Abstract
Background and aim
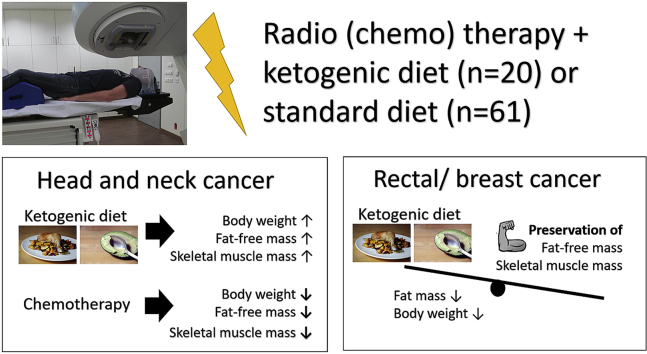
Ketogenic diets (KDs) have gained interest as a complementary treatment for cancer patients. Here we present first results of our ongoing KETOCOMP study (NCT02516501) concerning body composition changes among rectal, breast and head & neck cancer (HNC) patients who consumed a KD during curative radiotherapy (RT).
Experimental procedure
Sixty-one patients eating a non-ketogenic diet were compared to 20 patients on a KD supplemented with 10 g essential amino acids on RT days. Body composition was measured prior to and weekly during RT using 8-electrode bioimpedance analysis. Longitudinal body composition data were analyzed using linear mixed effects models.
Results and conclusion
Patients on the KD exhibited nutritional ketosis, defined as serum β-hydroxybutyrate levels ≥0.5 mmol/l, in a median of 69.0% of blood measurements (range 0–100%) performed in our clinic. In rectal and breast cancer patients, KD was significantly associated with a loss of 0.5 and 0.4 kg fat mass per week (p = 0.00089 and 8.49 × 10−5, respectively), with no significant changes in fat free and skeletal muscle mass. In HNC patients, concurrent chemotherapy was the strongest predictor of body weight, fat free and skeletal muscle mass loss during RT, while consuming a KD was significantly associated with a gain in these measures. These preliminary results confirm prior reports indicating that KDs are safe to consume during standard-of-care therapy. They also provide an important first indication that KDs with ample amino acid intake could improve body composition during RT in curative cancer patients.
Keywords: Bioimpedance analysis, Breast cancer, Head and neck cancer, Ketogenic diet, Rectal cancer
Abbreviations: BIA, Bioimpedance analysis; BHB, β-hydroxybutyrate; BW, Body weight; FM, Fat mass; FFM, Fat-free mass; KD, Ketogenic diet; SMM, Skeletal muscle mass
Graphical abstract
Highlights
-
•
Consumption of a ketogenic diet (KD) during radio(chemo-)therapy is feasible.
-
•
In rectal and breast cancer patients, the KD significantly reduced fat mass.
-
•
Fat-free mass and skeletal muscle mass were preserved by the KD.
-
•
In head and neck cancer patients a KD influenced body composition opposite to chemotherapy.
1. Introduction
Cancer patients frequently seek possibilities to support their standard therapies, improve their quality of life and positively influence their outcomes. One such supportive treatment approach is ketogenic therapy which comprises dietary interventions leading to nutritional ketosis such as ketogenic diets (KDs), short-term fasting and ketone body supplementation.1,2 Nutritional ketosis is a physiological state, usually defined as β-hydroxybutyrate (BHB) concentrations exceeding 0.5 mmol/l.3 KDs for cancer patients are of particular interest as they mimic certain aspects of fasting without necessarily inducing an energy deficit and have a variety of applications against other chronic diseases. While the first documented clinical application of KDs against cancer dates back to Wilhelm Brünings’ seminal trials in head and neck cancer patients published In 1941/1942,4 the scientific interest in using KDs for cancer treatment re-emerged only recently and is paralleled by an increasing interest on behalf of patients. For example, a recently published survey among high grade glioma patients revealed that almost three quarters (73%) of patients would be willing to try a KD for three months and 66% would participate in a clinical trial investigating the effectiveness of the KD.5
In a variety of preclinical tumor models, KDs have shown beneficial effects, including efficacy against tumor growth and a positive impact on body composition, although some counter-examples showing no or even tumor-promoting effects of KDs or ketone bodies exist.6,7 These contrasting findings concerning the efficacy of KDs against tumor growth are most likely explained by the metabolic phenotype of the particular tumor treated.8,9 However, a growing number of studies reveal synergistic effects of KDs with other therapies inducing oxidative stress in tumor cells such as radiotherapy (RT) or chemotherapy.2,10,11 In addition, mechanistic studies provide evidence for muscle-sparing effects of ketone bodies, especially under conditions of insulin resistance and inflammation often encountered in cancer patients.12,13 This makes sense from an evolutionary perspective, given that ketosis during starvation periods could have helped to maintain muscle mass which is indispensable for hunting and gathering foods.
Despite the growing number of preclinical in vitro and in vivo studies, research on the effects of KDs in humans is mostly limited to small pilot studies and case reports.6,14 The only randomized controlled trial on this topic was published recently and has shown that a KD in women with gynecological cancers had positive effects on body composition and quality of life when compared to a low-fat diet officially recommended for cancer patients.15,16 In an initial case series of patients undertaking a KD during RT in our clinic we also found some evidence that the diet could induce beneficial effects on body composition and quality of life.17 This lead to the initiation of a clinical phase I study with the main aim to investigate the impact of a KD intervention on body composition in cancer patients undergoing RT (the KETOCOMP study, ClinicalTrials.gov Identifier: NCT02516501).18 Here we report an interim analysis of the KETOCOMP study with the main aim to compare body composition changes in 20 patients who consumed a KD during RT to those of 61 patients consuming their standard diet. While the study is ongoing, these results are useful for providing first insights into the feasibility and effects of a KD during RT treatment of ambulatory patients.
