Abstract
Introduction
Hearing and vision loss are independently associated with dementia, but the impact of dual sensory impairment (DSI) on dementia risk is not well understood.
Methods
Self‐reported measures of hearing and vision were taken from 2051 participants at baseline from the Gingko Evaluation of Memory Study. Dementia status was ascertained using standardized criteria. Cox models were used to estimate risk of dementia associated with number of sensory impairments (none, one, or two).
Results
DSI was significantly associated with higher risk of all‐cause dementia (hazard ratio [HR] = 1.86; 95% confidence interval [CI] = 1.25‐2.76) and Alzheimer's disease (HR = 2.12; 95% CI = 1.34‐3.36). Individually only visual impairment was independently associated with an increased risk of all‐cause dementia (HR = 1.32; 95% CI = 1.02‐1.71).
Discussion
Older adults with DSI are at a significantly increased risk for dementia. Further studies are needed to evaluate whether treatments can modify this risk.
Keywords: Alzheimer's disease, dementia, dual sensory impairment (DSI), epidemiology, Gingko Evaluation of Memory Study, hearing, vision
1. INTRODUCTION
Hearing and visual impairments are common in older adults, with an estimated 33% of individuals age 70 years and older affected by hearing loss and 18% affected by vision impairment. 1 , 2 Because the incidence and prevalence of these sensory impairments increase with age, hearing and vision loss will affect a growing proportion of the population. 2 , 3 Sensory impairment is associated with increased risk of mortality and functional difficulties. 4 , 5 , 6 In addition, several prospective studies have found that hearing and visual impairments in older adults independently increase the risk of cognitive impairment and dementia. 7 , 8 , 9 , 10 , 11 Causal effects have been hypothesized due to sensory loss, precipitating social isolation, depression, and reduced physical activity. 12 , 13 Alternatively, sensory and cognitive impairment may share similar disease processes, such as cerebrovascular disease 14 , 15 , 16 or neurodegeneration. 17
Although most prior studies have focused on impairments in hearing and vision individually, the impact of having combined hearing and visual impairment, or dual sensory impairment (DSI), on dementia risk is unclear. In the United States, DSI affects up to 15% of adults, with the highest prevalence among adults age 80 years and older. 18 , 19 Studies examining both hearing and vision found that an increasing number of impairments may have a greater impact on health outcomes and increased mortality than a single sensory deficit. 6 , 20 Compared to the presence of only one impairment, DSI may also increase the risk of cognitive impairment. 20 Other studies, however, found no added risk of cognitive impairment 21 and suggested that sensory impairment in more than one domain may reflect a shared common aging process. 22 , 23 More recent work showed that a greater number of sensory impairments was associated with increasing risk of dementia in a graded fashion, which included a combination of hearing, smell, touch, and vision assessments. 24 Additional research is needed to quantify the effects of concurrent hearing and visual impairment on dementia risk. Few studies have characterized the impact of DSI specifically on dementia risk using a prospective study design with rigorous ascertainment of dementia and its major subtypes: Alzheimer's disease (AD) and vascular dementia (VaD).
In this study, we investigated associations between hearing and visual impairments in late life with incident dementia among participants of the Ginkgo Evaluation of Memory (GEM) Study. It included self‐reported hearing and vision at enrollment plus the rigorous evaluation of dementia and its subtypes during follow‐up in this cohort of older adults, who were either cognitively normal or met criteria for mild cognitive impairment (MCI). We hypothesized that DSI would be associated with an increased risk for dementia as compared to no or a single sensory impairment.
2. METHODS
The GEM Study was originally designed as a double‐blind randomized‐controlled trial to determine the efficacy of Ginkgo biloba in the prevention of dementia among older adults. 25 Although results of the GEM Study were negative, the data serve as a useful platform to address other scientific questions in older adults related to cognition and dementia. The institutional review boards of all universities involved in the study approved the study protocol, as did the National Institutes of Health. All participants and their proxies signed approved informed consent forms.
HIGHLIGHTS
Dual sensory impairment (DSI) increases the risk of dementia, primarily Alzheimer's disease (AD)
Dose‐response relationship between severity of DSI and dementia
Visual impairment, but not hearing impairment, is associated with incident dementia
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the published literature (eg, PubMed) on dual sensory impairment (DSI) and dementia. The impact of DSI on dementia risk is unclear, as most studies examined associations between DSI and cognitive decline or impairment, whereas other studies on DSI and dementia were cross‐sectional. No study has examined the association between DSI and dementia risk prospectively, and by dementia subtypes, such as Alzheimer's disease (AD).
Interpretation: DSI was independently associated with increased risk of all‐cause dementia and AD compared to single or no sensory impairment. There was also a dose‐response relationship between DSI severity and incident dementia. These novel results contribute to our understanding of the impact of sensory impairments on dementia risk in older adults.
