Abstract
Originating in China during December 2019, the novel corona-virus, SARS-CoV-2, has created mayhem worldwide in a very short time. The outbreak has been so rapid and widespread that the only option to treat the patients was administering drugs already available in the market like chloroquine/hydroxychloroquine (an antimalarial drug) and remedesivir. A large number of patients have been cured but the attribution to survival by these drugs has been controversial. Till date, we do not have any specific drug or vaccine available for COVID-19 and the pandemic seems to be far from over. To handle the current challenges posed by the outbreak effectively, we need to employ innovative interdisciplinary approaches. Organ-on-chip (OOC), particularly lung-on-chip, is one such approach which combines the potential of microfluidics, cell culture and molecular biology into a single miniaturised platform. The device is realized to be capable of simulating in-vivo physiological responses of an organ. In the current study, an OOC, which is a multichannel 3D cell culture microfluidic device, is made via soft lithography technique, using polydimethylsiloxane-polymer and diverse polymeric porous/semipermeable membranes. Several polymer membranes i.e. PDMS, polyvinylidene fluoride (PVDF), nitrocellulose, polyester etc., integrated into the microdevices, were efficiently explored to realize their better cell-adhesion and viability property. We also propose for the application of a simple, smart and cost-effective lung-on-chip platform to study the SARS-CoV-2 pathogenesis in humans, drug toxicity testing and provide insights into antigen–antibody interactions. This platform will enable us to study multiple phenomena at a micro-level generating more reliable data and a better understanding of the underlying mechanisms of SARS-CoV-2 infection and pathogenesis.
Keywords: SARS-CoV-2, COVID-19, Organ-on-chip, Lung-on-chip, Microfluidics, 3D-cell culture platform, Cell-adhesion, Polymeric-semipermeable-membrane
Introduction
On March 11, 2020, the WHO declared COVID-19 a pandemic considering the global apocalyptic effects of this disease. Although the scourge of this pandemic did not spare any nation, the top ten among most severely affected countries includes USA, Brazil, Russia, United Kingdom, Italy, Spain, India, Germany, France and Peru. As per the May 31, 2020 situation report of WHO, the total number of cases and deaths worldwide are 5,934,936 and 367,166, respectively.
Presently, no drug or vaccine for this disease is available. However, several potential drug and vaccine candidates are in various stages of clinical trials. Nevertheless, considering the lengthy procedures of conventional drug discovery pipeline it would take substantial time before these drugs reach the market. The rate at which COVID-19 spread across the globe has been alarming, and based on the initial information about the pathogenesis the only option was to administer existing drugs approved for other infectious diseases; however, their usage for COVID-19 has been controversial because of the associated side effects.
As the pandemic continues to spread, some important queries are imminent. Firstly, can existing drugs be repurposed for effective treatment of COVID-19? If so, how their efficacy can be evaluated rapidly? Secondly, what alternative options can be employed to reduce the time of clinical trials by rapid drug testing and approval?
The current need is a screening platform that can provide reliable results in less time. The organ-on-chip technology (OOC) is one such alternative. Though the field is still in its infancy, it has tremendous potential to revamp the conventional drug discovery pipeline. The OOC technology emerged with the pioneering work by Huh et al. at the Wyss Institute at the Harvard University in 2013 (Huh et al. 2011) in which they described a fabrication protocol for organ-on-chip using polydimethylsiloxane (PDMS). Since the inception of the concept, enormous efforts have gone into converting this proof-of-concept into actual working devices which are currently being globally used by researchers with some of the breakthrough results (Zhu et al. 2010; Neuzil 2012). Various startups have been set up as spin-offs from various research institutions across the globe with huge market potential and substantial revenue-earning. In India, however, this promising platform has not been harnessed. In the current pandemic situation, this technology may prove to be a valuable tool for not only for performing drug toxicity testing but may also be used for analysing the SARS-CoV-2 pathogenesis in human.
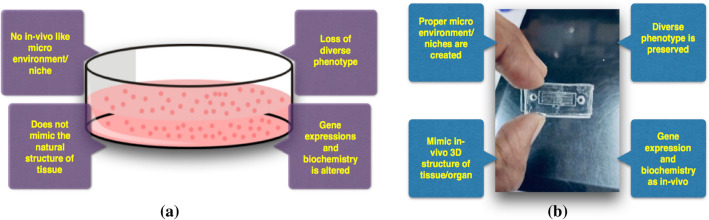
OOCs are a designed network of microfluidic channels intended for mimicking the smallest physiological and biochemical functional units of various organs. The chip dimensions are in the range of a few centimetres with microchannels network in the range of micrometres. These OOCs provide significant advantages over the traditional 2D cell culture (Fig. 1) in petri-plates like: (i) closely mimicking the cell’s natural microenvironment or niche, (ii) minimal requirements of reagents, (iii) options for flexible design to suit experiment needs and (iv) high throughput efficiency.
