Abstract
Background
The development of efficacious combination antiretroviral therapy (cART) has led to a dramatic decrease in mortality in HIV-positive patients. Specific data on the impact in HIV/hepatitis B virus (HBV)–coinfected patients are lacking. In this study, all-cause and cause-specific mortality risks stratified per era of diagnosis are investigated.
Methods
Data were analyzed from HIV/HBV-coinfected patients enrolled in the ATHENA cohort between January 1, 1998, and December 31, 2017. Risk for (cause-specific) mortality was calculated using Cox proportional hazard regression analysis, comparing patients diagnosed before 2003 with those diagnosed ≥2003. Risk factors for all-cause and liver-related mortality were also assessed using Cox proportional hazard regression analysis.
Results
A total of 1301 HIV/HBV-coinfected patients were included (14 882 person-years of follow-up). One-hundred ninety-eight patients (15%) died during follow-up. The adjusted hazard ratio (aHR) for all-cause mortality in patients diagnosed in or after 2003 was 0.50 (95% CI, 0.35–0.72) relative to patients diagnosed before 2003. Similar risk reduction was observed for liver-related (aHR, 0.29; 95% CI, 0.11–0.75) and AIDS-related mortality (aHR, 0.44; 95% CI, 0.22–0.87). Use of a tenofovir-containing regimen was independently associated with a reduced risk of all-cause and liver-related mortality. Prior exposure to didanosine/stavudine was strongly associated with liver-related mortality. Ten percent of the population used only lamivudine as treatment for HBV.
Conclusions
All-cause, liver-related, and AIDS-related mortality risk in HIV/HBV-coinfected patients has markedly decreased over the years, coinciding with the introduction of tenofovir. Tenofovir-containing regimens, in absence of major contraindications, should be strongly encouraged in this population.
Keywords: coinfection, hepatitis B virus, HIV, liver-related mortality, tenofovir
This ATHENA cohort based analysis shows a decline in the risk for all-cause and liver-related mortality in patients with a HIV/Hepatitis B virus coinfection diagnosed in the tenofovir era. However, physicians should strive to maintain optimal HBV suppression.
Approximately 38 million people are currently living with HIV worldwide [1], with hepatitis B virus (HBV) being a common coinfection in this population; an estimated 5%–20% of HIV-positive patients are coinfected with HBV, but these estimates vary between risk groups and geographical regions—with the highest prevalence in Sub-Saharan Africa and Asia [2]. In the Netherlands, 6% of all HIV-positive patients registered in the AIDS Therapy Evaluation in the Netherlands (ATHENA) observational cohort have ever tested positive for hepatitis B surface antigen (HBsAg) [3]. Data from the era during which effective anti-HBV therapy was not widely available show that patients with HIV/HBV coinfection display faster progression toward end-stage liver disease (ESLD) and have higher (liver-related) mortality rates compared with patients with either an HBV or HIV monoinfection [4]. A more recent study demonstrated that in the current antiretroviral era, coinfected subjects had no increased risk for ESLD compared with HBV-monoinfected patients, but their risk for all-cause and liver-related mortality was still significantly higher [5].
Lamivudine (3TC) became available in the mid-90s, and due to its dual activity against HIV and HBV, it was an ideal therapeutic option for HIV/HBV coinfection [6]. Nevertheless, viral resistance rapidly emerged with the use of this agent [7]. In 2003, tenofovir disoproxil fumarate (TDF) was introduced—also with potent dual efficacy, but with a higher genetic barrier to resistance [8]. Therefore, current guidelines recommend the use of a tenofovir plus either a lamivudine- or emtricitabine-containing regimen as preferential treatment in HIV/HBV-coinfected patients [9].
Earlier studies showed that the introduction of combination antiretroviral therapy (cART) led to a dramatic decrease in all-cause mortality in the general HIV-positive population [10]. Data focusing on changes in mortality among HIV/HBV-coinfected patients, particularly as more potent anti-HBV agents became available, are sparse. Considering the high prevalence and potential burden of liver-related disease, such data are of major interest. The main objective of this study was to describe mortality risk for HIV/HBV-coinfected patients stratified by calendar periods of HIV diagnosis in relation to changes in HIV/HBV treatment including the introduction of tenofovir and the declining use of more toxic antiretroviral drugs. Furthermore, we aimed to identify risk factors for all-cause and liver-related mortality in this specific population.
METHODS
Study Population
We performed a longitudinal analysis among HIV/HBV-coinfected patients from the ATHENA observational cohort, which was initiated in 1998. Data are collected by the HIV Monitoring Foundation and cover 98% of all patients with a confirmed HIV infection in care in the Netherlands. Medical history and data before 1998 were collected retrospectively. The structure of the cohort and procedures are described elsewhere [11]. All patients aged ≥18 years with HIV/HBV coinfection in care between January 1, 1998, and December 31, 2017, were included in the analysis. HBV infection was defined by 2 consecutive HBsAg-positive and/or HBV DNA detectable results during a period of ≥6 months. Patients with evidence of hepatitis C virus (HCV) infection (ie, a positive HCV RNA polymerase chain reaction [PCR]) were excluded from analysis.
Collected Variables
Patients’ demographic, clinical, and laboratory data were collected during follow-up. Laboratory data included HIV RNA viral load, HBV DNA viral load, CD4+ cell count, and alanine aminotransferase (ALT) levels. Laboratory data were retrieved time-updated per year. If multiple results were available during the yearly interval, the last available measurement was used. If CD4+ cell count and/or ALT was missing in a certain year, the last available observation was carried forward. Until April 2012, ALT levels were only collected if >3 times the upper limit of normal (ULN), and thus all missing ALT levels before this date were assumed to be ≤3× ULN. Due to varying levels of assay detection thresholds over the study period, undetectable HIV-RNA was defined as <400 copies/mL.
Treatment Data and Treatment Periods
Treatment data included past and current use of antiretroviral agents, based on information provided by the patients’ treating physicians in their medical record. We focused on the use of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) with activity against both HIV and HBV: tenofovir (either disoproxil fumarate or alafenamide; TDF/TAF) and 3TC. The use of the NRTIs stavudine (d4T) and didanosine (ddI) was also evaluated considering their hepatotoxic potential [12].
We defined 2 periods of HIV diagnosis calendar time based on both effectiveness of cART regimens and availability of potent anti-HBV treatment: diagnosis before 2003 (when cART was more readily available with only 3TC) and between 2003 and 2017 (with frequent use of more modern antiretroviral regimens and availability of tenofovir). In subsequent analysis, we further stratified the period 2003–2017: between 2003 and 2007—with less frequent use of TDF—and between 2008 and 2017—with the advent of integrase strand inhibitors (INSTIs) as recommended first-line backbone therapy and widespread TDF/TAF use in the Netherlands [13, 14].
