Abstract
Background
Symptomatic thoracic disc herniation (TDH) is a rare clinical entity and surgical intervention for it is even more uncommon. Despite several surgical techniques being described for thoracic discectomy, considering the unique surgical challenges, none of them have been accepted universally. Minimally invasive techniques have brought in a paradigm shift in the management of cervical/lumbar spinal disorders and similar techniques have been extrapolated to the thoracic region too. The purpose of this paper is to describe our technique, surgical experience, and the clinical results of transforaminal endoscopic thoracic discectomy (TETD).
Methods
Consecutive patients who underwent TETD (2001–2018) were reviewed. Patients who had a minimum of 6 months of follow-up, and without cervical and lumbar spine surgery or trauma during the follow-up period were included in the study. TETD was performed in patients who presented with symptomatic disc herniation of the thoracic spine and did not respond to conservative treatments. Patients with calcified disc herniation or concomitant ossification of the posterior longitudinal ligament (OPLL) were excluded. Under local anesthesia and intravenous sedation, a 4.7-mm endoscope (TESSYS, Joimax GmbH, Germany) was introduced via transforaminal approach with foraminoplasty using reamer. Patient outcome was evaluated using visual analogue scale (VAS) and Oswestry disability index (ODI) scores. Patient satisfaction was measured using Macnab’s criteria.
Results
Ninety-two consecutive patients (mean age was 48.9 years, 57 males) who underwent TETD from 2001 to 2018 met the inclusion criteria. Patients underwent surgery at different levels: 16 patients for T2–3 to T5–6 level, 41 cases for T6–7 to T8–9, and 35 patients for T9–10 to T12–L1. During follow-up for an average of 38.4 months, all patients showed a significant improvement of pain (7.6 to 1.6 in VAS and 68.2 vs. 13.2 for ODI, P<0.05 for both). There was one patient who had transient motor weakness.
Conclusions
TETD for soft, paramedian or lateral symptomatic TDH is a feasible and effective minimally invasive treatment option with favorable clinical results.
Keywords: Thoracic disc herniation (TDH), transforaminal endoscopic thoracic discectomy (TETD), foraminoplasty
Introduction
Symptomatic thoracic disc herniation (TDH) is a rare clinical entity and it is seldom treated with surgical intervention. The incidence of symptomatic TDH has been reported to be 0.25–0.5% of all the spinal disc diseases (1-4). However, diagnosis of TDH is increasing with the development of magnetic resonance image (MRI). Given the unique surgical challenges presented by thoracic discectomy, despite several surgical techniques being described they have not been accepted universally (5-9). Minimally invasive techniques have revolutionized the management of cervical/lumbar spinal conditions and these techniques have been applied to the thoracic region as well. This paper describes a technique we have developed, our surgical experience, and clinical outcome of transforaminal endoscopic thoracic discectomy (TETD).
Methods
Patient population
Consecutive patients who underwent TETD (2001–2018) were reviewed for this study. Subjects with minimum 6 months of follow-up, and without cervical and lumbar spine surgery or trauma during follow-up period were included. TETD were performed in patients who presented with symptomatic disc herniation on thoracic spine but did not respond to intensive, conservative treatment. Patients with calcified disc herniations, concomitant ossification of the posterior longitudinal ligament (OPLL) or ossification of the ligamentum flavum, profound neurological deficits due to significant myelopathy that showed definite signal change in the spinal cord, multi-level TDHs, history of trauma and worker’s compensation claim were excluded. Ninety-two consecutive patients (mean 48.9±15.6 years, 57 males) who underwent TETD from 2001 to 2018 were reviewed. With respect to surgical levels, the number of patients in each category were 16 for T2–3 to T5–6, 41 for T6–7 to T8–9, and 35 for T9–10 to T12–L1 (Figure 1). Review of the symptoms showed that 86 patients (93.5%) had axial dorsal back pain, 26 (28.3%) had radicular pain on chest wall, 11 (12.0%) visceral or somatic pain and 19 (20.7%) patients presented with thoracic myelopathy (sensory or motor deterioration). Symptomatic relief after selective epidural block was checked to confirm the diagnosis. Conservative treatment was given for at least 6 weeks before surgery.
