Abstract
Pediatric radiology departments across the globe face unique challenges in the midst of the current COVID-19 pandemic that have not been addressed in professional guidelines. Providing a safe environment for personnel while continuing to deliver optimal care to patients is feasible when abiding by fundamental recommendations. In this article, we review current infection control practices across the multiple pediatric institutions represented on the Society for Pediatric Radiology (SPR) Quality and Safety committee. We discuss the routes of infectious transmission and appropriate transmission-based precautions, in addition to exploring strategies to optimize personal protective equipment (PPE) supplies. This work serves as a summary of current evidence-based recommendations for infection control, and current best practices specific to pediatric radiologists.
Electronic supplementary material
The online version of this article (10.1007/s00247-020-04713-1) contains supplementary material, which is available to authorized users.
Keywords: Children, COVID-19, Infection control, Pediatric radiology, Personal protective equipment, Safety
Introduction
A cluster of patients with severe viral pneumonia was first described in Wuhan, China, in December 2019. The following month, genome sequencing of the virus isolated from a patient’s lower respiratory tract revealed the pathogen to be a novel coronavirus, now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the disease COVID-19 (coronavirus disease 2019) [1, 2]. Since first described, COVID-19 has spread rapidly across the globe; it was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [2].
As our understanding of COVID-19 evolves, hospitals around the world have been rapidly modifying practice guidelines. Each institution struggles with maintaining the critical balance between resource availability and safety for staff and patients. Pediatric radiology departments are inextricably linked to this struggle because urgent diagnostic imaging and image-guided procedures continue despite reduction in outpatient volume. The goal of the authors in this paper is to review current infection control practices in the literature and online across the multiple institutions that represent the Society for Pediatric Radiology (SPR) Quality and Safety committee. The discussion is informed by current evidence and societal guidelines, though these concepts may change with time. Additional information is available in the Online Supplementary Material regarding examples of institutional practices for personal protective equipment (PPE) usage depending on COVID-19 status, as well as tutorials for donning and doffing PPE.
Put succinctly, the current concern for most pediatric radiologists is this: what level of PPE is required for a mask-off, likely aerosol-generating procedure in a child of uncertain COVID-19 status? The answer is complex and varies based on institutional guidelines and equipment availability. This paper better informs the radiologist’s decision during such an encounter.
Routes of infectious transmission
Infections are commonly transmitted by contact, droplet and airborne routes (Tables 1 and 2) [3, 4]. Contact transmission occurs when infectious organisms are transferred from an infected person to a susceptible individual, either directly through physical contact, or indirectly via contaminated objects (e.g., US transducer, fluoroscopy table, doorknob, computer mouse); susceptible individuals could then inoculate themselves by touching their eyes, nose or mouth with contaminated fingers. Droplet transmission occurs when larger infectious particles (>5 μm) travel from the infected individual to the mucosal surfaces of a susceptible person’s eyes, nose or mouth; droplets might travel in the air as far as 6 ft. Airborne transmission occurs when smaller infectious particles (generally <5 μm), known as aerosols, remain suspended in the air for prolonged periods ranging from minutes to days; these particles might contact mucosal surfaces or be inhaled. Importantly, an organism might be spread by more than one of these routes. For example, there is strong evidence of influenza virus transmission by droplet, airborne and contact modes [5]. These pathogenic particles are absorbed via the respiratory mucosa and potentially across the conjunctivae.
Table 1.
Glossary of common terms and acronyms used in infection control
| Term | Definition |
|---|---|
| Airborne precautions | Precautions for diseases primarily transmitted by aerosolized particles through the air (e.g., tuberculosis, measles) |
| Aerosol transmission | Inhalation of an airborne pathogen through fine (<5 μm) respiratory droplets |
| Aerosol-generating procedure | Procedures that mechanically create and disperse aerosols, such as those that can result in coughing. This might also include the use of support apparatus such as ventilators and nebulizers |
| Contact precautions | Precautions for diseases primarily transmitted by direct patient contact, or with items in a patient’s environment (e.g., multidrug-resistant bacteria, C. difficile) |
| Droplet precautions | Precautions for diseases primarily transmitted by larger particulate droplets (e.g., adenovirus, Streptococcal pneumonia) |
| N95 | A tight-fitting respirator tested to filter at least 95% of very small (0.3 μm) aerosolized particles |
| Powered air purifying respirator (PAPR) | Protects the user by using a blower to filter ambient air through air-purifying elements |
| Personal protective equipment (PPE) | Includes, for example, masks, eye shields, gowns, gloves |
| Person under investigation (PUI) | An individual who has clinical features or epidemiologic risk (e.g., exposure to health care facility or individual with confirmed infection) that warrants further workup for COVID-19 or other infection |
Table 2.
Modes of infection transmission
| Mode of transmission | Description | Example pathogensa |
|---|---|---|
| Contact |
• Infectious organisms transferred from an infected person to a susceptible individual through direct physical contact, or indirectly via contaminated objects • Susceptible individual can self-inoculate by touching own eyes/nose/mouth with contaminated hands |
• Varicella-zoster virus • Norovirus • Respiratory syncytial virus • Methicillin-resistant S. aureus (MRSA) • C. difficile • SARS-CoV-2 |
| Droplet | • Larger infectious particles (>5 μm) generated by coughing or sneezing travel 3–6 ft from the infected individual to the mucosal surfaces of the susceptible person (conjunctivae and nasal/oral mucosa) |
• Adenovirus • Influenza virus • Rhinovirus • M. pneumoniae • SARS-CoV-2 |
| Airborne (aerosol) |
• Infected person generates smaller infectious particles, or aerosols (<5 μm) through coughing, sneezing, talking, exhalation and aerosol-generating procedures • Particles are suspended in air for longer periods of time than larger droplets, and therefore reach susceptible individuals through greater distances and time |
• Measles virus • M. tuberculosis • Varicella-zoster virus • Influenza virus (probable) • Aspergillus • SARS-CoV-2 |
aNote that our current understanding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suggests that the virus might transmit through all of these modes
Both droplets and aerosols can be generated during coughing, sneezing, talking and exhaling, which generates different numbers of respiratory particles. The particle size and infective capacity also varies among these activities. Coughing and sneezing expel a cloud of respiratory particles of many different sizes, ranging from 0.1 μm to greater than 500 μm [5–7].
A sneeze generally contains more particles than a cough [8]. Although particles are somewhat arbitrarily categorized as either aerosols or droplets, their behavior varies along a spectrum. For example, settling times (i.e. the time it takes particulate matter to fall 3 m, or approximately the height of a room) for particles of different diameters are 10 s for 100 μm, 4 min for 20 μm, 17 min for 10 μm, and 62 min for 5 μm [9]. This behavior can be further affected by environmental factors like airflow and humidity [8–10]. Aerosols typically travel longer distances in the air and are more likely to be inhaled deeper in the lungs, while larger droplets are typically trapped in the upper airways [8, 10].
Airflow dynamics of coughing, sneezing, breathing, speaking, toilet flushing and even vomiting have been studied and shown to generate aerosols [5], but there is little available evidence regarding airflow dynamics of many other processes that might be encountered by the pediatric radiologist, such as crying, burping and passing flatus.