2. Materials and methods
2.1. Study protocol
The KETOCOMP study has been approved by the ethics committee of the Bavarian Medical Association (Landesärztekammer Bayern) and registered under ClinicalTrials.gov Identifier NCT02516501. The detailed study protocol has been published previously.18 Briefly, patients between 18 and 75 years with rectal, breast or head and neck cancer (HNC) referred to our clinic for curative RT were principally eligible for participating. Main reason for non-eligibility was the presence of metallic body parts that interfere with bioimpedance analysis (BIA). Patients were assigned to one of three groups: (i) a standard diet group; (ii) a ketogenic breakfast group taking 50–225 ml of a medium-chain triglyceride (MCT) drink (betaquik®, vitaflo, Bad Homburg, Germany) plus 10 g crystalline essential amino acids (MyAmino®, dr. reinwald healthcare gmbh & co kg, Altdorf, Germany) in the morning of RT days; (iii) a complete KD group supplemented with 10 g MyAmino® on RT days. The study protocol stipulated to first fill the control group before filling the ketogenic breakfast group; the KD was offered to patients that appeared suitable or interested in parallel to the recruitment of these two groups. The composition of the MCT drink and amino acid supplement is shown in the appendix Table A1.
Table 1.
Baseline characteristics of all patients.
| Rectal cancer |
Head & neck cancer |
Breast cancer |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ketogenic diet (n = 8) | Control (n = 22) | p-value | Ketogenic diet (n = 5) | Control (n = 17) | p-value | Ketogenic diet (n = 7) | Control (n = 22) | p-value | |
| Age [years] | 54 (38–74) | 65 (43–76) | 0.05362 | 65 (61–68) | 64 (55–75) | 0.6366 | 45 (31–72) | 58 (41–68) | 0.1075 |
| Gender | Male: 6 Female: 2 |
Male: 14 Female: 8 |
0.6821 | Male: 4 Female: 1 |
Male: 13 Female: 4 |
1 | Female: 7 | Female: 22 | 1 |
| BMI [kg/m2] | 23.7 (20.7–32.3) | 27.5 (19.5–32.8) | 0.2373 | 20.7 (19.3–25.6) | 24.8 (17.8–35.6) | 0.08491 | 26.0 (20.0–36.0) | 26.6 (18.8–43.0) | 1 |
| Fasting glucose [mg/dl] | 96 (84–109) | 100 (66–265) | 0.140 | 106 (101–153) | 102 (83–188) | 0.5564 | 93 (82–114) | 95 (81–113) | 0.8008 |
| Fasting BHB [mmol/l] | 0.19 (0.03–0.81) | 0.11 (0.05–0.6) | 0.3463 | 0.1 (0.04–0.9) | 0.11 (0.03–0.42) | 0.556 | 0.19 (0.01–0.45) | 0.06 (0.02–0.29) | 0.325 |
| 50 kHz phase angle [°] | 5.65 (4.74–6.59) | 4.66 (3.31–5.97) | 0.02327 | 4.43 (4.22–4.74) | 4.5 (3.96–5.70) | 0.7836 | 4.96 (4.22–5.67) | 4.51 (3.72–5.88) | 0.2513 |
| PTV [ccm] | 1298 (943–1845) | 1467 (1076–2078) | 0.07043 | 821 (265–1278) | 755 (132–1147) | 0.6486 | 1066 (398–1296) | 1060 (622–2475) | 0.4003 |
| Chemotherapy | Yes: 8 | No: 1 Yes:21 |
1 | No: 2 Yes: 3 |
No: 7 Yes: 10 |
1 | No: 7 | No: 22 | 1 |
Continuous and categorical variables were compared using the Wilcoxon rank sum and Fisher's exact test, respectively. PTV: Planning target volume.
Body composition was supposed to be measured prior to and weekly during RT using the seca mBCA scale (seca Deutschland, Hamburg, Germany). Based on body weight (BW), height, age, gender and 5 kHz und 50 kHz resistance and reactance values, the scale estimates fat free mass (FFM), extracellular water and total body water and – using 50 kHz values only – skeletal muscle mass (SMM).19,20 Fat mass (FM) was calculated as FM=BW−FFM. In order to standardize each measurement, patients were advised to fast overnight, not to drink in the morning and to void their bladder; their RT appointments were accordingly scheduled in the morning so that they could receive RT after BIA and weighing. On three occasions, blood samples were supposed to be collected with the patient still in a fasting state immediately following BIA: once prior to, once in the middle of and once in the last week of RT.
2.2. Ketogenic and control diets
In most cases, the KD was started following baseline measurements prior to the first RT fraction and lasted until the final week of RT. Patients in the KD group received a popular book on the KD for cancer patients,21 handouts with brief descriptions which foods to consume and which to avoid, recipes and urinary ketone strips for self-assessment of ketosis. Most patients also had the opportunity to speak to the dietician (G.S.). The consumption of a whole food KD was promoted, with emphasis on high-quality protein (meat, eggs and fish), micronutrient-dense foods (vegetables to every meal, organ meats and bone broth), and avoidance of industrial and processed foods (with the exception of MCT oil), avoidance of vegetable oils (except virgin coconut and olive oil) and avoidance of foods rich in anti-nutrients and phytoestrogens (grains and legumes, in particular wheat and soy22, 23, 24, 25, 26). Dairy products were suggested only in moderation and preferably in the form of butter, cheese and fermented products. Due to the theoretically high micronutrient density and the moderate duration of the KD, no additional supplements were advised. Adherence to the KD was checked weekly by asking patients about their diet and measuring BHB concentrations through finger prick blood tests. Nutritional ketosis was defined as a BHB concentration ≥0.5 mmol/l. At the end of the study, patients also were required to provide their daily self-measured urinary ketone strip results. Patients in the control group received no dietary advice, but were also free to receive dietary counseling, in which case they obtained the official recommendations of the German Society for Nutrition (DGE). Adherence to a non-ketogenic diet in the control group was confirmed by asking each patient to classify his/her diet at the end of the study based on a multiple choice evaluation sheet. Besides diet, all patients were advised to maintain their habitual lifestyle habits during the duration of RT.
