which places posterior impingement and anterior dislocation if ante-inclination is too high in standing. The combination of PFA and AI is called the combined sagittal index (CSI), also termed the functional safe zone by Tezuka et al. [10, 24••]. They suggested CSI as a replacement for the Lewinneck safe zone, and that unexplained dislocations due to poor acetabular position is likely attributed to femoral motion. One caveat is abnormal CSI has not been validated for etiology of primary THA dislocation because the research is so recent. Most data will be to follow as studies unfold.
Spinopelvic Mechanics and Alignment
Lazennec et al. was one of the first to report that the acetabular position varied based on sitting and supine radiographs of the pelvis [17]. They evaluated 84 healthy patients in sitting, standing radiographs, and by supine computer tomography (CT) scans to define acetabular anteversion and how pelvic position in sitting and standing affect the anteversion. They reported that in standing, the pelvis is tilted anteriorly when looking at the patient in the frontal plane, due to the increased curvature of the lumbar lordosis. What this means is that if one is looking at a lateral radiograph with the sacrum and spine on the right-hand side, the pelvis rotates in a counterclockwise manner in standing with the coccyx moving more posteriorly and superiorly with standing and in a clockwise manner with the coccyx moving more anterior-inferiorly with sitting. The sacral slope is thus increased in the standing position and is decreased in the sitting position (Figs. 1 and 2 ). On average, this reduction in sacral slope from standing to sitting is about 20°. The reduction of sacral slope in sitting allows the femur to clear the pelvis and avoid impingement. At the same time, acetabular anteversion increases in sitting and the acetabular tilt, in the sagittal plane, increases in sitting by close to the same 20° (on average 17°) of change in sacral slope, to accommodate the position of the femur in the sitting position. If the sacral slope is changed from its normal in the standing position of around 40°, either due to spinal disease or spinal surgery such as spinal fusion or if the normal decrease in sacral slope in sitting is not possible for the same reasons, then the hip joint has to absorb more of the overall spinopelvic motion and can possibly lead to a dislocation even when the acetabular component is well-positioned according to the Lewennick safe zone criteria. They furthered this work by investigating behavior of the static pelvis in different positions utilizing CT as well as EOS with regard to total hip prostheses [18–21].
Fig. 2.
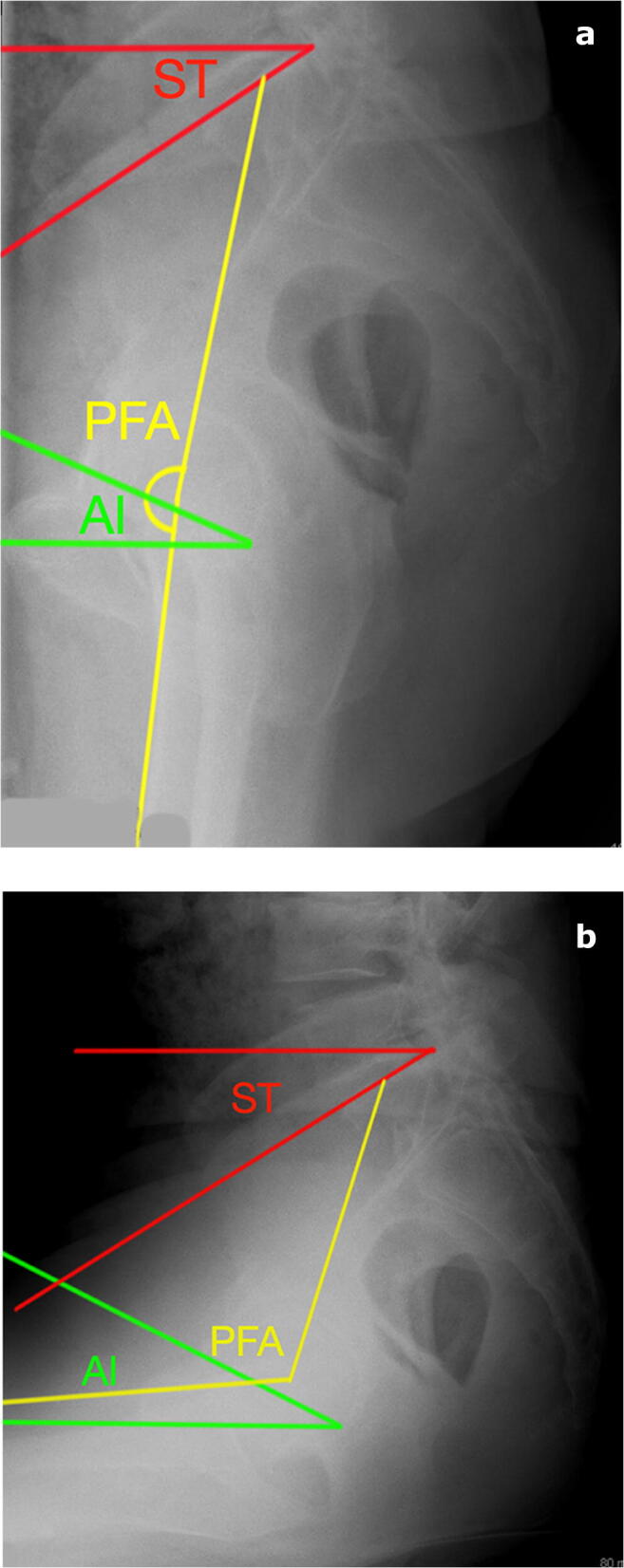
a, b Lateral radiographs of a stiff pelvis patient in a standing and b sitting positions. Notice the sacral tilt and ante-inclination do not change. Pelvic femoral angle changes in sitting and standing, which can result in impingement and dislocation if acetabular component position is improper
Dorr’s work on total hip computer navigation revealed a quantifiable link between sacral motion, acetabular anteversion, and subsequently spinopelvic motion. Initially reported by Lembeck et al. and confirmed by Zhu et al. [27•, 28, 29], 1 degree of decreased sacral slope increases acetabular anteversion by 0.7 degrees. This was implicated for computer navigated total hip arthroplasty, as acetabular anteversion was calculated utilizing a surrogate marker of the pelvis, called the anterior pelvic plane [30, 31]. With strong evidence of a connection between spine lumbar lordosis and sacral motion, solidifying the connection between lumbar lordosis, pelvic tilt, and acetabular cup position was simple [18, 22, 32].
