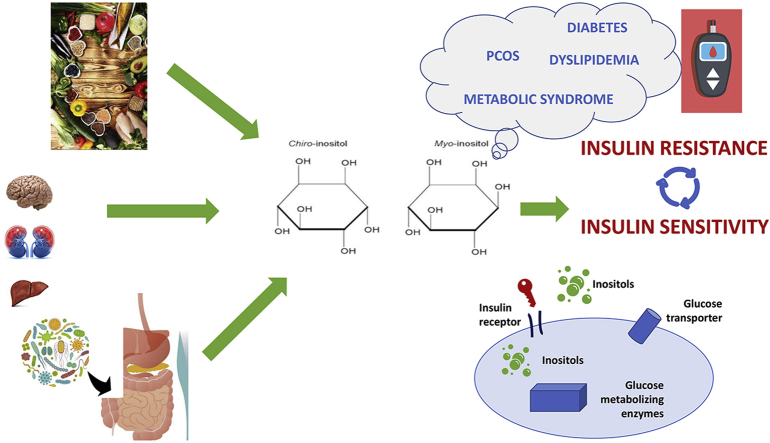
Abstract
Inositol and its derivates are catching interest in metabolism since taking part in several physiological processes, including endocrine modulation. Through several mechanisms mostly mediated by insulin signaling, these compounds regulate the activities of several hormones and are essential in oocytes maturation. It is interesting to point out the contribution of an inositol deficiency in the development of several diseases, mainly in the metabolic and endocrine setting. Inositols derive from both diet and endogenous production; among causes of inositol deficiency reduced dietary intake, increased catabolism and/or excretion, decreased biosynthesis, inhibition of gut and cellular uptake and altered microbiota could be considered. Mounting direct and indirect evidence suggests that the two main isoforms (Myo-inositol-inositol, D-chiro-inositol) are implied in glycemic and lipidic metabolism and supplementation yield a beneficial effect on these parameters without hazards for health. Moreover, they have a role in polycystic ovary syndrome, acting as insulin-sensitizing agents and free radical scavengers, helping to regulate metabolism and promoting ovulation. The aim of this narrative review is to discuss the role of inositols in metabolic function disorders paying attention to whether these compounds could be efficacious and safe as a therapeutic agent with a focus on dietary intake and the role of gut microbiota.
Keywords: Inositol, Diet, Food supplement, Metabolism, Microbiota, Type 2 diabetes, Ovary, Lipids
Graphical abstract
Highlights
-
•
Inositol deficiency is implicated in the development of metabolic and endocrine diseases.
-
•
Inositol compounds could be safe food supplement to restore metabolic imbalance.
-
•
Inositol compounds partly derive from microbiota phytases entering in bacterial metabolism.
-
•
Diet inositol content has a role in shaping gut microbiota and the host metabolism.
List of abbreviations
- ALA
alpha lipoic acid
- DCI
D-chiro-inositol
- DM
diabetes mellitus
- GDM
gestational diabetes mellitus HSC: high-sucrose diet
- HSR
High-Starch-Diet
- InsP
inositol phosphate
- IP3
inositol triphosphate
- IP6
inositol hexaphosphate
- IPG
inositol phosphoglycan
- MIOX
myo-inositol oxygenase
- MYO
Myo-inositol PA: phytic acid PCOS: Polycystic ovary syndrome
- PDHP
pyruvate dehydrogenase phosphatases
- PI3K
phosphoinositide 3 kinase
- RSOR
renal-specific oxidoreductase
1. Introduction
Inositol and its derivates are attracting increasing interest because they participate in several physiological processes, including calcium metabolism, proliferation, endocrine and metabolic modulation, stress response and signal transduction downstream neurotransmitter stimulation.1 Inositol is a hexahydroxycyclohexane alcohol of cyclohexane belonging to the glucose family.2 Nine stereoisomers are existing3; among them myo-inositol and D-chiro-inositol are the two main stereoisomers present in human body.1,3 Both D-chiro-inositol and myo-inositol have similar structures, differing in the stereochemistry for one hydroxyl group. The other isomers are scyllo-inositol, muco-inositol, L-chiro-inositol, neo-inositol, allo-inositol, epi-inositol, and ci-inositol.4,5
Although myo-inositol is synthesized both by prokaryote and eukaryote cells, it is provided to humans through dietary sources as free forms, as inositol-containing phospholipid, or as phytic acid.1,6
In humans, the biosynthesis of myo-inositol primarily occurs in the kidney, with a rate approaching 4 g/day. Extrarenal tissues like the brain, the testis and the liver also contribute to the endogenous production of inositol.7,8
Transport of inositol compounds from plasma to cells is an active and saturable carrier-mediated process, following the sodium gradient through different channels.1 The main transport system is represented by SMIT1 and SMIT2.9 However, also SGLT1/2 glucose transporter system is involved, at least in the liver.10
Inositol is incorporated into cell membranes as phosphatidyl-myo-inositol, the precursor of inositol triphosphate (IP3), which acts as second messenger.1,3,11,12
The best-known signaling function for inositol polyphosphate, is precisely IP3-mediated cytosolic calcium release from membranes of intracellular calcium storages such as the endoplasmic reticulum.13 IP3 regulates the activities of several hormones such as FSH, TSH and insulin.1,3,11,12 IP3 is also dephosphorylated to produce free myo-inositol which can be alternatively derived by recycling inositol phosphates (InsP).14 Moreover, myo-inositol is essential in oocytes maturation and it seems to play a role in testes, prostate, epididymis, seminal vesicles, and seminal fluid, that is one of the richest sources of inositol.1,3
Myo-inositol can be transformed into D-chiro-inositol by specific insulin dependent epimerases, and growing data suggest that these two inositol stereoisomers are both chemical mediators of insulin actions through different mechanism.1,3
The widespread role of inositols in cell functions, highlights the contribution of an inositol deficiency in the development of several diseases. The aim of this review is to discuss the role of inositols in metabolic function disorders paying attention on whether inositol could be an efficacious and safe therapeutic agent.