Future directions: DSI may serve to identify older adults at high risk of dementia. Future studies should characterize the exact role of sensory impairments and identify whether treatments that improve sensory function can modify this risk.
2.1. Study participants
Volunteers ages 75 years and older were recruited from September 2000 to June 2002 using voter registration and other purchased mailing lists of four U.S. communities with academic medical centers. Until the end of the study in 2008, a total of 3069 participants were assessed every 6 months for incident dementia. Although individuals with prevalent dementia were excluded from participation, participants with MCI were not. In addition participants were required to have adequate hearing and vision, based on the judgment of clinical study staff, in order to be included in the study. 26 Additional information on exclusion criteria and criteria for classification of MCI at baseline in the GEM Study have been described elsewhere. 25 , 26
2.2. Data collection
Data collected at baseline included vital signs, medication use, detailed medical history, physical function assessment, physical and neurologic examinations, and apolipoprotein E (APOE) genotype. Symptom and health habit questionnaires included self‐reported questions on cardiac, neurological, and gastrointestinal symptoms, as well as questions on smoking, alcohol use, and exercise. APOE ε4 carrier status was assayed from stored blood. Dropout and loss to follow‐up rates were low at 6.3%. 25
2.3. Dementia ascertainment
Cognitive function was evaluated by administering at baseline the Clinical Dementia Rating (CDR) scale, 27 Modified Mini‐Mental State Examination (3MSE), 28 and the Cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS‐Cog). 29 Participants also completed a neuropsychological battery of tests at baseline to determine whether they were free of dementia and to establish baseline cognitive scores. Participants with worsening scores on the neuropsychological battery due to poor hearing or vision continued follow‐up visits until there was evidence of decline on a broad range of tests not limited to those involving hearing or vision. 26 Incident dementia was determined in a standard fashion, as has been detailed previously. 25 This included taking into consideration the effects of hearing or visual problems at the time of dementia classification by the adjudication committee, which had a narrative from the examiner in each dementia case. All participants who were adjudicated as developing incident dementia, based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition 30 were assigned to one of the following specific categories: (1) Alzheimer's dementia only; (2) Alzheimer's dementia and VaD; (3) VaD only; or (4) dementia, other etiology (dementia with Lewy bodies, Creutzfeldt‐Jakob disease, and so on). Designation of specific dementia subtypes was based on the National Institute of Neurological and Communicative Disorders and Stroke—Alzheimer's Disease and Related Disorders Association, 31 National Institute of Neurological Disorders and Stroke—Association Internationale pour la Recherche et al. ’Enseignement en Neurosciences, 32 , 33 and AD Diagnostic and Treatment Centers 34 diagnostic criteria.
2.4. Hearing and visual impairment
Hearing and vision were assessed through self‐report at baseline of the GEM Study. For hearing, participants were asked: (1) Can you hear well enough (with or without) a hearing aid to listen to the radio; (2) Can you hear well enough (with or without) a hearing aid to use the telephone; and (3) Can you hear well enough (with or without) a hearing aid to carry on a conversation in a crowded room? For vision, participants were asked: (1) Can you see well enough (with or without) glasses to drive; (2) Can you see well enough (with or without) glasses to watch TV; (3) Can you see well enough (with or without) glasses to read the newspaper; and (4) Can you see well enough (with or without) glasses to recognize someone across the room? Hearing impairment was defined as a negative response to one or more of the three hearing questions. Visual impairment was defined as a negative response to one or more of the four vision questions. Participants classified with hearing impairment and visual impairment were defined as having DSI.
2.5. Statistical analysis
The primary analysis compared the time to all‐cause dementia and number of sensory impairments (0, 1, or 2) at baseline. Time to dementia was calculated as days from baseline to dementia onset, death, or the end of GEM Study follow‐up. The first visit was in September 2000 and the final visit was completed in April 2008. Participants were censored at the date of last contact, including at the end of the study, or date of death. The date of dementia onset was estimated as the date midway between the last clinic examination at which the participant was not demented and the examination at which the dementia diagnosis was made.