As one goes from standing to sitting, lumbar lordosis decreases, the sacrum and entire pelvis tilts posteriorly with sacrum and coccyx rotating posterior-inferiorly, resulting in increased acetabular cup anteversion to accommodate femoral flexion. If the spinopelvic junction is fused surgically or biologically, the pelvis will move very little, and the acetabular cup is fixed in space. With regard to dynamic movements of standing and sitting, the femur may impinge on the acetabulum depending on where the acetabular cup is positioned in space. If the pelvis and acetabulum are in a fused standing position (anteriorly tilted), when going from standing to sitting, the lumbar lordosis, sacrum, and acetabular cup will not change, the femur must flex more, and therefore may impinge on the anterior acetabulum and subsequently dislocate posteriorly.
Diagnosis of Spinopelvic Abnormalities
The first part to making a diagnosis of a spinopelvic abnormality is to measure the sacral slope on a lateral radiograph with the patient standing upright in a relaxed position with arms folded across the chest and sitting 90 degrees with thighs perpendicular to the trunk with no pressure of the trunk against the back of the chair in a relaxed position. The lateral radiograph should capture at least the L3 lumbar vertebrae (L1 preferred), and also part of the proximal femur. It is more important to understand the dynamics between the acetabulum and the femur than lumbar lordosis, as lordosis will not change if sacral tilt does not change between standing and sitting.
Next, calculate the difference in angles between sitting and standing and identify which behavior the spinopelvic mechanics belongs to (Table 2). Normal spinopelvic motion tends to have between 10 and 30° change in sacral slope from sitting to standing. Hypermobile motion has more than 30°. In both of these classes of pelvic motion, the acetabulum will accommodate femoral flexion by increasing its anteversion. Sacral motion less than 10° between standing and sitting is called the stiff pelvis. The stiff pelvis can be positioned a neutral position, or in varying degrees of anterior or posterior tilt (stuck in standing or stuck in sitting, respectively) which is important as this will dictate cup position. As a general rule of thumb, sacral tilt > 30° in both sitting and standing positions is usually in a stuck standing position, and < 10° sacral tilt is usually in a stuck sitting position. If the sacral motion is < 5°, this is considered surgically or biologically fused. Another important sacral position to note is kyphosis. Patients with a sitting sacral tilt < 5° are deemed kyphotic. These patients may be at risk for posterior bony impingement and subsequent anterior dislocation when standing. Kyphosis is dangerous for 3 reasons: low hip joint flexion ability requiring more posterior tilt of the pelvis in sitting, morbidly obese patients with large trunk mass inducing posterior pelvic tilt, and patients with neuromuscular imbalance. Kyphosis may result in posterior impingement and anterior dislocation in standing. In sitting, it may result in overly high ante-inclination angles and lack of inferior mechanical block, and drop out dislocation can occur, where in sitting dislocation can occur.
Table 2.