2.3. Study cohort
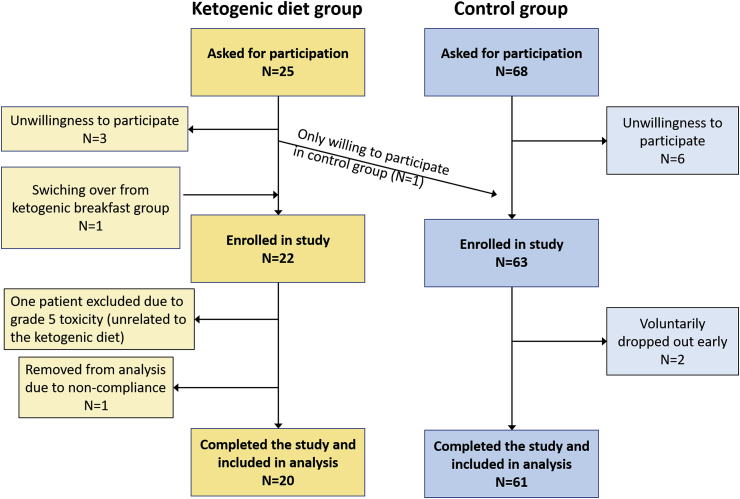
This analysis concentrates on a comparison between the KD and control groups only, since so far only eight patients (four with HNC, four with rectal cancer) were enrolled into the ketogenic breakfast group of which four were not able to tolerate the maximum MCT dose. A list of all patients enrolled into the KD and control group is given in the appendix in Table A2, and from now on individual patients will be referred to by their number given in that table. A total of 22 patients had been recruited into the KD group and 63 into the control group (Fig. 1). Four of the patients from the KD group have been described in more detail in a previous publication.17 Although being a deviation from the study protocol, we enrolled one rectal cancer (patient #2) and one HNC patient (#34) into the KD group despite having metallic implants; their data were used in the analysis of body weight changes. One rectal (patient #29) and one breast (patient #72) cancer patient from the control group quit the study after 20 and 11 days of RT, respectively, due to inconvenience with the weekly measurements; both were excluded from the analysis. In the KD group, one rectal cancer patient (patient #10) did not comply with our dietary advice and was removed from the analysis according to the study protocol. Furthermore, one patient (patient #6) was excluded from the analysis despite high compliance with the KD due to the development of an ultimately fatal Fournier's gangrene with sepsis which apparently also had an influence on his body composition as described in more detail in a separate case report.27
Table 2.
Regression coefficients for body composition changes in rectal cancer patients.
| Covariate | Body weight |
Fat free mass |
Fat mass |
Skeletal muscle mass |
50 kHz phase angle |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Time | −0.04 kg/week | 0.6266 | 0.04 kg/week | 0.5084 | −0.1 kg/week | 0.2291 | −0.03 kg/week | 0.2382 | −0.01°/week | 0.4703 |
| KD: yes | −1.4 kg | 0.0110 | −0.9 kg | 0.3942 | −0.4 kg | 0.6626 | −0.4 kg | 0.4774 | 0.27° | 0.0094 |
| Age | −0.5 kg/10 years | 0.0730 | −1.1 kg/10 years | 0.0056 | 0.4 kg/10 years | 0.2615 | −0.6 kg/10 years | 0.0109 | 0.01°/10 years | 0.8649 |
| Gender: female | −0.1 kg | 0.8789 | −3.5 kg | 0.0333 | 1.3 kg | 0.2378 | −1.7 kg | 0.0635 | −0.12° | 0.1654 |
| Baseline BMI | 1.1 kg/10 kg m−2 | 0.5226 | 2.3 kg/10 kg m−2 | 0.05135 | 3.9 kg/10 kg m−2 | 0.1663 | 1.1 kg/10 kg m−2 | 0.1661 | 0.01°/10 kg m−2 | 0.9510 |
| PTV | −0.3 kg/500 ccm | 0.5492 | 0.8 kg/500 ccm | 0.1964 | −0.3 kg/500 ccm | 0.6229 | 0.4 kg/500 ccm | 0.2627 | 0.08°/500 ccm | 0.2981 |
| Time × KD | −0.4 kg/week | 0.0118 | 0.0 | 0.9467 | −0.5 kg/week | 0.000889 | −0.02 kg/week | 0.7370 | −0.03 | 0.2078 |
KD: Ketogenic diet; PTV: Planning target volume.
Fig. 1.
Flow chart showing details of the patient recruitment for the study. The few patients (n = 8) that have been recruited into the ketogenic breakfast group are not the subject of this analysis and have been omitted.