2. Dietary sources of inositol
As introduced previously, inositol is a polyol, natural isomer of glucose, existing in nature under nine stereoisomeric forms depending on the spatial orientation of its six hydroxyl groups. It is a lipotropic compound essential for lipid metabolism, DNA methylation, and the production of nucleoproteins and membranes.4,5 Myo-inositol is the major nutritionally relevant form of inositol found in food. In the past, myo-inositol was considered to belong to the vitamin B family. However, because it is produced by the human liver and kidney starting from d-glucose at a production rate of up to 4–5 g/day, it is no more considered as an essential nutrient.15
Animal food contains mainly inositol in its free form or as inositol-containing phospholipid (phosphatidylinositol), diversely plant-derived food contains inositol preferentially as inositol hexaphosphate (IP6). IP6 is the primary storage compound of phosphorus in seeds. During germination, IP6 is hydrolyzed by endogenous phytases and other phosphatases to release phosphate, inositol and other micronutrients.4,5,15 A western diet has been estimated to provide about 1 g/day of myo-inositol.1,3 When a diet is poor in minerals, large quantities of IP6, but not of myo-inositol, could reduce the bioavailability of Ca, Fe, K, Mg, Mn and Zn because IP6 phosphates binds to these cations. This event is very rare when the dietary intake is around 1–2 g/day.15
Myo-inositol is present in greatest amount in fresh fruits and vegetables, and in seeds (beans, grain, pseudo-cereals and nuts), mainly in their aleurone layer.16, 17, 18 Whole-grain cereals are a source of myo-inositol.16, 17, 18 Oats and bran contain more myo-inositol than other cereals. Regarding nuts, walnuts, almonds and Brazilian nuts are the major source of IP6. Leafy vegetables are the poorest vegetable sources, whereas beans and peas are the richest. Melon and citrus fruits are also particularly rich in IP6.4 The IP6 in whole grain (0.1–1.5% on 100 g) is poorly absorbed in humans, being degraded the 54%–79% in subjects fed whole bread. Differently, the absorption of free myo-inositol and D-chiro-inositol is higher.18 However, the total amount of free myo-inositol absorbed with cereals is influenced by processing methods.4,16 In fact, the processing of food, like baking or even simple fermentation with yeast, seems to inactivate most of nutritional-derived phytases. Furthermore, in humans up to 66% of dietary phytates are hydrolyzed in the stomach and large intestine by microbial phytases.4,15,19 Hence, free myo-inositol and D-chiro-inositol are absorbed depending on the quantity not degraded by microbiota.
Collectively, many factors contribute to inositol deficiency, as reduced diet intake, increased catabolism and/or excretion, decreased biosynthesis, inhibition of gut and cellular uptake, altered microbiota.6 Some of these mechanisms are described in following chapters.
3. Inositol and the gut microbiota
All living cells (animals, plants, bacteria, fungi) contain inositol phospholipids in their membranes. Phytic acid, one of the inositol form, is the main form of phosphorus preservation in many plant tissues, mainly bran and seeds.5 Myo-inositol, starting from phytic acid (inositol hexaphosphate or IP6), can be released into the intestine of monogastric animals by the activity of enzymatic phytases, a process that occurs in the intestinal mucosa.4,15,20 Phytases (myo-inositol hexaphosphate phosphohydrolase) are found in plants, microorganisms, including bacteria, and animal tissues.16,17,18 It can release free inositol, orthophosphate and intermediate products including forms of inositol. Although inositol forms partly derive from bacterial phytases, scant data are available in the literature on microbiota modulation due to the inositol administration. To date, results are available from only three papers on rats fed different types of diet and from another study on laying hens. The first study21 described the effects of a diet supplemented with 2% sodium phytic acid 0.4% myo-inositol, or 1.% sodium phytic acid +0.2% myo-inositol given for 3 weeks, compared with a control diet. The authors documented that dietary phytic acid was able to up-regulate cecal production of microbial short chain fatty acids, particularly n-butyrate, significantly more than the phytic acid + myoinositol-containing diet. This response was associated with parallel changes in microbiota composition. In fact, the cecal ratio of Lactobacillales, cecal and fecal mucins, and fecal β-glucosidase activity were higher in rats fed a diet containing sodium phytic acid than those fed acontrol diet.21
A second study demonstrated that dietary phytic acid prevents fatty liver disease in rats fed a high-sucrose diet (HSC), and that the presence of undigested phytic acid products may have beneficial effects on gut microbiota.22 The effects are similar to those demonstrated in animals fed a diet rich in fiber or oligosaccharides. When compared to a high-starch diet (HSR), the HSC diet significantly increased liver weight, total lipid and triacylglycerol levels, as well as liver expression of lipogenic enzyme genes. However, these metabolic alterations were suppressed by dietary phytic acid. Moreover, the intake of phytic acid increased the fecal ratio of Lactobacillus spp., reduced Clostridium cocoides, suppressed the increase in the ratio of C. leptum induced by the HSC diet, and did not impact Bacteroides. Therefore, the authors22 suggested the existence of a relationship between the hepatic activity against fatty liver of dietary phytic acid and the changes of the intestinal microbiota, at least in rats fed a HSC diet.22 A third study23 confirmed that dietary phytic acid or myo-inositol suppresses the hepatic expression of lipogenic genes and their involvement in the modulation of intestinal microbiota in rats fed a HSC diet but not in those receiving a HSR diet. Cecal short chain fatty acids, which are produced by bacterial fermentation, influenced hepatic lipogenesis and cholesterogenesis, and increased fecal and cecal percentage of Lactobacillus spp., a finding that agrees with previous reports after a diet rich in phytic acid or myo-inositol.22,23 These results suggest that both dietary phytic acid and myo-inositol normalize hepatic triglycerides concentration as well as the activity and expression of hepatic lipogenic enzymes in rats fed a lipogenic diet through a concomitant improvement in microbiota composition. Because phytic acid and myo-inositol effects are parallel, the myo-inositol ring of phytic acid can be responsible for the effects of dietary phytic acid intake on the metabolism of liver lipids and on the intestinal microbiota.23
Interestingly, diet also has an essential influence on the establishment of the cecum microbial communities in poultry, so its supplementation with additives, such as probiotics (2 g/kg of addition of phytic acid), prebiotics, and symbiotics has been claimed to improve animal health.20 Olsenella spp., and Lactobacillus crispatus increased their abundance in prebiotic and symbiotic treatments in this animal model. Furthermore, metagenomes revealed that the genes encoding for the metabolism of butanoate, propanoate, InsP, and galactose were more abundant during a prebiotic diet. These findings supported a role for gut microbiota in the InsP degradation and phosphorous release derived by the diet.20
In exploring genes that clustered in the same structural genomic variant, Zeevi and coworkers24 uncovered several possible mechanistic links between the microbiome and its host: for example, a region in Anaerostipes hadrus that encodes a composite inositol catabolism-butyrate biosynthesis pathway, correlated with the host lower risk to have metabolic diseases. The positive energetic effect yielded by the catabolism of myo-inositol, in combination with glycolysis, the synthesis of Acetyl CoA, and the synthesis of butyrate was hypothesized to benefit the overall energy metabolism of A. hadrus.24 This interesting study suggests that what has been shown in studies in rats fed diets using different carbohydrate contents and supplemented with myo-inositol can be effectively confirmed in humans.
Together, these findings suggest that diet composition of the host, in terms of different carbohydrate contents as well as phytic acid and myo-inositol intake, has a metabolic role in concert with gut microbiota. However, given the few studies available in the literature, further studies will be needed to confirm the causal relationships between inositol uptake, microbiome modulation and metabolic effects.