Spinopelvic classification. Stiff Kyphotic: patients who are kyphotic have a sacral tilt angle less than 5° in sitting. There are 3 types of spinopelvic classes: normal, hypermobile, and stiff. There are 4 types of stiff pelvic: anterior tilt, posterior tilt, fused, and kyphotic
| Spinopelvic motion | Sacral tilt difference | Attributes | Recommendations |
|---|---|---|---|
| Normal | 10–30° |
Anteversion 15–25°, Inclination 35–45°, Combined anteversion 25–45° |
|
| Hypermobile | >30° |
Anteversion 15–20°, Inclination 35–40°, Combined anteversion 25–35° |
|
| Stiff-anterior acetabular tilt | < 10° | Typically standing ST < 30° (stuck standing), may cause anterior impingement, posterior dislocation |
Anteversion 20–25°, Inclination 45–50°, Combined anteversion 35–45° |
| Stiff-posterior acetabular tilt | < 10° | Typically sitting ST > 30° (stuck sitting), may cause posterior impingement, anterior dislocation |
Anteversion 20–25°, Inclination 45–50°, Combined anteversion 35–45° |
| Stiff-kyphotic | < 5° | < 5° sitting, suggests posterior acetabular tilt (stuck sitting) and may result in posterior impingement and anterior dislocation. Also, AI > 75° may risk dislocation |
Anteversion 15–20°, Inclination 35–40°, Combined anteversion 25–35° |
| Stiff-fused | < 5° |
A large amount of work regarding spinopelvic movement has been discovered using a biplanar radiograph imaging system called EOS (EOS Imaging, Paris, France)[12, 19, 20]. Using simultaneous 2D posteroanterior and lateral radiographs, a three-dimensional image is generated with low amounts of radiation [33, 34]. Studies have demonstrated both lateral radiographs and EOS imaging result in similar radiographic measures with no difference between angles in some studies [19]. Lazennec et al. performed both EOS and conventional lateral radiographs on 50 patients with THA and measured spinopelvic parameters. They reported differences up to 1–2° in pelvic and 2–3° in acetabular angles with higher variability in intra- and interobservability for conventional films than for EOS. They concluded EOS could be an alternative to conventional films. Various angles may be measured on conventional lateral radiographs or using EOS imaging with no significant difference in the measurements between the two techniques.
Indications for Evaluation
The concept behind spinopelvic imbalance is a loss of motion at the lumbar spine junction, resulting in compensatory motion at the hip articulation that may result in impingement and dislocation. Based on this principle, patients who may have decreased lumbar spine motion may be at risk. These patients include spondylolisthesis, spondylosis, facet joint arthritis, and degenerative disc disease to include a few. Epidemiology studies have reported risk factors such as older age, male, and Caucasian. Age appears to be variable depending on histologic studies, cadaveric, or radiographic. Overall, a strong majority of incidence studies report age over 70 may have a strong prevalence of lumbar spine pathology [35, 36].
Although some authors advocate obtaining lateral standing and sitting radiographs for all patients, it is important to understand which patients are at most risk. Multiple studies have reported increased instability in spinal fusion patients [16, 37] and revisions for dislocations and late dislocations [24••]. Some suggest patients develop a stiff spine by age 65 years with substantial worsening by age 75 years. Although by the amount of recent publications it may seem that spinopelvic disease is the leading contribution to primary THA dislocations, it is not [24••]. Component malpositioning is still the leading cause of primary THA instability and should be addressed as such [2]. However, for patients who suffer from late dislocations (> 1 year) or dislocations after revision THA, spinopelvic alignment may be a contributing factor for their instability [24••]. Heckmann et al. reported on 20 patients with late instability after THA. After measuring component positioning and spinopelvic parameters, they concluded that spinopelvic abnormalities contributed to 90% of the dislocations, in some combination with component malposition or soft tissue abnormality. Additionally, they briefly reported on 14 acute dislocations (< 1 year) during this same period and only one patient had evidence of spinopelvic abnormalities. If it is an early acute dislocation, consider other more common etiologies.
Management
The approach to managing a patient with spinopelvic abnormalities is first to diagnose them correctly (see previous section, Table 2). If the difference between their standing and sitting sacral tilt is 10° or higher, they do not have spinopelvic stiffness and routine component implantation is sufficient. If the difference is < 10°, additional radiographic measurements must be made to understand how the pelvis, acetabulum, and femur behave between one another in sitting and standing. Radiographic measurements (Table 1) of sacral tilt, sacral slope, pelvic incidence, ante-inclination, sacro-acetabular angle, and pelvic femoral angle can be performed in sitting and standing to further delineate where the acetabulum is in space, and how the femur behaves to accommodate for the lack of spinopelvic motion.
The acetabulum can be categorized into stuck standing, stuck sitting, or neutral. This is based off where the fixed sacral tilt is. Standing results in increased lumbar lordosis, increased sacral tilt, decreased ante-inclination, and thus decreased acetabular anteversion. The formula, SAA = ST (SS) + AI, accounts for this behavior. If the patient has low ante-inclination in sitting, the risk for anterior impingement and posterior dislocation is higher (Figs. 1, 2, 3, and 4). In that same vein of thinking, a posterior tilted acetabulum may result in posterior impingement and anterior dislocation. The key to placing the cup in the correct position is to understand how the pelvis behaves in the dynamic motion between standing and sitting, and to effectively place the cup in a functional impingement-free range of motion (i.e., more anteversion and inclination if pelvis is fixed in stuck standing, and less anteversion if fixed in stuck sitting; note that we did not say retroversion). Obtaining preoperative spinopelvic measurements and monitoring postoperative values may be the next step in understanding how intraoperative techniques can change these values [Ike JBJS 2019].
Fig. 3.