Understanding COVID-19
The most common symptoms of COVID-19 include fever, cough, dyspnea, fatigue and myalgia [1, 2]. Patients might also experience headache, loss of smell or taste, nasal congestion and gastrointestinal symptoms (e.g., vomiting, diarrhea) [2, 6]. About 15–29% of affected adults progress to severe pneumonia, adult respiratory distress syndrome (ARDS) and respiratory failure [2, 11]. Reported mortality rates among different countries range 0.8–12.7%, including an estimated 3.6% mortality rate in the United States, though these figures might be inaccurate because there could be a large number of people with the disease who have not been tested [12]. In children, COVID-19 is generally milder than in adults, and gastrointestinal symptoms are more prevalent [13, 14]. As of this writing, the etiology and pathophysiology of the newly identified multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 have not yet been elucidated (https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/children/mis-c.html). Children younger than 18 years account for only 2% of severely affected patients. However, of greater public concern, children might be asymptomatic viral carriers and transmit the disease to more vulnerable individuals [15].
The SARS-CoV-2 virus binds to the angiotensin-converting enzyme-2 (ACE2) receptor, which is abundant in respiratory epithelial cells [16], accounting for the high prevalence of respiratory symptoms in this disorder. Before it reaches the lungs, the virus must first come in contact with mucosal cells in the lips, nasal cavity, or conjunctivae that also express the ACE2 receptor [1]. ACE2 receptors are also expressed in the gastrointestinal tract, which might explain the gastrointestinal symptomatology occurring in 2–10% of patients. This might be of special interest in children in whom gastrointestinal symptoms are more common [13].
Our understanding of the virus is still growing, but early data suggest that SARS-CoV-2 is primarily spread through the respiratory droplets of sick individuals. There is still concern that airborne transmission occurs; data from the University of Nebraska have demonstrated aerosolization of the virus both within and outside the rooms of patients hospitalized with COVID-19 [17]. It is also clear that asymptomatic infection occurs. While it is uncertain to what degree asymptomatic people transmit the virus, these individuals can have high viral loads in their airway [18, 19], and the virus can be recovered from the environment that they inhabit [20, 21]. This potential for airborne transmission of SARS-CoV-2 is particularly concerning for pediatric radiology departments regarding aerosol-generating procedures (discussed later).
Although viral load for COVID-19 is certainly the highest in sputum and upper respiratory secretions, another potential route of transmission is through viral shedding in stool. Several studies demonstrated the presence of viral ribonucleic acid (RNA) in 15–53% of stool samples of COVID-19 patients, with persistence of viral RNA in the stool even after respiratory samples became negative. Furthermore, it was found that stool samples were positive at a higher rate in patients who experienced diarrhea [22–25]. Although viral RNA is present in COVID-19 patients’ stool, feco-oral transmission has not been documented, and there is no convincing evidence of viable pathogenic SARS-CoV-2 particles cultured from these stool samples.
Aerosol-generating procedures
Aerosol-generating medical procedures are increasingly recognized as a source of nosocomial infections that pose risk for health care professionals, particularly in the COVID-19 era. Many procedures performed by radiologists have the potential of inducing aerosol formation by patients either with coughing, or with aerosolization of bowel contents. Aerosol-generating procedures may be classified as: (1) procedures that mechanically create and disperse aerosols and (2) procedures that induce the patient to produce aerosols. The first classification includes nebulizer treatment, suctioning, manual ventilation and noninvasive ventilation (e.g., bilevel positive airway pressure, continuous positive airway pressure, and high-frequency oscillatory ventilation). The second classification includes endotracheal intubation, bronchoscopy, cardiopulmonary resuscitation, and sputum induction (produced by the patient coughing) [26].
Personal protective equipment (PPE) (Table 1)
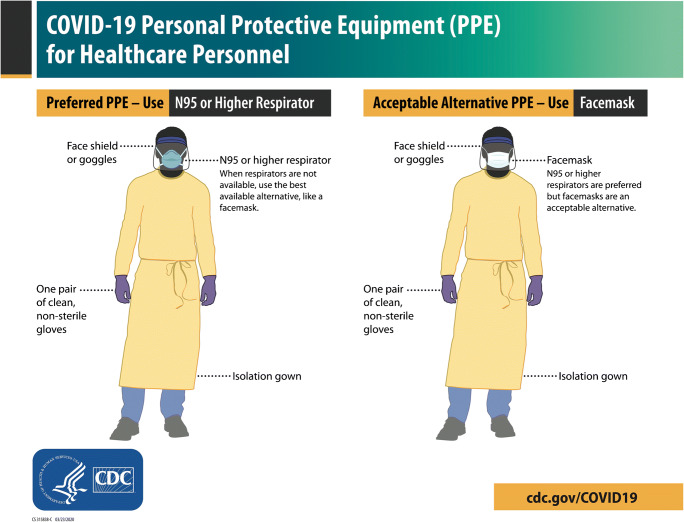
The purpose of wearing PPE is to minimize exposure to hazards that can cause injuries and illnesses in the workplace [27]. The use of PPE should meet standards specifically developed for each exposure risk level of a particular task. In the context of the current COVID-19 pandemic, it is of utmost importance that each workplace prepares for the corresponding levels of exposure defined by the Occupational Safety and Health Administration [28]. In pediatric radiology departments, the risk involved ranges from low (e.g., office workers, remote workers, telemedicine) to very high (e.g., workers performing aerosol-generating procedures on known or suspected COVID-19 patients), depending on the job task assigned [28, 29]. When caring for anyone with confirmed or suspected SARS-CoV-2 infection, health care personnel should adhere to standard and transmission-based precautions [30–32]. The preferred PPE for these COVID-19 precautions includes a face shield or goggles, a N95 or higher respirator, non-sterile gloves and an isolation gown (Fig. 1, Online Supplementary Material 1 and 2) [33].
Fig. 1.
Centers for Disease Control and Prevention (CDC) recommendations for personal protective equipment (PPE) in the context of COVID-19 patient care [33]
Face protection
The U.S. Department of Labor’s Occupational Safety and Health Administration has established the following standards for eye and face protection (these are designated as CFR 1910.133) [34].
Eye protection: Goggles or shields can be used to protect from splashes of blood and body fluids [35, 36]. Eye glasses and contact lenses do not meet requirements for eye protection but may be used underneath goggles or shield [37]. Reusable eye protection should be cleaned and disinfected prior to reuse [38].
Face shields: Face shields are used to protect the facial area and associated mucous membranes, and must cover the front and sides of the face [38, 39]. While there is no current standard for face/eye protection for airborne pathogens, the current recommendations by the Occupational Safety and Health Administration for bloodborne pathogens include “Masks in combination with eye protection devices, such as goggles or glasses with solid side shields, or chin-length face shields” [39, 40]. Face shields have been shown to reduce a respirator’s contamination by 97% and to block 68% of inhalational exposure immediately after a cough (3.4 μm particles at a distance of 18 in.) [41].
Surgical masks: Surgical masks are loose-fitting disposable devices. These masks protect the wearer’s mouth and nose with a physical barrier [42]. Surgical masks are fluid-resistant, and they guard others from the wearer’s respiratory emissions (>2 μm) [43]. These masks also protect against large droplets, splashes and sprays of bodily or other hazardous fluids.