2.4. Statistical analysis
Longitudinal body composition data were analyzed using linear mixed effects models with the intercept and slope of the variable “time” (since start of RT) as random effects varying by the individual patient. Time, intervention group (0 = control/1 = ketogenic), their interaction and the corresponding baseline body composition measure were included into each model. In addition, the following covariates were included based on their possible influence on body composition: Age, gender, baseline BMI, irradiated volume (planning target volume) for rectal cancer patients, and, for HNC patients, chemotherapy (0 = no/1 = yes) and PEG use in the timeframe prior to a particular measurement (0 = no/1 = yes). For HNC patients, a time × chemotherapy interaction was included because Akaike's information criterion (AIC) indicated an improvement in model fit. To ease interpretability of the regression coefficients, prior to model fitting, the covariates age and BMI were scaled to have mean zero and standard deviation 10 years or 10 kg m−2, respectively.
Differences between continuous and categorical variables were assessed using the Wilcoxon rank sum and Fisher's exact test, respectively. All analysis was carried out in R, version 3.5.0 with the software package lme4 for linear mixed effects modeling.
3. Results
Patient characteristics at baseline are given in Table 1. The KD and control groups were comparable with respect to most variables at baseline, although the intervention group in rectal cancer patients was younger and had significantly higher phase angle values. A minor deviation from the study protocol was that some patients received baseline measurements after RT had already started, but all within the first week of RT. The median study duration was 39 (KD) versus 35 (control) days in rectal cancer patients (p = 0.008936), 39 versus 40 days (p = 0.3425) in HNC patients and 35 versus 35 days (p = 0.4726) in breast cancer patients.
3.1. Ketogenic intervention
Median measured BHB concentrations were significantly higher in the KD versus control group during RT: 0.7 (range 0.12–2.1) mmol/l versus 0.1 (0.02–0.5) mmol/l (p = 2.865 × 10−13) in rectal cancer patients, 0.9 (0.05–4.2) mmol/l versus 0.17 (0.03–4.2) mmol/l (p = 0.0001769) in HNC patients and 0.71 (range 0.13–4.9) mmol/l versus 0.07 (0.02–2.59) mmol/l (p = 7.041 × 10−12) in breast cancer patients. Some patients in the control group apparently also achieved ketosis at certain time points. In HNC patients this was most frequently the consequence of insufficient energy intake due to therapy-induced dysphagia, xerostomia, anorexia and/or nausea developing towards the end of radio-chemotherapy. There were no grade>1 side effects associated with the KD, and no patient within the KD group voluntarily ended the study early. Based on weekly consultations, self-measured urinary acetoacetate concentrations and food diaries all patients included into this analysis appeared compliant to the KD. As an objective measure of diet adherence, we computed for each individual patient the percentage of BHB measurements that yielded concentrations ≥0.5 mmol/l during the KD. The median adherence rate thus defined was 50% (range 40–100%) in rectal cancer patients, 86% (29–100%) in HNC patients and 71% (0–100%) in breast cancer patients.
3.2. Body composition changes
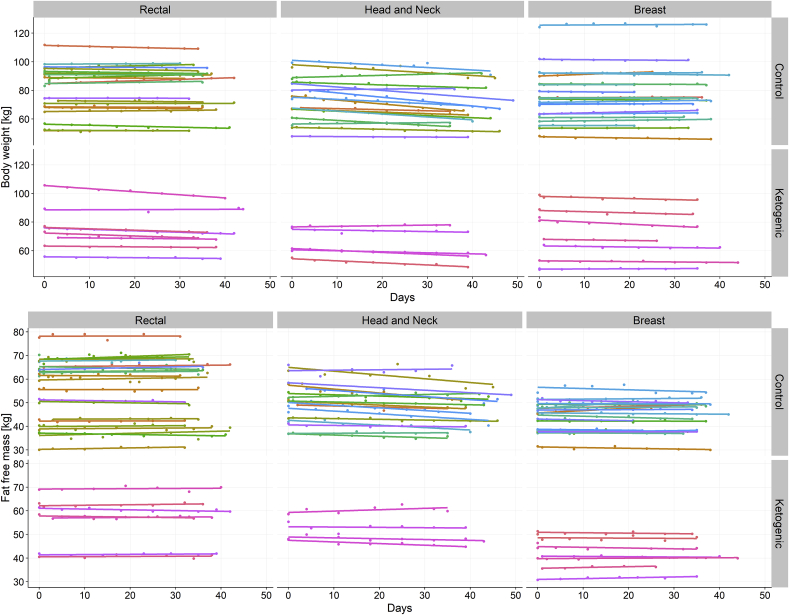
On average, 7 BIA measurements were performed per patient (range 2–9). Fig. 2 shows linear regression lines for each patient stratified according to intervention group and tumor entity. Visually it appears that linear regression against time gives an adequate fit to the data, so that for simplicity we did not include higher-order terms. In fitting all the data for each tumor entity together, we found mixed effects models with varying slope and intercept superior to varying intercept only or fixed effects models as judged by both the AIC and maximum likelihood ratio test (results not shown). The results are given in Table 2, Table 3, Table 4 for rectal, HNC and breast cancer patients, respectively.
Fig. 2.
Changes in body weight and fat free mass during radiotherapy, stratified according to tumor site and intervention group.
Table 3.