Fig. 1.
Comparative presentation of a 2D, and b 3D cell culture platfroms
Initially, the primary goal for developing the OOC was to expedite the conventional drug discovery pipeline (Wikswo 2014). In the current drug discovery and development paradigm, it takes about 10 years for a single drug to reach the market. About 90% of the drugs fail at various stages of clinical trial (Kola and Landis 2004). This culminates into severe loss in terms of money and time, and sometimes volunteer’s life. In addition, the present animal testing is deemed inhumane with organisations such as PETA protesting against it. There have also been many incidents where a novel drug performed excellently in animal models but subsequently failed to produce the same expected response in humans. Sometimes, this too resulted in life-threatening repercussions to the human clinical trial candidates (Suntharalingam et al. 2006; Strooper 2014). Opposed to all the above-mentioned drawbacks, OOC being an enclosed 3D cell culture platform mimics the smallest functional unit of an organ hence it provides reliable results as they are capable of generating in-vivo like environment on a chip in a comparatively lesser amount of time. One of the most important advantages is in its ability to recreate the cell microenvironment by precisely controlling the flow rate of fluids using pumping devices, such as syringe pumps. Some of the other pertinent advantages include reduced risk of contamination, resolution up to the single-cell level to study cellular morphology, mechanisms with the scope of parallelisation to study cellular reactions simultaneously. Figure 1 represents the comparison between 2 and 3d cell culture platforms (Halldorsson et al. 2015). Although it would be too early to say that these chips would replace animal testing, they will surely provide a platform to study diseases at the molecular level and for rapid drug screening. This would be a boon to the society in urgent situations such as in current pandemic (Fukumoto and Narasaiah 2013). Our group has been working on organ-on-chip technology that is primarily focussing on lung/liver-on-chip devices and searching for alternate ways of fabrication and application of versatile materials to lower the cost. In the liver-on-chip device, we tried to mimic the hepatic sinusoids which are the site for mixing of the oxygen-rich blood coming from the hepatic artery and the nutrient-rich blood from the portal vein. Further, we performed experiments in the search for the most optimal materials, by replacing the commonly used porous PDMS membrane that will be cell life-supporting, economical and easily accessible membrane to serve as an integral part of OOCs. We conducted a comparative study of cell adhesion on various easily available membranes to examine parameters of cell adhesion and viability properties. We also performed static cultures in the chip to assess for cell viability inside the chip. For this purpose, we conducted studies with human liver cell line HepG2 as they are a good representative model of hepatocytes. Although the ultimate aim of developing this platform is to assess the drug toxicity on the hepatocytes the protocol can be easily adapted to fabricate other organs too.
We illustrate the application of ‘Lung-on-chip’, a micro-engineered biomimetic platform to get a deeper insight into the infectious mechanism of the virus on the lung epithelial cells along with the evaluation of the efficacy of drug/vaccine and their toxicity testing. Our fabrication protocol takes inspiration from the protocol mentioned by Huh et al. but we have introduced some major changes in fabrication and materials to lower the cost of developing the chip. In addition, the bonding of membranes with upper and lower channel layers has been customized with a novel membrane-processing method.
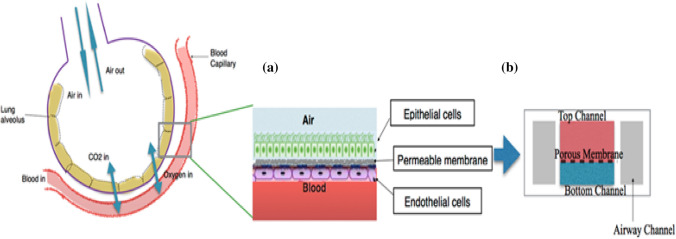
This ‘Lung-on-chip’ platform is basically a 3-dimensional alveolar-capillary interface (Fig. 2), which is the site of gaseous exchange between blood and alveolus (the functional unit of lungs). Under normal conditions, the alveolar membrane and the capillary boundary are close and hence optimal exchange of gases will occur to support life. Patients with a severe infection of COVID-19 develop acute respiratory distress syndrome (ARDS). In this syndrome, the alveolus sacs are filled with fluid and hence the interface between oxygen and blood capillary wall thickens. As a result of the fluid accumulation, the amount of oxygen in the alveoli decreases leading to less oxygen being diffused into the blood-stream. Since there is a decline in the amount of oxygen reaching the organs, the patient dies slowly due to multiple organ failure. Studies have shown that such a microfluidic platform can be effectively utilised to study ARDS (Viola et al. 2019).
Fig. 2.
