Abstract
Xanthohumol (XH), a plant flavonoid, was shown to attenuate cholangiocarcinoma (CCA) development induced by the liver fluke Opisthorchis viverrini (Ov) and N-dinitrosomethylamine (NDMA) in the hamster model. We investigated the possible involvement of autophagy, a self-degrading process dysregulated in cancer, in XH chemotherapeutic effect. During cholangiocarcinogenesis, the expression of LC3 (an autophagic marker) was increased in the precancerous stage and decreased in the cancerous stage. The XH-treated ON (Ov plus NDMA) group showed retarded progression of CCA along with increased expression of LC3. The possible relation between autophagy and cell death was investigated in cultured human CCA cells. XH induced apoptosis associated with reduced expression of BCL-2 and increased expression of BAX. In parallel, XH induced the autophagy flux, as testified by increased LC3-II and decreased p62, along with induction of BECLIN1 and Vps34. Inhibition of BECLIN1-dependent autophagy greatly limited XH toxicity in CCA cells. These data suggest that XH attenuates the development of CCA through overstimulation of autophagy which then precipitates apoptosis.
Keywords: Apoptosis, BECLIN1, Cancer, Cholangiocarcinoma, Herbal medicine, Flavonoids, LC3, Opistorchis viverrini, Spautin, Therapy
Abbreviations: XH, Xanthohumol; CCA, cholangiocarcinoma; OV, Opisthorchis viverrini; NDMA, N-dinitrosomethylamine; CIQ, chloroquine; SP-1, Spautin-1
Graphical abstract
1. Introduction
Xanthohumol (XH) is the principal flavonoid found in the hops plant Humulus lupulus L. (Cannabaceae).1 XH has been shown to possess anti-cancer properties,2,3 which have been associated with the abilities to scavenge free radicals4,5 and to induce apoptosis.6, 7, 8 XH toxicity has been associated also to induction of autophagy.9,10
Autophagy is a dynamic, self-degradation process in which intracellular proteins and organelles are engulfed within double-membrane autophagosomes that eventually fuse with lysosomes.11 Autophagy has a major role in macromolecular turnover and cell homeostasis and is dysregulated in cancer.12, 13, 14 Experimental evidence indicates that autophagy may play a dual-opposite role in cancer: it prevents and retards carcinogenesis by limiting the accumulation of harmful cellular components, yet it may provide a metabolic advantage to cancer cells experiencing hypoxia, lack of nutrients or exposed to chemotherapeutics.15, 16, 17 On the other hand, overstimulation of autophagy may precipitate into cell death with the characteristics of autophagy-associated apoptosis or of autophagic cell death.18, 19, 20, 21 Thus, modulation of autophagy may be curative or not depending on the stage at which it is induced or inhibited.
Cholangiocarcinoma (CCA) is a cancer arising from the biliary duct epithelia. In Southeast Asia CCA is primarily associated with liver fluke Opisthorchis viverrini (Ov) infection.22 Administration of Ov and N-dinitrosomethylamine (NDMA) at a sub-carcinogenic dose strongly induces CCA development in the hamster.23 Recently, it has been reported that XH suppresses inflammation and fibrosis resulting in a slow progression of CCA development in this experimental model.24 XH has been shown to induce apoptosis in CCA cells and to inhibit the growth of CCA xenograft via suppression of the Akt—NF—kB signaling pathway and inhibition of STAT3 expression.25,26 These data indicate that XH is a promising chemotherapeutics for CCA treatment. The present study intended to get a deeper insight into the anti-cancer mechanism of XH. Here we show that autophagy contributes to XH anti-cancer activity in CCA by slowing down cancer cell growth and promoting apoptosis.
2. Materials and methods
2.1. Animal model, treatments and histological analyses
CCA carcinogenesis was induced in Syrian golden hamsters by an infection of Ov metacercariae combined with NDMA treatment.24 The treatment protocol with XH in this experimental model has been reported previously.24 Animal experiments were approved by the Animal Ethics Committee of the Faculty of Medicine, Khon Kaen University (AEKKU 23/2555), Thailand. Syrian golden hamsters ranging from 6 to 8 weeks of age were divided into four groups (eight/group): (i) untreated; (ii) treated with XH; (iii) ON (Ov with NDMA administration); and (iv) XHON (Ov infection and NDMA administration and XH treatment). Overall survival in the subgroups was determined in parallel experiments and plotted in a Kaplan-Meier graph as survival percentage. Hamster subgroups were euthanized and the tumors removed at 60, 90, 120, and 180 days and liver tissue was collected for histology examination by the pathologists. Biliary epithelial alterations were graded as hyperplasia, dysplasia ducts, periductal fibrosis and CCA as described previously.23,24
2.2. Immunohistochemistry
Expression of the autophagy protein LC3 was analyzed in the tumor sections by immunohistochemistry. Sections of liver tissue were deparaffinized and antigen-retrieved in 0.05% Tween20 in 0.1M sodium citrate in microwave, then placed with 0.3% H2O2 to block endogenous peroxidase activity. Non-specific protein binding was blocked by 10% skim milk and the sections were incubated overnight with the primary antibody (rabbit anti-LC3 polyclonal antibody; Abcam; MA, UK; 1:500 dilution). After washing-out, the sections were incubated with peroxidase-conjugated Envision secondary antibodies and peroxidase activity was visualized with a diaminobenzidine solution. Hematoxylin was employed for counterstaining. The staining was observed under a light microscope (Carl Zeiss Axio Scope; A1 microscope) at 400x magnification. LC3 expression in the tissues was semiquantitatively scored based on the percentages of positive cells as follow: 0% = negative; 1–25% = +1; 26–50% = +2; and >50% = +3. The intensity of LC3 immunostaining was scored as weak = 1, moderate = 2, and strong = 3.