End Points
The primary end point in this study was mortality. The data were obtained from the ATHENA cohort database, which used the Cause of Death (CoDe) protocol to classify causes of death [15]. Causes of death were categorized into liver-related, AIDS-related, non-AIDS malignancy, cardiovascular disease (CVD), non-natural, unknown, or other. In addition, we assessed the occurrence of severe chronic liver disease (SCLD). In the ATHENA cohort, SCLD was categorized as either presumptive or definitive. In case of documented evidence of variceal bleeding, hepatic encephalopathy, hepatorenal syndrome, and/or portal hypertension or cirrhosis by radiography or endoscopy, the patient was considered to have presumptive SCLD. If the abovementioned conditions were present in combination with histological evidence of severe chronic liver disease (histopathological Metavir score F3-F4) or a transient elastography ≥8 kPa, patients were considered to have definitive SCLD.
Statistical Analyses
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). All reported P values were 2-sided, and P < .05 was considered to be statistically significant.
Follow-up began at the time patients first entered HIV care and consented to be enrolled into the ATHENA cohort [11]. As identification of HBV coinfection could be biased through failure to test for HBsAg, particularly in the earlier years of the ATHENA cohort, we decided to define the beginning of follow-up based on HIV diagnosis. Patients diagnosed with HIV before the start of the ATHENA cohort were left-censored on January 1, 1998. Follow-up continued until the date of death, date last seen if lost to follow-up (withdrawn from care for >1 year), date of moving abroad, or December 31, 2017, whichever occurred first. As HBsAg seroclearance was not systematically assessed across the entire study population, we decided not to censor after HBsAg loss. The cumulative incidence rates of progression to all-cause mortality were modeled across calendar periods using Cox proportional hazards regression. Hazards ratios (HRs) and their 95% confidence intervals (CIs) were estimated with HIV diagnosis <2003 as the reference group. The cumulative incidence rates of progression to the different causes of death were also modeled across calendar periods with proportional hazards regression, while taking into account competing risk of other causes of death using the method by Fine and Gray [16]. To account for patient differences across periods, HRs were adjusted by age at inclusion, mode of HIV transmission, and region of origin. Gender was not included in the adjustment due to its overlap with other demographic variables. In order to identify risk factors for all-cause and liver-related mortality, we used the proportional hazards models above to estimate differences in cumulative incidence between levels of risk factors. Variables with an associated P value ≤.2 in univariable analysis were included in the multivariable model without further selection. For this risk factor analysis, we included treatment variables that directly reflected certain calendar periods; hence these periods were not considered independent variables.
RESULTS
Study Population Characteristics
In the period ranging from January 1, 1998, until December 31, 2017, a total of 24 413 adult HIV-positive individuals were in care and registered in the ATHENA cohort. Of these, 1398 patients met the definition for chronic HBV infection; after excluding 97 patients with an HCV coinfection, we included 1301 individuals in our analysis. The vast majority were male, with the most common HIV/HBV transmission risk group being men who have sex with men (MSM) (Table 1). Description of the cohort at specific time points is also summarized in Table 1, showing that the cohort was aging and that there was a shift in cART composition.
Table 1.
Characteristics of Study Participants
| Characteristics at Cohort Entry & Death | Characteristics at Follow-up Dates | ||||
|---|---|---|---|---|---|
| Cohort Entry | Death | 1st of January 2003 | 1st of January 2008 | 31st of December 2017 | |
| No. of patients | 1301 (100) | 198 (100) | 425 (100) | 676 (100) | 931 (100) |
| Men | 1125 (86.5) | 178 (89.9) | 370 (87.1) | 583 (86.2) | 812 (87.2) |
|
Age, median (IQR), y
|
36.6 (30.8–43.7) 825 (63.4) 332 (25.6) 111 (8.5) 32 (2.5) |
50.0 (43.1–58.3) 35 (17.7) 64 (32.3) 56 (28.3) 43 (21.7) |
38.8 (33.7–44.0) 242 (56.9) 135 (31.8) 42 (9.9) 6 (1.4) |
42.1 (36.5–47.5) 271 (40.1) 278 (41.1) 104 (15.4) 23 (3.4) |
49.8 (43.0–55.4) 171 (18.4) 300 (32.2) 323 (34.7) 137 (14.7) |
|
HIV transmission route
|
810 (62.2) 383 (29.4) 26 (2.0) 82 (6.3) |
117 (59.1) 39 (19.7) 16 (8.1) 26 (13.1) |
294 (69.2) 112 (26.4) 5 (1.2) 14 (3.3) |
453 (67.0) 190 (28.1) 7 (1.0) 26 (3.8) |
623 (66.9) 259 (27.8) 8 (0.9) 41 (4.4) |
|
Region of origin
|
762 (58.6) 315 (24.2) 54 (4.2) 63 (4.9) 106 (8.1) |
141 (71.2) 30 (15.2) 4 (2.0) 6 (3.0) 17 (8.6) |
274 (64.0) 82 (19.3) 21 (4.9) 18 (4.2) 32 (7.5) |
409 (60.5) 147 (21.7) 35 (5.2) 31 (4.6) 54 (8.0) |
568 (61.0) 198 (21.3) 43 (4.6) 49 (5.3) 73 (7.8) |
|
HIV diagnosis era
|
401 (30.8) 261 (20.1) 332 (25.6) 307 (23.6) |
118 (59.6) 37 (18.7) 29 (14.6) 14 (7.1) |
243 (57.2) 182 (42.8) N/A N/A |
243 (35.9) 182 (26.9) 251 (37.1) N/A |
243 (26.1) 182 (19.5) 251 (27.0) 255 (27.4) |
|
CD4+ cell count, median (IQR), cells/mm3
|
310 (150–506) 408 (31.6) |
250 (110–490) 76 (38.3) |
470 (300–641) 63 (14.8) |
480 (340–650) 57 (8.4) |
630 (440–820) 34 (3.6) |
|
HIV viral load
|
973 (74.8) 327 (25.2) |
69 (34.8) 129 (65.2) |
162 (38.1) 263 (61.9) |
207 (30.6) 469 (69.4) |
48 (5.2) 883 (94.8) |
|
ALT level <3.0× ULNa ≥3.0× ULN |
1124 (86.4) 177 (13.6) |
178 (89.9) 20 (10.1) |
393 (92.5) 32 (7.5) |
631 (93.3) 45 (6.7) |
912 (98.0) 19 (2.0) |
|
History of ddI/d4T exposure
|
88 (44.4) 3 (1–5) 320 |
159 (37.4) 3 (2–5) 515 |
172 (25.4) 4 (2–6) 744 |
177 (19.0) 4 (2–6.5) 820 |
|
|
Previous treatment with mono- or dual therapy |
77 (38.9) |
126 (29.6) |
134 (19.8) |
140 (15.0) |
|
|
Ever TDF/TAF exposure
|
115 (57.5) 1 (0–4) 497 |
63 (14.8) 0 (0–0) 81 |
425 (62.9) 1 (0–4) 1352 |
866 (93.0) 8 (5–11) 7250 |
|
|
Time between HBV diagnosis and start TDF/TAF, median (IQR), y |
6 (0.5–10) |
2 (0–6) |
2.5 (0–7) |
2 (0–6) |
|
|
Ever exposure to other drugs with anti-HBV activity
|
145 (73.2) 60 (30.3) 4 (2.0) 2 (1.0) |
313 (73.6) 1 (0.2) 24 (15.6) 2 (0.5) |
456 (67.5) 111 (16.4) 33 (4.9) 2 (0.3) |
536 (57.6) 782 (84.0) 47 (5.0) 4 (0.4) |
|
|
Current ART use
|
73 (36.8) 10 (5.0) 44 (22.2) 43 (21.7) 6 (3.0) 22 (11.1) |
104 (24.5) 16 (3.7) 169 (39.7) 102 (24.0) N/A 34 (8.0) |
144 (21.3) 8 (1.1) 299 (44.2) 180 (26.6) N/A 45 (6.7) |
11 (1.2) 25 (2.8) 367 (39.4) 173 (25.7) 285 (30.6) 70 (7.5) |
|
Data are No. (%) unless otherwise specified.