Cox proportional hazards models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs). In the analysis, the time axis was participant age at randomization in the GEM Study to control more finely for the effect of age on dementia risk. Models were adjusted for baseline covariates, including sex, age, race, education, income, body mass index, smoking status, alcohol intake, physical activity, hypertension, diabetes, history of cardiovascular disease and cerebrovascular disease, and APOE ε4 allele status. Treatment status (G. biloba vs placebo) and clinic site were included as additional potential confounders. Separate models were developed for all‐cause dementia, AD only, and VaD (with or without AD). We included interactions between the number of sensory impairments and age, sex, and APOE genotype to test whether associations with all‐cause dementia differed in subgroups. We also stratified participants with MCI and without MCI (cognitively normal) at baseline to assess the possibility of reverse causation attributable to preclinical dementia. P < .05 was considered statistically significant, and all tests were two‐sided. Analyses were conducted using Stata version 14 (StataCorp, College Station, Texas, USA). Venn/Euler diagrams were created using eulerAPE, which accurately illustrate the overlap of hearing and visual impairments. 35
To evaluate whether a dose‐response relationship existed with risk for all‐cause dementia, we constructed a summary score of impairments as a best approximation for DSI severity. As a continuous measure, a score from 1 to 6 was used to define severity. A score of 1 represented impairment in a single question for vision and hearing each. A score of 6 represented impairment in all questions for vision and hearing. As a categorical measure, scores were classified into “high,” “intermediate,” and “low” groups. Based on the summary score scale, “high” severity was defined as having a score of 5 to 6; “intermediate” severity was defined as having a score of 3 to 4; “low” severity was defined as having a score of 1 to 2. No DSI was the reference group. In addition, we assessed associations between individual sensory impairments and risk of dementia, including AD and VaD, because associations may differ between types of sensory impairment (vision vs hearing). We first examined individual sensory impairments in separate models. Then, we included each sensory impairment in one model simultaneously to assess whether each sensory impairment was associated with dementia independently of each other.
Sensitivity analyses included the following: (1) restricting to diagnosis of probable AD only for models using (possible and probable) AD as the outcome; and (2) treating death as a competing risk, as well as non‐AD for analyses with AD as the outcome, and non‐VaD for analyses with VaD as the outcome. To evaluate potential bias due to missing covariate and predictor data, we used multiple imputation using chained equations to create 20 imputed data sets, 36 and compared results from the imputed data to the primary complete case analysis.
3. RESULTS
A total of 2051 participants without missing data constituted the analytic study sample. Most participants did not have self‐reported hearing or visual impairment at baseline, whereas 22.8% had hearing or visual impairment and 5.1% had DSI with both hearing and visual impairment (Figure 1). Compared to participants without sensory impairment, participants with DSI tended to be older, were more likely to be male and to have more co‐morbidities, smoke previously, and consume more alcohol (Table 1). Most missing data came from absent APOE genotype information (20.1%), which was due to lack of informed consent or technical problems with the blood samples from the clinical laboratory.
FIGURE 1.
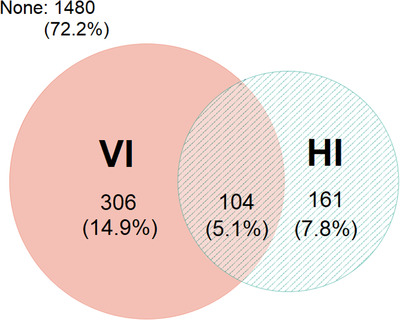
Co‐occurrence of visual impairment (VI) and hearing impairment (HI)
TABLE 1.
Baseline characteristics of study participants from the Gingko Evaluation of Memory (GEM) Study (2000‐2008) by number of sensory impairments
| Characteristic n (%) or mean (SD) | No sensory impairment (n = 1480) | Single sensory impairment (n = 467) | Dual sensory impairment (n = 104) |
|---|---|---|---|
| Age (years)a, mean (SD) | 78.4 (3.1) | 78.5 (3.2) | 79.1 (3.5) |
| Sex | |||
| Female | 653 (44.1) | 218 (46.7) | 34 (32.7) |
| Male | 827 (55.9) | 249 (53.3) | 70 (67.3) |
| Race | |||
| White | 1,438 (97.2) | 451 (96.6) | 100 (96.1) |
| Non‐white | 42 (2.8) | 16 (3.4) | 4 (3.9) |
| Education (years), mean (SD) | 14.6 (3.1) | 14.3 (3.4) | 14.0 (3.2) |
| Body mass indexb, mean (SD) | 27.1 (4.2) | 27.3 (4.2) | 27.1 (4.1) |
| Cardiovascular diseasec | 317 (21.5) | 105 (22.5) | 26 (25.0) |
| Cerebrovascular diseased | 112 (7.6) | 48 (9.2) | 11 (10.6) |
| Diabetes | 134 (9.1) | 43 (9.2) | 13 (12.5) |
| Hypertensione | 804 (54.3) | 241 (51.2) | 58 (55.8) |
| Smoking status | |||
| Current smoker | 71 (4.8) | 16 (3.4) | 5 (4.8) |
| Former smoker | 813 (54.9) | 276 (59.1) | 61 (58.7) |
| Never smoker | 596 (40.3) | 175 (37.5) | 38 (36.5) |
| Drinks per week, mean (SD) | 3.7 (6.7) | 3.5 (6.6) | 5.0 (8.0) |
| Blocks/miles walked per week, mean (SD) | 7.3 (8.6) | 7.8 (10.0) | 6.9 (7.9) |
| 3MSE scoref, mean (SD) | 93.6 (4.6) | 93.0 (4.7) | 91.9 (4.7) |
| APOE ε4g | |||
| ≥1 ε4 allele | 356 (24.1) | 109 (23.3) | 23 (22.1) |
| No ε4 allele | 1,124 (75.9) | 358 (76.7) | 81 (77.9) |
| Treatment status | |||
| G. biloba | 747 (50.5) | 233 (49.9) | 53 (51.0) |
| Placebo | 733 (49.5) | 234 (50.1) | 51 (49.0) |
Age = age at randomization.