Respirators: Respirators are used to reduce the risk of inhaling hazardous airborne particles, gases or vapors, and should cover at least the nose and mouth [38]. Respirators protect either by removing contaminants from the air or by supplying clean air from a different source [44]. They are certified by the Centers for Disease Control and Prevention (CDC) and the National Institute for Occupational Safety and Health (NIOSH) [45].
N95 respirators: These masks are filtering facepiece respirators (FFR) that efficiently filter out at least 95% of large and small (≥0.3 μm) airborne particles. They fit close to the face and are non-resistant to oil-based aerosols [38, 42, 43, 46]. Of note, most N95 respirators are not manufactured to be used in health care. Prior to patient care, N95 respirators should be fit-tested and seal-checked. The wearer should meet facial hair requirements because N95 masks cannot be used when facial hair comes between the sealing surface of the facepiece and the wearer’s face [38, 45, 47]. The wearer of an N95 should be medically cleared to use a respirator because it could prove hazardous for people with certain breathing conditions [48].
Powered air purifying respirators (PAPRs): Certified by Occupational Safety and Health Administration, PAPRs are battery-powered respirators that use a blower to force filtered ambient air to the inlet covering [45]. In contradistinction to N95 respirators, these are loose-fitting, provide eye protection, do not obscure the mouth, may be used with facial hair, and do not require a fit test. Challenges when using a PAPR might include impeded hearing for the user because of the sound of the fan, pediatric patient apprehension, and decontamination after use [38, 49, 50].
Body and hand protection
Current guidelines do not require gowns to conform to specific standards [51].The choice of gown depends on the risk level for contamination [52]. There should be enough fabric in the gown to wrap around the body and cover the back, even while sitting down or squatting [52]. Isolation gowns and surgical gowns, which are commonly used fluid-resistant and impermeable protective gowns, provide moderate to high barrier protection [51]. Surgical gowns should be prioritized for sterile procedures; disposable isolation gowns are sufficient for most patient encounters in pediatric radiology departments, even with high risk of contamination [51, 53].
Nonsterile disposable patient examination gloves are appropriate when caring for patients with suspected or confirmed COVID-19, similar to all contact precaution encounters [52]. Double gloves are not recommended for caring for COVID-19 patients [52].
Transmission-based precautions (Table 2)
Standard precautions to minimize the spread of infection within health care facilities from direct contact with contaminations include hand hygiene, use of PPE based on anticipated contact with contaminated material, respiratory hygiene/cough etiquette, cleaning and disinfection of the environment, and proper handling of patient care equipment and waste [10]. The WHO and the CDC provide guidelines for transmission-based precautions to be taken for patients with proven or suspected infection with certain pathogens [10, 31]. Transmission-based precautions are based on the mode of transmission of the pathogen and can be categorized as contact, droplet and airborne.
Contact precautions
These precautions are used for infections that can be transmitted through hand-to-hand contact and self-inoculation of nasal mucosa or conjunctiva [10]. Contact precaution measures include patient placement in a single room (if available), limiting the transport and movement of the patient outside the room only for medically necessary purposes, using disposable or dedicated patient-care equipment whenever possible, and frequent cleaning and disinfection of rooms. The appropriate PPE for contact precautions includes gloves and a gown, which must be worn for all interactions with the patient or the patient’s environment. Health care workers should wash their hands and don PPE before entering the room, and discard PPE before exiting and wash hands after doffing gloves.
Droplet precautions
Droplet precautions are used for patients who might be infected with pathogens transmitted via respiratory droplets. To control the source of pathogen spread, the infected patient should wear a surgical mask, be placed in a single room (if available), and instructed to follow respiratory hygiene and cough etiquette (e.g., covering mouth and nose with a tissue when coughing or sneezing, disposing the tissue in the nearest waste bin, and performing frequent hands hygiene). Transport and movement of the patient must be limited to medically necessary purposes. As per CDC recommendations, upon entry into a patient room or space, the health care worker’s eyes, nose and mouth should be covered with appropriate PPE, including a surgical mask and goggles. While recommendations regarding eye protection in the form of goggles or a face shield are still an “unresolved issue” as per the CDC, eye protection should be implemented during procedures and patient care activities that are likely to generate splashes or spray of body fluids or secretions [54].
Airborne precautions
These precautions are appropriate for patients who might be infected with pathogens transmitted by an airborne route, including SARS-CoV2, according to CDC guidelines. Other examples of common airborne infections include tuberculosis, measles and chickenpox. The patient must wear a mask to control the source of infection. The best placement for the patient is an airborne infection isolation room, which is a negative-pressure room with dedicated exhaust. If an airborne infection isolation room is not available, the patient should be placed in a negative-pressure room without dedicated exhaust, or a private room with the doors closed. If transport is necessary, the patient must wear a surgical mask and follow respiratory hygiene and cough etiquette. For health care workers caring for these patients, the CDC recommends a fit-tested N95 or higher-level respirator as PPE. The CDC also recommends restricting susceptible health care personnel from entering the room of the patient, and immunizing susceptible people as soon as possible following unprotected contact (if a vaccine is available for the particular pathogen).
Appropriate personal protective equipment usage stratified by COVID-19 status (Table 3)
Table 3.
Recommended use of personal protective equipment (PPE) stratified by COVID-19 status (based on authors’ multi-institutional experience and understanding as of April 2020)
| COVID-19 status | Recommended PPE |
|---|---|
| COVID-19 positive, confirmed | Respiratora, eye shield, gown and gloves |
|
COVID-19 unknown but patient is symptomatic and has one of the following (test may or may not be sent, presumed positive): • Clinical findings consistent with COVID-19 • Close contact with someone with COVID-19 • Traveled to high-risk area in last 14 days |
Respiratora, eye shield, gown and gloves |
| COVID-19 unknown, test results pending — presumed positive until proved otherwise | Respiratora, eye shield, gown and gloves |
| COVID-19 negative, confirmed | Patient-appropriate PPE — mask, eye shield and gloves common currently, gown as needed (e.g., spray expected) |
| COVID-19 unknown but presumed unlikelyb | Patient-appropriate PPE — mask, eye shield and gloves common currently, gown as needed (e.g., spray expected) — could consider respirator for AGP |
AGP aerosol-generating procedure
aBased on Centers for Disease Control and Prevention recommendations, if a respirator is unavailable a facemask should be worn
bThe categorization of “unlikely” is dependent on epidemiology (community level and patient contacts) and clinical findings
Because of the possibility of airborne transmission of the virus, the CDC recommends respirators for care of all patients with COVID-19 if adequate supplies are available. If respirators are not available, facemasks are a reasonable alternative. In contrast to the CDC guidelines, the WHO calls for airborne precautions only for aerosol-generating procedures. According to CDC guidance and general concepts of infection prevention, use of PPE in pediatric radiology departments should be determined by the principles underlying standard precautions (e.g., a basic risk assessment of the likelihood of contact with infectious material) and transmission-based precautions (e.g., routes of transmission of the proven or suspected pathogens). Because contact with bodily secretions is expected during aerosol-generating procedures, providers should at least wear a gown, gloves, a mask and eye protection.