Illustration showing a lung alveoli and blood capillary interface, and b the proposed lung-on-chip platform aiming to recapitulate this alveoli-capillary interface, as it is the place where the exchange of gases takes place
Recent studies (Chen et al. 2020; Shen et al. 2020) have suggested that the plasma from the patients, who have recovered from COVID-19, can be used to treat patients based on successful results of this therapy during SARS, MERS and EBOLA outbreak. The plasma of recovered patients develops antibody against the virus and these can be transfused to other patient’s body to help fight the infection. However, the FDA has only allowed it to be used as an experimental therapy for only a few critically ill patients (Tanne 2020). One of the major issue associated with this therapy is the risk of mild to severe allergic responses that could even be life-threatening (Roback and Guarner 2020). Utilising this platform we can also study the mechanism of convalescent plasma therapy on a micro-scale by introducing antibody-rich plasma into a virally infested chip. The results so obtained may be of immense importance for designing future therapies. In addition, there is immense flexibility in the design of OOC devices, it can be easily tailored to the needs of a single cell type culture or multiple cell type co-culture on the same chip (Yeo et al. 2011).
Device Design and Fabrication
While designing the ‘lung-on-chip’ device, the critical consideration is that the device assembly must resemble the alveolar-capillary interface as is found in the human lung. Besides, a mechanism to induce the stretching of the membrane should be possible as is the case with the alveolar membranes during breathing.
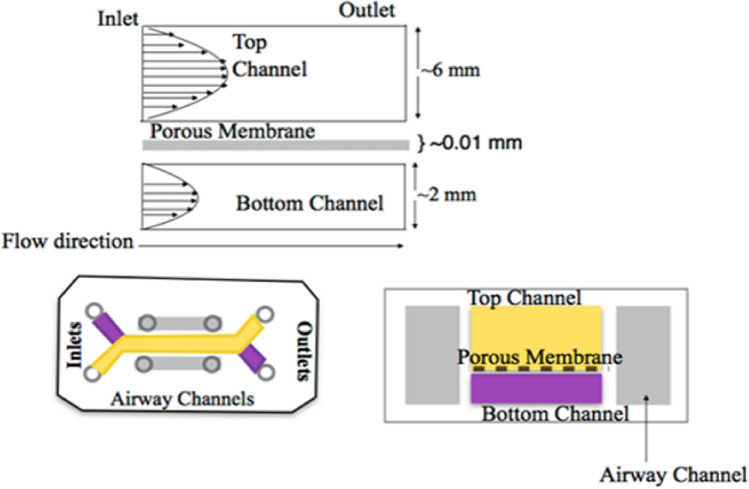
The device consists of three layers: an upper channel layer, lower channel and the side vacuum channels. A 3D microfluidic device replicating the alveolar-capillary interface has been described in Fig. 3. It consists of two channels separated by a porous membrane. In the original practice, a PDMS membrane was used; however, we have also explored the possibilities of other membranes easily available in the laboratory i.e. polyvinylidene fluoride (PVDF), nitrocellulose, polyester etc. The width of channels is selected after considering the average diameter of the human lung-alveolus.
Fig. 3.
Schematic of proposed microdevice presenting the prototype of device and the arrangement of different fluidic layers of the device: the top microchannel has a greater height as compared to the bottom microchannel. Lung epithelial cell lines is cultured on the membrane facing the top channel whereas the endothelial cells is cultured on the side facing bottom channel
Fabrication Steps
Initially, the device UV-mask were designed using AutoCAD and printed on transparency-sheet using the high-resolution printer. Further, the master-moulds of SU-8 polymer were fabricated using the photolithography technique. Later, the upper and the lower channels were fabricated separately using PDMS polymer, via the general soft-lithography technique. Afterwards, the upper and lower channels were bonded together using oxygen plasma, after proper alignment under the microscope, keeping the membrane sandwiched in between the two channels.
The microchannel network (Fig. 3) and its respective geometry and purpose are be defined below:
-
(i)
The upper channel: It is lined with the alveolar lung cells, and oxygen/air-flow is introduced through the channel. This channel has a greater depth as compared to the lower channel.
-
(ii)
The porous membrane: It is the membranous-interface, from where the diffusion of nutrients and waste material takes place, as well as, it is also the site for the cell–cell interactions. In general, this membrane needs to be very thin and stretchable. The porous membrane is present between the upper and lower cell culture channels.
-
(iii)
The lower channel: It represents the endothelial lining of the blood vessels that exchanges oxygen from alveolus in return of carbon dioxide.
-
(iv)
Side vacuum channels are for inducing relaxation and contraction as experienced by the human lung.
Result and Discussion
The above-mentioned fabrication process has resulted in the development of an efficient device with appropriate geometry and dimension, having ideal microchannel bonding strength. Both upper and lower channel is made of PDMS (polydimethylsiloxane) which is a transparent biocompatible polymer, along with polymer membrane, which is observed to be permeable to life-supporting gases.