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; cART, combination antiretroviral therapy; d4T, stavudine; ddI, didanosine; HBV, hepatitis B virus; IQR, interquartile range; IVD, intravenous drugs; MSM, men who have sex with men; NNRTI, non-nucleoside reverse transcriptase inhibitor; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate; ULN, upper limit of normal (35 IU/L).
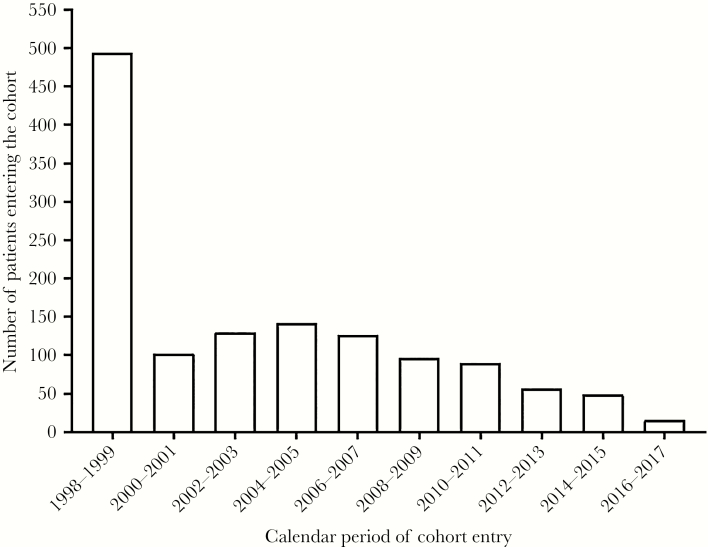
Median follow-up (interquartile range [IQR]) was 11 (6–17) years, totaling 14 882 person-years of follow-up—including 12 577 years of follow-up on antiretroviral treatment. The majority of the study population entered the cohort during 1998–2005, with a remarkable decline in new cases thereafter (Figure 1). The large number of individuals entering in 1998 was mostly due to left truncation. In 2017, no newly HBV-diagnosed patients entered the study cohort. Over the entire study period, 86 patients (7%) were lost to follow-up, and 85 patients (7%) moved abroad.
Figure. 1.
Bar graph displaying the number of patients entering the cohort over the time of follow-up per two years.
Antiretroviral Therapy and Efficacy
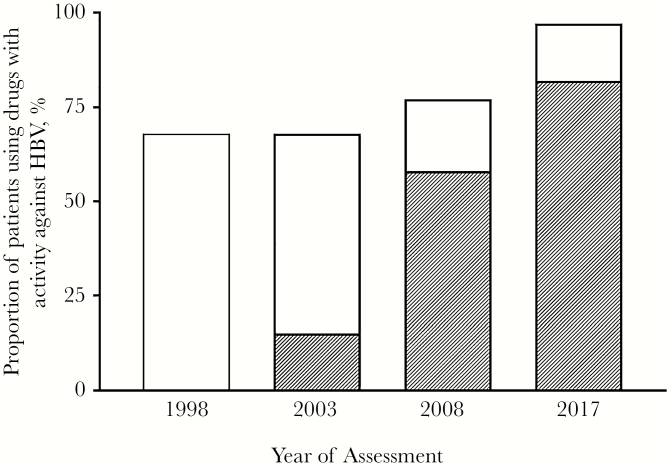
Over time, an increasing proportion of patients used antiretroviral therapy (ART), 76% on January 1, 2003, compared with almost everyone (98.8%) on December 31, 2017. Four hundred fifty-five patients (35%) did not start antiretroviral therapy in the first year they entered the cohort. Besides these patients, an additional 206 patients (16%) interrupted cART at some point during follow-up. In general, virological and immunological response was excellent at the end of follow-up, with 96% of the patients having a HIV viral load <400 copies/mL and a median CD4 cell count (IQR) of 630 (440–820) cells/mm3. Over time, 1095 patients (84%) were exposed to a TDF/TAF-containing regimen, accounting for 8233 person-years of tenofovir exposure. On December 31, 2017, 905 of the 931 patients (97%) remaining in follow-up were using drugs with activity against HBV (Figure 2). Most of them (n = 766, 83%) were on a TDF/TAF-containing regimen, and 16 (1%) patients were on entecavir. One-hundred twenty-three patients (10%) were using only lamivudine as the HBV-active component of their ART regimen. Of these patients, 62 (50%) were switched to dolutegravir/abacavir/lamivudine when this single-tablet regimen became available. Twenty-six patients did not use any anti-HBV therapy, 12 of whom displayed HBsAg seroclearance (ie, loss of HBsAg, not necessarily with acquisition of anti-HBsAg antibodies) during follow-up. HBV DNA monitoring was infrequent in our cohort, with only 210 (16%) patients having an HBV DNA viral load measurement in their last year of follow-up. The lack of monitoring was not only observed among patients using highly effective agents, such as TDF/TAF or entecavir, but also among those using 3TC as a single anti-HBV agent. Of the 123 patients with only 3TC for HBV treatment on December 31, 2017, 19 (15%) had an available HBV DNA viral load during the last year of follow-up. Of these 19 patients, 12 (63%) had an HBV DNA level <40 copies/mL.