Body mass index = kg/m2.
Cardiovascular disease = self‐reported history of the following conditions (heart attack, angina, heart failure, coronary bypass surgery, balloon angioplasty).
Cerebrovascular disease = self‐reported history of stroke, or transient ischemic attack/small stroke.
Hypertension = blood pressure >140/90, or use of anti‐hypertensives.
3MSE score = Modified Mini‐Mental State Examination.
APOE ε4 = apolipoprotein E ε4 allele.
3.1. Associations with dementia
Over 11,392 person‐years of follow‐up, 321 participants (15.6%) developed all‐cause dementia: 14.3% in participants with no sensory impairments, 16.9% in those with one sensory impairment, and 28.8% in those with DSI. An increasing number of sensory impairments was associated with risk of all‐cause dementia in a graded fashion based on crude Kaplan‐Meier estimates (Figure 2). In fully adjusted models, participants with DSI were 1.86 times more likely to develop all‐cause dementia (95% CI: 1.25‐2.76; P = .002) than those without any sensory impairments, whereas participants with DSI were 2.12 times more likely to develop AD (95% CI: 1.34‐3.36; P = .001) than those without any sensory impairments (Table 2). Trends across the three levels were also significant for all‐cause dementia and AD (Table 2). Significant associations were not present for single sensory impairment as the predictor or with VaD as the outcome. Interactions between an increasing number of sensory impairments and age, sex, and apoE were not significant. However, stratification revealed differences; for sex‐ and apoE‐stratified analyses, DSI was significantly associated with increased risk for all‐cause dementia only among female participants (HR: 2.21; 95% CI: 1.21‐4.06; P = .01) and participants without the APOE ε4 allele (HR: 2.21; 95% CI: 1.37–3.56; P = .001) (Table S1). Risk of all‐cause dementia associated with DSI appeared to increase with age (<80 years, 80–84 years, and ≥85 years), although estimates were borderline significant for all age groups (0.05 ≤ P ≤ .08). Stratifying on baseline cognitive status showed that DSI was associated with risk of all‐cause dementia only among participants who were cognitively normal at study entry (HR: 2.08; 95% CI: 1.29‐3.37; P = .003) (Table S2).
FIGURE 2.
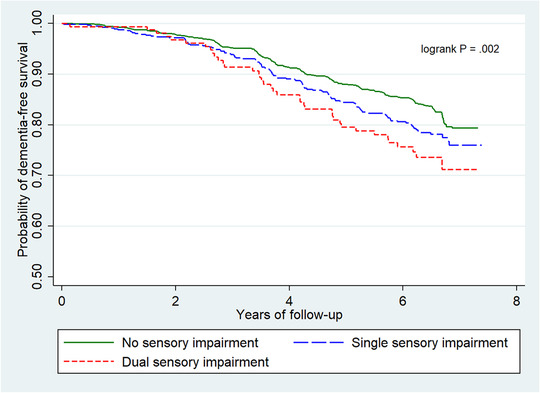
Kaplan‐Meier estimate of risk of all‐cause dementia by number of sensory impairments (hearing and vision)
TABLE 2.
Associations between total number of sensory impairments at baseline and risk of incident all‐cause dementia, Alzheimer's disease, and vascular dementia in the GEM Study (2000‐2008)
| All‐cause dementia (n = 321) | Alzheimer's disease (n = 220) | Vascular dementia (n = 86) | ||||
|---|---|---|---|---|---|---|
| Number of sensory impairments | Hazard ratio (95% CI)a | P | Hazard ratio (95% CI)a | P | Hazard ratio (95% CI)a | P |
| No sensory impairment | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Single sensory impairment | 1.11 (0.86‐1.44) | .43 | 1.10 (0.80‐1.50) | .58 | 1.19 (0.73–1.95) | .48 |
| Dual sensory impairment | 1.86 (1.25‐2.76) | .002 | 2.12 (1.34‐3.36) | .001 | 1.22 (0.52–2.89) | .65 |
| Test for trend | .009 | .01 | .46 | |||
CI, confidence interval.