2.3. Cell culture and treatment
The human CCA cell lines KKU-100 and KKU-213, deposited in the Japanese Collection of Research Bioresources (JCBR) Cell Bank, Osaka, Japan (http://cellbank.nibiohn.go.jp/~cellbank/en/search_res_list.cgi) were used in this study. All cell lines were cultured in Ham’s F12 medium and RPMI medium, respectively, supplemented with NaHCO3, 100 units/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum. Cells were maintained in a humidified incubator at 37 °C containing 5% CO2. Cells were seeded at a density of 1 × 105 cells/ml and left to adhere on plates for 16–18 h (KKU-213) and for 72 h (KKU-100) prior to the start of any treatment (at this time cell density had approximately doubled). Treatments included 30 μM of XH (kindly provided by Hopsteiner, Mainberge, Germany), 5 μM spautin-1 (SP-1, Sigma–Aldrich, St. Louis, MO, USA), and 30 μM chloroquine (ClQ; Sigma–Aldrich, St. Louis, MO, USA). In some cases, cells were exposed to ClQ for 8 h prior to harvesting.
2.4. Western blotting
Protein expression was evaluated by a standard immunoblotting procedure. Briefly, cells were homogenized in RIPA buffer and 20 μg of cell protein was separated by electrophoresis on a 12% polyacrylamide SDS gel and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% BSA, incubated with primary antibodies and subsequently with a secondary antibody at the indicated dilution. The rabbit anti-LC3 (1:1000), rabbit anti-p62/SQSTM1 (1:500), rabbit anti-Vps34 (1:200) and mouse anti-BECLIN1 (1:500) were purchased from Abcam (MA, US). The rabbit anti-BAX (1:500), and rabbit anti-BCL-2 (1:500) were purchased from Cell Signaling Technology, Inc. USA. Blots were also stained with anti-β-actin antibody (1:20,000, Cell Signaling Technology, Inc. USA) that served as an internal control of total protein loading. Immunoreactive bands were developed by Enhanced Chemiluminescence Plus solution (ECL; Perkin Elmer, Waltham, MA) with an ImageQuant LAS 4000 (GE Healthcare; Life Sciences). Intensity of the bands was estimated by densitometry (ImageJ software 1.48v; Wayne Rasband, USA). All western blotting experiments were performed in triplicate.
2.5. Immunofluorescence
Subcellular expression and localization of BECLIN1, Vps34, LC3 and p62 was assessed by immunofluorescence15 in CCA cells after fixation with 4% paraformaldehyde. The cells were stained with primary antibodies (same commercial source as for western blotting) against BECLIN1 (1:100 dilution), Vps34 (1:100 dilution), LC3 (1:100 dilution) and p62/SQSTM1 (1:100 dilution), followed by incubation with secondary antibodies as appropriate. The nuclei were stained with Hoechst 33342 (Invitrogen, Thermo Fisher Scientific, USA). The slides were mounted onto microscope slides using fluorescence mounting SlowFadeTMGold antifade (Invitrogen, Thermo Fisher Scientific, USA). Images were captured with a confocal scanning microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and image analysis was determined using Carl Zeiss ZEN software. At least five fields in each chamber were examined by two independent investigators. Representative images of selected fields are presented. Data were reproduced in at least three independent experiments.
2.6. Colony formation assay
CCA cells were seeded in 6-well plates (200 cells in complete HAM’s F-12 medium). The cells were treated with 30 μM of XH with or without 5 μM of Spautin-1 (SP-1) for up to 10 days. The medium and substances for treatment were renovated every 3 days. At the end of the treatment, the cells were washed with 1xPBS, fixed with 10% TCA (trichloroacetic acid), stained with a 0.05% crystal violet solution, and washed with tap water until excess dye was removed. The colony number was counted by photometric measurements using the CellCounter software (Nghia, Ho) version 0.2.1. Three independent experiments were performed for each assay condition.
2.7. Cell viability and cell cycle assay
Cell viability after exposure to XH with or without SP-1 was assessed by manual counting using the trypan blue dye to label necrotic cells. The results reflect the average (means ± SD) of four replicates. Cell cycle analysis was performed by flow cytofluorometry.15 CCA cells were stained with propidium iodide (PI, 50 μg/ml final concentration) (Alexis Laboratories, San Diego, CA, USA) and analyzed in a FacScan flow cytometer (Becton Dickinson, USA).
2.8. Statistical analysis
The data are presented as the mean ± SD from at least three independent experiments. The statistical significance of the difference between the control and treated groups was evaluated using the Student’s t-test via SPSS software version 17.0 (SPSS Inc., Chicago, IL). All analyses were two-tailed and P-values less than 0.05 (*) and 0.01 (**) were considered statistically significant.