Figure. 2.
Graph bar displaying the proportion of HIV/HBV co-infected patients using drugs with activity against HBV (eg, tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), lamivudine, entecavir, telbivudine, adefovir). The shaded area represents the proportion of patients using either TDF or TAF. The date of assessment was on 1 January 1998, 1 January 2003, 1 January 2008 and 31 December 2017.
Trends in Mortality Risk
A total of 198 patients (15%) died during follow-up—with the most common causes of death being AIDS-related (24%), liver-related (19%), and non-AIDS-related malignancies (19%) (Table 2). As shown in Table 2, patients diagnosed after 2002 were significantly less likely to die from any cause compared with those diagnosed before 2003 (adjusted HR [aHR], 0.50; 95% CI, 0.35–0.72), with similar effect sizes for the periods 2003–2007 (aHR, 0.53; 95% CI, 0.35–0.80) and 2008–2017 (aHR, 0.46; 95% CI, 0.26–0.81). A similar reduced risk after 2002 was observed with respect to liver-related mortality (aHR, 0.29; 95% CI, 0.11–0.75) and AIDS-related mortality (aHR, 0.44; 95% CI, 0.22–0.87) but not for the other causes of death. We observed decreasing trends in mortality for the 2003–2007 and 2008–2017 subcategories with respect to liver-related, AIDS-related, and non-AIDS-related malignancy death, but these results did not reach statistical significance.
Table 2.
Unadjusted and Adjusted Hazard Ratios of Underlying Cause of Death per Time Era of HIV Diagnosis
| <2003 (Reference) | Two-Period Analysis | Three-Period Analysis | ||
|---|---|---|---|---|
| ≥2003 | 2003–2007 | 2008–2018 | ||
| All-cause (n = 198) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.55 (0.38–0.78) 0.50 (0.35–0.72) | 0.53 (0.35–0.79) 0.53 (0.35–0.80) | 0.60 (0.34–1.05) 0.46 (0.26–0.81) |
| Liver-related (n = 38) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.30 (0.13–0.78) 0.29 (0.11–0.75) | 0.33 (0.11–0.94) 0.34 (0.12–0.97) | 0.21 (0.03–1.61) 0.17 (0.02–1.33) |
| AIDS-related (n = 48) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.48 (0.24–0.95) 0.44 (0.22–0.87) | 0.52 (0.24–1.12) 0.49 (0.22–1.06) | 0.39 (0.12–1.30) 0.33 (0.10–1.12) |
| Non-AIDS-defining malignancy (n = 38) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.95 (0.45–2.00) 0.78 (0.36–1.68) | 0.90 (0.40–2.07) 0.85 (0.37–1.95) | 1.09 (0.31–3.86) 0.61 (0.17–2.24) |
| Cardiovascular disease (n = 16) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.44 (0.12–1.62) 0.35 (0.09–1.32) | 0.67 (0.18–2.48) 0.66 (0.18–2.47) | b b |
| Non-natural (n = 10) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.84 (0.20–3.49) 0.90 (0.21–3.74) | 0.40 (0.05–3.37) 0.44 (0.05–3.72) | 2.27 (0.57–13.86) 2.19 (0.36–13.40) |
| Unknown (n = 18) • Nonadjusted •Adjusteda | 1.0 1.0 | 0.40 (0.11–1.42) 0.33 (0.09–1.21) | 0.38 (0.08–1.70) 0.34 (0.08–1.56) | 0.43 (0.05–3.51) 0.30 (0.04–2.52) |
| Other (n = 30) • Nonadjusted • Adjusteda | 1.0 1.0 | 0.65 (0.24–1.71) 0.72 (0.27–1.94) | 0.32 (0.07–1.43) 0.39 (0.09–1.76) | 1.41 (0.43–4.60) 1.34 (0.40–4.55) |
aAdjusted for demographic factors (baseline age, HIV transmission route, and region of origin).
bEstimates could not be calculated.
Risk Factors for Mortality
Lower age at baseline, being of non-European origin, deferral or interruption of antiretroviral therapy, having ALT levels <3.0× ULN, and higher time-updated CD4+ cell counts, as well as time-updated use of a TDF/TAF-containing regimen, were independently associated with a lower risk of all-cause mortality (Table 3). Patients using TDF/TAF had a significantly lower risk for all-cause mortality, with an aHR of 0.47 (95% CI, 0.34–0.64) when compared with those who did not receive TDF/TAF treatment. Factors associated with all-cause mortality also applied for liver-related mortality, with the exception of non-European origin and deferral or interruption of antiretroviral therapy. Cumulative exposure to d4T and/or ddI was strongly associated with liver-related mortality (aHR per additional year of exposure, 1.15; 95% CI, 1.02–1.29). The all-time risk for liver-related mortality among patients who were exposed to ddI/d4T was 7.4% vs 1.6% in patients who never used these agents (P < .001).
Table 3.
Adjusted Hazard Ratios for the Composite End Point of All-Cause Mortality and Liver-Related Mortality
| All-Cause Mortality, HR (95% CI), P Value | Liver-Related Mortality, HR (95% CI), P Value | |
|---|---|---|
| Age at baseline (per 5-y increase) | 1.36 (1.26–1.47), <.001 | 1.26 (1.06–1.51), .01 |
| HIV transmission route • MSM • Other (male) • Other (female) | 1.0 1.42 (1.01–1.98), .048 0.94 (0.55–1.62), .942 | 1.0 0.91 (0.38–2.18), .837 0.95 (0.27–3.33), .932 |
| Region of origin • European • Other | 1.0 0.66 (0.46–0.95), .024 | 1.0 0.53 (0.21–1.32), .173 |
| ALT (category)a • <3× ULN • ≥3× ULN | 1.0 2.38 (1.48–3.82), <.001 | 1.0 4.00 (1.62–9.89), .003 |
| CD4+ count square root (per unit increase)a | 0.88 (0.86–0.90), <.001 | 0.87 (0.82–0.92), <.001 |
| Use of TAF/TDFa • No • Yes | 1.0 0.47 (0.34–0.64), <.001 | 1.0 0.44 (0.22–0.88), .020 |
| Cumulative ddI/d4T use (per 1-y increase)a | 1.05 (0.98–1.11), .16 | 1.15 (1.02–1.29), .025 |
| Deferral or interruption of antiretroviral therapya • No • Yes | 1.0 1.98 (1.44–2.73), <.001 | 1.0 1.91 (0.57–2.48), .640 |
Adjusted for baseline age, transmission route, region of origin, time-updated CD4+ cell count, time-updated ALT levels, time-updated use of TDF/TAF, time-updated cumulative ddI/d4T use, and ever deferment or interruption of antiretroviral therapy.