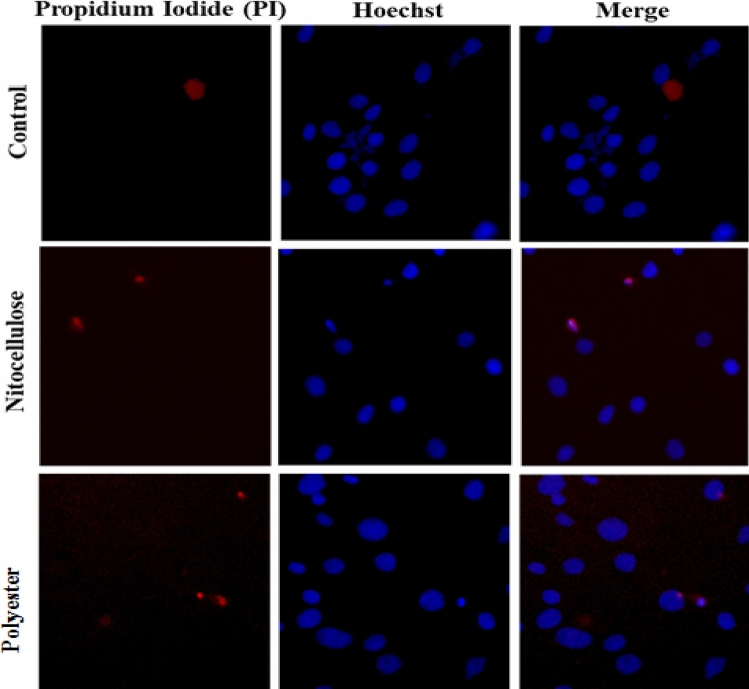
Once the device was fabricated after soft-lithography and bonding; the cells were seeded and immobilised inside the channels at 80% confluence. The cells were regularly observed under the phase-contrast microscope to assess for their normal morphology and health (Fig. 4). Initial results of staining and microscopy observations have shown excellent cell-viability inside the channels through live-dead cell staining.
Fig. 4.
Fluorescence images of HepG2 cells cultured on both nitrocellulose and polyester membrane and stained via PI/Hoechst dual-staining process performed for 1 h at 370 °C. Both nitrocellulose and polyester membranes exhibit cell adhesivity and viability similar to control. Cells were visualized for live/dead cells using live-cell imaging. Hoechst fluorescence images indicate that all cells have a normal healthy and intact nuclei; PI fluorescence images represent dead cells
Materials and Methods
In this section, we describe the overall methods used and intermediate results we obtained till now. We describe our work plan and timeline under the sub-heading ‘timeline envisaged’ for the future work through an illustrative diagram.
Cell Culture
Human hepatocellular carcinoma (HepG2) cells were procured from the National Cell Repository (NCSS, Pune, India) and were cultured and maintained according to the ATCC guidelines. Cells were maintained in DMEM (Sigma-Aldrich, Germany) supplemented with 10% fetal bovine serum (FBS) (PAN Biotech, Germany), 100 µg/ml of penicillin, streptomycin, and 0.25 μg/ml amphotericin. Cells were grown and maintained under conditions of 5% CO2 with 95% atmospheric air at 37 °C temperature in a humidified incubator.
Cell Viability and Adhesion Analysis Using PI and Hoechst Staining
For propidium iodide (PI) and Hoechst staining cells were seeded in 35 mm plate on both membranes (nitrocellulose and polyester) and control plate and allowed to grow to 60–70% of confluency. After 24 h, the cells were stained with a blue fluorescent nuclear dye Hoechst 33258 (Sigma-Aldrich, 0.25 µg/ml) and red fluorescent dye PI (Thermo Fisher Scientific, 4 µM) for dead cells and incubated for a minimum of 2 h at 37 °C. Subsequently, the cells were rinsed with PBS, and fresh media was added. Cells were observed for live-dead cells under a Nikon upright fluorescence microscope with water immersion objectives (model Evolution VF, Media Cybernetics, USA) microscope, and images were taken at 40 × magnification.
Intermediate Results
Cell Viability and Adhesion Analysis
In our experimental study, we envisage examining some of the cost-effective and commonly available synthetic biopolymer membranes as a substitute to the porous/semi-permeable PDMS polymer membrane that works similar to it in our OOC device and exhibits better cell-adhesion and viability. These membranes are also promising because they are chemically and mechanically stable, and biologically inert. In addition, its porous structure allows it for the exchange of biomolecules. In our study, we analysed the effect of nitrocellulose and the polyester membrane. These membranes were tested for its ability to support cell viability and adhesivity through dual staining with PI and Hoechst for live/dead cell nuclei using HepG2 cells as a cell model for hepatocytes followed by live-cell microscopy. Strikingly, we observed that both the membranes were efficient for adhesion of cells, and cells remained viable even after 24 h. In both membranes, high cell viability was maintained similarly to the control as shown in Fig. 4. Hoechst staining revealed that all cells have intact and evenly shaped nuclei. In terms of the PI staining, images displayed non-apoptotic changes in cells. This outcome confirms that both membranes are suitable for cell adhesivity and viability and might serve as promising candidates for the porous/semi-permeable polymeric membranes in our OCC device.