The conditions of the COVID-19 pandemic demand judicious use of limited PPE supplies. To that end, patients can be stratified into five groups. The group raising highest concern among providers is those with positive reverse transcription polymerase chain reaction (RT-PCR) tests. A second, similar group consists of patients who have not been tested but are symptomatic, and have traveled to a high-risk area in the last 14 days, or have had close contact with a person with COVID-19. The 14-day cut-off is based on the viral incubation period [55]. This group should be presumed and treated as though COVID-19-positive, and testing may or may not be sent for these individuals. Inpatient and emergency department settings might have the capacity for more widespread testing than outpatient environments, and might test mildly symptomatic or asymptomatic patients prior to an aerosol-generating procedure. Once a COVID-19 test has been sent, some consider this a third category, with the term “person under investigation” (PUI) applied. Turnaround time for these tests currently varies from 5 min to a few days. Therefore, patients with pending tests can be treated as presumed COVID-19 positive until test results return [56]. A fourth category is those who have been tested and whose RT-PCR test is negative. Finally, the fifth category is those who are presumed COVID-19-negative, in whom suspicion of COVID-19 is low and for whom no test is sent. Depending on hospital workflow, patients might pass through several of these categories during the course of an encounter.
Providing N95, eye protection, gloves and gowns to health care workers seeing all patients would be reasonable, but is not possible in most cases because of limitations on supplies [57]. Therefore, during this pandemic, PPE should be distributed where it will be most effective at preventing the spread of COVID-19. The highest risk of transmission arises during aerosol-generating procedures, especially those involving airway procedures or support. In the setting of limited PPE, respirators (N95 masks or PAPRs) should be reserved for these procedures, with PAPR used by those who cannot wear an N95. All COVID-19-positive patients need these expanded precautions during aerosolizing procedures. For emergent cases, patients with pending tests or presumed positive patients need similar precautions to those with confirmed disease. For less urgent cases, it might be possible to wait for a COVID-19 test to return.
A more difficult question is how to approach aerosolizing procedures on patients who are either COVID-19-negative or who have not been tested. Many practices require a COVID-19 test be sent prior to performing an aerosol-generating procedure. A provider might want to consider the sensitivity of that test [58] when deciding how heavily to rely on test results for categorizing risk [59, 60]. For example, while many of the laboratory-developed tests have high analytical sensitivity (>90–95%), some automated platforms and point-of-care tests are less sensitive. Clinical sensitivity of any test is difficult to confirm because there is no established gold standard. Ultimately, if the provider is uncomfortable with the possibility of a false-negative test, then the provider should don airborne precaution PPE and perform the aerosol-generating procedure without waiting for test results. Finally, for patients who test COVID-19-negative, standard PPE should be used.
Strategies to optimize personal protective equipment supply
The CDC has published strategies for optimizing the supply of PPE and ventilators, and for managing surge capacity. Three levels of surge capacity are described (Table 4): conventional — no change in normal daily practices; contingency — measures may change daily standard practices, but may not have significant impact on patient care or health care provider safety; and crisis — not commensurate with U.S. standards of care. These measures, alone or in combination, may be necessary during periods of shortages [61, 62].
Table 4.
Strategies to optimize the supply of personal protective equipment (PPE) and other equipment in different surge capacity settings
| Level of surge capacity | Strategy |
|---|---|
| Contingency |
• Use equipment beyond the manufacturer-designated shelf life for fit testing and training (e.g., N95 respirator, gown) • Extended use of equipment • Use of alternate equipment (e.g., cloth gowns, coveralls, equipment meeting international standards) • Selectively cancel elective and non-urgent procedures and appointments for which eye protection is typically required • Shift eye protection supplies from disposable to reusable devices such as goggles and face shields • Remove facemasks in public areas. Restrict facemasks to use by providers only |
| Crisis |
• Use of facemask and eye protection equipment beyond the manufacturer-designated shelf life for health care delivery • Use of equipment approved under standards used in other similar countries • Extended use and reuse of equipment • Selectively cancel elective and non-urgent procedures and appointments for which facemask, gown or eye protection is typically used by the provider • Prioritize use of facemask, gown and eye protection equipment by activity type (use during aerosol-generating procedures or other high-contact patient care activities) • Consider using safety glasses (e.g., trauma glasses) that have extensions to cover the side of the eyes • Reprocess eye protection with effective cleaning methods |
| When no equipment is available |
• Exclude provider at higher risk for severe illness from COVID-19 (e.g., immunocompromised) from contact with known or suspected COVID-19 patients • Designate convalescent provider for provision of care to known or suspected COVID-19 patients • Consider using gown alternatives that have not been evaluated as effective (preferably with long sleeves and closures such as snaps, buttons) • If facemask not available, consider: use of face shield that covers the entire front (extends to the chin or below) and sides of the face with no facemask; use of expedient patient isolation rooms for risk reduction; use of ventilated headboards, and provider use of homemade masks (e.g., bandana, scarf) |
Extended use of PPE is a contingency capacity strategy in which the same PPE is used by one provider when interacting with more than one patient. For respirators, this strategy has been used during previous outbreaks for patients housed in the same location (cohorted). The maximum recommended extended use period is 8–12 h. Reuse (“limited reuse”) of PPE is a crisis capacity strategy in which the same PPE is used by one provider for multiple encounters with different patients, but is removed after each encounter or periodically. For respirators, a maximum of five uses per device is recommended. PPE should be discarded if it is grossly contaminated with patient bodily fluids or if it loses structural integrity. If possible, the CDC proposes a strategy where five respirators are issued to each provider who might be caring for COVID-19 patients. The provider wears one per day, then stores the respirator in a breathable paper bag at the end of shift until the next week, allowing a minimum of five days between each use (the expected survival time of the SARS-CoV2 virus under these conditions is 72 h). A number of other reprocessing or sterilization strategies have been proposed and have been validated to varying extents [63, 64].
The increased demand for PPE and other medical devices has caused a breakdown in the supply chain.
Additive manufacturing (3-D printing) groups are addressing the resultant shortages. The first reported experience during the COVID-19 pandemic was from an Italian engineering team that re-created respirator parts [65]. Different sectors of the additive manufacturing industry have long shared their information through open-source file platforms, expanding their expertise into public and academic spaces, from forums like Thingiverse [66] to the National Institute of Health (NIH) 3-D Printing Exchange [67]. As an example, the 3-D printing team from the radiology department at Children’s Hospital of Philadelphia has partnered with supply chain management to produce or begin development of face shields and goggles, mask ear strap adaptors, PAPR hosing connectors, disposable exhalation ports, and reusable N95 respirators. On a local level, crowdsourced efforts might bring together additive manufacturing laboratories to share files, diversify machine styles and materials, collect limited raw materials, and ramp up production — such that a process that would usually take months, or even years, could be pared down to days or weeks. This could also reduce competition for raw materials in high demand, like thermoplastics and polymers. If distribution of these materials is also hampered by a supply chain breakdown, they could possibly be deemed non-essential and their production halted. In the near future, these efforts could be supported by industry partners with printing farms and large industrial machines.
