Abstract
Significant improvements have taken place in our understanding of classification neuroendocrine neoplasms of the pancreas in the past decade. These are now regarded in three entirely separate categories: (I) neuroendocrine tumors (PanNETs) are by definition well differentiated, the pancreatic counterpart of carcinoids; (II) neuroendocrine carcinomas, which are poorly differentiated (PDNEC), the pancreatic examples of small cell carcinomas or large cell neuroendocrine carcinomas; (III) other neoplasms that have neuroendocrine differentiation or a distinct neuroendocrine component. PanNETs are by far the most common. They are now regarded as malignancies (albeit often curable when low grade and low stage) with the exception of minute incidental proliferations (tumorlets, or dysplastic-like changes) seen in the setting of some syndromes like MEN. PanNETs are staged based on their size, and for small T1 tumors, watchful waiting is now being considered, although these tumors are also known to show about 10% metastatic rate and/or progression, creating concerns about this approach. PanNETs are graded into 3, based on the proliferative activity, mostly based on the Ki-67 index, and also partly mitotic activity, although the latter seldom if ever is the determinant of the final grade. Neuroendocrine neoplasms with well differentiated morphology but Ki-67 >20% are now regarded as PanNET Grade 3 (G3); they have been shown to have a prognosis significantly worse than lesser grade PanNETs but still incomparably better than frank PDNECs, the latter typically has Ki-67 >50% (often much higher) and require platinum-based chemotherapy. There are also cases that are ambiguous between PanNET-G3 and PDNEC, and very rarely transformation of the former to the latter appears to occur. For low grade (G1/G2) PanNETs, more refined criteria to further prognosticate this group are needed. Morphologic variants being recognized may bring new perspectives to this group.
Keywords: Pancreas, neuroendocrine neoplasm, tumor, carcinoma, pathology, terminology, classification, prognosis, WHO 2019
Introduction
The concepts regarding neuroendocrine neoplasms arising in the pancreas, their terminology, classification and prognostication have been ever-evolving (1-3). In the past decade, the efforts of European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Society (NANETS) have begun to merge with multiple international collaborative studies and consensus manuscripts (4-6), leading to several improvements and revisions, including the updated category designation of “Pancreatic Neuroendocrine Neoplasm (PanNEN)”, with the recognition of the rarer poorly differentiated pancreatic neuroendocrine carcinomas (PDNECs) as a separate tumor type different than conventional well-differentiated neuroendocrine tumors (PanNETs) of this organ, also included in the World Health Organization (WHO) 2019 classification (2,7). Consequently, a more unified approach to the grading and prognostication of these neoplasms have been achieved. Many of the molecular/genetic characteristics of these tumors have been elucidated (8-14). However, at the same time, many of the controversies and gaps regarding these tumors also still remain. In the ensuing text, an update regarding the current concepts and controversies in the pathology of “PanNENs” will be provided.
Evolution of terminology
Originally believed to be tumors arising from islets of Langerhans cells, these neoplasms had been initially designated “islet cell tumors” and “islet cell carcinomas” (15,16). However, because of their analogy with other endocrine neoplasms and carcinoids, and because the cell of origin is not necessarily provable to be the islets, the terminology of “endocrine tumor” was adopted instead (17). Later, the term was modified to “neuroendocrine” with the goal of distinguishing their identity from neoplasms of endocrine organs such as adrenal and thyroid.
Transformation of biologic perspective: all pancreatic neuroendocrine neoplasms are malignant
The indolent nature of these tumors was appreciated early on, and in fact, it was believed that smaller examples may be benign, thus the term “adenoma” was used for years (18). In the 1996 fascicle of Armed Forces Institute of Pathology, a category of “macroadenoma” was recognized for those <3 cm. Later, it became clear that all PanNETs with the exception of those “dysplastic-like” islet cells proliferations that are found in the setting of genetic syndromes like MEN1 or “adenomatosis” syndromes (19,20), or perhaps those diminutive incidental lesions (<0.5 cm), all PanNETs are malignant, albeit mostly slow-growing and often curable, especially if small and low grade (2,3,21,22). Please see below for prognostication and size issues.
There were slightly different views from Europe versus the US regarding the biology of neoplasms with neuroendocrine differentiation. The “adenoma” category used by European authors (23) was never really endorsed in the US, with most experts regarding all PanNETs as malignant, and in fact some proposing to call all of them carcinoma and to grade them as 1 to 3, with 1 corresponding the carcinoid, 2 to atypical carcinoids and 3 to full-blown small cell and large cell PDNECs (24,25); however, this approach, while a better reflection of biology and tumor categorization criteria, nevertheless was not adopted.