Based on the results obtained during this study and based on available literature we can assert that these membranes will also serve as excellent substrates for the cells lines used for the lung-on-chip device. Li et al. (2013) performed a study concluding that nitrocellulose membrane displays excellent cytocompatibility of different cell lines on nitrocellulose membrane for tissue culture. Due to its affinity towards membrane proteins, nitrocellulose membrane provides essential support for cell adhesion. Apart from being porous, this membrane can be rendered transparent enabling easy visibility of cells under microscopes. Hanke et al. (2012) in their elegant study demonstrated that excellent and immediate cell adhesion on both sides of the permeable polyester membrane by simple surface enhancement techniques of dielectrophoresis and electrostatic forces enabling a quick cell adhesion, even against gravity. Therefore, both these membrane can effectively be utilised for our intended lung-on-chip platform.
Proposed Technology Innovation
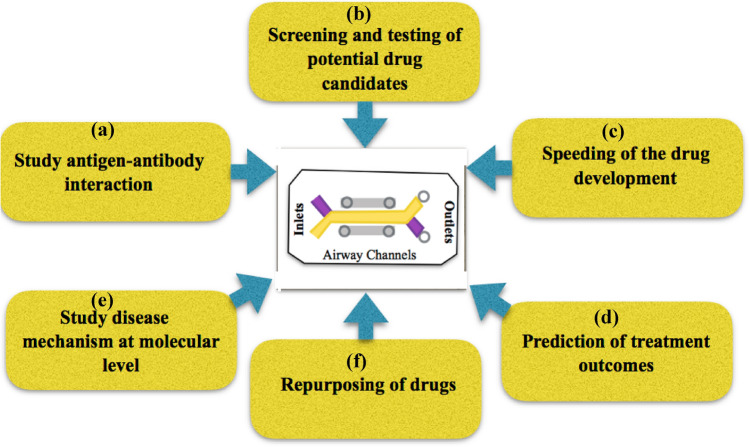
The technology innovation supports advanced in-depth insights concerning the COVID-19 and lung-cell interactions, requisite biomarker and transcription of various factors that need to be studied including Enzyme-linked immunosorbent assay (ELISA) for IL-6 and IL-8, mucus secretion, cell surface P-gp expression, sodium fluorescein permeation etc. The major goals of our study have been illustrated in Fig. 5.
Fig. 5.
Schematic for our objective of the proposed study. The major goals include: a studying antigen–antibody interaction, and b screening of the potential drug candidates for toxicity, which will aid in c speeding of drug development. d Prediction of any outcomes that may arise as a result of drug treatment. Further, it will be possible to e study disease mechanisms at micro levels which would give us useful insights in fighting the disease. f Other drugs that are available in the market and show potential to fight covid-19 can be tested and repurposed accordingly
There is immense flexibility in the plan of the proposed microfluidic device, as it can be customized, according to the specific needs of individual cell types and cell co-cultures, and can be implemented on the same chip. The reported ‘lung-on-chip’ devices closely mimic the cell's natural microenvironment; for example, by continuous culture perfusion or by creating chemical gradients. Further, there are possibilities of analysing low numbers of cells or single cells in high temporal and/or spatial resolution via automation, parallelization, on-chip analysis, as well as direct coupling to downstream analytical chemistry platforms. One of the major issue associated with drugs is hepatotoxicity, and hence by combining this platform with the liver-on-chip platform already developed by us, we can get a deeper insight into the pharmacokinetics of the drug.
Resource Envisaged (Materials)
This research documented herein requires an interdisciplinary approach as it requires the expertise of researchers working in the domain of microfluidics, mammalian cell culture and virology. To fabricate the chip using photolithography, appropriate consumable fabrication-polymer materials i.e. biocompatible polymer poly-dimethyl siloxane (PDMS) and SU-8 photoresist; however, other low-cost biocompatible polymers may also be explored. The basic laminar hood is essential to ensure the contamination-free fabrication of the cell-impregnated chip. The essential equipment set-up exclusively used for producing such contamination-free micro-devices, includes desiccator, centrifugal mixer, spin-coater, UV-curer, hot plate, hot air oven, plasma system, etc. In addition, while performing dynamic cell culturing, syringe-pumps and peristaltic-pumps would be required for continuous controlled flow of fluids at precise and very low flow rate. The entire chip can be moved from one place to another without any concern, ensuring that the surface remains contamination-free. For this purpose, the device needs to be packed suitably and kept in an appropriate thermally controlled atmosphere to maintain the cell viability.