Regression coefficients for body composition changes in head and neck cancer patients.
| Covariate | Body weight |
Fat free mass |
Fat mass |
Skeletal muscle mass |
50 kHz phase angle |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Time | −0.2 kg/week | 0.1064 | −0.01 kg/week | 0.9122 | −0.3 kg/week | 0.0388 | −0.02 kg/week | 0.6754 | 0.0°/week | 0.9078 |
| KD: yes | −1.0 kg | 0.1838 | 0.1 kg | 0.9432 | −0.9 kg | 0.1350 | −0.1 kg | 0.7627 | −0.10° | 0.5853 |
| Age | 0.5 kg/10 years | 0.4227 | 0.3 kg/10 years | 0.6710 | 0.1 kg/10 years | 0.8352 | 0.1 kg/10 years | 0.8494 | −0.06°/10 years | 0.7162 |
| Gender: female | −0.9 kg | 0.3168 | −1.5 kg | 0.3054 | 0.8 kg | 0.2401 | −0.3 kg | 0.6909 | −0.10° | 0.6400 |
| Baseline BMI | 3.7 kg/10 kg m−2 | 0.1044 | 1.7 kg/10 kg m−2 | 0.0632 | 4.3 kg/10 kg m−2 | 0.000363 | 0.6 kg/10 kg m−2 | 0.2008 | −0.10°/10 kg m−2 | 0.5505 |
| PEG use: yes | −0.7 kg | 0.2134 | −0.5 kg | 0.4181 | 0.4 kg | 0.4171 | −0.01 kg | 0.9662 | 0.13° | 0.2249 |
| Chemotherapy: yes | 1.0 kg | 0.1087 | 1.6 kg | 0.0389 | −0.5 kg | 0.2707 | 1.1 kg | 0.00419 | 0.27° | 0.1848 |
| Time × KD | 0.6 kg/week | 0.00823 | 0.4 kg/week | 0.03423 | 0.2 kg/week | 0.3296 | 0.2 kg/week | 0.00449 | 0.0°/week | 0.8692 |
| Time × Chemotherapy | −1.2 kg/week | 1.657 × 10−11 | −0.8 kg/week | 2.728 × 10−8 | −0.4 kg/week | 0.0108 | −0.5 kg/week | 2.909 × 10−14 | −0.08°/week | 0.00176 |
KD: Ketogenic diet; PEG: percutaneous endoscopic gastrostomy tube.
Table 4.
Regression coefficients for body composition changes in breast cancer patients.
| Covariate | Body weight |
Fat free mass |
Fat mass |
Skeletal muscle mass |
50 kHz phase angle |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Time | 0.04 kg/week | 0.3551 | −0.1 kg/week | 0.2473 | 0.1 kg/week | 0.1264 | −0.01 kg/week | 0.7239 | 0.02°/week | 0.0071 |
| KD: yes | −1.3 kg | 0.00014 | −1.6 kg | 0.00030 | 0.0 kg | 0.9941 | −0.5 kg | 0.0588 | 0.25° | 0.0044 |
| Age | −0.2 kg/10 years | 0.0975 | −0.5 kg/10 years | 0.0168 | 0.2 kg/10 years | 0.0225 | −0.2 kg/10 years | 0.1100 | 0.0 | 0.9126 |
| Baseline BMI | −1.1 kg/10 kg m−2 | 0.1036 | 0.8 kg/10 kg m−2 | 0.0881 | −0.5 kg/10 kg m−2 | 0.4462 | 0.8 kg/10 kg m−2 | 0.0014 | 0.05°/10 kg m−2 | 0.3786 |
| Time × KD | −0.3 kg/week | 0.00124 | 0.1 kg/week | 0.1655 | −0.4 kg/week | 8.490 × 10−5 | −0.1 kg/week | 0.3388 | −0.05°/week | 0.00051 |
KD: Ketogenic therapy.
3.2.1. Rectal cancer
In rectal cancer patients, those in the KD group lost significantly more BW between the first and the final measurement than those in the control group (median ΔBW = −2.9 kg versus −0.4 kg, p = 0.00781). There was also a significantly greater reduction in FM in the KD group (ΔFM = −2.5 kg versus −0.4 kg, p = 0.00164). Changes in FFM, SSM and phase angle between first and final measurement were not significantly different between groups.
In linear regression analysis, KD was associated with a significant gradual loss of 0.4 kg BW and 0.5 kg FM per week. No further significant associations with body composition changes over time were obtained (Table 2). As expected, greater age and being female were significantly associated with lower FFM at each time point.
3.2.2. Head and neck cancer
Most HNC patients tended to lose some BW over the course of RT. Average weight loss was 4.5 ± 4.4 kg in all patients and significantly greater in patients having received concurrent chemotherapy (7.3 ± 2.8 kg versus 0.5 ± 2.5 kg, p = 1.608 × 10−5). In linear regression modeling, the strongest predictor of gradual BW, FFM, FM and SMM loss as well as phase angle decline was chemotherapy, while KD was associated with a significant increase of BW, FFM and SMM (Table 3). Furthermore, there were significant associations between receiving chemotherapy and higher FFM as well as SMM which could reflect selection of fitter patients for chemotherapy.
3.2.3. Breast cancer
In breast cancer patients, consuming a KD was highly significantly associated with gradual BW (−0.3 kg/week) and FM (−0.5 kg/week) reductions (Table 4). Interestingly, there was a significant gradual decline in phase angle of 0.05°/week in the KD group; however, being in the KD group itself was associated with a 0.25° higher phase angle compared to the control group which might reflect patient selection into the KD group.
4. Discussion
In this interim analysis of the ongoing KETOCOMP study, we investigated the effect of a KD containing highly bioavailable essential amino acids on body composition changes during RT. The publication of these results appears justified given that this is a pilot study28 for which the feasibility of the design was not clear a priori. It is an important result that all patients enrolled in the KD group (Fig. 1) also finished the study with no serious diet-related side effects. This is in stark contrast to some previous studies. especially the KETOLUNG and KETOPAN studies in which ≥50% of patients did not tolerate a highly artificial KD containing only 8% energy from protein during RT and one patient developed a possibly diet-related grade 4 hyperuricemia.29 A systematic review by Sremanakova et al.14 estimated that only 49% of the patients undertaking a KD within the included studies were able to continue the diet until the respective study end, whereby the study duration varied considerably between 0.5 and 31 months. We think the fact that our patients had early tumor stages, were intrinsically highly motivated and advised to eat a diet based on natural foods could have contributed to the good compliance. However, the objectively measured adherence to the KD, quantified through the frequency of BHB measurements yielding ≥0.5 mmol/l, showed more variation, with a median at 69.0% and range from 0% to 100% per patient.