“Pancreatic Neuroendocrine Tumor (PanNET)” vs. “Poorly Differentiated Pancreatic Neuroendocrine Carcinoma (PDNEC)” as two distinct entities
In WHO-2017 endocrine blue book (26) and later in WHO-2019 for gastrointestinal tract (2), PanNENs were recognized as an over-arching conceptual category, but at the same time two very distinct tumor categories were recognized, determined solely on morphology: (I) PanNETs, which are the well differentiated neuroendocrine neoplasms representing what used to be known as “islet cell tumors/carcinomas”, or the pancreatic counterparts of “carcinoids”, with typically low proliferative activity, median Ki-67 (<5%) and only occasional examples above 20%, which are now graded as PanNET Grade 3 (see Figures 1-3); (II) PDNECs, which are the high-grade (poorly differentiated) carcinomas with clear-cut neuroendocrine lineage, are essentially the pancreatic counterparts of small cell lung cancers or large-cell neuroendocrine carcinomas of the lung, showing very high proliferative index (Ki-67 typically >50%), and thus much more aggressive and more responsive to platinum based treatments, albeit temporarily (see Figure 4) (27,28).
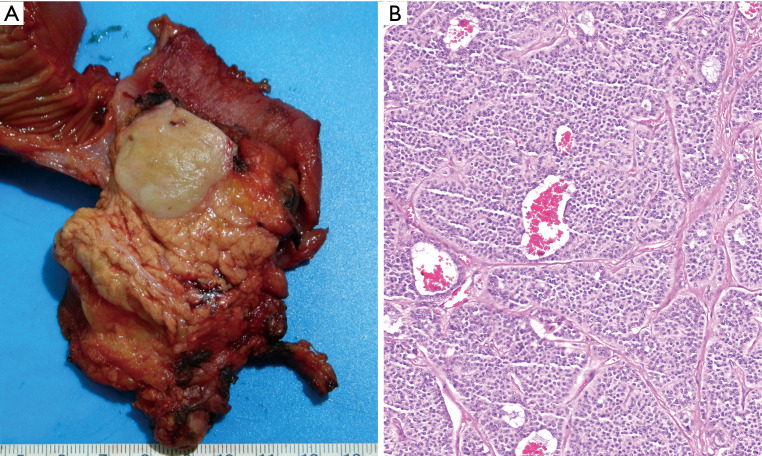
Figure 1.
Unlike pancreatic ductal adenocarcinomas which are scirrhous ill-defined lesions, the pancreatic neuroendocrine tumors typically form a well-demarcated, relatively homogenous and fleshy tumors (A). This is because they are fairly cellular neoplasia in which there is minimal or no stromal desmoplastic component but rather a delicate fibro-vascular stroma rich in capillaries (which are also often appreciated at the radiologic level), intervening in between the nests of cells (Hematoxylin & eosin, 40× magnification) (B).
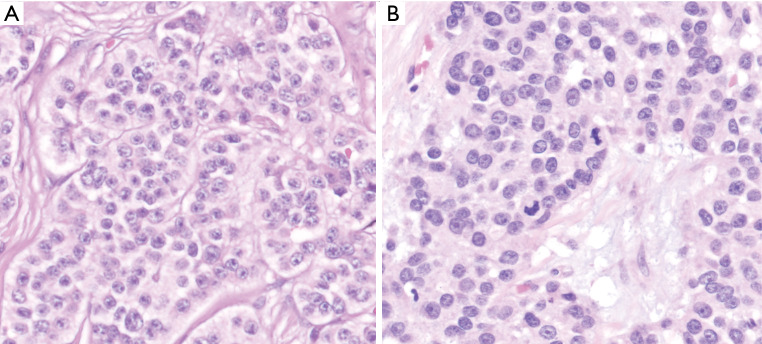
Figure 2.
At the high power microscopic examination, typical PanNETs form nested pattern (distinct, demarcated clusters) composed of uniform monotonous cells with fair amount of cytoplasm and the distinctive “salt and pepper” chromatin pattern characteristic of this tumor type (Hematoxylin & eosin, 100× magnification) (A). Although mitotic activity is generally low, they can be readily evident in some cases (B) which, if >2 per 10 high power fields, places the tumor in higher grade categories (Hematoxylin & eosin, 100× magnification).
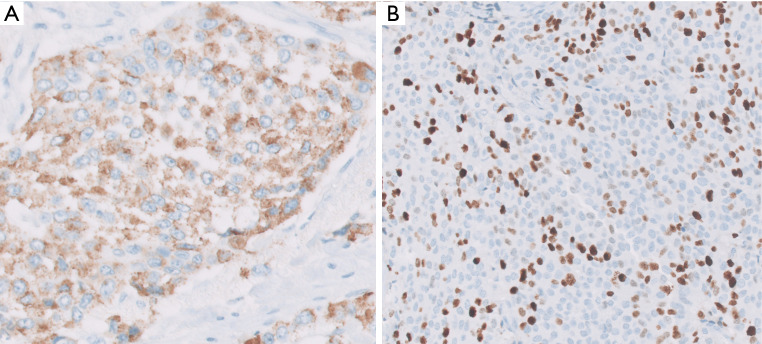
Figure 3.
Neuroendocrine markers of synaptophysin, chromogranin and CD56 are expressed in the vast majority of PanNETs, detectable by immunohistochemical analysis. Among these, chromogranin is the most specific, while others can be seen in other tumor types. Chromogranin immunostain typically shows a granular pattern (A) because it highlights the neurosecretory granules that are abundant in the tumor cells (Chromogranin immunostain, 100× magnification). Ki-67 immunostain is performed to assess the proliferative index of the tumor. In this case, the index was found to be >20% in the “hot spot” (B) placing the tumor into the “NET G3” category (previously called “grade discordant”) (Ki-67 immunostain, 40× magnification).