To mimic the alveolar-capillary interface, Calu-3 (cultured in Dulbecco's Modified Eagle's medium: F-12 supplemented with 10% FBS, 1% l-glutamine, 1% penicillin and 1% non-essential amino acids) and EGM-2MV human lung microvascular endothelial cell line will be required, along with other reagents such as the specific culture media, buffers, antibiotics, antimycotic solutions, PBS, FBS, trypsin. The types of equipment present in a standard mammalian cell culture lab are sufficient to transfer and incubate cells inside the device. To study the disease model and pathophysiology of COVID-19, the cells would have to be infested with the isolated virus. As this is potentially hazardous, therefore, BSL-3 and BSL-3 plus labs would be required to perform these studies. The Government of India has approved 126 labs, with BSL-3 and BSL-3 plus, across the country for COVID-19 testing and conducting research. Because the devices are portable, these can easily be transferred to distant laboratories for carrying out the requisite tests.
Work Plan and Timeline Envisaged
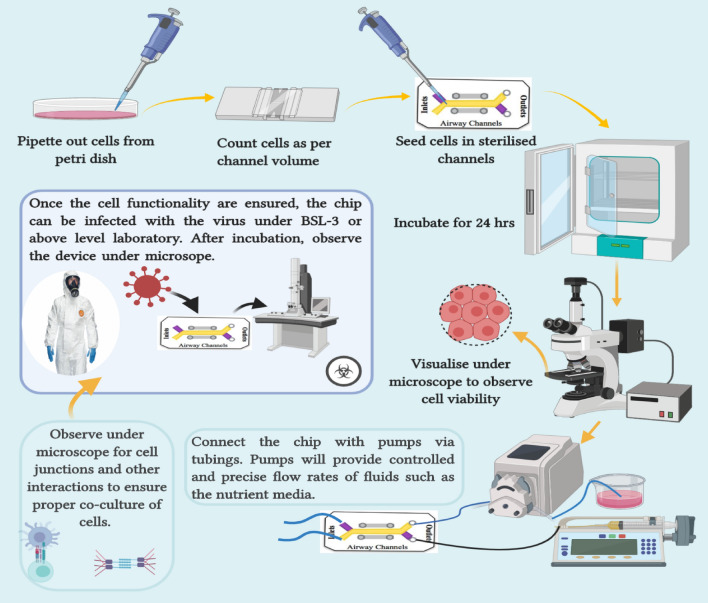
Figure 6 explains the entire work plan in a nutshell. The proposed study can be divided into three main stages:
- Design, fabrication and rapid prototyping of the device (15–30 days): The devices are proposed to be fabricated via the soft-lithography process. To begin with, the channels would be designed using the AutoCAD software. The mask would then be printed on transparent sheets with the help of high-resolution inkjet printer and would be left to dry in sterile conditions and cut in the appropriate size. Device fabrication will be done using optimised cost-effective photolithography process which does not necessarily require a clean-room facility. All the steps are performed under a laminar hood.
-
(i)SU-8 master fabrication: The first step in the fabrication is developing a master using SU-8 polymer, which is an epoxy-based negative photoresist.Substrate surface preparation: The glass slides (75 × 25 mm) would be used as the substrate for developing the SU-8 master. Initially, the glass slides will first be cleaned through acetone boiling using sonicator followed with detergent and DI water. Further, wiped with IPA to ensure perfect cleaning. Thermal treatment will be given via heating the slide at 200 °C for 5 min on a hotplateSpin coating: Since our device consists of two channels of varying depths, hence for each channel SU-8 mask will be required. As the thickness of the SU-8 layer varies with the rpm, hence, the final thickness of the microstructure will be defined by adjusting the rotational speed of spin coater chuck.Pre-bake: This is done on a hotplate at two different temperatures. First, at 65 °C to create slow evaporation of the solvent resulting in a uniform coating and enhanced adhesion to the substrate. The second cycle consists of gradually heating the substrate with the SU-8 layer up to 95 °C, to densify the SU-8.Exposure using UV-aligner: Placing the SU-8 polymer-coated glass-slide, beneath a predesigned UV-mask, and exposed with UV-aligner, for a defined period to suitably cross-link the polymer.Post-bake: This step is accountable for increasing the degree of cross-linking in the SU-8 irradiated areas (due to the photoacid generated in the UV exposure step) making it resistant to the action of solvents in the developing step. The temperature is applied gradually to reach two specific temperatures: 65 °C followed by 95 °C.Development of the SU-8 microstructure: For the final SU-8 microstructure, it is necessary to remove the non-polymerized SU-8. To achieve this, the microstructures are immersed in a container with SU-8 Developer from Microchem while continuously shaking the container to give a rigorous wash to the master mould.Rinse and dry: Once all the non-polymerised SU-8 is dissolved in the developer, it is wiped with a clean wipe and rinsed with a stream of DI water.Passivation of the SU-8 master: To make the SU-8 master more durable and resistant to peel off, the silanization process is necessary. To do this, the SU-8 mould is placed in the vacuum desiccator and few drops of APTES (silanizing agent) is placed, in a small open container using a disposable dropper. Further, a vacuum environment is quickly created and the lid of the desiccator was sealed. The vacuum pump is switched off and the setup kept stand like this for an hour so that a passivation monolayer is formed on the SU-8 mould. The process is always done inside the fume hood.