4. Inositol and insulin signaling
Insulin increases glucose uptake from the bloodstream and metabolism in peripheral tissues. The first step of insulin signaling is the phosphorylation of the tyrosine kinase receptor and its substrates. A cytoplasmic second messenger is generated in parallel with the phosphorylation events initiated by the tyrosine-kinase receptor. The phosphorylation network and the second messenger pathway are both required to fully account for insulin effects.25
In the classic model of insulin signaling phosphorylation of insulin receptor substrates, phosphoinositide 3 kinase (PI3K) and protein kinase B/Akt (PKB/Akt) account for the most intracellular actions of insulin. Moreover, insulin stimulates both cellular glucose uptake and glycogen synthesis, but these actions sometimes occur disconnectedly, suggesting other signaling pathways connecting activation of insulin receptor to glucose uptake. Consequently, second messengers of insulin as inositol phosphoglycans (IPG) have been discovered.5
Under insulin stimulation and activation of a phosphatidyl-inositol phospholipase C, liver plasma membranes release IPG containing either myo-inositol or D-chiro-inositol. While D-chiro-inositol (also named inositol phosphoglycan-phosphatase stimulator) promotes the activation of pyruvate dehydrogenase (PDH) phosphatases (PDHP) and PDH, myo-inositol (also named inositol phosphoglycan AMP kinase inhibitor) inhibits both protein kinase A and adenylyl cyclase. Both types of IPGs have been shown to exert an insulin-mimetic activity, acting as second messengers downstream of insulin receptors. Upon insulin receptor activation, IPGs are released outside the cell and re-uptaken by an ATP-dependent inositol glycan transporter, activating cytosolic phosphoprotein phosphatase 2C-α (PP2Cα) and mitochondrial PDH. This enhances oxidative glucose metabolism along the tetracarboxylic acid cycle. Activated PP2Cα stimulates glycogen synthase both directly and indirectly, through the PI3K/Akt pathway. Activation of Akt inactivates glycogen synthase kinase-3, enhancing glycogen synthase activity, and increases GLUT-4 translocation and glucose uptake. Upon insulin stimulation, myo-inositol is converted by a specizc epimerase into its D-chiro-inositol isomer.25,26 Inositol isomerization occurs at phospholipid level where (3H)-myo-inositol phospholipids are converted into (3H)-D-chiro-inositol in 15 min after insulin stimulation. Myo-inositol epimerization is severely impaired when muscle, fat, and liver become insulin resistant. Intriguingly, a reduced D-chiro-inositol/myo-inositol ratio has been proposed to reflect the degree of insulin resistance.27
D-chiro-inositol is depleted in muscles and reduced in urine of patients with type 2 diabetes (DM2). Differently, urinary loss of myo-inositol is increased, probably depending on glucose-mediated inhibition of renal myo-inositol re-absorption.13 A shortage of myo-inositol impacts negatively on D-chiro-inositol levels worsening insulin resistance.1,2
It is still unclear if insulin resistance depends on a reduced content of membrane-bound IPGs, a primary defect in IPG release, or impaired epimerase activation downstream of insulin stimulation leading to a reduced D-chiro-inositol intracellular availability. While insulin-resistant tissues suffer from a general depletion of D-chiro-inositol due to the reduced epimerization of myo-inositol, other tissues that retain an adequate insulin sensitivity, like the ovary, display increased levels of D-chiro-inositol but reduced levels of myo-inositol, due to its enhanced epimerization.25 The increased availability of D-chiro-inositol in these tissues leads to several biological dysfunctions that are partially unexplained. For example, polycystic ovary syndrome (PCOS) has been associated with the increase in the D-chiro-inositol/myo-inositol ratio within the ovary but not in other tissues.1,3,25
The relationship between different isomers of inositol and their glycan derivatives is still unclear and in-depth studies are warranted to fully understand the role of inositol and IPG in insulin signaling.25
5. Inositols in polycystic ovary syndrome and fertility
PCOS is a common disease that affects 5–21% of women during their reproductive age,28 and is characterized by two of these three conditions according to the Rotterdam criteria: (i) chronic anovulation disorder (oligo- or anovulation up to amenorrhea); (ii) clinical (acne, hirsutism) or biochemical signs of hyperandrogenism; and (iii) presence of micro polycystic ovaries at ultrasound or presence of 12 or more follicles with a diameter of 2 ± 9 mm in each ovary, and/or increased ovarian volume (>10 ml).29 It is the most common cause of infertility secondary to ovulatory dysfunction.
The exact etiology of this disorder remains largely unknown, although it is recognized that ovarian hyperthecosis, increased androgens, insulin resistance, genetic and environmental factors all play a role in the pathophysiology of this syndrome.29 Insulin resistance is a critical point in the pathogenesis of PCOS in a large subset of patients and it is associated with metabolic morbidities as well as reproductive dysfunction.29 Insulin contributes to hyperandrogenism in PCOS by stimulating androgen production and secretion by the ovarian theca cells and promoting a rise in glucose blood levels. The disorder coexists with a decreased hepatic synthesis of sex hormone-binding globulin (SHBG), which increases the circulating levels of free androgens. It has been postulated that a defect in the post-receptor transport of glucose and selective resistance to metabolic actions of insulin may be responsible for hyperinsulinemia in these patients.29,30 The well-known association between hyperinsulinemia, hyperandrogenism, and ovulatory dysfunction in PCOS formed the basis for pharmacological treatment with insulin-sensitizing agents such as inositol, which have been demonstrated effective in improving insulin resistance as well as ovarian functions in these women.29,30
The inability in PCOS tissues to synthesize or metabolize inositols adequately may contribute to insulin resistance and hyperinsulinaemia.31 Hyperinsulinemia, in particular when observed in lean PCOS patients, should be explained by a reduced function/expression of epimerases, which converts myo-inositol to D-chiro-inositol allowing post receptor transduction.26 These findings are supported by the evidence that also healthy siblings of patients with type 2 diabetes have an imbalance in myo-inositol conversion to D-chiro-inositol, expressed as myo-inositol-to-D-chiro-inositol ratio, suggesting that altered specific epimerase activity is a player in the development of insulin resistance and family predisposition to altered glucose levels.25,26 Since deficient IPG second messenger pathway could be at the basis of the hyperinsulinaemia, inositols are supposed to be therapeutic for PCOS because they act as insulin-sensitizing agents and free radical scavengers, helping to regulate metabolism while promoting ovulation.32 In fact, PCOS women have reduced serum level and increased urinary loss of D-chiro-inositol and urinary clearance of this compound was inversely correlated with insulin sensitivity.33,34 Following these assumptions, it has been established that PCOS is characterized by a dysregulation of inositol metabolism,35 providing a mechanistic link between inositol deficiency and insulin resistance in PCOS.35 Since IPG signaling pathways are involved in insulin-mediated thecal biosynthesis of androgens, a defective conversion of myo-inositol to D-chiro-inositol in PCOS patients may also contribute to hyperandrogenism.32 Indeed, administration of D-chiro-inositol at low doses has been shown to decrease insulin resistance and serum androgens and improve ovulatory frequency.31,32,36,37
In a first work, Nestler et al.37 administered 1200 mg of D-chiro-inositol to PCOS obese patients for 8 weeks demonstrating an improvement in insulin sensitivity as well as free testosterone concentrations. Ovulation was restored in a higher percentage of these patients, and similar results were observed also in lean PCOS patients.38 When higher doses of D-chiro-inositol were administered, no significant improvement was observed in PCOS patients, suggesting that it was not a simple problem of nutritional deficiency. However, when administered at a dose as high as 2400 mg/day the efficacy was again demonstrated.36