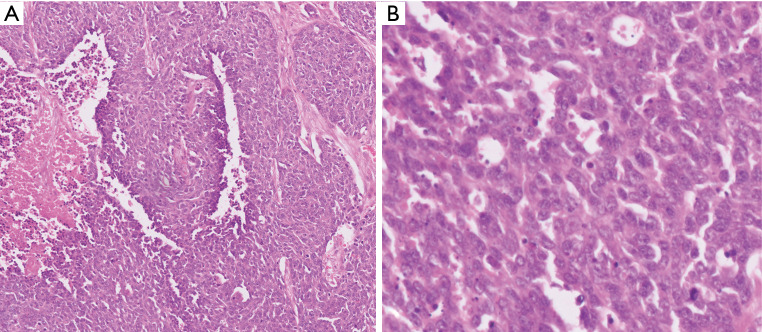
Figure 4.
PDNECs are high-grade carcinomas that often show more diffuse growth pattern and necrosis (A) and displaying overt signs of cytologic malignancy along with mitotic figures and apoptotic bodies (B) [Hematoxylin & eosin, 40× (A) and 100× (B) magnification].
Rather than being two ends of a spectrum, these two (PanNETs and PDNECs) are currently widely agreed to be two entirely independent categories with different origins and different biology, a belief that has been held by us and many others in the US for years (29). PDNECs are in essence neuroendocrine (or stem cell) counterparts of poorly differentiated carcinomas/adenocarcinomas, sometimes arising in association with them but almost never from well differentiated PanNETs, and often showing molecular/genetic alterations placing them phylogenetically closer to adenocarcinomas (30). In contrast, PanNETs are closer to endocrine neoplasia, not showing association with glandular epithelial malignancies, and showing molecular/genetic alterations of endocrine tumors like MEN1, Neurofibromatosis, or von-Hippel Lindau syndromes and not the molecular profile of ductal neoplasia (3,8-10). Additionally, expression of somatostatin receptor on the cell membranes is a typical feature of PanNETs, meanwhile PDNECs do not express this molecule.
Thus, it is now clear that PanNETs and PDNECs are not really a spectrum but distinct entities. Having said that, in a small number of cases that is referred to as “ambiguous category” (31), they can be impossible to distinguish from each other, moreover, there seems to be occasional cross-overs and transformations of a well differentiated PanNET to PDNEC. However, this is very unusual and does not negate the concept. This is an area that needs further clarification.
PanNET-lets: precursors and early/microscopic PanNETs
Precursor type PanNETs can be encountered in MEN1 patients or those with glucagon cell adenomatosis or insulin cell adenomatosis, consisting of proliferative islets of variable sizes and shapes (19,20,32). These can be regarded as “dysplastic” at least conceptually, if not diagnostically, when they form a clonal population forming a round demarcated nodule, often with a capsule. However, naturally, the definition of an autonomous/neoplastic process is vague in these cases, fully blown tumors that are <0.5 cm is acknowledged as “microadenomas”, although we report such cases as “incidental microscopic PanNET, microadenoma”. Microadenomas may exhibit loss of heterozygosity for the MEN1 gene, as opposed to their precursor lesions (32), and can also occur incidentally, in the absence of background changes (proliferative islets) or hereditary syndromes.
Prognostication of PanNENs
PanNET or PDNEC
All PanNENs are malignant, perhaps with the exception of the precursor type “dysplastic” lesions or the tumor-lets described above. PanNETs are slow growing neoplasia and those that are low grade and low stage are often curable. PDNECs, on the other hand, are very aggressive malignancies.
Therefore, the most important determination in the evaluation of a PanNEN case, after it is firmly established to be of neuroendocrine lineage, is whether it is a PanNET or a PDNEC. The prognosis of PanNETs overall is incomparably better (and largely in parallel with that of “carcinoids”) whereas PDNECs are highly aggressive and rapidly fatal cancers, as dismal, if not worse than ductal adenocarcinomas (33,34). Even the grade-3 PanNETs, which have higher proliferation index (Ki-67 >20% by definition, and sometimes even up to 50%) are in fact closer to lower grade PanNETs than they are to PDNECs (33). In some cases, however, it is difficult to make this determination and for such cases, immunohistochemical examination has been proposed to make the final classification with p53 overexpression, DPC4 (SMAD4) loss and Retinoblastoma loss pointing towards PDNEC, while loss of ATRX/DAXX in favor of a PanNET (1,3,29,35-37). Similarly, somatostatin receptor 2A expression is incomparably more common and abundant in PanNET than PDNEC, which is not only prognostically significant but also has great importance in terms of stratifying patients into proper management algorithms utilizing inhibitors of this molecule (35). However, this also fails to make the determination in rare occasions, and the case may have to be classified as “ambiguous or indeterminate”. It should be kept in mind tumor heterogeneity can lead to misclassifications and that in time the diagnosis may have to be modified in some cases, but these are very rare occurrences. In such cases, the close clinical evaluation is the only way to appreciate the true intent of the neoplasm.