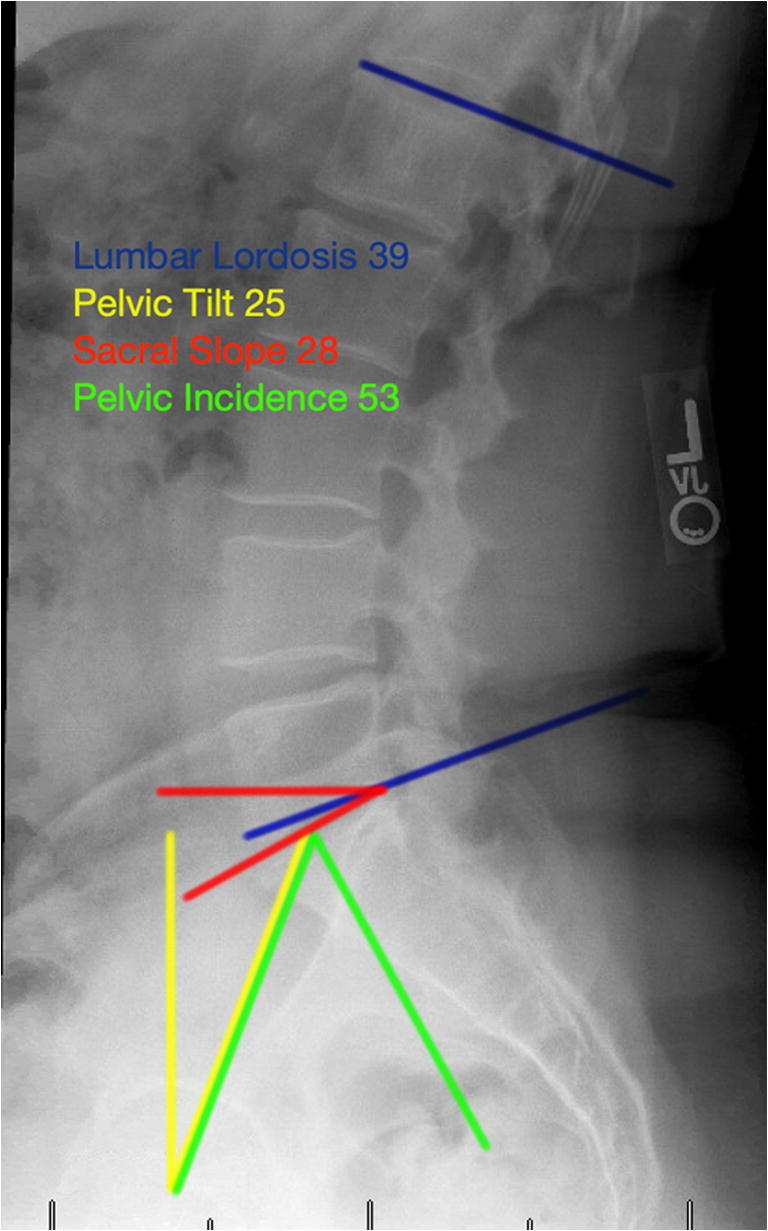
Radiographic measurements for pelvic incidence, sacral slope (aka sacral tilt), pelvic tilt, and lumbar lordosis
Fig. 4.
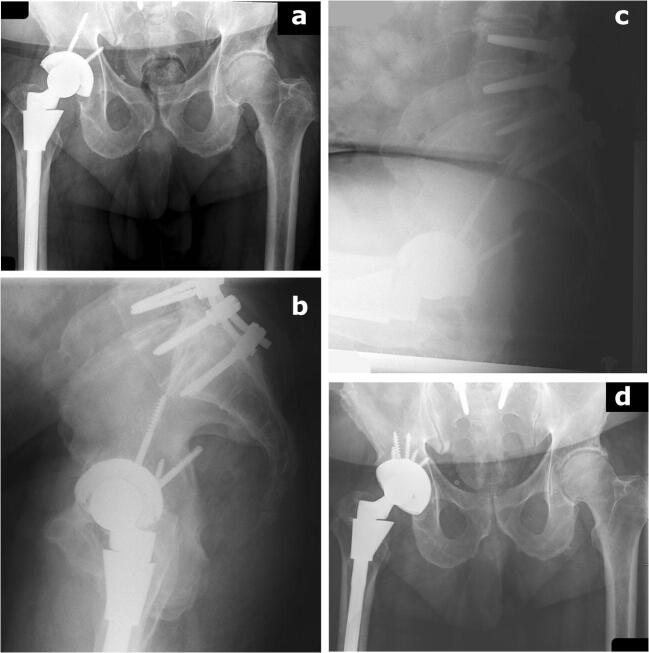
a–d This patient sustained a late dislocation 9 years from his index procedure. In the interim, he underwent spinopelvic fusion. He subsequently dislocated and underwent revision to adjust cup positioning according to his spinopelvic diagnosis, anterior tilt, or “stuck standing”
To re-iterate, a stuck standing position needs more cup anteversion and higher cup abduction. If a cup is too closed, and the pelvis does not rotate properly with sitting, the anterior superior edge of the cup will impinge and will lead to dislocation. This is why the cup abduction angle needs to be increased in this scenario. The opposite is true in a stuck sitting position, or in patients in reduced lumbar kyphosis. In sitting, the position of the pelvis will increase the relative anteversion of the acetabular component and may lead to increased stability in flexion, but in extension, the anteversion will not decrease and the patient will be at risk of anterior dislocation. This is why in such cases, the surgeon needs to reduce the anteversion and close the cup to avoid posterior impingement.