The combined administration of myo-inositol and D-chiro-inositol in a 40 to 1 ratio, which seems to be the physiological plasma ratio, ensures better clinical results in the setting of treatment of PCOS women.32
While several studies have analyzed the effectiveness of myo-inositol and D-chiro-inositol in PCOS, either given alone or in combination, only two studies have compared the effects of myo-inositol and D-chiro-inositol, finding that they seem to exert comparable effects.32,39
Focusing on metabolic parameters, inositol isoforms were shown effective in improving the glucose metabolism and the lipid profile of obese PCOS women thus reducing the cardiovascular risk.40,41 D-chiro-inositol was efficacious in decreasing blood pressure and triglyceride concentrations.37 Myo-inositol treatment was demonstrated to be effective in reducing metabolic and oxidative abnormalities in PCOS patients by improving insulin resistance with a significant reduction also in glucose and C-peptide levels.37,42,43 Minozzi et al.44 reported a higher reduction of homeostatic model assessment-insulin resistance (HOMA-IR) after a 12-month combination therapy with myo-inositol and an oral contraceptive as compared to the oral contraceptive alone. The combination of myo-inositol/D-chiro-inositol induced an improvement of LDL-cholesterol, HDL-cholesterol, triglycerides40 and the association seemed twice more effective in reducing HOMA-IR than myo-inositol alone.45
Results of inositols on body weight in PCOS patients are not so concordant. In fact, while some studies found significantly decreased BMI following myo-inositol treatment,32,46,47 Genazzani et al.42 showed a non-significant change of BMI, although insulin sensitivity and hormonal parameters were improved. Moreover, the positive effects of inositol on metabolic parameters were not observed in patients with morbid obesity (BMI>37 kg/m2) and an inverse relationship between BMI and treatment efficacy was described.32,48 However, the association of myo-inositol and D-chiro-inositol seems to accelerate weight loss and fat mass reduction with a slight increase of lean mass, and this treatment contributes in restoring of the menstrual cycle.49
Attention has recently focused on the combination of inositol treatment with alpha lipoic acid (ALA). Using doses as low as 1 gr of myo-inositol combined with 400 mg of ALA improved insulin sensitivity and hormonal parameters in PCOS, overcoming the blunted response observed in some PCOS individuals with family predisposition to DM2.50,51 Increasing evidence suggests that oxidative stress-related hyperglycemia downregulates the expression of the lipoic acid synthase which is responsible of the synthesis of ALA inside the mitochondria of mammalians. A reduced ALA synthesis contributes to insulin resistance by lowering glucose uptake through a decrease of AMPK and, therefore, GLUT-4 in skeletal muscles.50,51,52 Indeed, the choice of inositol supplementation and its co-administration with other food supplements active on insulin signaling should be carefully considered in respect to the phenotype of patients, and pursue further research on this topic.
By reducing insulin resistance, inositol is effective in reducing androgen concentrations and improving menstrual regularity. The efficacy of myo-inositol treatment on the improvement of gonadal parameters such as LH levels, the LH/FSH ratio, as well as prolactin and testosterone levels, has been repeatedly demonstrated.42,43 In a study on 46 hirsute women, Minozzi and colleagues40 observed that myo-inositol therapy given at a dose of 2 g/day for 6 months decreased hirsutism as well as total androgens, FSH and LH, while increase in estradiol concentrations. Some also suggested that myo-inositol could be more effective than metformin in reducing testosterone and hs-CPR levels, as well as the Ferriman-Gallway score, irrespective of circulating insulin levels.53 Favorable results have been also reported by Genazzani et al.54 in a group of overweight/obese PCOS patients receiving 500 mg/day D-chiro-inositol for 12 weeks; after treatment, improvements in LH, FSH and androstenedione levels, as well as in the GnRH-induced LH response was observed. Moreover, daily D-chiro-inositol administration for a maximum of 15 months increased the percentage of women reporting regular menstrual cycles, proportionally to the duration of the treatment. In the same study, D-chiro-inositol administration modulated the secretion of anti-mullerian hormone.55 Also when given in combination, myo-inositol and D-chiro-inositol treatments were associated with a decrease in free testosterone, LH, FSH, an increase of serum SHBG, and an improvement in skin conditions.56
Some studies have demonstrated that myo-inositol treatment in patients with PCOS improved fertility.57,58 The positive pro-ovulatory effect of inositol was demonstrated both when compared to placebo47,48 and metformin.57 In an observational study performed on more than 3000 infertile women with PCOS, a treatment with myo-inositol and folic acid restored ovulation in 70% of women and promoted 545 pregnancies.59 In fact, myo-inositol was shown to be essential for proper oocyte maturation, and a direct correlation was found between myo-inositol concentration in the follicular fluid and increasing oocyte quality.31 Additionally, myo-inositol increased the total number of retrieved oocytes and improved oocyte quality in assisted reproduction procedure, while reducing the average number of immature oocytes.31 Focusing on in vitro fertilization in PCOS, the combined treatment of myo-inositol and D-chiro-inositol was able to improve oocyte and embryo quality, as well as pregnancy rates.31,32 Although these clues are encouraging, it is worth mentioning that as the fertility-promoting effects of myo-inositol in some studies are possibly flawed by coadministration of other supplements, mainly folic acid.59 Indeed, larger studies are warranted to substantiate these promising evidences.
We can thus summarize that PCOS is an endocrine and reproductive disorder that is frequently associated with abdominal adiposity, insulin resistance, obesity, metabolic disorders and cardiovascular risk factors. The etiology of this syndrome remains largely unknown, but mounting evidence suggests that PCOS could be linked to a dysregulation of inositol metabolism, justifying the use of inositols in these patients.
6. Inositol and diabetes mellitus
Inositols have long been identified as possible mediators of insulin action and mechanism of insulin resistance in DM2 as well as gestational diabetes mellitus (GDM). Following its conversion in vivo from myo-inositol, D-chiro-inositol acts by enhancing the activity of proteins involved in intracellular glucose metabolism and activates rate-limiting enzymes (i.e., pyruvate dehydrogenase phosphatase and glycogen synthase) during insulin-mediated glucose metabolism, thereby accelerating insulin action in insulin-sensitive tissues.1,5,25,60 However, in the setting of insulin-resistance and DM2 a reduction of myo-inositol re-absorption is observed at the kidney level, which causes consequent urinary losses of myo-inositol.1,25 This urinary loss results from hyperactivation of renal-specific oxidoreductase/myo-inositol oxygenase (RSOR/MIOX) via transcriptional/translational events and reduced tubular uptake of myo-inositol in condition of hyperglycemia and oxidative stress.1,25,61, 62, 63 Hyperglycemia-related depletion of myo-inositol occurs also in insulin-sensitive tissues (liver, muscle, fat and kidney) as well as at the cellular level, where an impairment of physiological processes relating to myo-inositol uptake, de novo biosynthesis, phosphoinositide-cycle regeneration, efflux and degradation occurs.1,5,25,64 Moreover, a 2–3 fold decreased epimerase activity has been described in target tissues of insulin action in GK rats. This could justify the decrease of the content of D-chiro-inositol and of its ratio relative to myo-inositol that has been observed in animal tissues, in postmortem tissue specimens of diabetic individuals, and in relation to insulin resistance in siblings from families with predisposition to DM2.26,65,66 It has been additionally suggested that abnormality in myo-inositol metabolism could be linked to the development of microvascular complications, abnormal nerve conductivity and kidney fibrosis associated with DM2.