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; d4T, stavudine; ddI, didanosine; HR, hazard ratio; MSM, men who have sex with men; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ULN, upper limit of normal (35 IU/L).
aTime-updated variables.
Liver-Related Morbidity
Of the 1301 patients included in the cohort, 325 (25%) were classified as having SCLD, 61 (5%) with a definitive diagnosis and 264 (20%) with a presumptive diagnosis. The majority of the cases of definitive SCLD were established after 2003 (79% of the total), with the highest number of incident definitive SCLD in 2015 (n = 10). Of the 61 patients with definitive SCLD, 41 (67%) were still alive at the end of the study period. During follow-up, there were 17 cases (1%) of hepatocellular carcinoma—with the first case diagnosed in 2003 and the last in 2013. One patient in our cohort underwent a liver transplantation as a result of ESLD.
DISCUSSION
This is one of the first studies evaluating trends in risk of mortality in HIV/HBV-coinfected individuals during the cART era. We build on previous studies in the general HIV-positive population by assessing exclusively an HIV/HBV-coinfected population with extensive follow-up of up to 20 years. In this study, we found a marked decrease in risk of all-cause, AIDS-related, and liver-related mortality among patients diagnosed after 2002 compared with those diagnosed in earlier years. These findings are likely the result of a shift from moderately effective and potentially toxic antiviral therapy with limited anti-HBV activity toward highly potent and much less toxic antiretroviral drugs including agents with potent activity against HBV.
Several large cohort studies have established declining mortality rates in patients living or diagnosed with HIV during the modern cART era compared with earlier calendar periods. For example, the D:A:D Study Group showed a steep decline in mortality rates over the past decade for almost all underlying causes of death, with the exception of non-AIDS malignancy [10]. Although 11% of almost 50 000 HIV-positive individuals included in this study had HIV/HBV coinfection, no analysis of mortality rates in this subgroup of patients was reported. In another study conducted in HIV/HBV-coinfected patients, Klein et al. [17] failed to demonstrate a significant decline in ESLD-adjusted incidence rate ratios in the “late cART era” (2006–2010) compared with the “early cART era” (1996–2000). This may have been the result of a relatively short median follow-up time of 2.9 years and low uptake of anti-HBV treatment in the late cART era (only 65% of the HIV/HBV-coinfected patients received tenofovir-containing cART). With a much longer follow-up and increased uptake of TDF/TAF-containing regimens in the ATHENA cohort, we were able to establish that the use of tenofovir was one of the strongest factors associated with a decrease in both all-cause and liver-related mortality. It was remarkable that the mortality risk for the separate calendar periods 2003–2007 and 2008–2017 was not significantly reduced compared with patients diagnosed in the pretenofovir era; this was probably the result of a lack of power leading to wide confidence intervals. Our findings are in line with numerous reports demonstrating that the use of tenofovir diminishes the risk for hepatocellular carcinoma [18] and all-cause and liver-related mortality [19]. Taken together, tenofovir-containing regimens, in the absence of major contraindications, should be strongly encouraged in HIV/HBV coinfection.
In addition to the declining risk of mortality in this cohort, we observed that the influx of new HIV/HBV-coinfected patients in our cohort decreased drastically from 2005, with no such patients entering the cohort in the last year of the study period. The declining rate of new (acute) HBV infections matches trends observed in the general European population [20]. In the ATHENA cohort, the overall prevalence of chronic HBV coinfection among HIV-positive individuals has decreased from 9.8% in 1998 to 5.8% in 2018 [14]. These trends are likely the result of vaccination campaigns carried out by the Dutch Community Health Services in high-risk populations and awareness among HIV-treating physicians to offer HBV vaccination services to nonimmune patients [21]. Furthermore, there is increasing evidence for the prophylactic effects of TDF/TAF against HBV acquisition [22]. The extensive uptake of tenofovir-containing regimens provided a prophylactic benefit for HIV-monoinfected patients and virological suppression, leading to reduced onward transmission for HBsAg-positive patients, both of which probably contributed to fewer new cases.
We observed a strong association between the cumulative usage of ddI/d4T and the risk for liver-related mortality. The hepatotoxic potential of these drugs was already recognized in the 1990s after several case reports described patients developing fulminant hepatitis with microvesicular steatosis by histological examination [23]. However, later reports identified the use of these thymidine—and deoxyadenosine—analogues to be also associated with the development of liver fibrosis and cirrhosis [12]. Both d4T and ddI are strong inhibitors of the mitochondrial polymerase-γ, which is essential for mitochondrial DNA (mtDNA) replication. Inhibition of polymerase-γ leads to a loss of functional mitochondria and subsequently hepatic lipid accumulation and steatohepatitis [24]. The close interplay between these agents and mitochondrial toxicity could explain the increased risk of liver-related mortality with their use. Although d4T and ddI should no longer be used, clinicians should remain aware that patients ever exposed to these drugs may be at continued increased risk of liver-related disease.
Our data show that current treatment is highly successful, but challenges remain. A remarkable finding was that a significant part of the patients in our cohort did not receive any HBV-active agents or only lamivudine. A potential explanation may include patients having documented HBsAg clearance or controlled HBV infection with only lamivudine. Nonetheless, we found that several patients switched to a single-tablet regimen with possibly ineffective HBV-active agents. The introduction of tenofovir as part of ART may have reduced clinicians’ concern about HBV coinfection, including the need for regular HBV-DNA monitoring, given the virtual 0 risk of selecting HBV-resistant mutants on tenofovir [25]. Data from France have reported, however, that ~15% of patients on TDF-containing cART display persistent HBV viremia even after years of treatment [26]. Such patients are less likely to achieve HBsAg and HBeAg loss, but the impact on clinical end points is unknown. In addition, a recent study showed that adherence to HCC screening in patients with HIV/HBV coinfection with advanced fibrosis/cirrhosis was strikingly low [27]; in this cohort, only 5%–18% of the patients underwent biannual HCC screening in accordance with guidelines. Although treatment of HBV has become simpler in the tenofovir era, physicians need to remain vigilant on HBV management. Our HIV/HBV cohort is aging, with currently nearly half of the patients being ≥50 years—placing this population at risk for several age-related diseases. Currently, nonalcoholic fatty liver disease (NAFLD) is one of the most pervasive liver-related comorbidities in HIV-positive populations [28]. Even in the setting of optimal HIV/HBV treatment, a notable proportion of coinfected patients displayed significant fibrosis in Sterling et al. [29] and in the ATHENA cohort [30]. Therefore, the extra hit due to NAFLD could potentially lead to increased progression toward ESLD.