-
(ii)PDMS polymer casting and rapid prototyping:For making the upper and lower channel, first the PDMS elastomer was mixed with few drops of curing agent in the ratio 10:1 (wt/wt) or 9:1 (v/v) and vigorous mixing is done manually or via mixers for around 10 min.Once the mixing is completed, it is kept inside desiccator to remove all the bubble formed as a result of vigorous mixing. The duration of this degasification may range from 15 min to 1 h depending upon the number of bubbles. While degasification is in progress, the hot plate is maintained at 150 °C.The temporary boundary is made around the SU-8 master using aluminium foil, ensuring for no leakage and the bubble-free PDMS prepolymer mixture were poured/cast in it. Pouring/casting should not start from the region where the microstructures are present.The complete PDMS casting arrangement is placed on the hotplate, which is maintained at 150 °C for 10 min and the mould is removed from the hotplate.The foil boundary is removed and the PDMS layer is gently peeled off, and inlets and outlets holes were created at preplanned places using 1.5 mm hole puncher.The membrane to be used in between the channels is incised precisely by observing under a microscope. The width of the membrane should be slightly greater than the channel width and length equal to the length of the channel.The membrane is treated with corona treater for 1 min by doing sweeping motions and it is placed on top of the bottom PDMS layer under a microscope.The top PDMS layer is treated with corona treater for 1 min and it is placed on top of the membrane under a microscope to ensure proper alignment.The whole assembly is kept inside the oven at 60 °C overnight (7–8 h). Further, the assembly is taken out and brought at room temperature, and with a sharp scalpel, the edges of the device are trimmed and cleaned. Finally, it is kept in a clean closed box.
-
(i)
-
Cell seeding and culturing inside the channels (15–30 days):
The cells will be cultured following the standard protocol given by the manufacturer following all measures to avoid contamination. A confluency of 70–80% is required before seeding cells inside the device. The cell seeding density (cells/ml) is always done according to the volume of the channels and the days of continuous incubation of cells in the device. The counting of cells before seeding is done using a haemocytometer.
Before seeding the cells inside the channels, sterility of the channels should be ensured to avoid contamination. To do this, 70% ethanol (v/v) is perfused for 10 min followed by washing with autoclaved DI water. Further, the device is placed under UV light for 5–10 min. Once the device is sterilised completely, the cells are seeded as done routinely. The pipet tip the placed on the inlet of the channel and the suspension is dispensed swiftly. The device with the seeded cells is placed inside the incubator for 24 h. The cells are visualized under the microscope for cell adhesion. If the cells have adhered properly, the inlet/outlet ports of the top channel are closed and the device is reversed to seed cells inside the lower channel via repeating the same step.
To ensure a long term contamination-free health cell culture, the media may be changed every/alternate day depending on confluency.
- Immunological staining for cell viability and functionality testing (15–20 days):
-
(i)Staining with Hoechst and PI:To observe live/dead cells, the cells are stained with Hoescht and PI dye using the abovementioned process applied in the intermediate result section and subsequently observed under the microscope.
-
(ii)Enzyme-linked immunosorbent assay (ELISA) for IL-6 and IL-8:The media collected from the chips will be stored at − 80 °C until experimenting. After thawing them, the levels of secreted IL-6 and IL-8 will be analyzed according to the manufacturer’s instructions, using commercial human IL-6 and IL-8 ELISA kits to find the corresponding absorbance (Shrestha et al. 2020).
-
(iii)Immunofluorescence staining of intercellular junctions:At room temperature, the cells are fixed with 4% volume/volume (v/v) paraformaldehyde in PBS after washing three times with PBS. It is Incubated for 15 min and washed with PBS twice. Follow permeabilization of the cells with 0.3% (v/v) Triton X-100 for 10 min and blocking with 2% (w/v) bovine serum albumin (BSA) in PBS for 2 h. For staining of endothelial junctions, primary VE-Cadherin antibody is diluted in BSA solution at a dilution of 1:100, and introduced into the lower channel. The mixture is then incubated at 4 °C overnight. Antibody solution is removed by flushing the channels with D-PBS three times. Fluorescently labelled goat anti-rabbit antibody is diluted in 1:200 BSA solution and introduced into the lower channel. It is Incubated at room temperature in the dark for 1 h. Antibody solution is removed by flushing the channels with D-PBS three times. The device is now ready for visualization of endothelial adherens junctions using fluorescence microscopy. The whole process is repeated with fluorescently labelled anti-occludin antibody in the upper microchannel to visualize tight-junction formation in the epithelial cells (Huh et al. 2011).