3. Results
3.1. Effects of XH and tissue expression of LC3 in hamster during CCA development
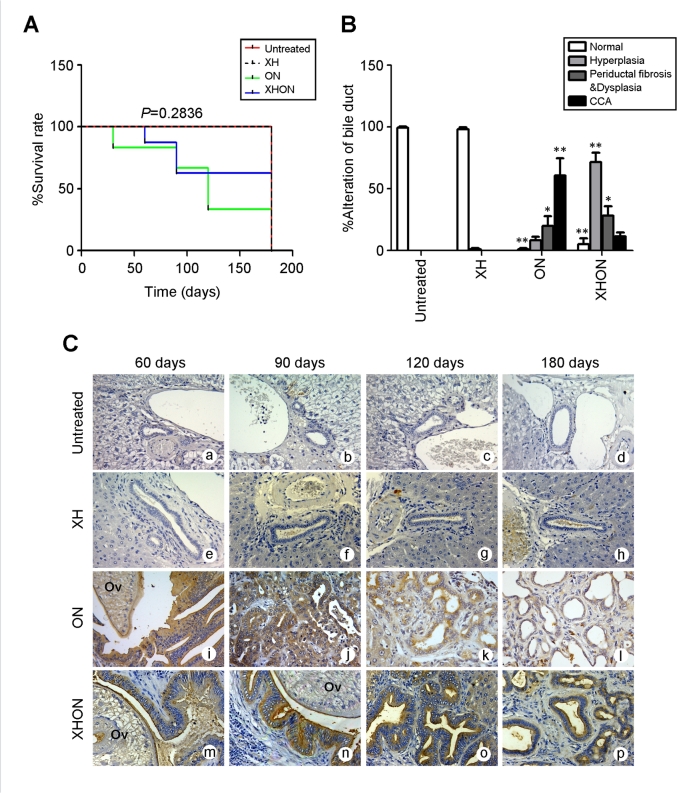
The outcome on hamster survival in the groups treated with or without ON (Ov plus NDMA; leading to CCA development) and co-treated or not with XH is shown in Fig. 1A. It is apparent that at 180 days XH can save hamster’s survival from ON effect (XHON group). The gross appearance and histopathological changes in hamster liver tissues caused by the combined treatments with ON in the absence or presence of XH has been reported in details in our previous study.24 Based on the morphometric analysis at day 180, the bile ducts in XH-treated and in control hamsters showed a similar morphology with negligible obvious alterations, while in the ON group the alterations of bile duct epithelial tissue increased in the order of normal < hyperplasia < periductal fibrosis and dysplasia < CCA (Fig. 1B). Strikingly, XH greatly prevented the development of CCA from ON (group XHON) and concomitantly increased the hyperplasia feature, though it did not attenuate the periductal fibrosis (Fig. 1B). These data suggest that XH may prevent cholangiocarcinogesis. Given the role of autophagy in tissue homeostasis and cancer prevention, we profiled the liver tissue expression of LC3, a marker of autophagic vacuoles, during the ON cholangiocarcinogenesis in the presence or absence of XH. Representative immunohistochemistry images are shown in Fig. 1C. The expression of LC3 was not detectable in the bile ducts of the untreated group (Fig. 1a-d) or the XH treated group at 60, 90, 120 and 180 days (Fig. 1e-h), indicating that in these tissues autophagy runs at low basal level. The highest level of LC3 expression was observed in all hyperplastic bile ducts at 60 and 90 days of the ON group (Fig. 1i-j), and it decreased from day 120 to day 180 (Fig. 1k-l), i.e. when CCA had frankly developed. Of note, LC3 expression maintained elevated in the XHON group (Fig. 1m-p) throughout the carcinogenesis process compared to the other groups. Thus, it seems that the progression from hyperplasia to cancer could be contrasted by keeping autophagy at levels above the baseline.
Fig. 1.
In vivo cholangiocarcinogenesis preventive effect of Xanthohumol and autophagy in hamster liver tissues. The potential involvement of autophagy in the chemopreventive and therapeutic effect of xanthohumol (XH) was investigated in hamster subjected to cholangiocarcinogenesis by treatment with Ov and NDMA (ON). (A) The Kaplan-Meier curves estimate the survival data and show that XH can save the life of treated hamsters (XHON group) by day 180. (B) Morphological alterations in the bile ducts as assessed by the pathologist. It is apparent that XH prevents the progression of hyperplastic nodules to frank CCA. (C) The expression of LC3 protein was profiled in hamster liver sections during the treatments. Representative images show undetectable LC3 staining in normal bile ducts in control groups (a–d) and in XH-treated group (e–h), intense LC3 staining at: 60–90 days (i–l), which declines by 180 days in ON group, and constant intense LC3 staining throughout the 60 days-180 days period in the hamsters treated with ON in the presence of XH (XHON group), (m-p). Magnification was × 400, scale bar 100 μm.