Figure 1.
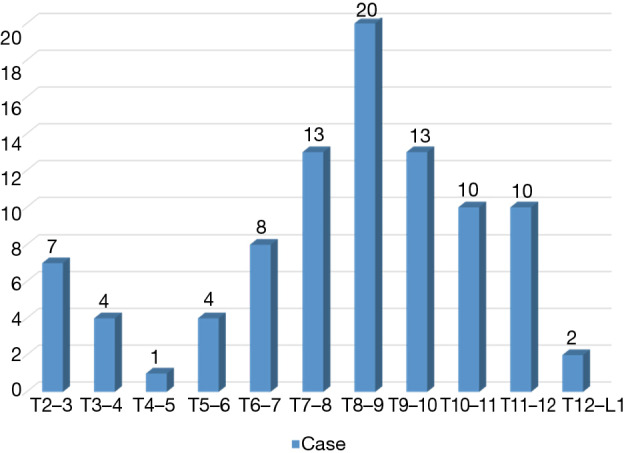
Distribution of surgical levels. Mid-thoracic spine was the most common index level (41 for T6–7 to T8–9) followed by lower thoracic spine (35 for T9–10 to T12–L1) and upper thoracic spine (16 for T2–3 to T5–6).
Surgical procedure
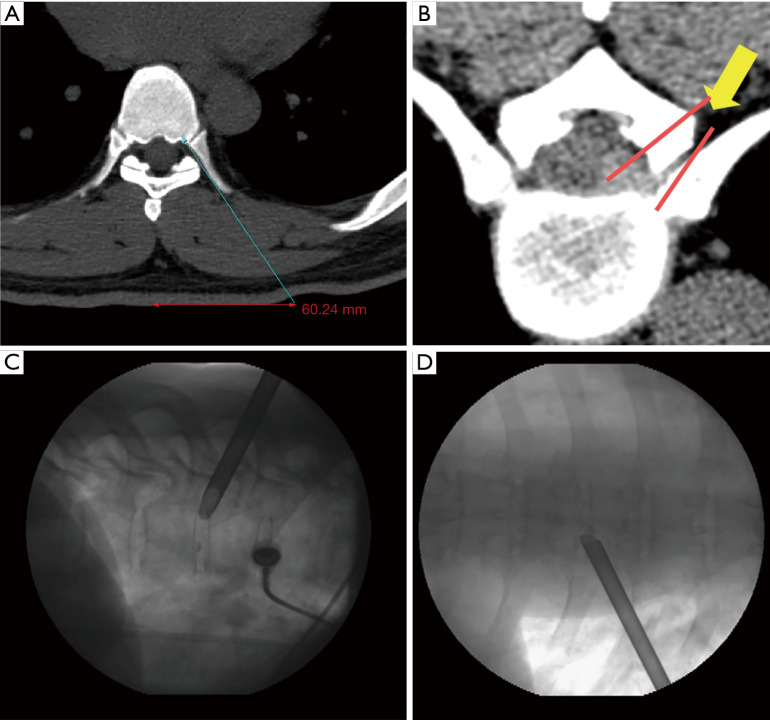
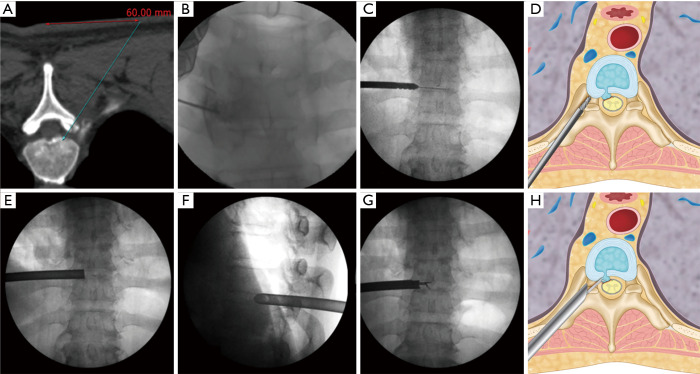
Surgery was performed in a prone position under local anesthesia, and conscious sedation with Midazolam. Skin entry point was determined by drawing a line from posterior annulus at the midpedicular level to the lateral margin of facet joint on axial computed tomography scan, usually located approximately 6 cm from the midline (Figure 2). After infiltration of the local anesthesia, a 6-inch long 18-G needle was inserted aimed at the midpedicular line on anteroposterior (AP) fluoroscopic view and the posterior vertebral body line on lateral fluoroscopic view. Epidurography was performed, followed by epidural anesthetic infiltration. The needle was advanced into the disc space following discography using a mixture of radiopaque dye (Telebrix; Guerbet, France), indigo carmine (Carmine; Korea United Pharmaceutical, Yoenki, Korea), and normal saline in a 2:1:2 ratio. A guide wire was inserted through the needle. Serial dilation and sequential reaming of ventral and lateral aspect of superior articular facet was performed over the wire, to expand the neural foraminal window (Figure 3). Then, the 4.7-mm spinal endoscope (TESSYS; Joimax GmbH, Karlsruhe, Germany) was introduced. Under direct visualization, a blue-stained herniated fragment could be identified. The posterior longitudinal ligament (PLL) was resected to expose the ventral epidural space. Holmium: yttrium-aluminum-garnet (Ho: YAG) laser (VersaPulse; Lumenis, Yokneam, Israel) was used to ablate the posterior annulus and the PLL with minimal thermal necrosis. The herniated fragment was removed with endoscopic forceps. After adequate decompression, skin was closed with subcuticular suture and sterile dressing. Patients were encouraged to ambulate immediately after surgery (case presentation: Figures 4,5).