It is increasingly recognized that BW per se is a poor indicator of nutritional status and health. BIA allows for an inexpensive, non-invasive tracking of body composition which has much more prognostic value since it is able to predict FFM and SMM. By directly measuring the electrical properties of body tissues, BIA also provides additional clues about the nutritional status on the cellular level. For example, De Luis et al. showed that HNC patients were characterized by lower reactance and phase angle than healthy control subjects despite normal weight and BMI and even without prior weight loss.30 On the metabolic side, these signs of cellular malnutrition manifest themselves as insulin resistance with increased lipid oxidation and impaired glucose tolerance.31, 32, 33 Hence, it has been argued that high fat diets with an appropriate supply of amino acids provide the best metabolic support for the cancer patient while minimizing tumor growth promoting stimuli.34, 35, 36
BIA is further useful to detect sarcopenia (degenerative SMM loss; to be distinguished from cachexia of which it is usually a component) which is not straightforward to detect with standard anthropometric assessments, yet can have significant adverse consequences in terms of treatment tolerability and overall survival.37 HNC patients represent a particularly frail population in this respect as they frequently develop sarcopenia during treatment which has been associated with poor quality of life and low physical performance status38 and occurs even under recommended energy and protein intake.39 FFM loss can account for 60–70% of total weight loss in these patients and has been correlated to increased inflammatory cytokine and C-reactive protein levels.38,39 It is therefore encouraging that our KD regime was associated with a significant increase of BW, FFM and SMM during RT, directly opposing the effects of concurrent chemotherapy to some extent.
In rat hearts and diaphragms, ketosis has been shown to inhibit oxidation of the branched chain amino acids40 and decrease the release of the gluconeogenic amino acid alanine.41 Consistent with these findings, Sherwin et al. measured decreased nitrogen excretion and hypoalaninemia in fasting men upon BHB infusion while most other amino acid concentrations remained stable.42 More evidence for anti-catabolic effects of BHB in muscle tissue is summarized in a brief overview by Koutnik and colleagues.13 Thus, in theory, nutritional ketosis could attenuate muscle protein breakdown while maintaining the availability of all amino acid precursors for muscle protein synthesis, leading to a net gain or at least maintenance of SMM despite lower insulin levels. Since it is availability of all essential amino acids that primarily drives muscle protein synthesis,43 the additional consumption of 10 g essential amino acids on radiation days could theoretically have further contributed to the attenuation of SMM loss in the HNC patients on a KD. The supplementation was generally well tolerated except for one HNC patient who ingested the amino acids dissolved in water via a PEG tube (patient #32).
In rectal and breast cancer patients, FFM and SMM appeared to be maintained irrespective of the study group. However, consuming a KD was significantly associated with a gradual decline of FM in both patient populations. This implies that KD would have increased the FFM-to-FM ratio in these patients. Such weight loss through a reduction of FM can be rated as beneficial since adipose tissue has a putative role in promoting growth and survival of colorectal and breast cancer cells44,45 and, accordingly, obesity has been found to be correlated with worse clinical outcomes in these patients.46, 47, 48 Unfortunately, many breast cancer patients experience weight gain during therapy.49,50 In this context low carbohydrate diets have been proposed as an optimal countermeasure since they reduce insulin und blood glucose spikes, decrease adipose tissue, increase HDL cholesterol and decrease triglycerides and inflammation.51
In summary, our data therefore confirm the hypothesis that KDs exert beneficial effects on body composition in (non-cachectic) cancer patients. The randomized controlled trial by Cohen et al. also revealed that a KD maintained over 12 weeks reduced total, android and visceral fat mass in women with gynecological cancers to a significantly greater extent than an officially recommended low-fat diet while preserving lean body mass.15 The KD was thereby composed of roughly 25% protein which could have contributed to maintenance of FFM. Beneficial effects on body composition were also found in a small Korean pilot study conducted in pancreatic cancer patients52; although only 10 out of 20 patients recruited into the KD arm finished the study (6 of them refused to eat the KD), those consuming a KD tended to lose less SMM during the weeks after pancreatectomy when compared with patients on a standard high-carbohydrate diet (p = 0.054). Since the majority of our subjects and those in the studies of Cohen et al.15 or Ok et al.52 did not engage in intense exercise, a contribution of exercise-stimulated muscle protein synthesis can be ruled out as an explanation for the observed maintenance of SMM. Rather, an anti-catabolic effect of ketosis per se, combined with the anabolic effects of an adequate amino acid intake appear as the most likely explanation for the maintenance of SMM in cancer patients on a KD despite low insulin levels and weight loss.
This preliminary analysis suffers from several limitations that we briefly discuss here. The small number of patients under a ketogenic regime for each tumor entity poses one of the largest limitations. However, with a median of 7 BIA measurements per patient we have collected enough data points for building mixed effects linear regression models with incorporation of several covariates with a putative influence on body composition. Another limitation is that it is not possible to separate the contributions of the amino acid supplement and ketosis to the observed beneficial effects on body composition. While we conceive the addition of crystalline essential amino acids to a KD regime as a good strategy to increase muscle protein synthesis without the need to increase the amount of food proteins which could interfere with ketosis, this supplementation strategy may limit the generalizability of our results to other KD regimes.