3.2. XH activates autophagy in cultured CCA cells
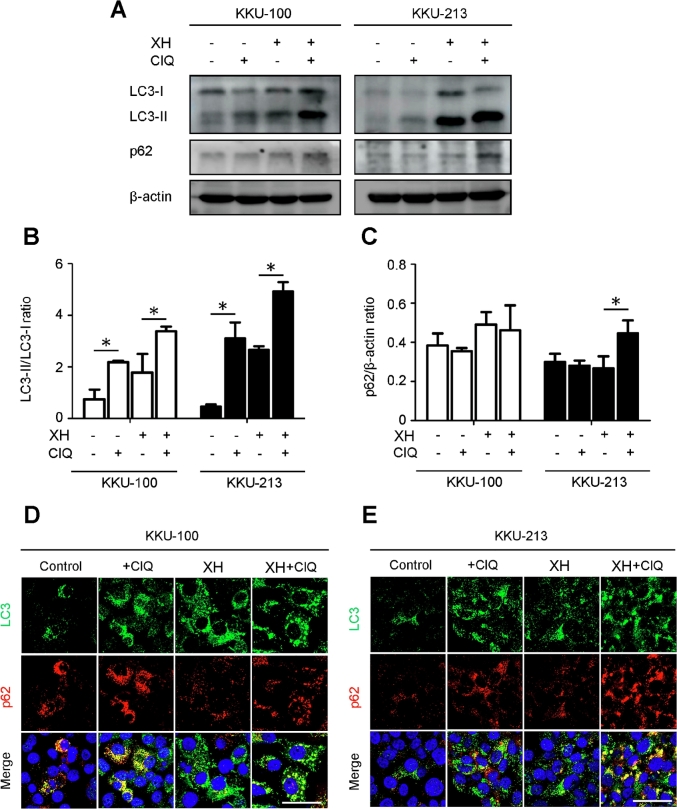
The modulation of autophagy by XH was further investigated in cultured human CCA cells. Two CCA cell lines, KKU-100 and KKU-213, were treated with 30 μM of XH for 24 h. Chloroquine (ClQ), an inhibitor of autophagosome degradation, was added for 8 h prior to sample collection in order to pause the autophagy flux.27,28 Western blotting analysis revealed that XH increased the cellular level of LC3-II, which is associated with autophagosomes, and concomitantly reduced the level p62, which reflects the degradation of autophagy substrates (Fig. 2A–C). Chloroquine (ClQ), known to interrupt the degradation of autophagosomes,27 caused an accumulation of LC3-II versus its precursor LC3-I and of p62 (Fig. 2A).27 The autophagy process induced by XH was further analyzed by immunofluorescence staining (Fig. 2D–E). The treatment of XH plus ClQ increased the number of the punctate fluorescent LC3 areas, consistent with the vacuolar localization of LC3. These were observed in the order of XH + ClQ > XH > ClQ > control. The expression of p62 was decreased in cells exposed to XH and accumulated, as expected, when ClQ was added.
Fig. 2.
Xanthohumol induces autophagy in CCA cells. The expression of the autophagy markers LC3 and p62 was assessed by western blotting (A) and immuonofluorescence (D,E) in KKU-100 and KKU-213 CCA cell lines exposed to XH. Chloroquine (ClQ), which impairs autophagosome degradation, was added to discriminate between true induction of autophagy and block of the autophagy flux. Densitometry of relevant protein bands from three independent western blotting experiments is reported in panels B and C. The ratio of LC3-II/LC3-I indicates the neoformation of autophagosomes and the level of p62/β-actin indicates the autophagy degradation efficiency. Data are expressed as mean ± standard deviation of three independent experiments. Statistical significance between experimental conditions is indicated (* denotes a significant p-value of less than 0.05).
3.3. Inhibition of BECLIN1-dependent autophagy attenuates apoptotic cell death induced by XH
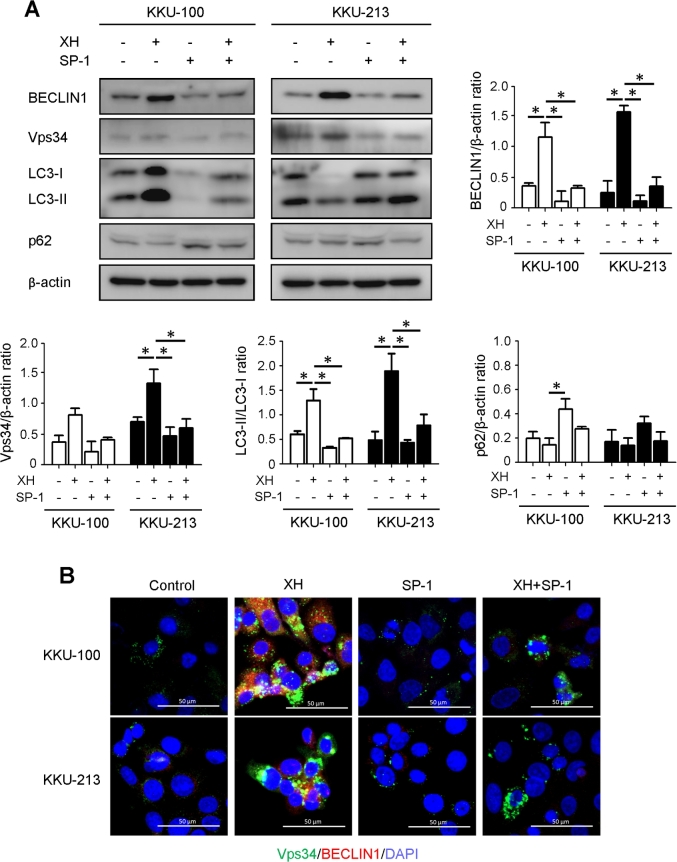
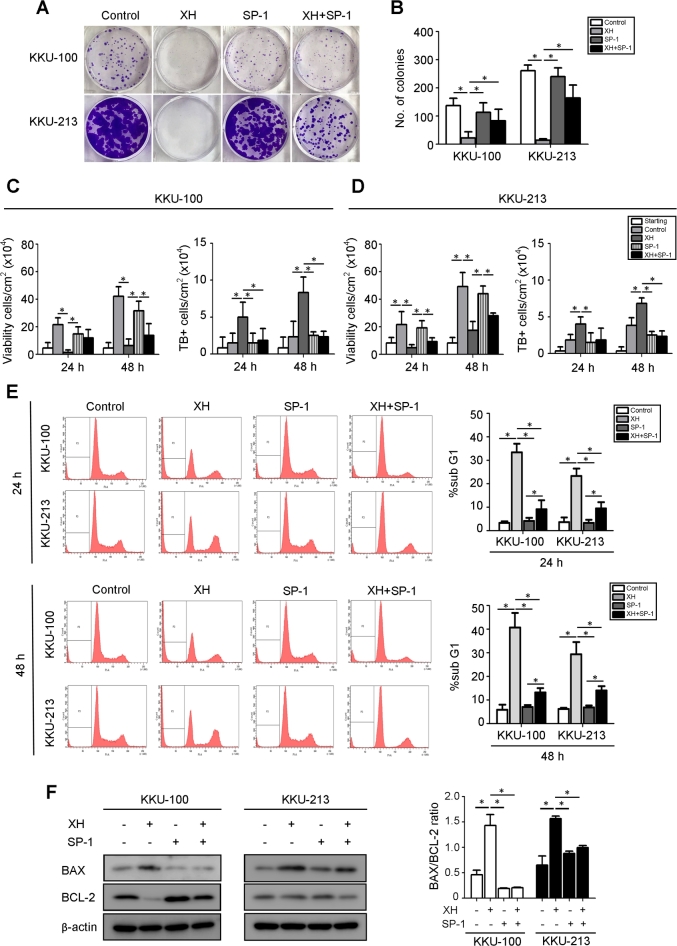