-
(i)
-
Drug efficacy and toxicity testing (10–15 days):
To study the drug efficacy/toxicity of various drug candidates, the primary requirement is to infest the chip with the isolated virus under BSL-3 laboratory. Once the chip is infested, the appropriate dosage of the drug would be given and drug toxicity study would be performed using MTS assay kit according to the manufacturer’s protocol.
-
Studying the mechanism of convalescent plasma therapy:
To study the underlying mechanism and the associated immune responses-like variations in cytokine activity at the cellular level, we will introduce plasma containing the antibody inside the infested channels.
Fig. 6.
Schematic diagram for the overall plan of the proposed work, illustrating the major planned steps in the entire study. Cells from petri dish is seeded inside the device channels (both upper and lower) at specified cell density (cells/ml). The device is then incubated overnight. To check cell viability, cells are stained with fluorescent dyes like Hoechst and PI. Further to perform studies under dynamic conditions, the platform is connected with pumps keepin the floe rates very precise and slow. The media is changed routinely and all assays are performed under controlled flow conditioned
Conclusion
The proposed ‘lung-on-chip’ platform is a simple yet innovative device containing a defined network of microfluidic channels lined by living human cells on the microchannel wall. These may replicate the smallest functional units of organs and organ-level physiology. The protocol followed here can be customised for other tissues or organs, as well, to study the effect of the drug on multiple organs. It is a rapid and low-cost alternative to the lengthy conventional drug testing pipeline that involves animal testing which would undoubtedly be an advantageous study tool in pandemic situations calling for urgent measures. We are in the initial experimental stages with very promising results. With further experimentation and validation, this platform would help tackle a resolve several issues associated with various aspects of COVID-19 resulting in designing therapies that are effective and elicit negligible side effects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- https://www.marketsandmarkets.com/Market-Reports/organs-on-chips-market-144117291.html
- https://www.icmr.gov.in/pdf/covid/labs/COVID_Testing_Labs_03072020.pdf
- Chen L et al (2020) Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 20(4):398–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B (2014) Lessons from a failed γ-secretase Alzheimer trial. Cell 159(4):721–726 [DOI] [PubMed] [Google Scholar]
- Fukumoto J, Narasaiah K (2013) Human lung on a chip: innovative approach for understanding disease processes and effective drug testing. Front Pharmacol 3:205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson S et al (2015) Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 6:218–231 [DOI] [PubMed] [Google Scholar]
- Hanke C, Dittrich PS, Reyes-Hernandez DR (2012) Generating cell co-cultures by rapid cell adhesion on opposite sides of polyester membranes. In: The 16th international conference on miniaturized systems for chemistry and life sciences 1639–1642
- Huh D, Hamilton GA, Ingber DE (2011) Trends Cell Biol 21:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug 3(8):711–716 [DOI] [PubMed] [Google Scholar]
- Li A, Wang Y, Deng L et al (2013) Use of nitrocellulose membranes as a scaffold in cell culture. Cytotechnology 65(1):71–81. 10.1007/s10616-012-9458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil P et al (2012) Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov 11:620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roback JD, Guarner J (2020) Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 323(16):1561–1562 [DOI] [PubMed] [Google Scholar]
- Shen C et al (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha J et al (2020) A rapidly prototyped lung-on-a-chip model using 3D-printed molds. Organs Chip 1:100001 [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD et al (2006) Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355:1018–1028 [DOI] [PubMed] [Google Scholar]
- Tanne JH (2020) Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ 368:m1256 [DOI] [PubMed] [Google Scholar]
- Viola H, Chang J, Grunwell JR, Hecker L, Tirouvanziam R, Grotberg JB, Takayama S (2019) Microphysiological systems modeling acute respiratory distress syndrome that capture mechanical force-induced injury-inflammation-repair. APL Bioeng 3(4):041503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo JP (2014) The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med 239:1061–1072. 10.1177/1535370214542068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation report, 132. World Health Organization. https://apps.who.int/iris/handle/10665/332280
- Yeo LY, Chang HC, Chan PP, Friend JR (2011) Small 7:12–48 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Chidekel A, Shaffer TH (2010) Cultured human airway epithelial cells (calu-3): a model of human respiratory function, structure, and inflammatory responses. Crit Care Res Pract. 2010:394578. 10.1155/2010/394578 [DOI] [PMC free article] [PubMed]