Our study has some limitations. Given the many changes in immunological recovery, viral suppression of both HIV and HBV, and improvement in antiretroviral medication occurring simultaneously over calendar periods, it is difficult to state which of these had a specific effect on liver-related mortality. Furthermore, the ATHENA cohort is a real-life cohort based on data that are gathered at different treatment sites during routine care. For this reason, other data related to liver-related or cause-specific mortality, such as time-updated alcohol use, liver-specific laboratory results, and HBV serological markers, are not collected in a standardized manner, and not all could be taken into account in the analyses.
In conclusion, we demonstrate that HIV/HBV-coinfected patients diagnosed after 2002 were far more likely to survive than patients diagnosed in the early cART era, coinciding with the introduction of safe and highly efficacious antiretroviral medications against HIV and HBV. Importantly, our data demonstrate a need for continued awareness by physicians to maintain optimal HBV suppression. Future research should focus on how the aging HIV/HBV-coinfected population is affected by comorbidities like NAFLD and how the decline in mortality risk compares to populations with HIV monoinfection.
Acknowledgments
Financial support. The ATHENA cohort is managed by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment.
Potential conflicts of interest. Berend van Welzen: no conflict of interest. Colette Smit: grants from the Netherlands Ministry of Health, Welfare and Sport, National Institute for Public Health and the Environment, Centre for Infectious Disease Control during the conduct of the study. Anders Boyd: grants from ANRS and Sidaction outside the submitted work. Faydra Lieveld: no conflict of interest. Tania Mudrikova: grant from Gilead and honoraria paid to the institution for scientific advisory board participation from Viiv outside the submitted work. Peter Reiss: grants from Gilead Sciences, Merck, and ViiV Healthcare and honoraria paid to the institution for scientific advisory board participation from Gilead Sciences, ViiV Healthcare, and Merck & Teva outside the submitted work. Annemarie Brouwer: no conflict of interest. Andy Hoepelman: no conflict of interest. Joop Arends: honoraria paid to the institution for scientific advisory board participation from Gilead, MSD, and ViiV healthcare outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Clinical centers. Amsterdam UMC, AMC site, Amsterdam: HIV treating physicians: M. van der Valk,* S. E. Geerlings, A. Goorhuis, J. W. Hovius, B. Lempkes, F. J. B. Nellen, T. van der Poll, J. M. Prins, P. Reiss, M. van Vugt, W. J. Wiersinga, F. W. M. N. Wit. HIV nurse consultants: M. van Duinen, J. van Eden, A. Hazenberg, A. M. H. van Hes, F. J. J. Pijnappel, S. Y. Smalhout, A. M. Weijsenfeld. HIV clinical virologists/chemists: S. Jurriaans, N. K. T. Back, H. L. Zaaijer, B. Berkhout, M. T. E. Cornelissen, C. J. Schinkel, K. C. Wolthers. Amsterdam UMC, VUmc site, Amsterdam: HIV treating physicians: E. J. G. Peters,* M. A. van Agtmael, R. S. Autar, M. Bomers, K. C. E. Sigaloff. HIV nurse consultants: M. Heitmuller, L. M. Laan. HIV clinical virologists/chemists: C. W. Ang, R. van Houdt, M. Jonges. Emma Kinderziekenhuis (Amsterdam UMC, AMC site): HIV treating physicians: T. W. Kuijpers, D. Pajkrt, H. J. Scherpbier. HIV nurse consultants: C. de Boer, A. van der Plas, A. M. Weijsenfeld. Admiraal De Ruyter Ziekenhuis, Goes: HIV treating physicians: M. van den Berge,* A. Stegeman. HIV nurse consultants: S. Baas, L. Hage de Looff. HIV clinical virologists/chemists: A. Buiting, A. Reuwer, J. Veenemans, B. Wintermans. Catharina Ziekenhuis, Eindhoven: HIV treating physicians: M. J. H. Pronk,* H. S. M. Ammerlaan. HIV nurse consultants: D. N. J. van den Bersselaar, E. S. de Munnik. HIV clinical virologists/chemists: B. Deiman, A. R. Jansz, V. Scharnhorst, J. Tjhie, M. C. A. Wegdam. DC Klinieken Lairesse—HIV Focus Centrum: HIV treating physicians: A. van Eeden,* J. Nellen, M. van der Valk. HIV nurse consultants: W. Brokking, L. J. M. Elsenburg, H. Nobel. HIV clinical virologists/chemists: C. J. Schinkel. ETZ (Elisabeth-TweeSteden Ziekenhuis), Tilburg: HIV treating physicians: M. E. E. van Kasteren,* M. A. H. Berrevoets, A. E. Brouwer. HIV nurse consultants: A. Adams, R. van Erve, B. A. F. M. de Kruijf-van de Wiel, S. Keelan-Phaf, B. van de Ven. Data collection: B. A. F. M. de Kruijf-van de Wiel, B. van der Ven. HIV clinical virologists/chemists: A. G. M. Buiting, J. L. Murck. Erasmus MC, Rotterdam: HIV treating physicians: T. E. M. S. de Vries-Sluijs,* H. I. Bax, E. C. M. van Gorp, N. C. de Jong-Peltenburg, M. de Mendonça Melo, E. van Nood, J. L. Nouwen, B. J. A. Rijnders, C. Rokx, C. A. M. Schurink, L. Slobbe, A. Verbon. HIV nurse consultants: N. Bassant, J. E. A. van Beek, M. Vriesde, L. M. van Zonneveld. Data collection: J. de Groot. HIV clinical virologists/chemists: C. A. B. Boucher, M. P. G Koopmans, J. J. A van Kampen. Erasmus MC–Sophia, Rotterdam: HIV treating physicians: P. L. A. Fraaij, A. M. C. van Rossum, C. L. Vermont. HIV nurse consultants: L. C. van der