Figure 2.
Illustration of surgical trajectory. (A) Skin entry point planning on axial computed tomography scan by drawing an imaginary line from posterior annulus at the midpedicular level to lateral margin of facet joint; (B) imaginary line for foraminoplasty reaming of ventral and lateral aspect of superior facet; (C,D) placement of beveled working cannula on the posterior disc space through annular window at the posterior disc space—lateral view (C) and at the midpedicular level—AP view (D). AP, anteroposterior.
Figure 3.
Surgical technique. (A) Skin entry point planning on axial computed tomography scan by drawing an imaginary line from posterior annulus at the midpedicular level to lateral margin of facet joint; (B) placement of needle tip at the midpedicular level; (C,D) enlargement of foramen by serial dilation and sequential reaming of ventral and lateral aspect of superior facet over guidewire; (E) placement of beveled 7.5-mm working cannula on the posterior disc space through annular window at the midpedicular level—AP view; (F) placement of beveled 7.5-mm working cannula on the posterior disc space through annular window at the midpedicular level—lateral view; (G,H) removal of herniated disc fragment using microforceps. AP, anteroposterior.
Figure 4.
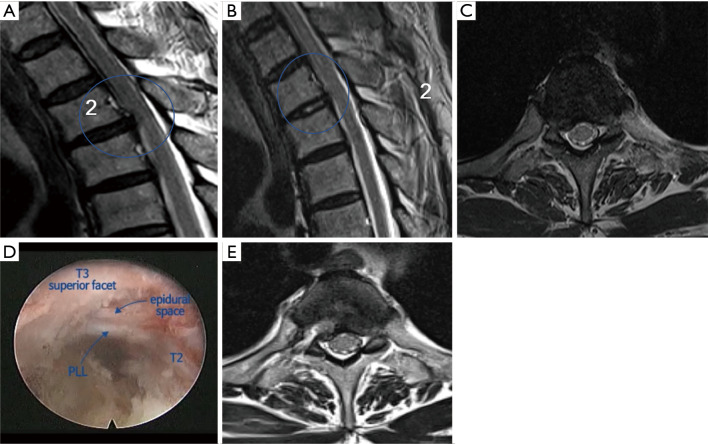
A 55-year-old male patient: (A) Preoperative sagittal view showing paramedian TDH at the T2–3 level; (B) postoperative sagittal view showing successful removal of disc herniation; (C) preoperative axial view showing right paramedian TDH at the T2–3 level; (D) intraoperative video image shows full decompression of epidural space; (E) postoperative axial view showing successful removal of disc herniation. TDH, thoracic disc herniation.