The speed of production in additive manufacturing is certainly an advantage, but it is essential to consider safety, both in quality control of the processes and in regulatory aspects of the products. A quality-control method entails documentation of manufacturing (e.g., confirming materials, printed files, and resolution) and use to ensure consistent output (e.g., inspecting and fixing burrs, delamination gaps, and cracks). It also establishes checkpoints for inspection and cleaning before each part enters the general supply. These methods are particularly important in efforts to solicit public donations. From a regulatory standpoint, now might be an opportune time to test the boundaries of approved applications like those in Emergency Use Authorization [68]. However, it must be done in a controlled fashion defined by specific conditions (e.g., the FDA Enforcement Discretion policy [69]) to prevent a free-for-all beyond the scope of the situation. Other considerations with additive manufacturing in this setting are: the limited supply of some of the necessary raw materials, such as clear polymers for face shields; and prioritizing design plans that result in products that can be cleaned and are durable enough for reuse.
Appropriate personal protective equipment usage specific to pediatric radiology (Table 5, Online Supplementary Material 3, 4 and 5)
Table 5.
Common pediatric diagnostic and interventional aerosol-generating procedures in which personal protective equipment (PPE) for airborne (aerosol) and contact precautions is recommended (modified guidelines from the Society of Interventional Radiology [70] and authors’ multi-institutional experience and understanding as of April 2020)
| Aerosol-generating proceduresa | Recommended PPE: airborne precautionsb |
|---|---|
|
Procedure itself potentially aerosolizing • Intussusception reduction (either air or liquid contrast) • Nasogastric or nasoenteric tube placements • Scintigraphic ventilation scan • Gastrostomy or gastrojejunostomy tube placements or exchanges • Bronchial stenting |
• Respirator • Eye shield • Gown • Gloves |
|
Procedure with risk of cough and aerosolization • Feeding studies, esophagrams and upper gastrointestinal studies when higher risk of aspiration • Nasogastric or nasoenteric tube placements • Scintigraphic gastric emptying study • Gastrostomy or gastrojejunostomy placements or exchanges • Pleural drain placement or drainage • Lung biopsies • Bronchial stenting | |
|
Airway manipulation and potential aerosolization • Requiring intubation or extubation • Receiving ventilator support that might result in mechanical aerosolizaton • Requiring airway suctioning |
aBold font procedures are commonly performed in diagnostic radiology
bThese recommendations are primarily for patients who are of COVID-19-positive or indeterminate status. If they are COVID-19-negative, then patient-appropriate PPE can be used (e.g., no respirator)
Pediatric radiology staff can be exposed to COVID-19 while performing fluoroscopic or interventional procedures, scintigraphy or exams associated with anesthesia use. For these exams, there is increased risk from direct contact with body fluids, either in droplet or aerosolized form, to unprotected mucous membranes of the eyes, nose or mouth. At many institutions, all patients presenting for a radiology exam from the emergency department or as outpatients receive COVID-19 tests. However, at the time of exam, test results from emergency department patients are often unavailable because test results can take up to 8 days. Despite the lack of evidence-based standards related to radiology procedures in the setting of COVID-19, many evolving practices are similar across the authors’ institutions.
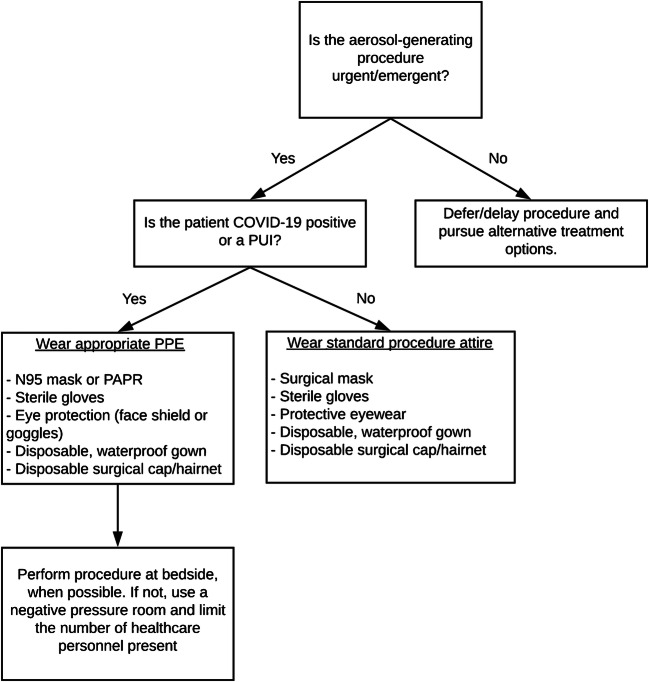
Lists of aerosol-generating procedures have been compiled by professional societies, but none is specific to pediatric radiology (Fig. 2) [70]. Standard aerosol-generating procedures remain undefined for many areas of practice, and the debate continues in the setting of the COVID-19 pandemic. Based on our collective experience, as well as recent guidelines published by the Society of Interventional Radiology (SIR) and Society for Nuclear Medicine and Molecular Imaging (SNMMI), common pediatric fluoroscopic, scintigraphic, and interventional procedures requiring PPE for airborne (aerosol) precautions are described in Table 5. Nasoenteric tube placements and exchanges are common for urgent or emergent fluoroscopic procedures performed in pediatric patients. Both types are considered aerosol-generating because of the potential for sneeze or cough induction. Upper gastrointestinal exams can also lead to aerosol formation in the setting of aspiration and cough. Air enemas for intussusception reduction are typically considered aerosol-generating procedures, given their similarity to lower endoscopic procedures where the colon is insufflated and that they can lead to generation of aerosols containing fecal material while gas is evacuated [71]. Some argue that liquid contrast agent might be safer for intussusception reductions because it might decrease risk of aerosolization compared with droplets. However, given that luminal pressure is still elevated in combination with increased intraabdominal pressure, and that there is evidence that viral shedding in stool may be found 4 weeks after resolution of fever in COVID-19-positive patients [72], many think that aerosolization remains a risk in all intussusception reductions, regardless of contrast agent, because of the risk of spraying fecal material. Discussions about aerosol-generating procedure risk between air- and liquid-contrast intussusception reductions should also incorporate safety profiles, which tend to favor air reductions because of their comparable success rate with lower radiation [73, 74].
Fig. 2.
Proposed triage mechanism for resource allocation for aerosol-generating procedures (reprinted with permission from the Society of Interventional Radiology). PAPR powered air purifying respirator, PPE personal protective equipment, PUI person under investigation
For all aerosol-generating procedures in children who have either unknown or confirmed positive COVID-19 status, radiologists should adhere to the highest level of respiratory protection available: a respirator, an eye shield, a disposable gown and gloves. Additional measures to augment safety might include requiring the child to also wear a mask. Only essential personnel should be present in the fluoroscopy suite during the procedure. If the COVID-19 test is negative, appropriate PPE for the specific patient encounter should be used for aerosol-generating fluoroscopy exams, which might include precautions against viral droplets or spray of bodily fluids (following CDC Standard Precautions philosophy) [32].