Models adjusted for age, sex, race, education, income, body mass index, alcohol intake, smoking status, physical activity, cardiovascular disease, cerebrovascular disease, diabetes, hypertension, clinic site, treatment status, and APOE.
3.2. Estimated severity of sensory impairments and differential associations by vision and hearing
In models evaluating baseline summary scores of DSI and risk of all‐cause dementia, significant associations were found for continuous measures (summary score 1 to 6) and categorization based on three ordered groups of DSI severity (Table 3). In the fully adjusted model comparing participants with no DSI, those with “high” severity of DSI (defined as having the highest summary score of 5 to 6) were at the greatest risk of all‐cause dementia (HR: 5.09; 95% CI: 1.24‐20.85; P = .02). Participants with “low” severity of DSI (defined as having the lowest summary score of 1 to2) were also at significantly increased risk of all‐cause dementia (HR: 1.74; 95% CI: 1.04‐2.90; P = .03) compared to participants with no DSI. The dose‐response relationship between continuous measures of DSI severity and risk of all‐cause dementia was also significant (P trend = .02).
TABLE 3.
Associations between estimated baseline levels of dual sensory impairment severity and risk of all‐cause dementia
| Severity of DSI | Hazard ratio (95% confidence interval)a | P‐value |
|---|---|---|
| DSI severity (continuous)b | 1.15 (1.02‐1.29) | .02 |
| DSI severity categories | ||
| No DSI (n = 1947) | 1.00 (reference) | |
| Low (1‐2) (n = 62) | 1.74 (1.04‐2.90) | .03 |
| Intermediate (3‐4) (n = 30) | 1.30 (0.64‐2.65) | .46 |
| High (5‐6) (n = 12) | 5.09 (1.24‐20.85) | .02 |
| Test for trend | .02 | |
DSI, dual sensory impairment.
Models adjusted for age, sex, race, education, income, body mass index, alcohol intake, smoking status, physical activity, cardiovascular disease, cerebrovascular disease, diabetes, hypertension, clinic site, treatment status, and APOE.
A summary score of hearing and visual impairments from 1 to 6 was used to define DSI severity. A score of 1 represented impairment in a single question for vision and hearing each. A score of 2 represented impairments in two questions for hearing and in a single question for vision, or impairments in a single question for hearing and in two questions for vision. A score of 3 represented impairments in all three questions for hearing and in a single question for vision, impairments in two questions for hearing and in two questions for vision, or impairments in a single question for hearing and in three questions for vision. A score of 4 represented impairments in a single question for hearing and in all four questions for vision, impairments in two questions for hearing and in three questions for vision, impairments in all three questions for hearing and in two questions for vision. A score of 5 represented impairments in all three questions for hearing and in three questions for vision, or impairments in two questions for hearing and in all four questions for vision. A score of 6 represented impairments in all questions for hearing and vision.
Although single sensory impairment was not associated with risk of dementia as compared with no sensory impairment, associations differed between the type of sensory deficit and dementia risk. Visual impairment was individually associated with risk of all‐cause dementia (HR: 1.34; 95% CI: 1.04‐1.71; P = .02) and AD (HR: 1.39; 95% CI: 1.03‐1.87; P = .03) after adjusting for demographics, health conditions, lifestyle factors, APOE genotype, treatment status, and clinic site, but not with risk of VaD (Table 4). With simultaneous inclusion of visual and hearing impairments in one model, visual impairment remained significantly associated with risk of all‐cause dementia (HR: 1.32; 95% CI: 1.02‐1.71; P = .04), but not with risk of AD or VaD. Hearing impairment was not associated with any dementia outcome whether considered individually or after adjusting for visual impairment in the single model.
TABLE 4.
Associations between individual sensory impairments (vision and hearing) and risk of incident all‐cause dementia, Alzheimer's disease, and vascular dementia
| All‐cause dementia | Alzheimer's disease | Vascular dementia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual modelsa | Combined modelb | Individual modelsa | Combined modelb | Individual modelsa | Combined modelb | |||||||
| Sensory impairment | HR (95% CI)c | P | HR (95% CI)c | P | HR (95% CI)c | P | HR (95% CI)c | P | HR (95% CI)c | P | HR (95% CI)c | P |
| Vision | 1.34 (1.04‐1.71) | .02 | 1.32 (1.02‐1.71) | .04 | 1.39 (1.03‐1.87) | .03 | 1.32 (0.97–1.80) | .08 | 1.24 (0.76–2.03) | .38 | 1.36 (0.82–2.25) | .23 |
| Hearing | 1.18 (0.88‐1.59) | .28 | 1.20 (0.88‐1.63) | .25 | 1.30 (0.92‐1.85) | .14 | 1.31 (0.92–1.89) | .14 | 0.86 (0.46–1.61) | .64 | 0.90 (0.48–1.69) | .74 |
CI, confidence interval; HR, hazard ratio; P, P value.