Next, we determined whether XH induced BECLIN1-dependent canonical autophagy and whether autophagy served a pro-survival or pro-death function during CCA development. XH strongly induced the expression (Fig. 3A) and co-localization (Fig. 3B) of BECLIN1 and Vps34 (also known as PI3K class III, PI3KC3), which is suggestive of activation of the canonical pathway. As a further proof, we co-treated the cells with Spautin-1 (SP-1), which promotes the proteasome degradation of BECLIN1.29 As shown in Fig. 3, SP-1 effectively reduced the cellular level of BECLIN1 even in the cells exposed to XH. More importantly, SP-1strongly limited the conversion of LC3-I into LC3-II by XH (Fig. 3A), further confirming that XH induces BECLIN1-dependent autophagy. Taking advantage of this finding, we inhibited autophagy with SP-1 to better assess the pro-survival or pro-death function of autophagy in XH-treated CCA cells. Cell growth and cell death were determined through a clonogenic assay and cell counting. Apoptosis was assessed by determining the SubG1 (hypodiploid) peak via flow cytometry and by western blotting analysis of the expression of BCL-2 and BAX. Data in Fig. 4A–B shows that XH effectively inhibited the growth of CCA cells. Of note, SP-1 partially rescued cell survival in XH-treated CCA colonies. Cell counting data (Fig. 4C–D) confirmed this trend. The number of viable cells was reduced while that of Trypan blue-positive increased in the cultures exposed to XH, and the concomitant treatment with SP-1 attenuated both these effects. Combined with the previous data showing inhibition of autophagy by SP-1 (Fig. 3), these data suggest a pro-death function of XH-induced autophagy. Cytofluorometric analysis of propidium iodide (PI)-stained cells revealed that hypodiploid-subG1 peak was five to eight times higher in XH than in controls (Fig. 4E). The proportion of hypodiploid cells was however much reduced in the population co-treated with XH and SP-1 (histograms in Fig. 4E). Finally, we assayed how the treatments impacted the expression of pro- and anti-apoptotic proteins. XH promoted the aggregation of BAX, and to some extent the degradation of BCL-2, and SP-1 counteracted these effects (Fig. 4F). Taken together, these data show that XH may induce cell death in CCA through a coordinated autophagy-apoptosis pathway.
Fig. 3.