Figure 5.
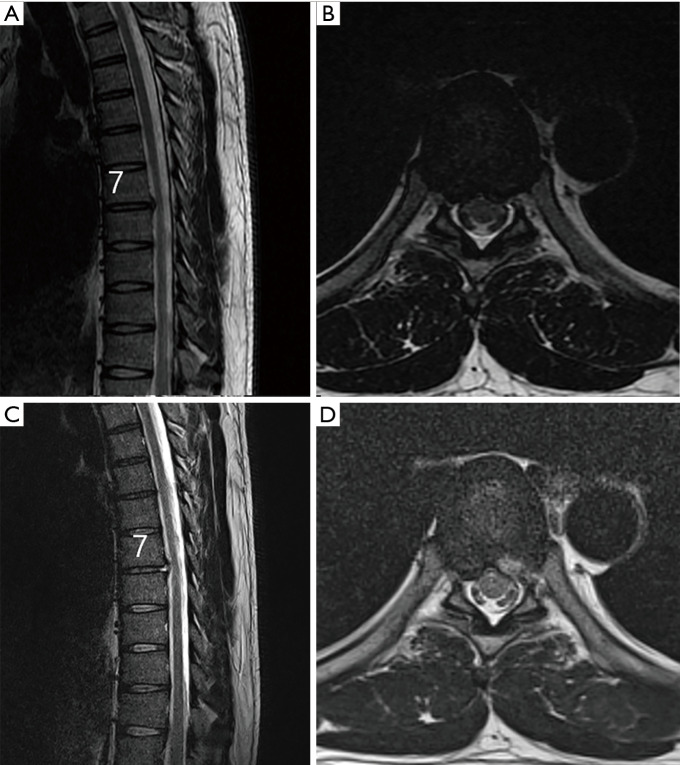
A 36-year-old male patient: (A) Preoperative sagittal view showing paramedian TDH at the T7–8 level; (B) preoperative axial view showing left paramedian TDH at the T3–4 level; (C) postoperative sagittal view showing successful removal of disc herniation; (D) postoperative axial view showing successful removal of herniated disc. TDH, thoracic disc herniation.
Data analysis
Clinical outcomes were assessed by the visual analogue scale (VAS; 0–10, with 0= no pain), and functional outcomes were scored with the Oswestry disability index (ODI; 0–100%). Patient satisfaction was measured with modified MacNab criteria. SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A P value of <0.05 was considered statistically significant.
Results
After 38.4±32.3 [mean ± standard deviation (SD)] months of follow-up, all patients showed a significant improvement in pain (7.6 to 1.6 for VAS and 68.2% to 13.2% for ODI, P<0.05 for both). According to the modified Macnab’s criteria, 44 patients reported an excellent outcome (47.8%), 39 (42.4%) showed good outcomes, 8 (8.7%) of them had a fair outcome, and only 1 patient (1.1%) had a poor outcome. There was one patient who had transient motor weakness, while 3 patients complained of paresthesia on the lower extremities. Two patients had symptomatic recurrent herniation. Of these, one patient underwent TETD and the other patient improved with conservative treatment.
Discussion
The clinical symptoms of TDH often manifest by various types of severe, incapacitating pain and any surgical approach that will cause excessive surgical morbidity may be counter-productive (10). This makes the choice of the least morbid surgical approach for thoracic discectomy even more significant. However, rarity of symptomatic thoracic discogenic conditions combined with relative surgical inexperience in dealing with the unique anatomy of the thoracic region has hampered the development of a universally accepted gold standard; the choice of thoracic discectomy technique is mostly dictated by the surgeon’s training, clinical experience, and personal preference (2,6,10,11).