Vision and hearing examined individually in separate models.
Vision and hearing examined together in one model.
Models adjusted for age, sex, race, education, income, body mass index, alcohol intake, smoking status, physical activity, cardiovascular disease, cerebrovascular disease, diabetes, hypertension, clinic site, treatment status, and APOE.
3.3. Sensitivity analyses
Restricting the analysis to probable AD resulted in slightly attenuated estimates for DSI, although the interpretation did not change (Table S3). Including death as a competing risk produced similar associations between DSI and all‐cause dementia (Table S4). DSI remained significantly associated with AD when including non‐AD as a competing risk (Table S4). Neither DSI nor single sensory impairment was significantly associated with VaD when including non‐VaD as a competing risk. Conducting multiple imputation for missing data resulted in attenuated, but still significant, estimates as compared with the complete case analysis (Table S5).
4. DISCUSSION
In this prospective cohort of 2051 older adults, we found significant associations between DSI and risk of all‐cause dementia and AD, but not VaD. In fully adjusted models, DSI was associated with an 86% increased risk for all‐cause dementia and an 112% increased risk for AD compared with having no sensory impairments. Greater extent of DSI was also significantly associated with increasing risk of all‐cause dementia. These findings were robust after adjustment for multiple potential confounders and in sensitivity analyses. Together, our findings suggest that older adults with DSI represent a high‐risk population that could be a target for intervention prior to the onset of dementia.
Previous studies have suggested that associations similar to those reported here might be found. In a cross‐sectional study of over 250,000 older adults in China, those with combined vision and hearing impairments had greater odds of dementia than those with no sensory impairment. 37 A longitudinal study with participants from the United States and Europe found that older adults with DSI had lower episodic memory scores and remembered fewer words compared with those with no sensory impairment. 38 Another study from Europe examining nursing home residents showed that those with DSI had greater cognitive decline than those with either vision or hearing impairment and those without sensory impairment. 39 A population‐based cohort study from Japan using administrative health care data reported that DSI among long‐term care recipients was associated with cognitive impairment compared to individuals with normal sensory function. 40 Our study adds to this body of knowledge on the impact of DSI in relation to cognitive impairment by using prospectively collected data in participants followed over 8 years, which includes rigorous evaluation of dementia and its main subtypes: AD and VaD.
Several hypotheses can be advanced to explain the link between sensory and cognitive function. Greater impairment in hearing and vision may accelerate cognitive decline because of the association of sensory impairment with social isolation, depression, reduced physical, and mental activities, and functional limitations. 13 At a neural level, these impairments may limit or stress the neural resources needed for optimal performance of cognitive tasks by increasing cognitive load. 22 Loss of sensory input can also lead to reduced activation in central sensory pathways, resulting in changes to brain structure, and function, such as deafferentation‐induced atrophy in frontal brain regions and stressing brain circuitry due to resource demands to address poor signal‐to‐noise ratios. 3 , 41 Sensorial stimulation, on the other hand, may have beneficial effects on neuronal development and function, such as formation of new synapses and survival. 42 Alternatively, common pathological processes including vascular disease, inflammation, or some combination of these may be responsible for the relationship between DSI and dementia. 14 , 23 Hearing and vision loss are associated with vascular pathology, such as white matter hyperintensities and microvascular lesions, 43 , 44 which are also important contributors to VaD. DSI may also be a marker of underlying neurodegeneration. We found that the impact of DSI was strongest for AD. Other studies support a connection between sensory dysfunctions and AD, 45 but not all. 46 , 47 Although we accounted for MCI in our analysis, given the long and insidious development of AD, which is also the case for hearing and vision loss, we cannot rule out that reverse causation from early neurodegenerative stages of the AD disease process contributed to the development of DSI. Our findings do not allow a conclusive choice between these hypotheses, which are not mutually exclusive. Additional studies are necessary to determine the mechanisms underlying these associations. Further research is also needed to evaluate whether these mechanisms are shared or differ between hearing and vision as we found that individually visual impairment, but not hearing impairment, was significantly associated with increased risk of all‐cause dementia. Either mechanism has implications for potential clinical interventions.