Pediatric interventional radiology procedures are often performed under sedation or anesthesia. Accordingly, all such procedures are considered aerosol-generating because of airway manipulation from intubation and airway rescue or suctioning during the exam. Many institutions, such as Seattle Children’s Hospital, require all patients undergoing anesthesia or sedation to have a COVID-19 test performed within 72 h prior to the procedure. For patients with positive COVID-19 test results, the highest level of respiratory protection is required for all health care workers involved throughout the duration of the procedure. For sterile procedures, scrubbed personnel close to the sterile field should use PAPR shrouds to prevent air blown into the sterile field.
In nuclear medicine, ventilation scans use Xenon-133 or, less commonly, aerosolized technetium-99 m-diethylenetriamine pentaacetate (Tc-99 m-DTPA). If a ventilation/perfusion (V/Q) scan is requested, aerosol-generating procedure risk can be mitigated by performing perfusion only [75]. Scintigraphic gastric emptying, esophageal reflux, and salivary gland exams can also induce vomiting or coughing in children, and therefore aerosol-generating procedure precautions might be taken. Because of the length of time required for many scintigraphic exams, patients should wear a mask if possible.
Because of the broad net cast by the SIR in classifying sedated procedures as aerosol-generating procedures [70], further clarifications are warranted regarding the true risks of airborne transmission in what would inherently be a non-aerosol-generating procedure. For example, one might reasonably question whether a sedated voiding cystourethrogram in a child with unknown COVID-19 status should necessitate airborne PPE precautions because of the low risk of airway rescue. While the authors think that many such procedures are not necessarily aerosol-generating procedures because of the low risk of additional airway manipulation and subsequent aerosolization, evidence to support or dispute this rationale has not been established. Such nonurgent examinations are uncommon during this pandemic, but speak to the need to establish clear guidelines around aerosol-generating procedures as outpatient imaging volumes return to normal levels.
For all children undergoing examinations in the radiology department, PPE usage by patients should be consistent with the appropriate level of transmission precautions required for their care, following CDC Standard Precautions [32]. All patients should wear masks and follow basic respiratory hygiene and cough etiquette principles if possible, if they are symptomatic for a viral upper respiratory infection [32]. Wearing a mask might not be possible for children undergoing an aerosol-generating procedure that requires access to nose or mouth (e.g., upper gastrointestinal series or nasogastric tube placement), for infants and young children, or for cognitively impaired children. The accompanying caregiver may be encouraged or required to wear a mask, even when asymptomatic, depending on the particular hospital’s policies. Symptomatic caregivers should be asked to leave and find an asymptomatic caregiver to accompany the child whenever possible. Limiting the number of caregivers in these encounters minimizes the possibility of exposure between an asymptomatic adult carrier of the virus and health care provider.
Risk of exposure is particularly high for technologists, who perform a wide variety of radiology exams across the department and have direct contact with patients. Consequently, radiologists should be sensitive to and supportive of their technologists’ workflow. Technologists should wear the highest level of protection when interacting with emergency department patients who are symptomatic for viral infection, regardless of a verified COVID-19 status. For patients who are asymptomatic, technologists should take respiratory (droplet) precautions (mask and face shield), with or without additional contact precautions (gown and gloves). Technologists also have more interaction with other health care staff while performing portable exams or receiving patient care teams at the scanner. It is important that the PPE worn by radiology technologists is similar to that worn in the patient care environment, with increased protection as necessary depending on the technologist’s task. Similarly, other critical support staff in the radiology department, such as nurses, should adhere to PPE precautions commensurate with each encounter because of their close contact with patients. Of note, the presence of child life specialists to optimize chances for a successful study should be balanced with the need to minimize exposure between staff and patient. The PPE available to radiology staff might be limited by hospital supply chains. Radiologists should advocate for safe PPE for department personnel, as those distributing hospital PPE might have a limited understanding of the varied roles technologists have.
Finally, we return to the initial question posed regarding appropriate PPE usage for the pediatric radiologist about to perform an aerosol-generating procedure on a child with unknown COVID-19 status. A conservative approach, and one that is backed by current CDC guidelines, would recommend that radiologist don airborne and contact transmission precautions, which include a respirator if available, eye protection, gown and gloves. However, droplet and contact precautions — eye protection, surgical mask, gown and gloves — might be a reasonable alternative depending on PPE availability.
Where to find additional resources
Health care providers are faced with an overwhelming amount of data and constantly evolving recommendations regarding the COVID-19 pandemic. It can be challenging to remain current with evolving guidelines while also providing optimal patient care and fulfilling other professional obligations. First and foremost, each radiology department should align with institutional guidelines regarding infection control. Current versions of these materials should be distributed to all radiology personnel. The CDC is also actively adapting PPE recommendations as the situation evolves [76]. Professional societies, including the American Academy of Pediatrics and the Society for Healthcare Epidemiology of America, refer directly to the CDC for guidance on the recommended use of PPE. Health care organizations with early experience in managing COVID-19 patients have also developed extensive policies and protocols, including PPE recommendations, which are available for review. Additional resources and clinical guidelines are provided by the University of Washington Medicine COVID-19 Resource Site [77] and the Brigham and Women’s Hospital [78].
Conclusion
The COVID-19 pandemic has presented an array of unique and daunting challenges, not the least of which is maintaining the safety of health care providers while they care for patients. Although respiratory droplet transmission of the virus is most likely, our current understanding indicates health care providers should nonetheless don a respirator, if available, whenever caring for a COVID-19-positive patient. In pediatric radiology departments, this is particularly true for aerosol-generating procedures that might result in cough or spray of fecal matter, such as intussusception reductions. This precaution also applies to procedures involving intubated and sedated children. Fundamental knowledge of PPE and infectious transmission is crucial for pediatric radiologists as we navigate through this pandemic and enter a world of heightened awareness of infection control.
Electronic supplementary material
Decision tree for PPE usage in patients with confirmed or concern for COVID-19 at Seattle Children’s Hospital (as of April 2020). Please note that the time of this algorithm’s publication, “Lower GI procedure” only referred to gastroenterology procedures with viral ribonucleic acid (RNA) retention in stool in mind, and specifically did not include intussusception reductions. CAPR controlled air-purifying respirator (type of powered respirator), GI gastroenterology, OTO otolaryngology, PAPR powered air-purifying respirator, PPE personal protective equipment (PNG 236 kb)
Modified guidelines for PPE usage in the emergency department, stratified by COVID-19 status, in the Department of Radiology at Children’s Hospital of Philadelphia (as of April 2020). Times needed for room turnover are institution-specific. Consultation of local facility policies is needed to determine appropriate lengths of time at each institution, and depends on facility- and room-specific air exchange rates. CT computed tomography, Fluoro fluoroscopy, IR interventional radiology, MR magnetic resonance imaging, PAPR powered air-purifying respirator, PPE personal protective equipment, US ultrasound, XR radiography (PNG 557 kb)
Guidelines for PPE usage, stratified by COVID-19 status, at Texas Children’s Hospital (as of April 2020). BAL bronchoalveolar lavage, BiPAP bilevel positive airway pressure, CAPR controlled air-purifying respirator (type of powered respirator), CDC Centers for Disease Control and Prevention, CPAP continuous positive airway pressure, CPR cardiopulmonary resuscitation, CPT chest physical therapy, PEP positive expiratory pressure, WHO World Health Organization (PNG 382 kb)
Acknowledgments
The authors wish to thank Lydia Sheldon for her editorial contributions to this manuscript.