The role of inositols, particularly myo-inositol, in regulating pathways related to DM2 has been also investigated in interventional studies. In animals and humans studies, inositol supplementation demonstrated that the risk of DM2 is significantly reduced by diets rich in fibers as well as by meal plans rich in fresh fruit/vegetables, legumes, whole grains, nuts and seeds, all of which are high in myo-inositol content.16,67 Myo-inositol has been shown to inhibit intestinal glucose absorption and promote muscle glucose uptake in rats,68 while a D-chiro-inositol-derivative glycan originally isolated in beef liver has been shown to act as an insulin mimetic and sensitizer, decreasing body weight and food intake when administered intracerebroventricularly in mice.69 In humans, clinical trials demonstrated that both myo-inositol and D-chiro-inositol possess insulin-mimetic properties and improve insulin sensitivity in metabolic conditions associated with insulin resistance other than PCOS. At doses ranging between 2 and 10 g/day with or without D-chiro-inositol supplementation, myo-inositol has favorable effects on glucose and insulin homeostasis, lipid profile and arterial blood pressure in people with prediabetes or dyslipidemia, as well as in women with GDM or postmenopausal metabolic syndrome. A pilot study in patients with incompletely controlled DM2 found that myo-inositol and D-chiro-inositol, given at relatively small doses (550 mg and 13.8 mg/day, respectively) for three months, observed a significant reduction in fasting glucose and HbA1c levels compared to baseline, devoid of changes in arterial blood pressure, lipid profile, and BMI levels.70 In a recent meta-analysis and systematic review, myo-inositol given at doses ranging 600–4000 mg showed to promote a reduction in fasting and post-OGTT plasma glucose, as well as improvements in glucose intolerance and insulin resistance independent of effects on body weight, HbA1c and insulin needs.71 Also, myo-inositol supplementation has been proven to be particularly useful in non-pharmacological management of GDM. A secondary analysis of 3 randomized controlled trials found that myo-inositol supplementation reduced the risk for GDM diagnosis by 64% and decreased the risk for macrosomia and preterm birth by 62% and 56%, respectively, without harms for the fetuses.72 Because urinary myo-inositol loss occurs in DM2 and insulin resistance,1,6,25,61,62 a further application of myo-inositol is diabetic nephropathy that is characterized by thickening of basement membranes, mesangial expansion with progression to glomerulosclerosis, tubular atrophy, and interstitial fibrosis, finally resulting in renal failure. Although convincing studies on the involvement of myo-inositol depletion in diabetic nephropathy are present, results of trials in humans are still lacking.
These direct and indirect evidences suggest that myo-inositol (and D-chiro-inositol) supplementation yields a beneficial effect on glycemic parameters of people at risk for or with DM2 without hazards for health.
7. Inositols and dyslipidemia
As mentioned above, the insulin-mimetic properties of myo-inositol and D-chiro-inositol supplementation could also represent a potential tool to control lipid profile in combination with lifestyle modification and pharmacological therapy.
Obesity, metabolic syndrome and PCOS are well known to be associated with hypertriglyceridemia and hypercholesterolemia, and hypertriglyceridemia is per se well correlated with insulin resistance and DM2.
Many studies have been conducted to determine whether inositol supplementation had any effect on lipid profile in populations with metabolic diseases.73 Even though most studies demonstrated the beneficial effects of inositol supplementation on lipid profile in patients with metabolic syndrome or PCOS, findings are still controversial.37,38,74,75
Several randomized controlled trial (RCTs) have been so far conducted to investigate the role of both myo-inositol and D-chiro-inositol supplements on lipids.73,76, 77, 78
A decrease in triglycerides and an increase in HDL-cholesterol levels were shown in postmenopausal women with metabolic syndrome taking a combination of inositol and alpha lipoic acid,76 as well as in early postmenopausal women affected by metabolic syndrome taking myo-inositol.76 Moreover, Kim et al.77 demonstrated that soybean-derived pinitol (D-3-O-methyl-Chiro-Inositol) supplementation in patients with DM2 decreased total-, LDL-, LDL/HDL-cholesterol ratio, and increased HDL-cholesterol, but did not affect triglycerides levels.
A recent systematic review of RCTs73 conducted in populations with metabolic diseases showed that inositols supplementation results in an improvement in triglycerides, total- and LDL-cholesterol levels, with no apparent effects on HDL-cholesterol levels. Noticeably, favorable effects of inositols supplementation on HDL-cholesterol levels were also observed in patients with PCOS.73 Moreover, Shokrpour et al.,78 recently compared the effects of 12 week myo-inositol and metformin administration on glycemic control, lipid profile, as well as gene expression related to insulin and lipid metabolism in women with PCOS, and found beneficial effects on triglycerides and VLDL-cholesterol levels, without modifications of LDL- and HDL-cholesterol or LDLR expression.
Several mechanisms have been proposed to explain the possible effects of inositols on lipid profile.
First, the decrease in insulin resistance following the intake of inositol. In addition, lipid metabolism might improve through lowering visceral fat weight, hepatic lipid accumulation and insulin secretion, as well as by increasing adiponectin concentrations.73 In fact, 8-weeks myo-inositol supplementation in women with GDM increased adiponectin levels79; moreover, Gerli et al.48 described a significant weight loss and leptin reduction following myo-inositol administration, that might led to an improvement in lipid profile.
8. Inositols as food supplements
Based on the findings above commented, it seems that consuming extra inositols has therapeutic potentials in several metabolic diseases.
Studies investigating this topic have employed different forms, combinations, and dosages of inositols with variable results. With regard to dosing, supplements usually contained no more than 2–4 g/day of inositol content, although studies in patients with depressive disorders used much larger doses of up to 12–18 g/day, without reporting significant adverse events while showing additional clinical benefits.80 The main side effects reported were nausea, flatus, and diarrhea secondary to the highest dose of myo-inositol (starting from 12 g/day).71,80 Some authors seem to favor supplements containing D-chiro-inositol, while others question this option and, rather, stress the disadvantage relating to D-chiro-inositol inability to be converted in myo-inositol.81 It has been argued that a myo-inositol/D-chiro-inositol ratio of 40 : 1 represents more closely the physiological condition because it reflects what we can find in the plasma.70 However, uncertainties still remain relatively to intracellular balance, target tissue distribution, clearance, interactions with food minerals, and plasmatic and urinary measurements of the different inositol forms. Furthermore, most of the trials did not consider the daily intake of inositols and phytates with the diet, making difficult to understand if a threshold effect for different forms exists. It can be speculated that low and high dietary intake of inositol could have an impact, especially in studies using relatively low supplementation doses (1.2–2 g/day). Also, food supplements containing inositol frequently include combinations of functional molecules such as folic acid, melatonin, vitamin D, vitamin E, or ALA making it difficult to decipher the specific action of inositol itself.