Stage
As in any malignancy, the stage at presentation is one of the most important determinants of outcome for PanNENs. There used to be different staging systems from different societies (21,38), and until recently, PanNENs were also staged significantly different than other cancers; however, with updated staging systems, the differences have become minimized (2,26).
Lymph node metastasis or distant metastasis which is almost always to liver are perhaps the most significant prognosticators. For the primary tumors, size of the tumor is an important factor and thus is the main parameter in the T-stage. As discussed previously, minute Langerhans islet proliferations can be encountered in the context of some syndromes like MEN, and these can be viewed conceptually as “tumorlets” or “dysplastic like” or the “hyperplastic” islets. Full-blown PanNETs may also be discovered incidentally during workup and resection for other disorders. It has also been shown that PanNETs with small sizes are often curable. All these observations have led to the ‘watchful waiting’ approach, which is now widely advocated for small PanNETs (39-41). However, it should be kept in mind that that even the studies advocating this illustrate about 10% of the full-blown PanNETs show progression within up to 5 years, and if the follow-up is extended to 10 years, this figure is likely to increase significantly. Therefore, until this issue is further clarified, caution is warranted in careful case selection and close follow-up.
Grade
As discussed previously, the high-grade PDNECs—as defined in the lung and that can occur in the pancreas albeit rarely—are now regarded as a distinct tumor category (2,3,5,34). There is no sub-grading system for this group; they are uniformly very aggressive cancers from what we know so far. They are classified as small versus large cell; this is not a grading but rather a classification scheme as in the lungs, but small cell cases may be more aggressive (2,34).
PanNETs, i.e., the well-differentiated neuroendocrine tumors, on the other hand, are graded into 3 based on the proliferative activity of the neoplastic cells as illustrated by Ki-67 and mitotic rate. Of the two, Ki-67 labeling index seems to be more crucial and somewhat more objective and reliable (42). In the United States, many experts were reluctant to use a continuous variable in grading a tumor by calculation, and it is still not employed in the lungs because of this reluctance. However, especially in the absence of any other reliable parameter to stratify the patients, Ki-67 fills the vacuum quite well (43). It is now part of the requirements of College of American Pathologists cancer synoptic protocol and thus routinely used also in the US (44).
There are various aspects of Ki-67 index calculation that needs to be carefully considered. First, per the current WHO criteria, it is the “hot spot” that must be counted (2). This is important because studies have shown that, due to the intratumoral heterogeneity of labeling in the tumors, about a third of the cases are placed in a different grade if “random” area count is performed (45). It should be kept in mind that studies using tissue microarrays are not assessing the “hot spot” and thus would yield very different results (46). Same principles also apply to the interpretation of Ki-67 in small biopsy or cytology specimens, in which the tumor is partially represented.
One problematic issue in calculating the Ki-67 index is whether to count lightly staining nuclei as well or not. Many experts believe that, since Ki-67 is capturing a wide segment of the cell cycle, any labeling is meaningful and should be counted; however, there is no consensus on this matter.
There are also various approaches in counting Ki-67 (47): eyeballing method, which is the most widely used one, has been proven to be highly irreproducible and should be avoided unless the findings are overtly in the extremes, like clearly <1% or obviously >60%. Unfortunately, about 60% of the cases have an index too close to the cut-off zone for the eye to clearly determine the precise grade (47-49). Automated (digital) counting systems are clearly the future, but currently, they need substantial software modification for each case for the instrument to distinguish lymphocytes, endothelial cells and hemosiderin from the true neuroendocrine cells (48,50,51). We have found that currently the most reliable and reproducible approach is to count manually from the image-captured print of the hot-spot. In this approach, after the identification of the hot spot by low power screening of the slide, a photograph of this hot spot is taken and printed, and the positive and negative cells are counted, and final index is given as positives divided by positives + negatives. This allows the calculation of the index in decimals and should be reported as such (47). A Ki-67 index given as a range is usually product of eyeballing calculation and should be considered suspect as a miscalculation.
Mitotic count is the second determinant of the grade in the current grading system. Whichever of the two is higher trumps for the final grade. Mitotic count is given as number of mitotic figures per field area, unlike the Ki-67 index, which is given as percent of cells labeling. In addition to being extremely cumbersome, mitotic count and the literature on it, are fraud with challenges that lead to erroneous impressions. Most of the earlier studies/literature provided the mitotic count result as “per high-power field”, referring to the high-power objective of the microscopes such as 5 mitosis per high-power field. However, it was later recognized that the “power fields” (high-power objectives) of different microscopes vary greatly, often up to 2 folds, meaning a tumor calculated as 5 in one microscope could yield 10 per high-power field in another. In the WHO 2017/2019 (2), it is now mandated that the results are provided by field area, which requires the calculation of the field area of the microscope that the measurement is being taken on. There are other challenges about mitotic count; for example, even what constitutes a mitotic figure is highly subjective. Moreover, counting the stroma poor versus stroma rich areas of the very same tumor with same mitotic activity may yield vastly different results. These render mitotic activity as a very weak partner in this grading scheme. Thankfully, with a few exceptions (42), Ki-67 trumps mitotic count for the final grade in the vast majority (33,52), even when mitotic figures were highlighted by phosphohistone H3 immunohistochemistry (53).