It is also obvious that some patients in the KD group were not able to reach high blood BHB levels when measured in our clinic; although all patients subjectively appeared more or less compliant based on weekly consultations, self-measured urinary acetoacetate concentrations and food diaries, this poses a major limitation for attributing the observed effects to ketosis per se. In future patients assigned to the KD group we will place even more emphasis on the early adjustment of dietary “mistakes” with the aim to achieve consistent nutritional ketosis.
Finally, the validity of BIA for estimating body composition is limited by assumptions relating to body shape. Comparing the estimates of our BIA device to those derived from Dual-energy X-ray Absorptiometry and MRI, Bosy-Westphal et al. calculated the coefficients of determination (R2) for the FFM and SMM prediction equations as 0.98 and 0.97, respectively, and the root mean square errors as only 1.9 kg and 1.2 kg, respectively.19,20 Since we were mainly interested in changes of body composition and not their exact absolute values, our conclusions should be robust against any systematic deviations from the true body composition values within individual patients.
5. Conclusion
In this preliminary analysis we observed beneficial effects of a KD supplemented with essential amino acids during RT on body composition: Rectal and breast cancer patients lost adipose tissue while preserving lean body mass, and HNC patients lost significantly less BW, FFM and SMM compared to the control group. The KD was safe, and so far no patient in the KD group ended the study voluntarily. While these early results from the ongoing KETOCOMP study should be interpreted with caution, they already provide some degree of justification for using KDs alongside RT for patients who are interested in taking self-responsibility to support their therapy.
Conflicts of interest
RJK has received an honorarium from the company vitaflo for giving a talk about the objectives and preliminary results of the KETOCOMP study. The other authors declare that there are no potential conflicts of interest relating to this analysis. The products used in this study were kindly provided by the manufacturing companies. These companies had no influence on the design, data collection and analysis of this study.
Authorship statement
RJK and RAS designed the study and collected the data. RJK analyzed the data and wrote the initial manuscript draft. GS helped with conducting the dietary intervention. All authors read, edited and approved the final manuscript.
Funding
The study is funded solely by our clinic.
Acknowledgments
We thank our colleagues Dr. Katharina Brauner, Dr. Sami Ok and Dr. Irina Ok for helping with patient recruitment into this study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.03.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Winter S.F., Loebel F., Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev Oncol Hematol. 2017;112:41–58. doi: 10.1016/j.critrevonc.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Klement R.J. The influence of ketogenic therapy on the 5 R's of radiobiology. Int J Radiat Biol. 2017 doi: 10.1080/09553002.2017.1380330. [DOI] [PubMed] [Google Scholar]
- 3.Miller V.J., Villamena F.A., Volek J.S. Nutritional ketosis and Mitohormesis : potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645. doi: 10.1155/2018/5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klement R.J. Wilhelm Brünings’ forgotten contribution to the metabolic treatment of cancer utilizing hypoglycemia and a very low carbohydrate (ketogenic) diet. J Tradit Complement Med. 2018 doi: 10.1016/j.jtcme.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-McGill K.J., Marson A.G., Smith C.T., Jenkinson M.D. The modified ketogenic diet in adults with glioblastoma: an evaluation of feasibility and deliverability within the national health service. Nutr Cancer. 2018;70(4):643–649. doi: 10.1080/01635581.2018.1460677. [DOI] [PubMed] [Google Scholar]
- 6.Klement R.J. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol. 2017;34:132. doi: 10.1007/s12032-017-0991-5. [DOI] [PubMed] [Google Scholar]
- 7.Weber D.D., Aminazdeh-Gohari S., Kofler B. Ketogenic diet in cancer therapy. Aging (N Y) 2018;10(2):164–165. doi: 10.18632/aging.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia S., Lin R., Jin L. Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell Metabol. 2017;25(2):358–373. doi: 10.1016/j.cmet.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Jia P.-P., Liu Q.-L. Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo. J Lipid Res. 2018;59(4):625–634. doi: 10.1194/jlr.M082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klement R.J., Champ C.E. Calories, carbohydrates, and cancer therapy with radiation: exploiting the five R's through dietary manipulation. Cancer Metastasis Rev. 2014;33(1):217–229. doi: 10.1007/s10555-014-9495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen B.G., Bhatia S.K., Anderson C.M. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla S.K., Gebregiworgis T., Purohit V. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metabol. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutnik A.P., D'Agostino D.P., Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metabol. 2019 doi: 10.1016/j.tem.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Sremanakova J., Sowerbutts A.M., Burden S. A systematic review of the use of ketogenic diets in adult patients with cancer. J Hum Nutr Diet. 2018;31(6):793–802. doi: 10.1111/jhn.12587. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C.W., Fontaine K.R., Arend R.C. A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr. 2018;148(8):1253–1260. doi: 10.1093/jn/nxy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen C., Fontaine K., Arend R., Soleymani T., Gower B. Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: a randomized, controlled trial. Nutrients. 2018;10(9):1187. doi: 10.3390/nu10091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klement R.J., Sweeney R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes. 2016;9:143. doi: 10.1016/j.clnesp.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klement R.J., Sweeney R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: II. Protocol of a randomised phase I study (KETOCOMP) Clin Nutr ESPEN. 2016;12:e1–e6. doi: 10.1016/j.clnesp.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Bosy-Westphal A., Schautz B., Later W., Kehaylas J., Gallagher D., Müller M. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr. 2013;67(Suppl 1):S14–S21. doi: 10.1038/ejcn.2012.160. [DOI] [PubMed] [Google Scholar]