9. Conclusions
Isoforms of inositol are polyols present in both prokaryotic and eukaryotic cells and are components of many molecules and second messengers, making them essential for numerous biological processes. Inositol-deficient diets or alterations in its metabolism are potentially associated with several diseases, particularly metabolic disorders where inositol is implicated in insulin function. Since inositol isoforms are putative mediators of insulin action, their deficit or unbalance in insulin target tissues could participate in the development or progression of insulin resistance. Some tasks should be focused on future research. Investigations are required to decipher the exact molecular mechanisms of action of isoforms, mainly myo-inositol and D-chiro-inositol. The interaction with diet and microbiota is also a critical challenge to determine supplementation rate, isomers and dose range to use. Moreover, shaping microbiota to potentiate inositol production by a daily natural diet is an interesting field. Yet another challenging question in investigating the functional inositol benefits is constituted by gender medicine, since inositols have been more investigated in females than males, and open issues remain on gender-dependent dosing and efficacy. Larger appropriately designed studies on both genders are warranted to thoroughly exploiting the potentiality of inositol in the diet or as a food supplement for the prevention or treatment of insulin-related metabolic diseases.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The Authors declare that there is no financial/personal interest or belief that could affect their objectivity.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bizzarri M., Fuso A., Dinicola S., Cucina A., Bevilacqua A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expet Opin Drug Metabol Toxicol. 2016;12(10):1181–1196. doi: 10.1080/17425255.2016.1206887. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui N., Singh V., Deshmukh M.M., Gurunath R. Structures, stability and hydrogen bonding in inositol conformers. Phys Chem Chem Phys. 2015;17(28):18514–18523. doi: 10.1039/c5cp02690c. [DOI] [PubMed] [Google Scholar]
- 3.Bizzarri M., Carlomagno G. Inositol: history of an effective therapy for polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2014;18:1896–1903. [PubMed] [Google Scholar]
- 4.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? 2010; Nutr Res Rev. 23(1):65-134. [DOI] [PubMed]
- 5.Croze M.L., Soulage C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95(10):1811–1827. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Holub B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa R., Eisenberg F., Jr. Selective hormonal control of myo-inositol biosynthesis in reproductive organs and liver of the male rat. Proc Natl Acad Sci U S A. 1981;78(8):4863–4866. doi: 10.1073/pnas.78.8.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitkänen E. Changes in serum and urinary myo-inositol levels in chronic glomerulonephritis. Clin Chim Acta. 1976;71(3):461–468. doi: 10.1016/0009-8981(76)90097-8. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois F., Coady M.J., Lapointe J.Y. Determination of transport stoichiometry for two cation-coupled myo-inositol cotransporters: SMIT2 and HMIT. J Physiol. 2005;563(pt2):333–343. doi: 10.1113/jphysiol.2004.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider S. Inositol transport proteins. FEBS Lett. 2015;589(10):1049–1058. doi: 10.1016/j.febslet.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Thomas R.M., Nechamen C.A., Mazurkiewicz J.E., Ulloa-Aguirre A., Dias J.A. The adapter protein APPL1 links FSH receptor to inositol 1,4,5-trisphosphate production and is implicated in intracellular Ca(2+) mobilization. Endocrinology. 2011;152:1691–1701. doi: 10.1210/en.2010-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu T.T., Rogers M.S., Briton-Jones C., Haines C. Effects of myo-inositol on the invitro maturation and subsequent development of mouse oocytes. Hum Reprod. 2003;18:408–416. doi: 10.1093/humrep/deg113. [DOI] [PubMed] [Google Scholar]
- 13.Streb H., Irvine R.F., Berridge M.J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306(5938):67–69. doi: 10.1038/306067a0. Nov 3-9. [DOI] [PubMed] [Google Scholar]
- 14.Loewus M.W., Loewus F.A., Brillinger G.U., Otsuka H., Floss H.G. Stereochemistry of the myo-inositol-1-phosphate synthase reaction. J Biol Chem. 1980;255(24):11710–11712. [PubMed] [Google Scholar]
- 15.Dinicola S., Minini M., Unfer V., Verna R., Cucina A., Bizzarri M. Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102187. Pii:E2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements R.S., Jr., Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am J Clin Nutr. 1980;33(9):1954–1967. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- 17.Reddy N.R., Sathe S.K., Salunke D.K. Phytates in legumes and cereals. Adv Food Res. 1982;28:1–89. doi: 10.1016/s0065-2628(08)60110-x. [DOI] [PubMed] [Google Scholar]
- 18.Schlemmer U., Frølich R.M., Prieto R.M., Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53(Suppl. 2):S330–S375. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- 19.Schlemmer U., Jany K.D., Berk A., Schulz E., Rechkemmer G. Degradation of phytate in the gut of pigs-pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch Tierernahr. 2001;55(4):255–280. doi: 10.1080/17450390109386197. [DOI] [PubMed] [Google Scholar]
- 20.Pineda-Quiroga C., Borda-Molina D., Chaves-Moreno D., Ruiz Atxaerandio R., Camarinha-Silva A., García-Rodríguez A. Microbial and functional profile of the ceca from laying hens affected by feeding prebiotics, probiotics, and synbiotics. Microorganisms. 2019;7(5):E123. doi: 10.3390/microorganisms7050123. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okazaki Y., Katayama T. Dietary phytic acid modulates characteristics of the colonic luminal environment and reduces serum levels of proinflammatory cytokines in rats fed a high-fat diet. Nutr Res. 2014;34(12):1085–1091. doi: 10.1016/j.nutres.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Sekita A., Okazaki Y., Katayama T. Dietary phytic acid prevents fatty liver by reducing expression of hepatic lipogenic enzymes and modulates gut microflora in rats fed a high-sucrose diet. Nutrition. 2016;32(6):720–722. doi: 10.1016/j.nut.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki Y., Sekita A., Katayama T. Intake of phytic acid and myo-inositol lowers hepatic lipogenic gene expression and modulates gut microbiota in rats fed a high-sucrose diet. Biomed Rep. 2018;8(5):466–474. doi: 10.3892/br.2018.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeevi D., Korem T., Godneva A. Structural variation in the gut microbiome associates with host health. Nature. 2019;568(7750):43–48. doi: 10.1038/s41586-019-1065-y. [DOI] [PubMed] [Google Scholar]