Adjunct prognosticators
For neuroendocrine neoplasia, in various organs, it has been documented that necrosis is a sign of a more rapidly growing tumor and aggressive behavior. In the pancreas, necrosis is a common feature of PDNECs, and in fact is often one of the diagnostic characteristics. However, for PanNETs, the significance of necrosis as a prognostic factor has been somewhat controversial. In the study by Hochwald and Klimstra in 2002, necrosis had appeared as one of the best prognosticators but this could not be verified in other studies (54-56). Also, it is not clear whether necrosis is independent of Ki-67 and mitotic activity, which are all indicators of high proliferative rate. Nevertheless, we believe necrosis should be duly recorded and taken into consideration in management of PanNETs as well.
Along those lines, vascular invasion and perineural invasion are presumed to be indicators of aggressive behavior; however, this presumption has yet to be proven in large-scale studies with long-term follow-up. Currently, their value as prognostic factors is mostly intuitive with variable results in the literature (53,57,58). It is also true for various biological markers. At the immunohistochemical level, especially CK19 and CD117 (C-kit), and more recently ATRX/DAXX have been implicated as signs of aggressive behavior among various other markers, but none have yet proven (9,59-61). A variety of molecular markers are also under investigation for this purpose. As mentioned previously, some of these such p53, loss of Retinoblastoma and loss of SMAD4 which are incomparably more common in PDNECs, and virtually non-existing in PanNETs and are already in clinical use for ambiguous cases.
One important prognostic determinant appears to be the underlying genetic disease if there is one. It has been documented that the PanNETs that develop in the background of syndromes like MEN1 or “glucagon cell adenomatosis”, which are often multifocal and in the background of “dysplastic” like islet cell proliferations, are in fact more benevolent in general (19,20,32,62). If there is exclusive hormone production by the tumor, which also seems to be an indicator of behavior, but it is not clear how much of this is attributable to the cell biology and how much to early detection phenomenon. For example, the vast majority of “insulinomas” are benevolent but they are also often small at the time of diagnosis due to the hormonal manifestations leading to early diagnosis.
Morphologic variants
Microscopic examination of PanNETs can reveal a wide spectrum of histomorphologic variants. The biologic and prognostic significance of these is gradually becoming unraveled. Evolving observations including our recent studies indicate that cases with oncocytic, hepatoid, rhabdoid phenotype, all of which are characterized by rich cytoplasm and nucleolar prominence, which indicates metabolic activity, are associated with significantly larger size, higher grade and higher stage, and higher frequency of metastasis and progression. In contrast, cases showing severe pleomorphism and bizarre nuclei appear to represent a symplastic phenomenon and paradoxically more benevolent characteristics (63,64). Same is also true for paraganglioma-like pattern. There other variants for which the clinicopathologic characteristics have yet to be determined. Of note, these variants can create a substantial diagnostic challenge for pathologists, they are prone to be misdiagnosed as other tumor types (64). Additionally, some histologic variants and findings are found to be associated with specific endocrine expressions; for example serotonin-producing examples seem to be prone to lead to more sclerosis and located on the duct walls (65).
Other pancreatic tumors with neuroendocrine differentiation
Signs of neuroendocrine differentiation are commonly encountered in non-neuroendocrine neoplasms of the pancreas. Solid pseudopapillary neoplasms morphologically resemble PanNETs very closely, and typically also express the most reliable “neuroendocrine marker” synaptophysin, and therefore often misdiagnosed (66). Diffuse nuclear beta-catenin expression and lack of keratins and chromogranin are the markers diagnostic of the former. Acinar cell carcinoma also not only have close histopathologic mimicry of PanNETs but also often show true neuroendocrine differentiation, and in many cases, clear-cut mixed neuroendocrine component (34,67). The prevailing view is to classify and manage such tumors as acinar cell carcinoma provided that acinar nature of the tumor is clearly demonstrated by acinar markers (trypsin, chymotrypsin and BCL10). Similarly, pancreatoblastoma is a tumor of multiphenotypic nature and neuroendocrine differentiation is a characteristic component, detectable in many examples. The presence of morules (so called squamoid corpuscles) is diagnostic of pancreatoblastoma (68). Occasionally a ductal adenocarcinoma can be seen in a mixture with neuroendocrine carcinoma (previously called “MANEC”, i.e., mixed adeno-neuroendocrine carcinoma; now termed as “MINEN”, i.e., mixed non-neuroendocrine-neuroendocrine neoplasm); however, these are exceedingly uncommon (2). Typically, the neuroendocrine component is high-grade, although rare cases with well differentiated tumor are also on record.