BECLIN 1 is an essential mediator of XH-induced autophagy. A: Western blotting analysis of BECLIN1, Vps34, LC3 and p62 expression in KKU-100 and KKU-213 exposed or not to XH in the absence or presence of Spautin-1 (SP-1) for 24 h. The filter was stripped and probed for β-Actin as a marker of protein loading. Densitometry analysis of the bands from three independent western blotting is reported in the histogram. *p < 0.05 compared to control. (B) Double immunostaining for BECLIN1 (red fluorescence) and Vps34 (green fluorescence) in CCA cells treated with XH ± SP-1 as indicated. Nuclei were counterstained with Hoechst 33342 (blue fluorescence). Scale bar = 20 μM; magnification = 63x. Representative images of three independent experiments. Data in this figure demonstrate that SP-1-induced degradation of BECLIN-1 abrogates autophagy induced by XH.
Fig. 4.
Abrogation of BECLIN-1-dependent autophagy by Spautin-1 decreases the cytotoxic effect of Xanthohumol in CCA cells. (A) KKU-100 and B: KKU-213 CCA cells (2000 cells/well) were treated with XH for 24 h in the absence or presence of SP-1. Medium was replaced and substances re-added every 3 days for 10 days. At the end, the plates were stained and photographed. (B) The number of clones was determined using the CellCounter software (means ± SD of triplicate experiments) *p < 0.05 vs. control. (C–D) Cell counting of cultures growth for up to 48 h in the presence of XH and SP-1 as indicated. Cell viability was evaluated by trypan blue (TB positive) dye exclusion assay. (E) Cytofluorometric analysis of CCA cells treated with XH and SP-1 as indicated for 24 h and 48 h and labeled with propidium iodide. Representative cytofluorogram are shown. Quantitative analysis of the cytofluorometric data is shown in the histograms. The SubG1 peak represents the apoptotic cells. Data indicate that SP-1 reduces the population of apoptotic cells in the cultures treated with XH. (F) Western blotting of the pro-apoptotic BAX and anti-apoptotic BCL-2 proteins in the cells treated or not with XH in the presence or absence of SP-1. The filter was stripped and probed for β-Actin as marker of protein loading. Densitometry data of three separate experiments are reported as mean ± SD. Statistical significance *p < 0.05 compared to control.
4. Discussion
Autophagy, the catabolic process of cellular self-digestion, is a housekeeping survival mechanism with a protective function against stress conditions. However, when the severity or duration of stress increases, it may promote cell death.30 Malfunction of autophagy may contribute to certain diseases, including cancer.31 The ratio of LC3-II to LC3-I is a measure of autophagosome biogenesis, while a low level of p62 reflects the efficiency of autophagy degradation. The actual level of LC3-II and p62 in the cell can be determined by interrupting the autophagosome-lysosome fusion step, referring to autophagy flux.31 In this study, we provide a time profile of the expression of molecules related to autophagy appearing during CCA genesis generated by the combined administration of Ov plus NDMA, including XH treatment. Previous data showed that a combination of Ov infection and NDMA administration synergistically induces fibrosis and CCA development in hamsters in a time-dependent manner.24 The results from the current study show an increased accumulation of LC3-II protein in hyperplastic bile duct epithelial cells at the precancerous stage (60 and 90 days), and a reduction in the cancerous stage (120 and 180 days). These findings indicate that autophagy is activated during the precancerous stage and is suppressed once CCA had developed, suggesting the role of autophagy as a tumor suppressor. Induction of autophagy in CCA carcinogenesis is possibly caused by oxidative stress,32 starvation33 and hypoxia,34 whereas it is suppressed in fully developed CCA possibly due to the activation of the PI3K/AKT pathway.35,36 Consistent with this interpretation, XH was shown to induce autophagy in glioma9 and prostate cancer cells10 through the inhibition of Akt/mTOR/S6K and the activation of the MAPK signaling pathways. Another possible cause of autophagy suppression is the inflammation that associates with carcinogenesis. It is known that pro-inflammatory cytokines released by cancer associated fibroblasts in the tumor microenvironment can inhibit autophagy in cancer cells.21,37 XH could restore autophagy in cancer cells by suppressing the inflammation and the synthesis of pro-inflammatory cytokines in the tumor environment acting in a similar way as Resveratrol, another polyphenol nutraceutical.21 Indeed, XH has been reported to dampen inflammation and fibrosis in liver.38,39 Further supporting this possibility, XH was shown to inhibit the STAT3 inflammatory signaling pathway in CCA.26 XH has thus been claimed to be a potential chemopreventive agent in several types of cancer.4 Particularly, XH was shown to prevent the formation of preneoplastic lesions in livers and colons of rats exposed to chemical carcinogen.40 The possible involvement of autophagy in such cancer preventing effect of XH has not been fully investigated. Here, we found that autophagy was upregulated in the early stage of cholangiocarcinogenesis, while it was downregulated in frank CCA. XH saved the CCA-bearing animals and this associated with induction of autophagy and reduced progression of hyperplastic nodules to malignant tumors. Interestingly, XH did not raise basal autophagy in normal bile duct epithelium. We further investigated the pro-survival or pro-death function of XH-induced autophagy in cultured human CCA cells. We found that LC3 accumulated while p62 was degraded in CCA cells exposed to XH. Western blotting confirmed the increasing conversion of LC3-I to LC3-II and the decreasing p62 levels associated with the autophagy induction by XH. Our data indicate that the hyper induction of autophagy by XH could lead to apoptosis of CCA cell. In fact, this effect was abolished when autophagy was inhibited by Spautin-1, a chemical that promotes the proteasome degradation of BECLIN-1.29 This observation resembles our previous study where autophagy-dependent apoptosis of CCA cells was obtained with Dihydroartemisinin.41 In that study we showed that both Spautin-1 and direct silencing of the BECN1 gene translation could prevent CCA cell death by Dihydroartemisinin, supporting the view that hyper induction of BECLIN-1-dependent autophagy may be deleterious for cancer cells.