Knaap, E. Visser. Flevoziekenhuis, Almere: HIV treating physicians: J. Branger,* R. A. Douma. HIV nurse consultant: A. S. Cents-Bosma, C. J. H. M. Duijf-van de Ven. HagaZiekenhuis, Den Haag: HIV treating physicians: E. F. Schippers,* C. van Nieuwkoop. HIV nurse consultants: J. M. van IJperen, J. Geilings. Data collection: G. van der Hut. HIV clinical virologist/chemist: N. D. van Burgel. HMC (Haaglanden Medisch Centrum), Den Haag: HIV treating physicians: E. M. S. Leyten,* L. B. S. Gelinck, F. Mollema. HIV nurse consultants: S. Davids-Veldhuis, C. Tearno, G. S. Wildenbeest. HIV clinical virologists/chemists: E. Heikens. Isala, Zwolle: HIV treating physicians: P. H. P. Groeneveld,* J. W. Bouwhuis, A. J. J. Lammers. HIV nurse consultants: S. Kraan, A. G. W. van Hulzen, M. S. M. Kruiper. Data collection: G. L. van der Bliek, P. C. J. Bor. HIV clinical virologists/chemists: S. B. Debast, G. H. J. Wagenvoort. Leids Universitair Medisch Centrum, Leiden: HIV treating physicians: F. P. Kroon,* M. G. J. de Boer, H. Jolink, M. M. C. Lambregts, A. H. E. Roukens, H. Scheper. HIV nurse consultants: W. Dorama, N. van Holten. HIV clinical virologists/chemists: E. C. J. Claas, E. Wessels. Maasstad Ziekenhuis, Rotterdam: HIV treating physicians: J. G. den Hollander,* R. El Moussaoui, K. Pogany. HIV nurse consultants: C. J. Brouwer, J. V. Smit, D. Struik-Kalkman. Data collection: T. van Niekerk. HIV clinical virologists/chemists: O. Pontesilli. Maastricht UMC+, Maastricht: HIV treating physicians: S. H. Lowe,* A. M. L. Oude Lashof, D. Posthouwer, M. E. van Wolfswinkel. HIV nurse consultants: R. P. Ackens, K. Burgers, J. Schippers. Data collection: B. Weijenberg-Maes. HIV clinical virologists/chemists: I. H. M. van Loo, T. R. A. Havenith. Medisch Centrum Leeuwarden, Leeuwarden: HIV treating physicians: M. G. A. van Vonderen,* L. M. Kampschreur. HIV nurse consultants: S. Faber, R. Steeman-Bouma. HIV clinical virologists/chemists: A. Al Moujahid. Medisch Spectrum Twente, Enschede: HIV treating physicians: G. J. Kootstra,* C. E. Delsing. HIV nurse consultants: M. van der Burg-van de Plas, L. Scheiberlich. Noordwest Ziekenhuisgroep, Alkmaar: HIV treating physicians: W. Kortmann,* G. van Twillert,* R. Renckens. HIV nurse consultant and data collection: D. Ruiter-Pronk, F. A. van Truijen-Oud. HIV clinical virologists/chemists: J. W. T. Cohen Stuart, E. R. Jansen, M. Hoogewerf, W. Rozemeijer, W. A. van der Reijden, J. C. Sinnige. OLVG, Amsterdam: HIV treating physicians: K. Brinkman,* G. E. L. van den Berk, W. L. Blok, K. D. Lettinga, M. de Regt, W. E. M. Schouten, J. E. Stalenhoef, J. Veenstra, S. M. E. Vrouenraets. HIV nurse consultants: H. Blaauw, G. F. Geerders, K. Hoeksema, M. J. Kleene, M. Kok, M. Knapen, I. B. van der Meché, E. Mulder-Seeleman, A. J. M. Toonen, S. Wijnands, E. Wttewaal. HIV clinical virologists: D. Kwa. Radboudumc, Nijmegen: HIV treating physicians: R. van Crevel,* K. van Aerde, A. S. M. Dofferhoff, S. S. V. Henriet, H. J. M. ter Hofstede, J. Hoogerwerf, M. Keuter, O. Richel. HIV nurse consultants: M. Albers, K. J. T. Grintjes-Huisman, M. de Haan, M. Marneef, R. Strik-Albers. HIV clinical virologists/chemists: J. Rahamat-Langendoen, F. F. Stelma. HIV clinical pharmacology consultant: D. Burger. Rijnstate, Arnhem: HIV treating physicians: E. H. Gisolf,* R. J. Hassing, M. Claassen. HIV nurse consultants: G. ter Beest, P. H. M. van Bentum, N. Langebeek. HIV clinical virologists/chemists: R. Tiemessen, C. M. A. Swanink. Spaarne Gasthuis, Haarlem: HIV treating physicians: S. F. L. van Lelyveld,* R. Soetekouw. HIV nurse consultants: L. M. M. van der Prijt, J. van der Swaluw. Data collection: N. Bermon. HIV clinical virologists/chemists: W. A. van der Reijden, R. Jansen, B. L. Herpers, D. Veenendaal. Medisch Centrum Jan van Goyen, Amsterdam: HIV treating physicians: D. W. M. Verhagen, F. N. Lauw. HIV nurse consultants: M. C. van Broekhuizen, M. van Wijk. Universitair Medisch Centrum Groningen, Groningen: HIV treating physicians: W. F. W. Bierman,* M. Bakker, J. Kleinnijenhuis, E. Kloeze, A. Middel, D. F. Postma, E. H. Schölvinck, Y. Stienstra, C. L. A. R. Verhage, M. Wouthuyzen-Bakker. HIV nurse consultants: A. Boonstra, H. de Groot-de Jonge, P. A. van der Meulen, D. A. de Weerd. HIV clinical virologists/chemists: H. G. M. Niesters, C. C. van Leer-Buter, M. Knoester. Universitair Medisch Centrum Utrecht, Utrecht: HIV treating physicians: A. I. M. Hoepelman,* J. E. Arends, R. E. Barth, A. H. W. Bruns, P. M. Ellerbroek, T. Mudrikova, J. J. Oosterheert, E. M. Schadd, B. J. van Welzen. HIV nurse consultants: K. Aarsman, B. M. G. Griffioen-van Santen, I. de Kroon. Data collection: M. van Berkel, C. S. A. M. van Rooijen. HIV clinical virologists/chemists: R. Schuurman, F. Verduyn-Lunel, A. M. J. Wensing. Wilhelmina Kinderziekenhuis, UMC Utrecht, Utrecht: HIV treating physicians: L. J. Bont, S. P. M. Geelen, Y. G. T. Loeffen, T. F. W. Wolfs. HIV nurse consultants: N. Nauta. Sint Elisabeth Hospital, Willemstad, Curaçao: HIV treating physicians: E. O. W. Rooijakkers, H. Holtsema, R. Voigt, D. van de Wetering. HIV nurse consultants: A. Alberto, I. van der Meer. HIV clinical virologists/chemists: A. Rosingh, T. Halaby.