Compliance with ethical standards
Conflicts of interest
None
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiatong S, Lanqin L, Wenjun L (2020) COVID-19 epidemic: disease characteristics in children. J Med Virol. 10.1002/jmv.25807 [DOI] [PMC free article] [PubMed]
- 2.Park SE (2020) Epidemiology, virology, and clinical features of severe acute respiratory syndrome — coronavirus-2 (SARS-CoV-2; coronavirus disease-19). Clin Exp Pediatr. 10.3345/cep.2020.00493 [DOI] [PMC free article] [PubMed]
- 3.Mirza SK, Tragon TR, Fukui MB, et al. Microbiology for radiologists: how to minimize infection transmission in the radiology department. Radiographics. 2015;35:1231–1244. doi: 10.1148/rg.2015140034. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (2016) How infections spread. https://www.cdc.gov/infectioncontrol/spread/index.html. Accessed 13 April 2020
- 5.Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57:501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 6.Workman AD, Welling DB, Carter BS et al (2020) Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 10.1002/alr.22577 [DOI] [PubMed]
- 7.Gralton J, Tovey E, McLaws M-L, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Inf Secur. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook TM (2020) Personal protective equipment during the COVID-19 pandemic — a narrative review. Anaesthesia. 10.1111/anae.15071
- 9.Tellier R. Review of aerosol transmission of influenza a virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Liu X, Xiong L, Cai K (2020) Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed]
- 12.Worldometer (2020) Coronavirus update (live): 1,997,666 cases and 126,597 deaths from COVID-19 virus pandemic. https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1? Accessed 15 April 2020
- 13.Han Y-N, Feng Z-W, Sun L-N et al (2020) A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J Med Virol. 10.1002/jmv.25835 [DOI] [PubMed]
- 14.Mallineni SK, Innes NP, Raggio DP et al (2020) Coronavirus disease (COVID-19): characteristics in children and considerations for dentists providing their care. Int J Paediatr Dent. 10.1111/ipd.12653 [DOI] [PMC free article] [PubMed]
- 15.Molloy EJ, Bearer CF (2020) COVID-19 in children and altered inflammatory responses. Pediatr Res. 10.1038/s41390-020-0881-y [DOI] [PubMed]
- 16.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santarpia JL, Rivera DN, Herrera V et al (2020) Transmission potential of SARS-CoV-2 in viral shedding observed at the university of Nebraska medical center. medRxiv. 10.1101/2020.03.23.20039446
- 18.Kam K-Q, Yung CF, Cui L et al (2020) A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 10.1093/cid/ciaa201 [DOI] [PMC free article] [PubMed]
- 19.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility — King County, Washington, march 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriarty LF, Plucinski MM, Marston BJ, et al. Public health responses to COVID-19 outbreaks on cruise ships — worldwide, February-march 2020. MMWR Morb Mortal Wkly Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yung CF, Kam K-Q, Wong MSY et al (2020) Environment and personal protective equipment tests for SARS-CoV-2 in the isolation room of an infant with infection. Ann Intern Med. 10.7326/M20-0942 [DOI] [PMC free article] [PubMed]
- 22.Cai J, Xu J, Lin D et al (2020) A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed]
- 23.Bonato G, Dioscoridi L, Mutignani M (2020) Faecal-oral transmission of SARS-COV-2: practical implications. Gastroenterology. 10.1053/j.gastro.2020.03.066 [DOI] [PMC free article] [PubMed]
- 24.Xiao F, Tang M, Zheng X et al (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed]
- 25.Cheung KS, Hung IF, Chan PP et al (2020) Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed]
- 26.Judson SD, Munster VJ (2019) Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 10.3390/v11100940 [DOI] [PMC free article] [PubMed]
- 27.United States Department of Labor Occupational Safety and Health Administration (2020) Personal protective equipment. https://www.osha.gov/SLTC/personalprotectiveequipment/. Accessed 13 April 2020
- 28.United States Department of Labor Occupational Safety and Health Administration (2020) COVID-19: control and prevention. https://www.osha.gov/SLTC/covid-19/controlprevention.html#healthcare. Accessed 14 April 2020
- 29.United States Department of Labor Occupational Safety and Health Administration (2020) COVID-19: hazard recognition. https://www.osha.gov/SLTC/covid-19/hazardrecognition.html#risk_classification. Accessed 13 April 2020
- 30.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): using PPE. https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html. Accessed 13 April 2020
- 31.Centers for Disease Control and Prevention (2016) Transmission-based precautions. https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html. Accessed 14 April 2020
- 32.Centers for Disease Control and Prevention (2016) Standard precautions for all patient care. https://www.cdc.gov/infectioncontrol/basics/standard-precautions.html. Accessed 14 April 2020
- 33.Centers for Disease Control and Prevention (2020) Use personal protective equipment (PPE) when caring for patients with confirmed or suspected COVID-19. CDC infographic. https://www.cdc.gov/coronavirus/2019-ncov/downloads/A_FS_HCP_COVID19_PPE.pdf. Accessed 6 May 2020
- 34.United States Department of Labor Occupational Safety and Health Administration (2016) Standard number 1910.133 — eye and face protection. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.133. Accessed 13 April 2020
- 35.World Health Organization . Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Geneva: WHO; 2020. [Google Scholar]
- 36.World Health Organization . Infection prevention and control of epidemic — and pandemic-prone acute respiratory infections in health care. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 37.Wisconsin Department of Health Services (2018) Infection control and prevention — personal protective equipment (PPE). https://www.dhs.wisconsin.gov/ic/ppe.htm. Accessed 14 April 2020
- 38.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): infection control guidance: summary of changes to the guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html. Accessed 14 April 2020
- 39.Roberge RJ. Face shields for infection control: a review. J Occup Environ Hyg. 2016;13:235–242. doi: 10.1080/15459624.2015.1095302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United States Department of Labor Occupational Safety and Health Administration (2012) 1910.1030. Toxic and hazardous substances: bloodborne pathogens. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10051&p_table=STANDARDS. Accessed 14 April 2020
- 41.Lindsley WG, Noti JD, Blachere FM, et al. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Food and Drug Administration (2020) N95 respirators and surgical masks (face masks). https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-masks-face-masks. Accessed 13 April 2020
- 43.Paxton NC, Forrestal DP, Desselle M et al (2020) N95 respiratory masks for COVID-19: a review of the literature to inform local responses to global shortages. https://research.qut.edu.au/biofabrication/wp-content/uploads/sites/62/2020/04/N95_COVID-19_LiteratureReview_2020_Submission.pdf. Accessed 6 May 2020
- 44.Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) (2020) Respirators: overview. https://www.cdc.gov/niosh/topics/respirators/. Accessed 14 April 2020
- 45.United States Department of Labor Occupational Safety and Health Administration (2011) 1910.134. Personal protective equipment: respiratory protection. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134. Accessed 14 April 2020