Conclusions
All PanNENs are malignant neoplasms, although incipient precursor lesions do occur but difficult to define. Poorly differentiated (high-grade) neuroendocrine carcinomas (PDNECs) are a totally separate entity then PanNETs (the ordinary, well-differentiated, pancreatic counterparts of carcinoids, previously called islet cell neoplasms) both by biology, molecular origins and prognosis. The previous impression that PanNETs are benevolent tumors with a subset recognizable as benign (“adenoma”) is being discredit increasingly with better studies with long-term follow-up. PanNETs should be regarded as malignant neoplasms although low-grade and low-stage tumors are often curable if resected. Per WHO 2019, neuroendocrine neoplasms with Ki-67 >20% are now regarded in two distinct groups, PanNET Grade 3 or PDNEC, based on the morphologic characteristics. In prognostication of PanNETs, in addition to the established parameters of grade (which is predominantly based on the proliferative activity as determined by Ki-67 index) and stage (mostly based on size and metastatic status), other factors such as morphologic patterns, immunohistochemical and molecular markers are under investigation.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Callisia N. Clarke, Douglas B. Evans) for the series “Pancreatic Neuroendocrine Tumors” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.03.07). The series “Pancreatic Neuroendocrine Tumors” was commissioned by the editorial office without any funding or sponsorship. CNC and DBE served as the unpaid Guest Editors of the series. The other authors have no other conflicts of interest to declare.
References
- 1.Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med 2017;33:324-30. 10.1159/000481390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill A, Klimstra D, Lam A, et al. Tumors of the pancreas. In: WHO Classification of Tumors: Digestive System Tumours. 5th edition. Lyon (France): International Agency for Research on Cancer, 2019;295-376. [Google Scholar]
- 3.Reid MD, Balci S, Saka B, et al. Neuroendocrine tumors of the pancreas: Current concepts and controversies. Endocr Pathol 2014;25:65-79. 10.1007/s12022-013-9295-2 [DOI] [PubMed] [Google Scholar]
- 4.Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol 2010;34:300-13. 10.1097/PAS.0b013e3181ce1447 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186-94. 10.1159/000443172 [DOI] [PubMed] [Google Scholar]
- 6.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017;46:707-14. 10.1097/MPA.0000000000000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018;31:1770-86. 10.1038/s41379-018-0110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017;543:65-71. 10.1038/nature21063 [DOI] [PubMed] [Google Scholar]
- 9.Pea A, Yu J, Marchionni L, et al. Genetic Analysis of Small Well-differentiated Pancreatic Neuroendocrine Tumors Identifies Subgroups With Differing Risks of Liver Metastases. Ann Surg 2020;271:566-73. 10.1097/SLA.0000000000003022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mafficini A, Scarpa A. Genomic landscape of pancreatic neuroendocrine tumours: the International Cancer Genome Consortium. J Endocrinol 2018;236:R161-7. 10.1530/JOE-17-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keutgen XM, Kumar S, Gara S, et al. Transcriptional alterations in hereditary and sporadic nonfunctioning pancreatic neuroendocrine tumors according to genotype. Cancer 2018;124:636-47. 10.1002/cncr.31057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackeng WM, Hruban RH, Offerhaus GJA, et al. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol 2016;11:47. 10.1186/s13000-016-0497-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Rosa S, Marando A, Gatti G, et al. Achaete-scute homolog 1 as a marker of poorly differentiated neuroendocrine carcinomas of different sites: a validation study using immunohistochemistry and quantitative real-time polymerase chain reaction on 335 cases. Hum Pathol 2013;44:1391-9. 10.1016/j.humpath.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Feng Z, Modica I, et al. Gene amplifications in well-differentiated pancreatic neuroendocrine Tumors inactivate the p53 Pathway. Genes Cancer 2010;1:360-8. 10.1177/1947601910371979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanno HA, Banks RW. Islet Cell Carcinoma of Pancreas, with Metastasis. Ann Surg 1943;117:437-49. 10.1097/00000658-194303000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray LM. Functioning Islet Cell Carcinoma with Metastases to Liver. Am J Pathol 1942;18:633-43. [PMC free article] [PubMed] [Google Scholar]
- 17.Heitz PU, Komminoth P, Perren A, et al. Pancreatic endocrine tumors: introduction. In: DeLellis RA, Lloyd RV, Heitz PU et al. editors. Pathology and Genetics: Tumors of Endocrine Organs. WHO Classification of Tumors. Lyon (France): IARC, 2004:177-82. [Google Scholar]