During the early part of 21st century, thoracoscopy was well established as a procedure of choice for thoracic discectomy (2). The successful use of posterior/postero-lateral endoscopic techniques in the lumbar region prompted surgeons to apply similar techniques in the thoracic region also (12). Both thoracoscopy and postero-lateral endoscopy techniques are similar, being needle based procedures utilizing endoscopic visualization to address the compressive disc pathology without causing instability. However, thoracoscopy is associated with more surgical insult with the use of general anesthesia, larger working channels and retraction of thoracic viscera (9,13). Transforaminal thoracic endoscopic discectomy offers the advantages of a more natural access to dorsal intervertebral disc space with minimal removal or destruction of surrounding anatomical structures, use of smaller working channels which facilitates mid-line decompression of ventral thecal sac pathology under direct vision without any retraction or contact of the thecal sac itself (10,14). Due to direct visualization, surgeons can assess adequate decompression with the following: (I) the ventral dural sac is directly identified in the endoscopy field, (II) the herniectomy size is assessed before surgery, and (III) fluctuation of the PLL may be observed in case of contained herniation.
Similar to other techniques, TETD has its own limitations. The constrained position of endoscope and instruments, and use of small, less powerful instruments contraindicates its use in the presence of discs which are calcified or sequestrated. Unfortunately, a large number of thoracic disc pathologies show calcification limiting the widespread application of TETD (2,10). Side firing Ho: YAG laser is useful for adequate decompression via the constrained position of endoscopy. The Ho: YAG laser can be introduced through the working canal of the endoscopy. Under direct visualization, a small amount of laser energy is able to cut and ablate tissues in an aqueous medium by creating a vapor bubble at the tip of the fiber (15).
Choi et al. (7) published a report on fourteen patients with soft lateral or central TDH with successful outcomes. Nie and Lui (14) also reported good to excellent outcomes with the use of TETD in 13 patients as compared to other techniques such as thoracoscopic microdiscectomy (5) in the management of selected TDH. Indeed, symptomatic TDH may be much more frequent than previously reported. Presentation with predominant (axial and/or bandlike) pain in TDH may not convince most surgeons less familiar with the disease to propose surgical intervention. This leaves the patients in severe pain which is often refractory to conservative and even invasive pain therapy. In the present study, 93.4% of the patients presented with axial dorsal back pain. The current series of 92 cases include discectomies performed at various levels from T2–3 to T12–L1. Pain, either axial or radicular, was the chief complaint of all the patients and showed a significant improvement during the immediate post-operative period. The initial improvements in the clinical symptoms either improved or remained stable over a period of 38.4 months as indicated by 13.2% ODI. The overall complication rate in the current series was 6.5%, with lower extremity paresthesia being the most common complication occurring in 3 patients. The combined advantages of local anesthesia, minimal surgical approach morbidity without affecting the main goal of surgery (removal of compressive disc pathology), and avoidance of hospitalization clearly translated into excellent clinical outcomes in the current series. All surgeries in the present study were performed under conscious sedation. Since the patients’ response can be checked, TETD could be performed safely without the need of neuromonitoring.
It cannot be emphasized enough that successful use of TETD relies upon careful selection of suitable cases and sufficient surgical skills/experience in the use of endoscopes. The use of endoscopy requires special visuomotor surgical skills (16). The surgeons who are relatively new to this technique are advised to gain considerable experience in spinal endoscopy by first operating in more familiar and less challenging terrains like the lumbar spine. The endoscopy skills can be comfortably acquired and maintained by formal training and performing a sufficient number of surgeries on a regular basis.
The overall high rate of satisfaction in patients (90.2%) with a low complication rate (6.5%) achieved in the current series indicates the potential for universal application of TETD in the treatment of symptomatic TDHs from T2–3 to T12–L1 level. Furthermore, this study also paves the way for larger, more sophisticated, multi-center, randomized controlled studies to evaluate the usefulness of TETD in management of TDHs.