Recommendations for cup implantation are as follows (Table 2):
Normal spinopelvic motion: anteversion 15–25°, inclination 35–45°, combined anteversion 25–45°
Hypermobile spinopelvic motion: anteversion 15–20°, inclination 35–40°, combined anteversion 25–35°
Stiff stuck standing (anterior tilt): anteversion 20–25°, inclination 45–50°, combined anteversion 35–45°
Stiff stuck sitting (posterior tilt): anteversion 20–25°, inclination 45–50°, combined anteversion 35–45°
Stiff kyphotic: anteversion 15–20°, inclination 35–40°, combined anteversion 25–35°
The femoral component is an important factor as impingement must occur first, prior to dislocation. Dorr and colleagues [10] suggested a combined sagittal index measurement to incorporate femoral motion with acetabular cup position. Some authors anecdotally recommend high offset to combat the risk for impingement; however, results have not demonstrated clear benefit with regard to dislocation [38–43]. Robinson, Gerhardt, and Cogan et al. all reported no clinical difference in dislocation rates between patients with low vs high femoral offset. However, these studies were likely underpowered to detect the difference in dislocation rates. Brown, Shoji, and Hayashi suggested in their studies that higher offset may provide a greater ROM until impingement studies that without deleterious effects. Although controversial, higher femoral offset may lower the risk for impingement. More studies looking at this role are needed.
While time and more research will elucidate whether spinal fusion or THA should be performed first in patients with concurrent disease, Malkani et al. [14] suggested that patients who underwent THA first and spinal fusion second at 1–2 years after had less dislocations than patients who had spinal fusion first and THA second. Similarly, time and research will provide a better understanding of the success of these management concepts. We have yet to validate these strategies, because there has not been enough time, and the current dislocation rate is low by modern standards.
Treatment Options and Outcomes
Currently, there are several surgical options for managing a patient with spinopelvic abnormalities.
The concept of constrained liners is to physically prevent the femoral head from dissociating with the acetabular cup by placement of a locking mechanism incorporated into the elevated acetabular liner. Usually identified radiographically by a metal ring around the head, this captured device requires more force to dislocate the femoral head by more than 7 times an unconstrained liner [44]. Hernigou et al. reported good success with 97% survivorship at 7 years [45], while Berend et al. initially reported 98.8% survivorship at 6 months, but dropped to 51.7% at 10.7 years follow up [46]. The main problem with constrained liners is the subsequent mid- to long-term failure rate, and theoretically, these devices result in earlier impingement and loss of functional range of motion. Since spinopelvic abnormalities are due to low functional ROM, we advise against its use in these situations due to lowering range of motion even more.
The dual articulating design originated from Pr. Giles Bousquet and Pr. Andre Rambert in Saint Etienne and Lyon, France, respectively, in the 1970s and 1980s. Complimenting the low-torque friction arthroplasty of the Charnley design with large head stability of the McKee-Farrar design, the bipolar prosthesis underwent many designs until the successful Novae cup (Serf, Decines-Charpieu, France) was finalized [47]. Survivorship for primary THA without risks has been very high with 90–97% early survivorship and 89.2% and 73.9% long-term survivorship at 15 and 22 years, respectively [48, 49]. Specifically for high-risk primary THA patients, several studies have reported less than 1% dislocation rates in small clinical studies and large registry databases [50–52]. Plummer et al. [53] reported on dual mobility in the revision use setting including recurrent instability or infection with an 11% revision rate at 2-year follow up, none for instability. While no studies have specifically investigated dual mobility designs for spinopelvic abnormalities, one study investigated a 39-patient subset of 1046 patients who underwent primary THA who were at extremely high risk for instability including paraplegia, cerebral palsy, neuromuscular disease, abductor insufficiencies, trisomy 21, and other extraordinary instability risk factors [52]. They reported no dislocations at 2-year minimum follow up. This may suggest abnormal spinopelvic parameters may be suitable for dual-mobility constructs. Lastly, complications specific to the dual mobility design such as intra-prosthetic dissociation, backside corrosion wear, or increase polyethylene particle wear should caution the surgeon to be judicious with their use [54, 55]. It should be re-emphasized that a dual-mobility bearing is not an excuse for an in-attentive cup position.
Spinopelvic pathology causes impingement and may cumulate into the worst result: dislocation. To prevent impingement, increasing the distance between both articulating parts of the femur and the acetabulum may help [56–58]. Matsushita et al. performed a cadaveric study investigating varying femoral offset and femoral head size on total hip arthroplasty impingement and dislocation. They concluded increasing offset would delay impingement, while increased head size would only increase jump distance. McGrory et al. evaluated 86 THAs in 64 patients and after checking hip range of motion and abduction strength concluded that greater femoral offset increased both of these measures [58]. Similarly, Innmann found better Harris Hip Scores (HHS) in patients with higher combined acetabular and femoral offset compared with normal or slightly decreased offset, suggesting a higher acetabular and femoral offset may provide some benefit [59]. Not all studies have reproduced this result, and while we understand lowering global offset leads to worse outcomes, increasing offset should be considered with caution as higher femoral offset may lead to more pain [60].