- 20.Bosy-Westphal A., Jensen B., Braun W., Pourhassan M., Gallagher D., Müller M.J. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr. 2017:1–7. doi: 10.1038/ejcn.2017.27. (July 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kämmerer U., Schlatterer C., Knoll G. 2012. Krebszellen Lieben Zucker - Patienten Brauchen Fett. 1st ed. Systemed. [Google Scholar]
- 22.Gupta Y.P. Anti-nutritional and toxic factors in food legumes: a review. Plant Foods Hum Nutr. 1987;37(3):201–228. doi: 10.1007/BF01091786. [DOI] [PubMed] [Google Scholar]
- 23.Cordain L. Cereal grains: humanity's double-edged sword. In: Simopoulos A.P., editor. first ed. vol. 84. Karger; Basel: 1999. pp. 19–73. (World Review of Nutrition and Dietetics). [DOI] [PubMed] [Google Scholar]
- 24.Du M., Yang X., Hartman J.A. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33(4):895–901. doi: 10.1093/carcin/bgs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Punder K., Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. 2013;5:771–787. doi: 10.3390/nu5030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan L., Farouk M.H., Qin G., Zhao Y., Bao N. The influences of soybean agglutinin and functional oligosaccharides on the intestinal tract of monogastric animals. Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klement R.J., Schäfer G., Sweeney R.A. A fatal case of Fournier's gangrene during neoadjuvant radiotherapy for rectal cancer. Strahlenther Onkol. 2018 doi: 10.1007/s00066-018-1401-4. [DOI] [PubMed] [Google Scholar]
- 28.Supak Smolcic V. Salami publication: definitions and examples. Biochem Med. 2013;23(3):237–241. doi: 10.11613/BM.2013.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahra A., Fath M.A., Opat E. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of Iowa experience of two phase 1 clinical trials. Radiat Res. 2017;187(6):743–754. doi: 10.1667/RR14668.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Luis D.A., Aller R., O I., Terroba M., Cabezas G., Cuellar L. Tissue electric properties in head and neck cancer patients. Ann Nutr Metab. 2006;50(1):7–10. doi: 10.1159/000089484. [DOI] [PubMed] [Google Scholar]
- 31.Muck B.R., Trotnow S., Egger H., Hommel G. Altered carbohydrate metabolism in breast cancer and benign breast affections. Arch Gynakol. 1976;221(1):83–91. doi: 10.1007/BF00667684. [DOI] [PubMed] [Google Scholar]
- 32.Hansell D.T., Davies J.W.L., Burns H.J.G., Shenkin A. The oxidation of body fuel in cancer patients. Ann Surg. 1986;204(6):637–642. doi: 10.1097/00000658-198612000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Körber J., Pricelius S., Heidrich M., Müller M.J. Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur J Clin Nutr. 1999;53(9):740–745. doi: 10.1038/sj.ejcn.1600843. [DOI] [PubMed] [Google Scholar]
- 34.Klement R.J., Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab. 2011;8:75. doi: 10.1186/1743-7075-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holm E., Kämmerer U. Lipids and carbohydrates in nutritional concepts for tumor patients. Aktuelle Ernährungsmed. 2011;36:286–298. [Google Scholar]
- 36.Bozzetti F., Zupec-Kania B. Toward a cancer-specific diet. Clin Nutr. 2016;35(5):1188–1195. doi: 10.1016/j.clnu.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Prado C.M., Cushen S.J., Orsso C.E., Ryan A.M. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–198. doi: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 38.Silver H.J., Dietrich M.S., Murphy B.A. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherpay. Head Neck. 2007;29(10):893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 39.Jager-Wittenaar H., Dijkstra P.U., Vissink A. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck. 2011;33(6):863–870. doi: 10.1002/hed. [DOI] [PubMed] [Google Scholar]
- 40.Buse M.G., Biggers J.F., Friderici K.H., Buse J.F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972;247(24):8085–8096. http://www.ncbi.nlm.nih.gov/pubmed/4640937 [PubMed] [Google Scholar]
- 41.Palaiologos G., Felig P. Effects of ketone bodies on amino acid metabolism in isolated rat diaphragm. Biochem J. 1976;154(3):709–716. doi: 10.1042/bj1540709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherwin R.S., Hendler R.G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Investig. 1975;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpi E., Kobayashi H., Sheffield-Moore M., Mittendorfer B., Wolfe R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–258. doi: 10.2144/000113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz B., Yehuda-Shnaidman E. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Front Oncol. 2014;4:164. doi: 10.3389/fonc.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picon-Ruiz M., Pan C., Drews-Elger K. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76(2):491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 46.Sinicrope F.A., Foster N.R., Yothers G. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson P.J., Bell R.J., Davis S.R. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014 doi: 10.1016/j.maturitas.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Azrad M., Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: a review of the recent literature. Curr Nutr Rep. 2014;3(1):9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demark-Wahnefried B.W., Winer E.P., Rimer B.K. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11(7):1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- 50.Genton L., Kyle U.G., Balmer Majno S., Pichard C. Body composition changes in breast cancer patients during curative radiation therapy. e-SPEN Eur E J Clin Nutr Metab. 2006;1(1):2–8. doi: 10.1016/j.eclnm.2006.07.005. [DOI] [Google Scholar]
- 51.Champ C.E., Volek J.S., Siglin J., Jin L., Simone N.L. Weight gain, metabolic syndrome, and breast cancer recurrence: are dietary recommendations supported by the data? Int J Breast Cancer. 2012;2012:506868. doi: 10.1155/2012/506868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ok J.H., Lee H., Chung H.-Y. The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy. Anticancer Res. 2018;38(11):6519–6527. doi: 10.21873/anticanres.13017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.