In summary, our findings indicate a biphasic pattern of autophagy regulation in cholangiocarcinogenesis, likely eliciting a preventive effect in the early phase (60–90 days), where it is up-regulated, and being thereafter suppressed and thus unable to avoid the transformation of hyperplastic nodules into cancer (from 120 to 160 days). The ON promoting CCA in association with deregulation of autophagy was attenuated by XH. Our in vitro data demonstrate that autophagy contributes to the anti-cancer ability of XH in CCA, supporting its possible employment for the prevention and treatment of CCA. In this respect, a recent clinical trial has shown that XH can prevent DNA damage in humans naturally exposed to dietary carcinogens.42
Declarations of interest
None.
Acknowledgments
This work was supported by Khon Kaen University and the Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. PHD/0048/2557) to ST and NN, a grant from a Mid-Career Grant (RSA5980012) of Thailand Research Fund to NN, a grant from the Faculty of Medicine, Khon Kaen University to NN (IN60341), a grant from Khon Kaen University to N.N., and a grant from CASCAP Program to NN.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Ciro Isidoro, Email: ciro.isidoro@med.uniupo.it.
Nisana Namwat, Email: nisana@kku.ac.th.
References
- 1.Colgate E.C., Miranda C.L., Stevens J.F. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2007;246:201–209. doi: 10.1016/j.canlet.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Dietz B.M., Kang Y.-H., Liu G. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens J.F., Page J.E. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Gerhauser C., Alt A., Heiss E. Cancer chemopreventive activity of xanthohumol, a natural product derived from hop 1. Mol Canc Therapeut. 2002;1:959–969. [PubMed] [Google Scholar]
- 5.Stevens J.F., Miranda C.L., Frei B. Inhibition of peroxynitrite-mediated LDL oxidation by prenylated flavonoids: the α, β-unsaturated keto functionality of 2 ‘-hydroxychalcones as a novel antioxidant pharmacophore. Chem Res Toxicol. 2003;16:1277–1286. doi: 10.1021/tx020100d. [DOI] [PubMed] [Google Scholar]
- 6.Festa M., Capasso A., D’Acunto C.W. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J Nat Prod. 2011;74:2505–2513. doi: 10.1021/np200390x. [DOI] [PubMed] [Google Scholar]
- 7.Monteghirfo S., Tosetti F., Ambrosini C. Antileukemia effects of xanthohumol in Bcr/Abl-transformed cells involve nuclear factor-κB and p53 modulation. Mol Canc Therapeut. 2008;7:2692–2702. doi: 10.1158/1535-7163.MCT-08-0132. [DOI] [PubMed] [Google Scholar]
- 8.Pan L., Becker H., Gerhäuser C. Xanthohumol induces apoptosis in cultured 40-16 human colon cancer cells by activation of the death receptor-and mitochondrial pathway. Mol Nutr Food Res. 2005;49:837–843. doi: 10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 9.Lu W.-J., Chang C.-C., Lien L.-M. Xanthohumol from Humulus lupulus L. induces glioma cell autophagy via inhibiting Akt/mTOR/S6K pathway. J Funct Foods. 2015;18:538–549. [Google Scholar]
- 10.Deeb D., Gao X., Jiang H. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010;30:3333–3339. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhi X., Zhong Q. Autophagy in cancer. F1000Prime Rep. 2015;7 doi: 10.12703/P7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi K.S. Autophagy and cancer. EMM. 2012;44:109. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalby K., Tekedereli I., Lopez-Berestein G. Targeting the pro-death and pro-survival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das C.K., Mandal M., Kögel D. Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev. 2018:1–18. doi: 10.1007/s10555-018-9727-z. [DOI] [PubMed] [Google Scholar]
- 15.Morani F., Phadngam S., Follo C. PTEN deficiency and mutant p53 confer glucose-addiction to thyroid cancer cells: impact of glucose depletion on cell proliferation, cell survival, autophagy and cell migration. Genes Canc. 2014;5:226. doi: 10.18632/genesandcancer.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eales K., Hollinshead K., Tennant D. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2015.50. e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuvayeva G., Bobak Y., Igumentseva N. Single amino acid arginine deprivation triggers prosurvival autophagic response in ovarian carcinoma SKOV3. BioMed Res Int. 2014;2014 doi: 10.1155/2014/505041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castino R., Bellio N., Follo C. Inhibition of PI3k class III–dependent autophagy prevents apoptosis and necrosis by oxidative stress in dopaminergic neuroblastoma cells. Toxicol Sci. 2010;117:152–162. doi: 10.1093/toxsci/kfq170. [DOI] [PubMed] [Google Scholar]