This study has a number of strengths, including its prospective and longitudinal design, large sample size, rigorous classification of dementia and its subtypes, and standardization of data collection for other covariates. Important limitations exist, however, such as misclassification of sensory impairments. The questions used to assess hearing and visual function in the GEM Study were asked at baseline only, and their accuracy is unknown. In addition, information on date of onset and cause of sensory loss were unavailable. The low prevalence of hearing impairment in our sample was likely a result of excluding those with severe hearing loss from participating in the GEM Study and misclassifying some participants who had hearing loss as not having hearing loss. Even with misclassification of hearing impairment that would bias our results toward the null, we still found a significantly increased risk of dementia associated with DSI, which also accounted for sensory problems in the diagnosis of dementia. Had hearing impairment status been more accurately classified in our study, we would expect an even stronger association between DSI and dementia than what was observed. AD and VaD were classified using standardized clinical criteria and cranial magnetic resonance imaging, but neuropathological information was not available, and again misclassification is possible. However, for the AD outcome, when restricting only to probable AD, which has a higher predictive value of neuropathologic changes in AD as compared with possible AD, 48 we did not find substantially different results compared to the primary findings with probable and possible AD included. Another limitation is that our findings may not be generalizable to other populations because participants in the GEM Study were highly selected to be healthy at baseline and were mostly white.
DSI was independently and strongly associated with increased risk of all‐cause dementia and AD in this cohort of older adults, but not VaD. Further research is needed to characterize the exact role of sensory impairments and whether treatments that improve sensory function, such as surgery or sensory aids, devices, and prostheses, can modify this risk. Because the public health burden of dementia will increase over the next three decades, evaluation of vision and hearing function in older adults may help identify patients at elevated risk of developing dementia.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
This work was supported by grant U01 AT000162 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements and National Institute on Aging; National Heart, Lung, and Blood Institute; University of Pittsburgh Alzheimer's Disease Research Center (P50AG05133); Roena Kulynych Center for Memory and Cognition Research; Wake Forest University School of Medicine; and the National Institute of Neurological Disorders and Stroke.
Hwang PH, Longstreth WT, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimer's Dement. 2020;12:e12054 10.1002/dad2.12054
Institution of origin: Department of Epidemiology, University of Washington, Seattle, WA 98195, United States of America (USA)
REFERENCES
- 1. Crews JE, Campbell VA. Vision impairment and hearing loss among community‐dwelling older Americans: Implications for health and functioning. Am J Public Health. 2004;94:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell V, Crews J, Moriarty D, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health related quality of life among older adults—United States, 1993‐1997. MMWR CDC Surveillance Summaries. 1999;48:131‐156. [PubMed] [Google Scholar]
- 3. Whitson HE, Cronin‐Golomb A, Cruickshanks KJ, et al. American Geriatrics Society and National Institute on Aging Bench‐to‐Bedside Conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018;66:2052‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lupsakko T, Mantyjarvi M, Kautiainen H, Sulkava R. Combined hearing and visual impairment and depression in a population aged 75 and older. Int J Geriatr Psych. 2002;17:808‐813. [DOI] [PubMed] [Google Scholar]
- 5. Schneider JM, Gopinath B, McMahon CM, Leeder SR, Mitchell P, Wang JJ. Dual sensory impairment in older age. J Aging Health. 2011;23:1309‐1324. [DOI] [PubMed] [Google Scholar]
- 6. Schubert CR, Fischer ME, Pinto AA, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2016;72:710‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deal JA, Betz J, Yaffe K, et al.; Health ABC Study Group . Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72:703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ward ME, Gelfand JM, Lui LY, et al. Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann Neurol. 2018;83:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies‐Kershaw HR, Hackett RA, Cadar D, Herbert A, Orrell M, Steptoe A. Vision impairment and risk of dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2018;66(9):1823‐1829. [DOI] [PubMed] [Google Scholar]
- 10. Davies HR, Cadar D, Herbert A, Orrell M, Steptoe A. Hearing impairment and incident dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2017;65:2074‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB. Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis A, McMahon CM, Pichora‐Fuller KM, et al. Aging and hearing health: the life‐course approach. Gerontologist. 2016;56(suppl 2):S256‐S267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer ME, Cruickshanks KJ, Klein BE, Klein R, Schubert CR, Wiley TL. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009;16:346‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis. 2015;238:344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell P, Gopinath B, McMahon CM, et al. Relationship of Type 2 diabetes to the prevalence, incidence, and progression of age‐related hearing loss. Diabet Med. 2009;26:483‐488. [DOI] [PubMed] [Google Scholar]