Conclusions
The present study shows the possible utilization of spinal endoscopy techniques in the management of TDHs. It is also necessary to understand the clinical presentation of symptomatic TDH without significant myelopathy. TETD is a feasible, effective, minimally invasive treatment option with favorable clinical results in carefully selected (soft, paramedian to lateral disc herniations) patients, when performed by well experienced surgeons.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Wooridul Hospital (WRDIRB-2018-10-009), and informed consent was obtained from the patients for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.11.19). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Arce CA, Dohrmann GJ. Herniated thoracic disks. Neurol Clin 1985;3:383-92. 10.1016/S0733-8619(18)31043-0 [DOI] [PubMed] [Google Scholar]
- 2.Gille O, Soderlund C, Razafimahandri HJ, et al. Analysis of hard thoracic herniated discs: review of 18 cases operated by thoracoscopy. Eur Spine J 2006;15:537-42. 10.1007/s00586-005-1014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gokcen HB, Erdogan S, Gumussuyu G, et al. A rare case of T1-2 thoracic disc herniation mimicking cervical radiculopathy. Int J Spine Surg 2017;11:30. 10.14444/4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan H, Abood C. Disc herniation at T1-2. Report of four cases and literature review. J Neurosurg 1998;88:148-50. 10.3171/jns.1998.88.1.0148 [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal D, Rosenthal R, de Simone A. Removal of a protruded thoracic disc using microsurgical endoscopy. A new technique. Spine (Phila Pa 1976) 1994;19:1087-91. 10.1097/00007632-199405000-00018 [DOI] [PubMed] [Google Scholar]
- 6.Cho JY, Lee SH, Jang SH, et al. Oblique paraspinal approach for thoracic disc herniations using tubular retractor with robotic holder: a technical note. Eur Spine J 2012;21:2620-5. 10.1007/s00586-012-2438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi KY, Eun SS, Lee SH, et al. Percutaneous endoscopic thoracic discectomy; transforaminal approach. Minim Invasive Neurosurg 2010;53:25-8. 10.1055/s-0029-1246159 [DOI] [PubMed] [Google Scholar]
- 8.Malham GM, Parker RM. Treatment of symptomatic thoracic disc herniations with lateral interbody fusion. J Spine Surg 2015;1:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YY, Huang TJ, Liu HP, et al. Thoracic disc herniation treated by video-assisted thoracoscopic surgery: case report. Changgeng Yi Xue Za Zhi 1998;21:453-7. [PubMed] [Google Scholar]
- 10.Bouthors C, Benzakour A, Court C. Surgical treatment of thoracic disc herniation: an overview. Int Orthop 2019;43:807-16. 10.1007/s00264-018-4224-0 [DOI] [PubMed] [Google Scholar]
- 11.Bae J, Chachan S, Shin SH, et al. Percutaneous endoscopic thoracic discectomy in the upper and midthoracic spine: a technical note. Neurospine 2019;16:148-53. 10.14245/ns.1836260.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner R, Telfeian AE, Iprenburg M, et al. Transforaminal endoscopic foraminoplasty and discectomy for the treatment of a thoracic disc herniation. World Neurosurg 2016;90:194-8. 10.1016/j.wneu.2016.02.086 [DOI] [PubMed] [Google Scholar]
- 13.Sasani M, Fahir Ozer A, Oktenoglu T, et al. Thoracoscopic surgery for thoracic disc herniation. J Neurosurg Sci 2011;55:391-5. [PubMed] [Google Scholar]
- 14.Nie HF, Liu KX. Endoscopic transforaminal thoracic foraminotomy and discectomy for the treatment of thoracic disc herniation. Minim Invasive Surg 2013;2013:264105. [DOI] [PMC free article] [PubMed]
- 15.Li Y, Wang B, Lü G, et al. Effects of Ho:YAG laser ablation on postoperative low back pain and functional status after transforaminal endoscopic lumbar discectomy: minimum of 2-year follow-up. World Neurosurg 2019;127:e793-8. 10.1016/j.wneu.2019.03.265 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon’s training level of minimally invasive spine surgery. Clin Neurol Neurosurg 2013;115:1987-91. 10.1016/j.clineuro.2013.06.008 [DOI] [PubMed] [Google Scholar]