Abstract
Background and aim
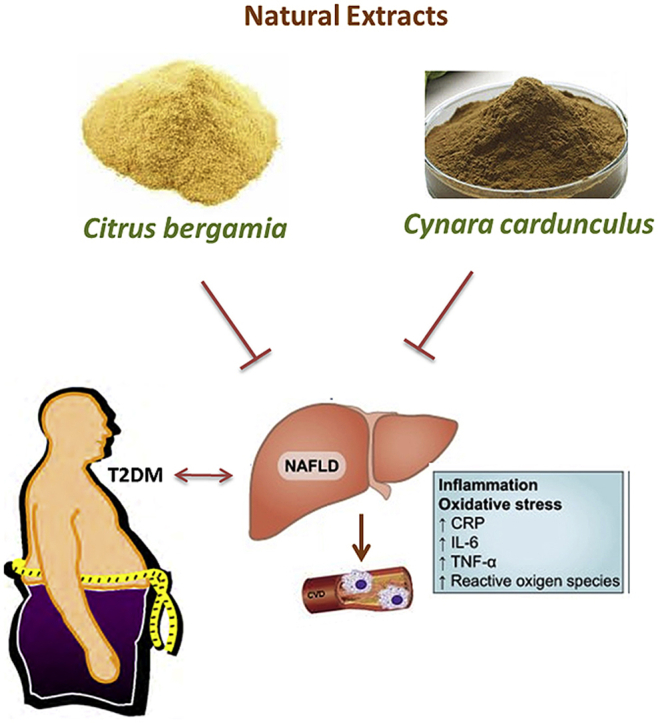
Non-Alcoholic Fatty Liver Disease (NAFLD) represents a risk factor for cardiovascular diseases. NAFLD is worsened by the simultaneous occurrence of type 2 diabetes mellitus (T2DM) causing an enhancement of inflammatory and fibrotic processes. Although insulin resistance appears the link between NAFLD and T2DM, current pharmacological treatments of T2DM failed to produce relevant benefits in preventing T2DM-related liver dysfunction. In this randomized, double blind, placebo-controlled clinical study, we evaluated the effect of Bergacyn, an innovative formulation originating from the combination of Bergamot Polyphenolic Fraction (BPF) and Cynara cardunculus (CyC).
Experimental procedure
80 adult patients with a history of at least 12 months of T2DM and NAFLD received orally BPF (300 mg/daily) Cyc (300 mg/daily), separately or formulated in combination 50/50% (Bergacyn; 300 mg/daily), or placebo all containing 300 mg of bergamot albedo fibers micronized and co-grinded as excipients.
Results and conclusion
Serum measurements and liver ultrasound analyses showed that concomitant administration of BPF and CyC produced significant improvement of NAFLD biomarkers in patients with T2DM. This effect was associated with a substantial reduction of oxidative stress/inflammatory biomarkers, thus contributing to a significant improvement of NO-mediated reactive vasodilation. Furthermore, the effect of Bergacyn showed a synergistic effect of both extracts, thus suggesting that this peculiar formulation represents a novel therapeutic strategy to counteract vascular inflammation and endothelial dysfunction in patients suffering from T2DM and NAFLD. Further studies in larger cohort of diabetic patients are required to better identify the potential of Bergacyn on metabolic disorders accompanying T2DM and NAFLD.
Keywords: Bergamot polyphenols, Cynaropicrin, NAFLD, T2DM, Vascular injury, Endothelial impairment
Graphical abstract
Highlights
-
•
Concomitant administration of BPF and CyC improves NAFLD in patients with T2DM.
-
•
Co-administration of BPF and CyC reduces oxidative stress and inflammation.
-
•
Co-administration of BPF and CyC improves NO-mediated reactive vasodilation.
List of Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- T2DM
type 2 diabetes mellitus
- BPF
Bergamot Polyphenolic Fraction
- CyC
Cynara cardunculus
- FPG
fasting plasma glucose
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
- BJ
Bergamot Juice;
- HRI-diff
Hepato- Renal Index Difference
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma-glutamyl transpeptidase
- ALP
alkaline phosphatase
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- MDA
malondialdehyde
- TNF-α
Tumor necrosis factor-α;
- RHI
hyperaemia index
- PAIx
augmentation index
- HA
hyaluronic acid
- PC III
type III precollagen
- IV-C
type IV collagen
1. Introduction
Growing evidence suggests that Non-Alcoholic Fatty Liver Disease (NAFLD) represents the most relevant form of liver dysfunction occurring in industrialized countries, mostly due to sedentary lifestyle and an excess of dietary calories intake.1 In addition, the occurrence of NAFLD represents per se a consistent risk factor of developing cardiovascular diseases including arterial hypertension, stroke and coronary artery disease, being liver dysfunction associated with an accelerated atherosclerosis development,2,3 though the pathophysiological mechanisms and the appropriate treatment remain to be better assessed.