Coordinating center. Director: P. Reiss. Deputy director: S. Zaheri. Data analysis: A. C. Boyd, D. O. Bezemer, A. I. van Sighem, C. Smit, F. W. M. N. Wit. Data management and quality control: M. Hillebregt, A. de Jong, T. Woudstra. Data monitoring: D. Bergsma, R. Meijering, L. van de Sande, T. Rutkens, S. van der Vliet. Data collection: L. de Groot, M. van den Akker, Y. Bakker, A. El Berkaoui, M. Bezemer, N. Brétin, E. Djoechro, J. Geerlinks, M. Groters, E. Kruijne, K. J. Lelivelt, C. Lodewijk, E. Lucas, R. van der Meer, L. Munjishvili, F. Paling, B. Peeck, C. Ree, R. Regtop, Y. Ruijs, M. Schoorl, P. Schnörr, A. Scheigrond, E. Tuijn, L. Veenenberg, K. M. Visser, E. C. Witte. Patient registration: Y. Ruijs.
*Denotes site coordinating physician.
References
- 1. UNAIDS. UNAIDS data 2019 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf. Accessed 1 April 2020.
- 2. Singh KP, Crane M, Audsley J, et al. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017; 31:2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Sighem AI, Wit FWNM, Boyd A, Smit C, Matser A, Reiss P.. Monitoring Report 2019. Human Immunodeficiency Virus (HIV) Infection in the Netherlands. Amsterdam: Stichting HIV Monitoring; 2019. [Google Scholar]
- 4. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009; 48:1763–71. [DOI] [PubMed] [Google Scholar]
- 5. Lieveld FI, Smit C, Richter C, et al. Liver decompensation in HIV/hepatitis B coinfection in the combination antiretroviral therapy era does not seem increased compared to hepatitis B mono-infection. Liver Int 2019; 39:470–83. [DOI] [PubMed] [Google Scholar]
- 6. Prescott LM. Lamivudine useful against hepatitis B-HIV co-infection. J Int Assoc Physicians AIDS Care 1995; 1:34. [PubMed] [Google Scholar]
- 7. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006; 20:863–70. [DOI] [PubMed] [Google Scholar]
- 8. Benhamou Y, Tubiana R, Thibault V. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N Engl J Med 2003; 348:177–8. [DOI] [PubMed] [Google Scholar]
- 9. European AIDS Clinical Society (EACS). Guidelines. Version 10.0.2019. Available at: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf.
- 10. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet (London, England) 2014; 384:241–248. [DOI] [PubMed] [Google Scholar]
- 11. Boender TS, Smit C, Sighem AV, et al. ; ATHENA national observational HIV cohort AIDS Therapy Evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018; 8:e022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anadol E, Lust K, Boesecke C, et al. Exposure to previous cART is associated with significant liver fibrosis and cirrhosis in human immunodeficiency virus-infected patients. PLoS One 2018; 13:e0191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/. Accessed 1 March 2020. [Google Scholar]
- 14. van Sighem A, Boender TS, Wit FWNM, Smit C, Matser A, Reiss P. HIV monitoring report. Human immunodeficiency virus (HIV) infection in the Netherlands 2018. 2018. Available at: http://www.hiv-monitoring.nl. Accessed 15 March 2020.
- 15. Kowalska JD, Friis-Møller N, Kirk O, et al. ; CoDe Working Group; D:A:D Study Group The Coding causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496– 509. [Google Scholar]
- 17. Klein MB, Althoff KN, Jing Y, et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016; 63:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wandeler G, Mauron E, Atkinson A, et al. ; Swiss HIV Cohort Study, Athena Observational Cohort Study, EuroSIDA, ANRS CO3 Aquitaine Cohort Incidence of hepatocellular carcinoma in HIV/HBV-coinfected patients on tenofovir therapy: relevance for screening strategies. J Hepatol 2019; 71:274–80. [DOI] [PubMed] [Google Scholar]
- 19. Tsai W-C, Hsu W-T, Liu W-D, et al. Impact of antiretroviral therapy containing tenofovir disoproxil fumarate on the survival of patients with HBV and HIV coinfection. Liver Int 2019; 39:1408–17. [DOI] [PubMed] [Google Scholar]
- 20. Ott JJ, Horn J, Krause G, Mikolajczyk RT. Time trends of chronic HBV infection over prior decades—a global analysis. J Hepatol 2017; 66:48–54. [DOI] [PubMed] [Google Scholar]
- 21. Mangen MJ, Stibbe H, Urbanus A, et al. ; National Working Group of hepatitis B behavioural risk-groups vaccination program Targeted outreach hepatitis B vaccination program in high-risk adults: the fundamental challenge of the last mile. Vaccine 2017; 35:3215–21. [DOI] [PubMed] [Google Scholar]
- 22. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014; 28:999–1005. [DOI] [PubMed] [Google Scholar]
- 23. Lai KK, Gang DL, Zawacki JK, Cooley TP. Fulminant hepatic failure associated with 2’,3’-dideoxyinosine (ddI). Ann Intern Med 1991; 115:283–4. [DOI] [PubMed] [Google Scholar]
- 24. Walker UA, Bäuerle J, Laguno M, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology 2004; 39:311–7. [DOI] [PubMed] [Google Scholar]
- 25. de Vries-Sluijs TEMS, Reijnders JGP, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology 2010; 139:1934–1941. [DOI] [PubMed] [Google Scholar]
- 26. Boyd A, Gozlan J, Maylin S, et al. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: virological and clinical implications. Hepatology 2014; 60:497–507. [DOI] [PubMed] [Google Scholar]
- 27. Willemse S, Smit C, Sogni P, et al. ; Hepatocellular Carcinoma Screening Project Working Group for the Collaboration of Observational HIV on behalf of Epidemiological Research Europe (COHERE) in EuroCoord. Low compliance with hepatocellular carcinoma screening guidelines in hepatitis B/C virus co-infected HIV patients with cirrhosis. J Viral Hepat 2019; 26:1224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Welzen BJ, Mudrikova T, El Idrissi A, et al. A review of non-alcoholic fatty liver disease in HIV-infected patients: the next big thing? Infect Dis Ther 2019; 8:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterling RK, Wahed AS, King WC, et al. Spectrum of liver disease in hepatitis B virus (HBV) patients co-infected with human immunodeficiency virus (HIV): results of the HBV-HIV Cohort Study. Am J Gastroenterol 2019; 114:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smit C, Boyd A, Van Der Valk M, Arends J, Reiss P. HIV monitoring report. Chapter 4: viral hepatitis 2019. Available at: https://www.hiv-monitoring.nl/application/files/5615/7622/5185/197778_HIV_RAPPORT_2019-IA_Chapter_4.pdf. Accessed 1 April 2020.