- 16. Haan M, Espeland MA, Klein BE, et al. Women's Health Initiative Memory Study and the Women's Health Initiative Sight Exam . Cognitive function and retinal and ischemic brain changes: the Women's Health Initiative. Neurology. 2012;78:942‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 2019;15:11‐24. [DOI] [PubMed] [Google Scholar]
- 18. Heine C, Browning C. Dual sensory loss in older adults: a systematic review. Gerontologist. 2015;55:913‐928. [DOI] [PubMed] [Google Scholar]
- 19. Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med . 2013;173:312‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin MY, Gutierrez PR, Stone KL, et al. Study of Osteoporotic Fractures Research Group . Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996‐2002. [DOI] [PubMed] [Google Scholar]
- 21. Hong T, Mitchell P, Burlutsky G, Liew G, Wang JJ. Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the Blue Mountains Eye Study. PLoS One. 2016;11:e0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gadkaree SK, Sun DQ, Li C, et al. Does sensory function decline independently or concomitantly with age? Data from the Baltimore longitudinal study of aging. J Aging Res. 2016;2016:1865038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinto JM, Wroblewski KE, Huisingh‐Scheetz M, et al. Global sensory impairment predicts morbidity and mortality in older US adults. J Am Geriatr Soc. 2017;65:2587‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci. 2019;74(6):890‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo Evaluation of Memory (GEM) Study Investigators . Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeKosky ST, Fitzpatrick A, Ives DG, et al. GEMS Investigators . The Gingko Evaluation of Memory (GEM) study: Design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238‐253. [DOI] [PubMed] [Google Scholar]
- 27. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. [DOI] [PubMed] [Google Scholar]
- 28. Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314‐318. [PubMed] [Google Scholar]
- 29. Mohs RC. The Alzheimer's disease assessment scale. Int Psychogeriatr. 1996;8:195‐203. [DOI] [PubMed] [Google Scholar]
- 30. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 31. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939‐944. [DOI] [PubMed] [Google Scholar]
- 32. Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS‐AIREN criteria. Dementia. 1994;5:189‐192. [DOI] [PubMed] [Google Scholar]
- 33. Rockwood K, Parhad I, Hachinski V, et al. Diagnosis of vascular dementia: consortium of Canadian centres for clinical cognitive research consensus statement. Can J Neurol Sci. 1994;21:358‐364. [DOI] [PubMed] [Google Scholar]
- 34. Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of California Alzheimer's disease diagnostic and treatment centers. Neurology. 1992;42:473‐480. [DOI] [PubMed] [Google Scholar]
- 35. Micallef L, Rodgers P. eulerAPE: Drawing Area‐proportional 3‐Venn Diagrams Using Ellipses. PLoS ONE. 2014;9:e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377‐399. [DOI] [PubMed] [Google Scholar]
- 37. Luo Y, He P, Guo C, Chen G, Li N, Zheng X. Association between sensory impairment and dementia in older adults: evidence from China. J Am Geriatr Soc. 2018;66:480‐486. [DOI] [PubMed] [Google Scholar]
- 38. Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Sense‐Cog WP1 group . Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing. 2018;47:575‐581. [DOI] [PubMed] [Google Scholar]
- 39. Yamada Y, Denkinger MD, Onder G, et al. Dual sensory impairment and cognitive decline: the results from the Shelter study. J Gerontol A Biol Sci Med Sci. 2016;71:117‐123. [DOI] [PubMed] [Google Scholar]
- 40. Mitoku K, Masaki N, Ogata Y, Okamoto K. Vision and hearing impairments, cognitive impairment and mortality among long‐term care recipients: a population‐based cohort study. BMC Geriatr. 2016;16:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age‐related hearing loss to late‐life depression and cognitive decline. Am J Psychiatry. 2018;175:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swaab DF, Dubelaar EJ, Hofman MA, Scherder EJ, van Someren EJ, Verwer RW. Brain aging and Alzheimer's disease: use it or lose it. Prog Brain Res. 2002;138:343‐273. [DOI] [PubMed] [Google Scholar]
- 43. Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, Dubno JR. White matter hyperintensities predict low frequency hearing in older adults, J Assoc Res Otolaryngol. 2013;14:425‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prins D, Hanekamp S, Cornelissen FW. Structural brain MRI studies in eye diseases: are they clinically relevant? a review of current findings. Acta Opthalmol. 2016;94:113‐121. [DOI] [PubMed] [Google Scholar]
- 45. Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11:70‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deal JA, Rawlings A, Sharrett AR, et al. Hearing impairment, cognitive performance, and beta‐amyloid deposition in the ARIC‐PET amyloid imaging study. Innov Aging. 2019;3(S1):551. [Google Scholar]
- 47. Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer's disease. Neurology. 1993;43:779‐785. [DOI] [PubMed] [Google Scholar]
- 48. Lim A, Tsuang D, Kukull W, et al. Clinico‐neuropathological correlation of Alzheimer's disease in a community‐based case series. J Am Geriatr Soc. 1999;47:564‐569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