- 18.Whipple AO, Frantz VK. Adenoma of Islet Cells with Hyperinsulinism. Ann Surg 1935;101:1299-335. 10.1097/00000658-193506000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klöppel G, Anlauf M, Perren A, et al. Hyperplasia to neoplasia sequence of duodenal and pancreatic neuroendocrine diseases and pseudohyperplasia of the PP-cells in the pancreas. Endocr Pathol 2014;25;181-5. 10.1007/s12022-014-9317-8 [DOI] [PubMed] [Google Scholar]
- 20.Henopp T, Anlauf M, Schmitt A, et al. Glucagon Cell Adenomatosis: A Newly Recognized Disease of the Endocrine Pancreas. J Clin Endocrinol Metab 2009;94:213-7. 10.1210/jc.2008-1300 [DOI] [PubMed] [Google Scholar]
- 21.Klimstra D, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the pancreas. In: Bosman F, Carneiro F, Hruban R, et al. editors. WHO Classification of Tumours: Digestive System Tumours. 3rd edition. Lyon (France): International Agency for Research on Cancer, 2010:322-6. [Google Scholar]
- 22.Vollmer CM. Incidentally, It’s Still Cancer. Arch Surg 2011;146:539. 10.1001/archsurg.2011.105 [DOI] [PubMed] [Google Scholar]
- 23.Solcia E, Capella C, Klöppel G. Atlas of Tumor Pathology: Tumors of the Pancreas. 3rd edition. Washington, DC: AFIP, 1997. [Google Scholar]
- 24.Wick MR, Graeme-Cook FM. Pancreatic Neuroendocrine Neoplasms. Am J Clin Pathol 2001;115:S28-45. 10.1309/9DVM-V8C4-JFL0-BCLY [DOI] [PubMed] [Google Scholar]
- 25.Wick MR. Neuroendocrine Neoplasia. Am J Clin Pathol 2000;113:331-5. 10.1309/ETJ3-QBUK-13QD-J8FP [DOI] [PubMed] [Google Scholar]
- 26.Lloyd R, Osamura R, Klöppel G, et al. WHO classification of tumours of the endocrine organs. 4th edition. Lyon (France): International Agency for Research on Cancer, 2017. [Google Scholar]
- 27.Raj N, Valentino E, Capanu M, et al. Treatment Response and Outcomes of Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology. Pancreas 2017;46:296-301. 10.1097/MPA.0000000000000735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152-60. 10.1093/annonc/mds276 [DOI] [PubMed] [Google Scholar]
- 29.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173-84. 10.1097/PAS.0b013e3182417d36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girardi DM, Silva ACB, Rêgo JFM, et al. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat Rev 2017;56:28-35. 10.1016/j.ctrv.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 31.Tang LH, Untch BR, Reidy DL, et al. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res 2016;22:1011-7. 10.1158/1078-0432.CCR-15-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anlauf M, Perren A, Klöppel G. Endocrine Precursor Lesions and Microadenomas of the Duodenum and Pancreas with and without MEN1: Criteria, Molecular Concepts and Clinical Significance. Pathobiology 2007;74:279-84. 10.1159/000105810 [DOI] [PubMed] [Google Scholar]
- 33.Basturk O, Yang Z, Tang LH, et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. Am J Surg Pathol 2015;39:683-90. 10.1097/PAS.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basturk O, Tang L, Hruban RH, et al. Poorly Differentiated Neuroendocrine Carcinomas of the Pancreas. Am J Surg Pathol 2014;38:437-47. 10.1097/PAS.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konukiewitz B, Schlitter AM, Jesinghaus M, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod Pathol 2017;30:587-98. 10.1038/modpathol.2016.217 [DOI] [PubMed] [Google Scholar]
- 36.Chan CS, Laddha SV, Lewis PW, et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 2018;9:4158. 10.1038/s41467-018-06498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. 10.1126/science.1200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J Natl Cancer Inst 2012;104:764-77. 10.1093/jnci/djs208 [DOI] [PubMed] [Google Scholar]
- 39.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2013;98:4784-9. 10.1210/jc.2013-2604 [DOI] [PubMed] [Google Scholar]
- 40.Jung JG, Lee KT, Woo YS, et al. Behavior of small, asymptomatic, nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs). Med (United States) 2015;94:e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assi HA, Mukherjee S, Kunz PL, et al. Surgery Versus Surveillance for Well-Differentiated, Nonfunctional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist 2020;25:e276-83. 10.1634/theoncologist.2019-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuncel D, Reid M, Ohike N, et al. Do We Still Really Need to Count Mitoses for PanNETs?: Proper Ki67 Counting Negates the Need for the Cumbersome and Problematic Mitotic Count Required in the Current WHO-2017 Grading Scheme. Mod Pathol 2019;12:51. [Google Scholar]
- 43.Adsay V. Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: To count or not to count is not the question, but rather how to count. Am J Surg Pathol 2012;36;1743-6. 10.1097/PAS.0b013e318272ff77 [DOI] [PubMed] [Google Scholar]
- 44.CAP (2019) College of american pathologists cancer protocols and checklists. College of American Pathologists. Available online: http://www.cap.org/. Accessed 31/10/2019.