- 46.Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) (1996) NIOSH guide to the selection and use of particulate respirators: certified under 42 CFR 84. https://www.cdc.gov/niosh/docs/96-101/default.html. Accessed 6 May 2020
- 47.Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) (2018) Filtering out confusion: frequently asked questions about respiratory protection — user seal check. https://www.cdc.gov/niosh/docs/2018-130/default.html. Accessed 6 May 2020
- 48.United States Department of Labor Occupational Safety and Health Administration (2020) Transcript for the OSHA training video entitled medical evaluations for workers who use respirators. https://www.osha.gov/video/respiratory_protection/medevaluations_transcript.html. Accessed 17 April 2020
- 49.Centers for Disease Control and Prevention National Institute for Occupational Safety and Health (NIOSH) (2013) Infection control. https://www.cdc.gov/niosh/topics/eye/eye-infectious.html. Accessed 14 April 2020
- 50.Wong J, Goh QY, Tan Z et al (2020) Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 10.1007/s12630-020-01620-9 [DOI] [PMC free article] [PubMed]
- 51.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): gowns. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/isolation-gowns.html. Accessed 14 April 2020
- 52.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): FAQ about PPE: gowns. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirator-use-faq.html. Accessed 14 April 2020
- 53.American National Standard (2012) ANSI/AAMI PB70:2012: liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities. https://my.aami.org/aamiresources/previewfiles/pb70_1206_preview.pdf. Accessed 6 May 2020
- 54.Centers for Disease Control and Prevention (2019) Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings (2007). https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Accessed 17 April 2020
- 55.Ng K, Poon BH, Kiat Puar TH et al (2020) COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 10.7326/L20-0175 [DOI] [PMC free article] [PubMed]
- 56.Ong SWX, Tan YK, Sutjipto S et al (2020) Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol:1–6. 10.1017/ice.2020.91 [DOI] [PMC free article] [PubMed]
- 57.Leung NHL, Chu DKW, Shiu EYC et al (2020) Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed]
- 58.Johns Hopkins Bloomberg School of Public Health Center for Health Security (2020) Serology-based tests for COVID-19. https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html. Accessed 15 April 2020
- 59.Wang W, Xu Y, Gao R et al (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed]
- 60.Kim H, Hong H, Yoon SH (2020) Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 10.1148/radiol.2020201343 [DOI] [PMC free article] [PubMed]
- 61.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): N95 respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html. Accessed 9 April 2020
- 62.Centers for Disease Control and Prevention (2020) Coronavirus disease 2019 (COVID-19): Optimize PPE supply. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Accessed 15 April 2020
- 63.Lin TH, Tseng CC, Huang YL et al (2020) Effectiveness of N95 facepiece respirators in filtering aerosol following storage and sterilization. Aerosol Air Qual 20
- 64.Lauer SA, Grantz KH, Bi Q et al (2020) The incubation period of 2019-nCoV from publicly reported confirmed cases: estimation and application. medRxiv. 10.1101/2020.02.02.20020016 [DOI] [PMC free article] [PubMed]
- 65.3D Printing Media Network (2020) [Updateing] Italian hospital saves Covid-19 patients [sic] lives by 3D printing valves for reanimation devices. https://www.3dprintingmedia.network/covid-19-3d-printed-valve-for-reanimation-device/. Accessed 6 May 2020
- 66.Thingiverse (2020) Challenge 2: face covering. https://www.thingiverse.com/. Accessed 13 April 2020
- 67.National Institutes of Health (2020) NIH 3D print exchange. https://3dprint.nih.gov/. Accessed 13 April 2020
- 68.Food and Drug Administration (2020) Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed 13 April 2020
- 69.Food and Drug Administration (2020) Enforcement policy for ventilators and accessories and other respiratory devices during the coronavirus disease 2019 (COVID-19) public health emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-ventilators-and-accessories-and-other-respiratory-devices-during-coronavirus. Accessed 6 May 2020
- 70.Society of Interventional Radiology (2020) Aerosol generating procedures performed by interventional radiology clinical notification from the Society of Interventional Radiology. https://www.sirweb.org/practice-resources/covid-19-resources/covid-19-clinical-notification-3-26-20/. Accessed 7 April 2020
- 71.Sultan S, Lim JK, Altayar O et al (2020) AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 10.1053/j.gastro.2020.03.072 [DOI] [PMC free article] [PubMed]
- 72.Xing Y-H, Ni W, Wu Q et al (2020) Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 10.1016/j.jmii.2020.03.021 [DOI] [PMC free article] [PubMed]
- 73.Kaplan SL, Magill D, Felice MA, et al. Intussusception reduction: effect of air vs. liquid enema on radiation dose. Pediatr Radiol. 2017;47:1471–1476. doi: 10.1007/s00247-017-3902-1. [DOI] [PubMed] [Google Scholar]
- 74.Sadigh G, Zou KH, Razavi SA, et al. Meta-analysis of air versus liquid enema for intussusception reduction in children. AJR Am J Roentgenol. 2015;205:W542–W549. doi: 10.2214/AJR.14.14060. [DOI] [PubMed] [Google Scholar]
- 75.Society of Nuclear Medicine & Molecular Imaging (2020) COVID-19 and ventilation/perfusion (V/Q) lung studies. https://www.snmmi.org/NewsPublications/NewsDetail.aspx?ItemNumber=33543. Accessed 17 April 2020
- 76.Centers for Disease Control and Prevention (2020) Coronavirus (COVID-19). How to protect yourself. https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 17 April 2020
- 77.The University of Washington Medicine (2020) UW Medicine COVID-19 resource site. https://www.covid-19.uwmedicine.org. Accessed 6 May 2020
- 78.Brigham and Women’s Hospital (2020) Brigham and Women's Hospital COVID-19 clinical guidelines. https://www.covidprotocols.org. Accessed 6 May 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Decision tree for PPE usage in patients with confirmed or concern for COVID-19 at Seattle Children’s Hospital (as of April 2020). Please note that the time of this algorithm’s publication, “Lower GI procedure” only referred to gastroenterology procedures with viral ribonucleic acid (RNA) retention in stool in mind, and specifically did not include intussusception reductions. CAPR controlled air-purifying respirator (type of powered respirator), GI gastroenterology, OTO otolaryngology, PAPR powered air-purifying respirator, PPE personal protective equipment (PNG 236 kb)
Modified guidelines for PPE usage in the emergency department, stratified by COVID-19 status, in the Department of Radiology at Children’s Hospital of Philadelphia (as of April 2020). Times needed for room turnover are institution-specific. Consultation of local facility policies is needed to determine appropriate lengths of time at each institution, and depends on facility- and room-specific air exchange rates. CT computed tomography, Fluoro fluoroscopy, IR interventional radiology, MR magnetic resonance imaging, PAPR powered air-purifying respirator, PPE personal protective equipment, US ultrasound, XR radiography (PNG 557 kb)
Guidelines for PPE usage, stratified by COVID-19 status, at Texas Children’s Hospital (as of April 2020). BAL bronchoalveolar lavage, BiPAP bilevel positive airway pressure, CAPR controlled air-purifying respirator (type of powered respirator), CDC Centers for Disease Control and Prevention, CPAP continuous positive airway pressure, CPR cardiopulmonary resuscitation, CPT chest physical therapy, PEP positive expiratory pressure, WHO World Health Organization (PNG 382 kb)