Lastly, when undergoing hip arthroplasty surgery, it is important to consider what type of spinopelvic pathology the patient has and how the acetabulum and femur behave when going through a standard range of motion. Primary total hip arthroplasty in the setting of a stiff spine requiring increased femoral flexion may benefit from a dual mobility articulation or a high offset component. More care must go into evaluating and understanding the revision total hip arthroplasty as spinopelvic abnormalities play a larger role in these patients [24••]. In addition to dual mobility and high offset components, constrained liners may be needed, especially in the setting of abductor insufficiency. An important concept, impingement-free range of motion, will ensure a satisfactory outcome.
Conclusion
Technology, surgical technique, and implant design have dramatically lowered the dislocation rates of total hip arthroplasty. To lower them even more, it is important to understand how the pelvis behaves linked between the spine and the femur. Diagnosis and classification of spinopelvic motion will help the arthroplasty surgeon categorize the position of the cup and femur in sitting and standing positions and help implant these components to achieve an impingement-free range of motion. High offset femoral and acetabular liners, dual-mobility articulations, and constrained liners may lower the dislocation risk.
Compliance with Ethical Standards
Conflict of Interest
Zachary Lum, John Meehan, Mauro Giordani declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, Stiehl JB, Mont MA. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32(5):597–604. doi: 10.1007/s00264-007-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wera GD, Ting NT, Moric M, Paprosky WG, Sporer SM, Della Valle CJ. Classification and management of the unstable total hip arthroplasty. J Arthroplast. 2012;27(5):710–715. doi: 10.1016/j.arth.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295–1306. [PubMed] [Google Scholar]
- 4.Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop Relat Res. 1983;173:151–158. [PubMed] [Google Scholar]
- 5.Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop Relat Res. 1998;355:144–151. doi: 10.1097/00003086-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217–220. [PubMed] [Google Scholar]
- 7.Esposito CI, Gladnick BP, Lee YY, Lyman S, Wright TM, Mayman DJ, Padgett DE. Cup position alone does not predict risk of dislocation after hip arthroplasty. J Arthroplast. 2015;30(1):109–113. doi: 10.1016/j.arth.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2016;474(2):386–391. doi: 10.1007/s11999-015-4432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorr LD, Callaghan JJ. Death of the Lewinnek “safe zone”. J Arthroplast. 2019;34(1):1–2. doi: 10.1016/j.arth.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Tezuka T, Heckmann ND, Bodner RJ, Dorr LD. Functional safe zone is superior to the Lewinnek safe zone for total hip arthroplasty: why the Lewinnek safe zone is not always predictive of stability. J Arthroplast. 2019;34(1):3–8. doi: 10.1016/j.arth.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Salib CG, Reina N, Perry KI, Taunton MJ, Berry DJ, Abdel MP. Lumbar fusion involving the sacrum increases dislocation risk in primary total hip arthroplasty. Bone Joint J. 2019;101-B(2):198–206. doi: 10.1302/0301-620X.101B2.BJJ-2018-0754.R1. [DOI] [PubMed] [Google Scholar]
- 12.DelSole EM, Vigdorchik JM, Schwarzkopf R, Errico TJ, Buckland AJ. Total hip arthroplasty in the spinal deformity population: does degree of sagittal deformity affect rates of safe zone placement, instability, or revision? J Arthroplast. 2017;32(6):1910–1917. doi: 10.1016/j.arth.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Luthringer TA, Vigdorchik JM. A preoperative workup of a “hip-spine” total hip arthroplasty patient: a simplified approach to a complex problem. J Arthroplast. 2019;34(7S):S57–S70. doi: 10.1016/j.arth.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Malkani AL, Himschoot KJ, Ong KL, Lau EC, Baykal D, Dimar JR, Glassman SD, Berry DJ. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates? J Arthroplast. 2019;34(5):907–911. doi: 10.1016/j.arth.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Malkani AL, Garber AT, Ong KL, Dimar JR, Baykal D, Glassman SD, Cochran AR, Berry DJ. Total hip Arthroplasty in patients with previous lumbar fusion surgery: are there more dislocations and revisions? J Arthroplast. 2018;33(4):1189–1193. doi: 10.1016/j.arth.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Buckland AJ, Puvanesarajah V, Vigdorchik J, Schwarzkopf R, Jain A, Klineberg EO, Hart RA, Callaghan JJ, Hassanzadeh H. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99-B(5):585–591. doi: 10.1302/0301-620X.99B5.BJJ-2016-0657.R1. [DOI] [PubMed] [Google Scholar]
- 17.Lazennec JY, Charlot N, Gorin M, Roger B, Arafati N, Bissery A, Saillant G. Hip-spine relationship: a radio-anatomical study for optimization in acetabular cup positioning. Surg Radiol Anat. 2004;26(2):136–144. doi: 10.1007/s00276-003-0195-x. [DOI] [PubMed] [Google Scholar]