- 45.Reid M, Ohike N, Basturk O, et al. Diagnostic Impact of Intratumoral Heterogeneity of Ki67 Labeling Index in Pancreatic Neuroendocrine Tumors (NETs): Analysis of 91 Cases Shows Hot Spot Counting Correlates Better With Signs of Aggressiveness Than Counting Random Areas (Abstract) Mod Pathol 2015;28:446A.25216229 [Google Scholar]
- 46.Lopez-Aguiar AG, Ethun CG, Postlewait LM, et al. Redefining the Ki-67 Index Stratification for Low-Grade Pancreatic Neuroendocrine Tumors: Improving Its Prognostic Value for Recurrence of Disease. Ann Surg Oncol 2018;25:290-8. 10.1245/s10434-017-6140-8 [DOI] [PubMed] [Google Scholar]
- 47.Reid MD, Bagci P, Ohike N, et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 2015;28:686-94. 10.1038/modpathol.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang LH, Gonen M, Hedvat C, et al. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol 2012;36:1761-70. 10.1097/PAS.0b013e318263207c [DOI] [PubMed] [Google Scholar]
- 49.Papathomas TG, Pucci E, Giordano TJ, et al. An International Ki67 Reproducibility Study in Adrenal Cortical Carcinoma. Am J Surg Pathol 2016;40:569-76. 10.1097/PAS.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 50.Goodell PP, Krasinskas AM, Davison JM, et al. Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol 2012;137:576-82. 10.1309/AJCP92UCXPJMMSDU [DOI] [PubMed] [Google Scholar]
- 51.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 2011;35:853-60. 10.1097/PAS.0b013e31821a0696 [DOI] [PubMed] [Google Scholar]
- 52.McCall CM, Shi C, Cornish TC, et al. Grading of Well-differentiated Pancreatic Neuroendocrine Tumors Is Improved by the Inclusion of Both Ki67 Proliferative Index and Mitotic Rate. Am J Surg Pathol 2013;37:1671-7. 10.1097/PAS.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozturk Sari S, Taskin OC, Gundogdu G, et al. The Impact of Phosphohistone-H3-Assisted Mitotic Count and Ki67 Score in the Determination of Tumor Grade and Prediction of Distant Metastasis in Well-Differentiated Pancreatic Neuroendocrine Tumors. Endocr Pathol 2016;27:162-70. 10.1007/s12022-016-9424-9 [DOI] [PubMed] [Google Scholar]
- 54.Hochwald SN, Zee S, Conlon KC, et al. Prognostic Factors in Pancreatic Endocrine Neoplasms: An Analysis of 136 Cases With a Proposal for Low-Grade and Intermediate-Grade Groups. J Clin Oncol 2002;20:2633-42. 10.1200/JCO.2002.10.030 [DOI] [PubMed] [Google Scholar]
- 55.Ferrone CR, Tang LH, Tomlinson J, et al. Determining Prognosis in Patients With Pancreatic Endocrine Neoplasms: Can the WHO Classification System Be Simplified? J Clin Oncol 2007;25:5609-15. 10.1200/JCO.2007.12.9809 [DOI] [PubMed] [Google Scholar]
- 56.La Rosa S, Klersy C, Uccella S, et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol 2009;40:30-40. 10.1016/j.humpath.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 57.Gao Y, Gao H, Wang G, et al. A meta-analysis of Prognostic factor of Pancreatic neuroendocrine neoplasms. Sci Rep 2018;8:7271. 10.1038/s41598-018-24072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchegiani G, Landoni L, Andrianello S, et al. Patterns of recurrence after resection for pancreatic neuroendocrine tumors: who, when, and where? Neuroendocrinology 2018;108:161-71. [DOI] [PubMed] [Google Scholar]
- 59.Sahan EK, Erdogan N, Ulusoy I, et al. P53, KI-67, CD117 expression in gastrointestinal and pancreatic neuroendocrine tumours and evaluation of their correlation with clinicopathological and prognostic parameters. Turk J Gastroenterol 2015;26:104-11. 10.5152/tjg.2015.1965 [DOI] [PubMed] [Google Scholar]
- 60.Han X, Zhao J, Ji Y, et al. Expression of CK19 and KIT in resectable pancreatic neuroendocrine tumors. Tumour Biol 2013;34:2881-9. 10.1007/s13277-013-0850-8 [DOI] [PubMed] [Google Scholar]
- 61.Gilbert JA, Adhikari LJ, Lloyd RV, et al. Molecular Markers for Novel Therapeutic Strategies in Pancreatic Endocrine Tumors. Pancreas 2013;42:411-21. 10.1097/MPA.0b013e31826cb243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garbrecht N, Anlauf M, Schmitt A, et al. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: Incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr Relat Cancer 2008;15:229-41. 10.1677/ERC-07-0157 [DOI] [PubMed] [Google Scholar]
- 63.Xue Y, Reid M, Pehlivanoglu B, et al. Morphologic Repertoire of Well-Differentiated Pancreatic Neuroendocrine Tumors (Pannets): A Clinicopathologic Analysis of 139 Cases (Abstract). United States and Canadian Academy of Pathology (USCAP) (Platform). Mod Pathol 2018;31:693. [Google Scholar]
- 64.Zee SY, Hochwald SN, Conlon KC, et al. Pleomorphic pancreatic endocrine neoplasms: A variant commonly confused with adenocarcinoma. Am J Surg Pathol 2005;29:1194-200. 10.1097/01.pas.0000164370.81132.25 [DOI] [PubMed] [Google Scholar]
- 65.McCall CM, Shi C, Klein AP, et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum Pathol 2012;43:1169-76. 10.1016/j.humpath.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: Immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 2000;24:1361-71. 10.1097/00000478-200010000-00005 [DOI] [PubMed] [Google Scholar]
- 67.La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: Insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol 2012;36:1782-95. 10.1097/PAS.0b013e318263209d [DOI] [PubMed] [Google Scholar]
- 68.Reid MD, Bhattarai S, Graham RP, et al. Pancreatoblastoma: Cytologic and histologic analysis of 12 adult cases reveals helpful criteria in their diagnosis and distinction from common mimics. Cancer Cytopathol 2019;127:708-19. 10.1002/cncy.22187 [DOI] [PMC free article] [PubMed] [Google Scholar]