- 25.Bevilacqua A., Bizzarri M. Inositols in insulin signaling and glucose metabolism. Internet J Endocrinol. 2018;2018 doi: 10.1155/2018/1968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genazzani AD Inositol as putative integrative treatment for PCOS. Reprod Biomed Online. 2016;33(6):770–780. doi: 10.1016/j.rbmo.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Larner J., Craig J.W. Urinary myo-inositol-to-chiro-inositol ratios and insulin resistance. Diabetes Care. 1996;19(1):76–78. doi: 10.2337/diacare.19.1.76. [DOI] [PubMed] [Google Scholar]
- 28.Azziz R., Woods K.S., Reyna R., Key T.J., Knochenhauer E.S., Yildiz B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 29.Fauser B.C., Tarlatzis B.C., Rebar R.W. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Dunaif KR Insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2006;86(1):S13–S14. doi: 10.1016/j.fertnstert.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Facchinetti F., Bizzarri M., Benvenga S. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol. 2015;195:72–76. doi: 10.1016/j.ejogrb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Nestler J.E., Unfer V. Reflections on inositol(s) for PCOS therapy: steps toward success. Gynecol Endocrinol. 2015;31(7):501–505. doi: 10.3109/09513590.2015.1054802. [DOI] [PubMed] [Google Scholar]
- 33.Baillargeon J.P., Diamanti-Kandarakis E., Ostlund R.E., Jr., Apridonidze T., Iuorno M.J., Nestler J.E. Altered D-chiro- inositol urinary clearance in women with polycystic ovary syndrome. Diabetes Care. 2006;29(2):300–305. doi: 10.2337/diacare.29.02.06.dc05-1070. [DOI] [PubMed] [Google Scholar]
- 34.Baillargeon J.P., Nestler J.E., Ostlund R.E., Apridonidze T., Diamanti-Kandarakis E. Greek hyperinsulinemic women, with or without polycystic ovary syndrome, display altered inositols metabolism. Hum Reprod. 2008;23(6):1439–1446. doi: 10.1093/humrep/den097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baillargeon J.P., Iuorno M.J., Apridonidze T., Nestler J.E. Uncoupling between insulin and release of a d-chiro-inositol- containing inositolphosphoglycan mediator of insulin action in obese women with polycystic ovary syndrome. Metab Syndr Relat Disord. 2010;8(2):127–136. doi: 10.1089/met.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheang K.I., Baillargeon J.P., Essah P.A. Insulin- stimulated release of d-chiro-inositol-containing inositolphosphoglycan mediator correlates with insulin sensitivity in women with polycystic ovary syndrome. Metabolism. 2008;57(10):1390–1397. doi: 10.1016/j.metabol.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestler J.E., Jakubowicz D.J., Reamer P., Gunn R.D., Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340(17):1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]
- 38.Iuorno M.J., Jakubowicz D.J., Baillargeon J.P. Effects of D-chiro-inositol in lean women with the poly- cystic ovary syndrome. Endocr Pract. 2002;8:417–423. doi: 10.4158/EP.8.6.417. [DOI] [PubMed] [Google Scholar]
- 39.Pizzo A., Laganà A.S., Barbaro L. Comparison between effects of myo-inositol and D-chiro-inositol on ovarian function and metabolic factors in women with PCOS. Gynecol Endocrinol. 2014;30(3):205–208. doi: 10.3109/09513590.2013.860120. [DOI] [PubMed] [Google Scholar]
- 40.Minozzi M., Nordio M., Pajalich R. The combined therapy myo-inositol plus D-chiro-inositol, in a physiological ratio, reduces the cardiovascular risk by improving the lipid profile in PCOS patients. Eur Rev Med Pharmacol. 2013;17:537–540. [PubMed] [Google Scholar]
- 41.Benelli E., Del Ghianda S., Di Cosmo C., Tonacchera M. A combined therapy with myo-inositol and D-chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Internet J Endocrinol. 2016;2016 doi: 10.1155/2016/3204083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genazzani A.D., Lanzoni C., Ricchieri F., Jasonni V.M. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in over- weight patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:139–144. doi: 10.1080/09513590801893232. [DOI] [PubMed] [Google Scholar]
- 43.Artini P.G., Di Berardino O.M., Papini F. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol Endocrinol. 2013;29:375–379. doi: 10.3109/09513590.2012.743020. [DOI] [PubMed] [Google Scholar]
- 44.Minozzi M., Costantino D., Guaraldi C., Unfer V. The effect of a combination therapy with myo-inositol and a combined oral contraceptive pill versus a combined oral contraceptive pill alone on metabolic, endocrine, and clinical parameters in polycystic ovary syndrome. Gynecol Endocrinol. 2011;27:920–924. doi: 10.3109/09513590.2011.564685. [DOI] [PubMed] [Google Scholar]
- 45.Nordio M., Proietti E. The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo -inositol supplementation alone. Eur Rev Med Pharmacol Sci. 2012;16(5):575–581. [PubMed] [Google Scholar]
- 46.Venturella R., Mocciaro R., De Trana E., D’Alessandro P., Morelli M., Zullo F. Assesment of the modification of the clinical, endocrinal and metabolical profile of patients with PCOS syndrome treated with Myo-inositol. Minerva Ginecol. 2010;64:239–243. [PubMed] [Google Scholar]
- 47.Gerli S., Mignosa M., Di Renzo G.C. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double-blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2003;7:151–159. [PubMed] [Google Scholar]
- 48.Gerli S., Papaleo E., Ferrari A., Di Renzo G.C. Randomized, double blind placebo- controlled trial: effects of myo -inositol on ovarian function and metabolic factors in women with PCOS. Eur Rev Med Pharmacol Sci. 2007;11:347–354. [PubMed] [Google Scholar]
- 49.Le Donne M., Metro D., Alibrandi A., Papa M., Benvenga S. Effects of three treatment modalities (diet, myo-inositol or myo-inositol associated with D-chiro-inositol) on clinical and body composition outcomes in women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2019;23(5):2293–2301. doi: 10.26355/eurrev_201903_17278. [DOI] [PubMed] [Google Scholar]
- 50.Genazzani A.D., Prati A., Marchini F., Petrillo T., Napolitano A., Simoncini T. Differential insulin response to oral glucose tolerance test (OGTT) in overweight/obese polycystic ovary syndrome patients undergoing to myo-inositol (MYO), alpha lipoic acid (ALA), or combination of both. Gynecol Endocrinol. 2019;35(12):1088–1093. doi: 10.1080/09513590.2019.1640200. [DOI] [PubMed] [Google Scholar]
- 51.Genazzani A.D., Prati A., Simoncini T., Napolitano A. Modulatory role of D-chiro-inositol and alpha lipoic acid combination on hormonal and metabolic parameters of overweight/obese PCOS patients. Eur Gynecol obstetr. 2019;1(1):29–33. [Google Scholar]
- 52.Gomes M.B., Negrato C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndrome. 2014;6(1):80. doi: 10.1186/1758-5996-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamilian M., Farhat P., Foroozanfard F. Comparison of myo -inositol and metformin on clinical, metabolic and genetic parameters in polycystic ovary syndrome: a randomized controlled clinical trial. Clin Endocrinol. 2017;87:194–200. doi: 10.1111/cen.13366. [DOI] [PubMed] [Google Scholar]