- 18.Lazennec JY, Boyer P, Gorin M, Catonné Y, Rousseau MA. Acetabular anteversion with CT in supine, simulated standing, and sitting positions in a THA patient population. Clin Orthop Relat Res. 2011;469(4):1103–1109. doi: 10.1007/s11999-010-1732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazennec JY, Rousseau MA, Rangel A, Gorin M, Belicourt C, Brusson A, Catonné Y. Pelvis and total hip arthroplasty acetabular component orientations in sitting and standing positions: measurements reproductibility with EOS imaging system versus conventional radiographies. Orthop Traumatol Surg Res. 2011;97(4):373–380. doi: 10.1016/j.otsr.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Lazennec JY, Brusson A, Rosseau MA. Hip-spine relations: an innovative paradigm in THR surgery, recent advances in arthroplasty, Dr. Samo Fokter (Ed.) 2012, ISBN: 978–953–307-990-5, InTech, Available from: http://www.intechopen.com/books/recent-advances-in-arthroplasty/hip-spine-relations-an-innovative-paradigm-in-thr-surgery
- 21.Lazennec JY, Riwan A, Gravez F, Rousseau MA, Mora N, Gorin M, Lasne A, Catonne Y, Saillant G. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(Suppl 5):S91–104. [PubMed] [Google Scholar]
- 22.Lum ZC, Coury JG, Cohen JL, Dorr LD. The current knowledge on spinopelvic mobility. J Arthroplast. 2018;33(1):291–296. doi: 10.1016/j.arth.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Kanawade V, Dorr LD, Wan Z. Predictability of acetabular component angular change with postural shift from standing to sitting position. J Bone Joint Surg Am. 2014;96(12):978–986. doi: 10.2106/JBJS.M.00765. [DOI] [PubMed] [Google Scholar]
- 24.•• Heckmann N, Stefl M, Trasolini N, McKnight B, Ike H, Dorr LD. The influence of spinopelvic motion on acute and late dislocation following total hip arthroplasty. J Bone Joint Surg Am. 2018. This study found late dislocations and revision THAs were at higher risk to have spinopelvic abnormalities and impingement and dislocation risk factors.
- 25.Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99–103. doi: 10.1007/s005860050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ike H, Dorr LD, Trasolini N, Stefl M, McKnight B, Heckmann N. Spine-pelvis-hip relationship in the functioning of a total hip replacement. J Bone Joint Surg Am. 2018;100(18):1606–1615. doi: 10.2106/JBJS.17.00403. [DOI] [PubMed] [Google Scholar]
- 27.Stefl M, Lundergan W, Heckmann N, Mcknight B, Ike H, Murgai R, Dorr LD. Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone Joint J. 2017;99-B(1 supple a):37–45. doi: 10.1302/0301-620X.99B1.BJJ-2016-0415.R1. [DOI] [PubMed] [Google Scholar]
- 28.Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005;76(4):517–523. doi: 10.1080/17453670510041501. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Wan Z, Dorr LD. Quantification of pelvic tilt in total hip arthroplasty. Clin Orthop Relat Res. 2010;468(2):571–575. doi: 10.1007/s11999-009-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Z, Malik A, Jaramaz B, Chao L, Dorr LD. Imaging and navigation measurement of acetabular component position in THA. Clin Orthop Relat Res. 2009;467(1):32–42. doi: 10.1007/s11999-008-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaramaz B, DiGioia AM, 3rd, Blackwell M, Nikou C. Computer assisted measurement of cup placement in total hip replacement. Clin Orthop Relat Res. 1998;354:70–81. doi: 10.1097/00003086-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schwab FJ, Blondel B, Bess S, Hostin R, Shaffrey CI, Smith JS, Boachie-Adjei O, Burton DC, Akbarnia BA, Mundis GM, Ames CP, Kebaish K, Hart RA, Farcy JP, Lafage V, International Spine Study Group (ISSG) Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976) 2013;38(13):E803–E812. doi: 10.1097/BRS.0b013e318292b7b9. [DOI] [PubMed] [Google Scholar]
- 33.Luo TD, Stans AA, Schueler BA, Larson AN. Cumulative radiation exposure with EOS imaging compared with standard spine radiographs. Spine Deform. 2015;3(2):144–150. doi: 10.1016/j.jspd.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 34.Lazennec JY, Rousseau MA, Brusson A, Folinais D, Amel M, Clarke I, Pour AE. Total hip prostheses in standing, sitting and squatting positions: an overview of our 8 years practice using the EOS imaging technology. Open Orthop J. 2015;9:26–44. doi: 10.2174/1874325001509010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farfan HF. The pathological anatomy of degenerative spondylolisthesis. A cadaver study. Spine (Phila Pa 1976) 1980;5(5):412–418. doi: 10.1097/00007632-198009000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34(2):199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedard NA, Martin CT, Slaven SE, Pugely AJ, Mendoza-Lattes SA, Callaghan JJ. Abnormally high dislocation rates of total hip arthroplasty after spinal deformity surgery. J Arthroplast. 2016;31(12):2884–2885. doi: 10.1016/j.arth.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 38.Gerhardt DMJM, Bisseling P, de Visser E, van Susante JLC. Modular necks in primary hip arthroplasty without anatomical deformity: no clear benefit on restoration of hip geometry and dislocation rate. An exploratory study. J Arthroplast. 2014;29:1553–1558. doi: 10.1016/j.arth.