- 19.Trincheri N.F., Follo C., Nicotra G. Resveratrol-induced apoptosis depends on the lipid kinase activity of Vps34 and on the formation of autophagolysosomes. Carcinogenesis. 2007;29:381–389. doi: 10.1093/carcin/bgm271. [DOI] [PubMed] [Google Scholar]
- 20.Rouschop K., Wouters B.G. Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med. 2009;9:417–424. doi: 10.2174/156652409788167131. [DOI] [PubMed] [Google Scholar]
- 21.Thongchot S., Ferraresi A., Vidoni C. Resveratrol interrupts the pro-invasive communication between Cancer Associated Fibroblasts and Cholangiocarcinoma cells. Cancer Lett. 2018 Aug 28;430:160–171. doi: 10.1016/j.canlet.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Srivatanakul P., Sriplung H., Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev APJCP. 2004;5:118–125. [PubMed] [Google Scholar]
- 23.Yongvanit P., Pinlaor S., Loilome W. Risk biomarkers for assessment and chemoprevention of liver fluke-associated cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:309–315. doi: 10.1002/jhbp.63. [DOI] [PubMed] [Google Scholar]
- 24.Jamnongkan W., Thanee M., Yongvanit P. Antifibrotic effect of xanthohumol in combination with praziquantel is associated with altered redox status and reduced iron accumulation during liver fluke-associated cholangiocarcinogenesis. PeerJ. 2018;6 doi: 10.7717/peerj.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dell’Eva R., Ambrosini C., Vannini N. AKT/NF-κB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007;110:2007–2011. doi: 10.1002/cncr.23017. [DOI] [PubMed] [Google Scholar]
- 26.Dokduang H., Yongvanit P., Namwat N. Xanthohumol inhibits STAT3 activation pathway leading to growth suppression and apoptosis induction in human cholangiocarcinoma cells. Oncol Rep. 2016;35:2065–2072. doi: 10.3892/or.2016.4584. [DOI] [PubMed] [Google Scholar]
- 27.Klionsky D.J., Abdalla F.C., Abeliovich H. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizushima N., Yoshimori T., Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Xia H., Kim M. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain K., Paranandi K.S., Sridharan S. Autophagy in breast cancer and its implications for therapy. Am J Cancer Res. 2013;3:251. [PMC free article] [PubMed] [Google Scholar]
- 31.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 32.Karantza-Wadsworth V., Patel S., Kravchuk O. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Y.-J., Dong L.-W., Tan Y.-X. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Invest. 2011;91:1146. doi: 10.1038/labinvest.2011.97. [DOI] [PubMed] [Google Scholar]
- 34.Thongchot S., Yongvanit P., Loilome W. High expression of HIF-1α, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev APJCP. 2014;15:5873–5878. doi: 10.7314/apjcp.2014.15.14.5873. [DOI] [PubMed] [Google Scholar]
- 35.Yothaisong S., Dokduang H., Techasen A. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumor Biol. 2013;34:3637–3648. doi: 10.1007/s13277-013-0945-2. [DOI] [PubMed] [Google Scholar]
- 36.Yothaisong S., Thanee M., Namwat N. Opisthorchis viverrini infection activates the PI3K/AKT/PTEN and Wnt/beta-catenin signaling pathways in a Cholangiocarcinogenesis model. Asian Pac J Cancer Prev APJCP. 2014;15:10463–10468. doi: 10.7314/apjcp.2014.15.23.10463. [DOI] [PubMed] [Google Scholar]
- 37.Ferraresi A., Phadngam S., Morani F. Resveratrol inhibits IL-6-induced ovarian cancer cell migration through epigenetic up-regulation of autophagy. Mol Carcinog. 2017 Mar;56(3):1164–1181. doi: 10.1002/mc.22582. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-García C., Rancan L., Paredes S.D. Xanthohumol exerts protective effects in liver alterations associated with aging. Eur J Nutr. 2018 Mar 13 doi: 10.1007/s00394-018-1657-6. [DOI] [PubMed] [Google Scholar]
- 39.Dorn C., Heilmann J., Hellerbrand C. Protective effect of xanthohumol on toxin-induced liver inflammation and fibrosis. Int J Clin Exp Pathol. 2012;5(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- 40.Ferk F., Huber W.W., Filipic M. Xanthohumol, a prenylated flavonoid contained in beer, prevents the induction of preneoplastic lesions and DNA damage in liver and colon induced by the heterocyclic aromatic amine amino-3-methyl-imidazo[4,5-f]quinoline (IQ) Mutat Res. 2010 Sep 10;691(1-2):17–22. doi: 10.1016/j.mrfmmm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Thongchot S., Vidoni C., Ferraresi A. Dihydroartemisinin induces apoptosis and autophagy-dependent cell death in cholangiocarcinoma through a DAPK1-BECLIN1 pathway. Mol Carcinog. 2018 Dec;57(12):1735–1750. doi: 10.1002/mc.22893. [DOI] [PubMed] [Google Scholar]
- 42.Pichler C., Ferk F., Al-Serori H. Xanthohumol prevents DNA damage by dietary carcinogens: results of a human intervention trial. Cancer Prev Res (Phila) 2017 Feb;10(2):153–160. doi: 10.1158/1940-6207.CAPR-15-0378. [DOI] [PubMed] [Google Scholar]