- 54.Genazzani A.D., Santagni S., Rattighieri E. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol Endocrinol. 2014;30(6):438–443. doi: 10.3109/09513590.2014.897321. [DOI] [PubMed] [Google Scholar]
- 55.La Marca A., Grisendi V., Dondi G., Sighinolfi G., Cianci A. The menstrual cycle regularization following D-chiro-inositol treatment in PCOS women: a retrospective study. Gynecol Endocrinol. 2015;31(1):52–56. doi: 10.3109/09513590.2014.964201. [DOI] [PubMed] [Google Scholar]
- 56.Januszewski M., Issat T., Jakimiuk A.A., Santor-Zaczynska M., Jakimiuk A.J. Metabolic and hormonal effects of a combined Myo-inositol and d-chiro-inositol therapy on patients with polycystic ovary syndrome (PCOS) Ginekol Pol. 2019;90(1):7–10. doi: 10.5603/GP.2019.0002. [DOI] [PubMed] [Google Scholar]
- 57.Raffone E., Rizzo P., Benedetto V. Insulin sensitiser agents alone and in co-treatment with r-FSH for ovulation induction in PCOS women. Gynecol Endocrinol. 2010;26:275–280. doi: 10.3109/09513590903366996. [DOI] [PubMed] [Google Scholar]
- 58.Papaleo E., Unfer V., Baillargeon J.P., Fusi F., Occhi F., De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. 2009;91:1750–1754. doi: 10.1016/j.fertnstert.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 59.Regidor P.A., Schindler A.E. Myo-inositol as a safe and alternative approach in the treatment of infertile PCOS women: a German observational study. Internet J Endocrinol. 2016;2016 doi: 10.1155/2016/9537632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakkar J.K., Raju M.S., Kennington A.S., Foil B., Caro J.F. [3H] Myo-inositol incorporation into phospholipids in liver microsomes from humans with and without type II diabetes. The lack of synthesis of glycosylphosphatidylinositol, precursor of the insulin mediator inositol phosphate glycan. J Biol Chem. 1990;265(10):5475–5481. [PubMed] [Google Scholar]
- 61.Nayak B., Xie P., Akagi S. Modulation of renal-specific oxidoreductase/myo-inositol oxygenase by high-glucose ambience. Proc Natl Acad Sci U S A. 2005;102(50):17952–17957. doi: 10.1073/pnas.0509089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nayak B., Kondeti V.K., Xie P. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J Biol Chem. 2011;286(31):27594–27611. doi: 10.1074/jbc.M110.217141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma I., Tupe R.S., Wallner A.K., Kanwar Y.S. Contribution of myo-inositol oxygenase in AGE: RAGE-mediated renal tubulointerstitial injury in the context of diabetic nephropathy. Am J Physiol Ren Physiol. 2018;314(1):F107–F121. doi: 10.1152/ajprenal.00434.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung T.S., Hahm J.R., Kim J.J. Determination of urinary Myo-inositol-/chiro-inositol ratios from Korean diabetes patients. Yonsei Med J. 2005;46(4):532–538. doi: 10.3349/ymj.2005.46.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asplin I., Galasko G., Larner J. chiro-inositol deficiency and insulin resistance: a comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc Natl Acad Sci U S A. 1993;90(13):5924–5928. doi: 10.1073/pnas.90.13.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun T.H., Heimark D.B., Nguygen T., Nadler J.L., Larner J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem Biophys Res Commun. 2002;293(3):1092–1098. doi: 10.1016/S0006-291X(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 67.Martínez-González M.A., De La Fuente-Arrillaga C., Nunez-Cordoba J.M. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336(7657):1348–1351. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chukwuma C.I., Ibrahim M.A., Islam M.S. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: a dual approach study. J Physiol Biochem. 2016;72(4) doi: 10.1007/s13105-016-0517-1. 791-80. [DOI] [PubMed] [Google Scholar]
- 69.Jeon Y., Aja S., Ronnett G.V., Kim E.K. D-chiro-inositol glycan reduces food intake by regulating hypothalamic neuropeptide expression via AKT-FoxO1 pathway. Biochem Biophys Res Commun. 2016;470(4):818–823. doi: 10.1016/j.bbrc.2016.01.115. [DOI] [PubMed] [Google Scholar]
- 70.Pintaudi B., Di Vieste G., Bonomo M. The effectiveness of myo-inositol and D-chiro inositol treatment in type 2 diabetes. Internet J Endocrinol. 2016 doi: 10.1155/2016/9132052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miñambres I., Cuixart G., Gonçalves A., Corcoy R. Effects of inositol on glucose homeostasis: systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1146–1152. doi: 10.1016/j.clnu.2018.06.957. [DOI] [PubMed] [Google Scholar]
- 72.Santamaria A., Alibrandi A., Di Benedetto A. Clinical and metabolic outcomes in pregnant women at risk forgestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. Am J Obstet Gynecol. 2018;219(3):300.e1–300.e6. doi: 10.1016/j.ajog.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Tabrizi R., Ostadmohammadi V., Lankarani K.B. The effects of inositol supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018;17:123. doi: 10.1186/s12944-018-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capasso I., Esposito E., Maurea N. Combination of inositol and alpha lipoic acid in metabolic syndrome-affected women: a randomized placebo controller trial. Trials. 2013;14:273. doi: 10.1186/1745-6215-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pundir J., Psaroudakis D., Savnur P. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomized trials. BJOG. 2018;125:509–510. doi: 10.1111/1471-0528.14996. [DOI] [PubMed] [Google Scholar]
- 76.Giordano D., Corrado F., Santamaria A. Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: a perspective, randomized, placebo-controlled study. Menopause. 2011;18:102–104. doi: 10.1097/gme.0b013e3181e8e1b1. [DOI] [PubMed] [Google Scholar]
- 77.Kim J.I., Kim J.C., Kang M.J., Lee M.S., Kim J.J., Cha I.J. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: a randomized controlled study. Eur J Clin Nutr. 2005;59(3):456–458. doi: 10.1038/sj.ejcn.1602081. [DOI] [PubMed] [Google Scholar]
- 78.Shokrpour M., Foroozanfard F., Ebrahimi F. Comparison of myo-inositol and metformin on glycemic control, lipid profiles, and gene expression related to insulin and lipid metabolism in women with polycystic ovary syndrome: a randomized controlled clinical trial. Gynecol Endocrinol. 2019;35:406–411. doi: 10.1080/09513590.2018.1540570. [DOI] [PubMed] [Google Scholar]
- 79.Unfer V., Carlomagno G., Dante G., Facchinetti F. Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials. Gynecol Endocrinol. 2012;28:509–515. doi: 10.3109/09513590.2011.650660. [DOI] [PubMed] [Google Scholar]
- 80.Mukai T., Kishi T., Matsuda Y., Iwata N. A meta-analysis of inositol for depression and anxiety disorders. Hum Psychopharmacol. 2014;29:55–63. doi: 10.1002/hup.2369. [DOI] [PubMed] [Google Scholar]
- 81.Michell R.H. Do inositol supplements enhance phosphatidylinositol supply and thus support endoplasmic reticulum function? Br J Nutr. 2018:1–16. doi: 10.1017/S0007114518000946. [DOI] [PubMed] [Google Scholar]