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Cogan A, Klouche S, Mamoudy P, Sariali E. Total hip arthroplasty dislocation rate following isolated cup revision using Hueter’s direct anterior approach on a fracture table. Orthop Traumatol Surg Res. 2011;97:501–505. doi: 10.1016/j.otsr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Robinson M, Bornstein L, Mennear B, Bostrom M, Nestor B, Padgett D, Westrich G. Effect of restoration of combined offset on stability of large head THA. Hip Int. 2012;22:248–253. doi: 10.5301/HIP.2012.9283. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi S, Nishiyama T, Fujishiro T, Hashimoto S, Kanzaki N, Nishida K, Kuroda R, Kurosaka M. Excessive femoral offset does not affect the range of motion after total hip arthroplasty. Int Orthop. 2013;37(7):1233–1237. doi: 10.1007/s00264-013-1881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoji T, Yamasaki T, Izumi S, Hachisuka S, Ochi M. The influence of stem offset and neck shaft angles on the range of motion in total hip arthroplasty. Int Orthop. 2016;40(2):245–253. doi: 10.1007/s00264-015-2826-3. [DOI] [PubMed] [Google Scholar]
- 43.Brown TD, Elkins JM, Pedersen DR, Callaghan JJ. Impingement and dislocation in total hip arthroplasty: mechanisms and consequences. Iowa Orthop J. 2014;34:1–15. [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard SM, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Design factors influencing performance of constrained acetabular liners: finite element characterization. J Biomech. 2006;39(5):885–893. doi: 10.1016/j.jbiomech.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 45.Hernigou P, Filippini P, Flouzat-Lachaniette CH, Batista SU, Poignard A. Constrained liner in neurologic or cognitively impaired patients undergoing primary THA. Clin Orthop Relat Res. 2010;468:3255–3262. doi: 10.1007/s11999-010-1340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berend KR, Lombardi AV, Jr, Mallory TH, Adams JB, Russell JH, Groseth KL. The long-term outcome of 755 consecutive constrained acetabular components in total hip arthroplasty examining the successes and failures. J Arthroplast. 2005;20(7 suppl 3):93–102. doi: 10.1016/j.arth.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. Survival of cementless dual mobility socket with a mean 17 years follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:e23–e27. doi: 10.1016/j.rco.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2012;36:511–518. doi: 10.1007/s00264-011-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones CW, De Martino I, D'Apolito R, Nocon AA, Sculco PK, Sculco TP. The use of dual-mobility bearings in patients at high risk of dislocation. Bone Joint J. 2019;101-B(1_Supple_A):41–45. doi: 10.1302/0301-620X.101B1.BJJ-2018-0506.R1. [DOI] [PubMed] [Google Scholar]
- 51.Kreipke R, Rogmark C, Pedersen AB, Kärrholm J, Hallan G, Havelin LI, Mäkelä K, Overgaard S. Dual mobility cups: effect on risk of revision of primary total hip arthroplasty due to osteoarthritis: a matched population-based study using the Nordic arthroplasty register association database. J Bone Joint Surg Am. 2019;101(2):169–176. doi: 10.2106/JBJS.17.00841. [DOI] [PubMed] [Google Scholar]
- 52.Kaiser D, Kamath AF, Zingg P, Dora C. Double mobility cup total hip arthroplasty in patients at high risk for dislocation: a single-center analysis. Arch Orthop Trauma Surg. 2015;135(12):1755–1762. doi: 10.1007/s00402-015-2316-5. [DOI] [PubMed] [Google Scholar]
- 53.Plummer DR, Christy JM, Sporer SM, Paprosky WG, Della Valle CJ. Dual-mobility articulations for patients at high risk for dislocation. J Arthroplast. 2016;31(Suppl):131–135. doi: 10.1016/j.arth.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Tarity TD, Koch CN, Burket JC, Wright TM, Westrich GH. Fretting and corrosion at the backside of modular cobalt chromium acetabular inserts: a retrieval analysis. J Arthroplast. 2017;32(3):1033–1039. doi: 10.1016/j.arth.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 55.De Martino I, D'Apolito R, Waddell BS, McLawhorn AS, Sculco PK, Sculco TP. Early intraprosthetic dislocation in dual-mobility implants: a systematic review. Arthroplast Today. 2017;3(3):197–202. doi: 10.1016/j.artd.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89(8):1832–1842. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 57.Matsushita A, Nakashima Y, Jingushi S, Yamamoto T, Kuraoka A, Iwamoto Y. Effects of the femoral offset and the head size on the safe range of motion in total hip arthroplasty. J Arthroplast. 2009;24(4):646–651. doi: 10.1016/j.arth.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 58.McGrory BJ, Morrey BF, Cahalan TD, An KN, Cabanela ME. Effect of femoral offset on range of motion and abductor muscle strength after total hip arthroplasty. J Bone Joint Surg (Br) 1995;77(6):865–869. [PubMed] [Google Scholar]
- 59.Innmann MM, Maier MW, Streit MR, Grammatopoulos G, Bruckner T, Gotterbarm T, Merle C. Additive influence of hip offset and leg length reconstruction on postoperative improvement in clinical outcome after total hip arthroplasty. J Arthroplast. 2018;33(1):156–161. doi: 10.1016/j.arth.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Liebs TR, Nasser L, Herzberg W, Rüther W, Hassenpflug J. The influence of femoral offset on health-related quality of life after total hip replacement. Bone Joint J. 2014;96-B(1):36–42. doi: 10.1302/0301-620X.96B1.31530. [DOI] [PubMed] [Google Scholar]