A clear relationship exists between NAFLD and type 2 diabetes mellitus (T2DM), which could lead to additional cardio-metabolic risk (NAFLD may be found in more that 65–70% of patients with T2DM) due to the enhanced pro-inflammatory and pro-fibrotic profile of both diseases when they occur simultaneously.4, 5, 6, 7
NAFLD represents a predictor for the development of T2DM and emphasizes its complications.8,9 Indeed, NAFLD is associated with early impairment of fasting glucose and insulin resistance in patients with T2DM.10, 11, 12, 13, 14 On the other hand, experimental and clinical studies show that T2DM leads to detrimental effects on the consequences of pre-existing or concomitant NAFLD with accelerated development of steato-hepatitis (NASH), liver fibrosis and, at the late stages, hepatocarcinoma.8,9,14
Although insulin resistance seems to represent the common link between NAFLD and T2DM, current treatment of T2DM with hypoglycemic drugs failed to produce relevant benefits in preventing T2DM -related liver dysfunction. In particular, studies carried out with metformin, pioglitazone and GLT-1 agonists showed not significant changes in biomarkers of NAFLD associated to T2DM.15, 16, 17
Recently, we found that the bergamot polyphenolic fraction (BPF) is able to produce significant benefits in preventing NAFLD both in preclinical studies and in clinical trials. The BPF effects were associated with a significant improvement of glycemic control and a reduction of inflammatory biomarkers associated with NAFLD.18, 19, 20, 21 Bergamot derivatives represent a very peculiar source of dietary polyphenols,22 which have been found to produce consistent anti-dyslipidemic and hypo-glycemic effects, mostly via improvement of packaging of lipoproteins in the liver.18,20,23 In particular oral adminstration of BPF in patients with metabolic syndrome and NAFLD produced a significant reduction of major hyperlipemia serum biomarkers, an effect associated with an improvement of both ultrasound and metabolic biomarkers of NAFLD. This suggests that bergamot extract improves NAFLD biomarkers, therefore reducing cardiometabolic risks, though the effect on hyperglicemia-related vascular damage and its consequences on liver dysfunction complicating NAFLD is still to be assessed.
A significant contribution of diet supplementation with Cynara cardunculus (CyC) derivatives in reducing insulin resistance has recently been assessed, suggesting that polyphenols found in CyC extracts may be consistent in ameliorating glycemic control in T2DM.24, 25, 26, 27, 28, 29
On the other hand, CyC extracts are rich in sesquiterpenes such as cynaropicrin and it is likely that the administration of this extract alone or in combination with BPF may produce significant choleretic30 and hepatoprotective effect thereby improving liver dysfunction in T2DM,31 though the potential reduction of cardiometabolic risk still remains to be identified.
The present randomized, double blind, placebo-controlled clinical study has been performed in order to verify the efficacy of oral administration of BPF and CyC, alone or formulated in combination, in serum biomarkers of NAFLD associated with T2DM. Furthermore, the effect of the combination of both plant extract on reactive vasodilatation, and on vascular oxidative stress and inflammation has also been assessed.
2. Material and methods
2.1. Study design
A 16-week, randomized, double-blind, placebo-controlled study was conducted to assess in patients with type 2 diabetes and NAFLD: 1) the hepato-protective effect of Bergamot Polyphenolic Fraction (BPF) and CyC extract (CyC) given alone or in combination (Bergacyn; 50/50%); 2) its correlation with reduction of oxidative stress/inflammation biomarkers; 3) the related improvement of endothelial dysfunction.
80 patients were enrolled at the San Raffaele IRCCS, Rome. The inclusion criteria were adult patients with a history of at least 12 months of T2DM and NAFLD with no excessive alcohol consumption (less than 20 g/day for men and 10 g/day for women). A fatty liver was diagnosed by abdominal ultrasonography and identified by characteristic echo patterns, including a diffuse increase in the echogenicity of the liver compared with that of the kidney. Only patients with a hepato-renal index in a range between 2.5 and 3.5 were admitted to the study.
T2DM was diagnosed according to the criteria defined by the American Diabetes Association (fasting plasma glucose (FPG) levels higher than 126 mg/dl, or impaired glucose tolerance (IGT) (2-h values in the oral glucose tolerance test (OGTT) of up to 200 mg/dl).31 The exclusion criteria were: i) a history of viral hepatitis (including hepatitis B and/or C infection), autoimmune hepatitis or other liver disease; ii) the use of any medicine or dietary supplementation, in the last 6 months, that could influence NAFLD and glucose metabolism; iii) gastrointestinal disease, severe chronic disease, kidney dysfunctions, or malignant tumors; iv) any acute or chronic infectious disease or injury; v) any surgical procedure. This study was performed according to the guidelines established in the Declaration of Helsinki, and all procedures involving human participants were approved by the Local Ethic Committee and registered in the ISRCTN Registry with Reg. N. ISRCTN33165861. All participants were given and signed an informed consent.
Potential interested participants were first screened by phone, followed by a secondary screening to perform liver ultrasound examination after an overnight fast. Eligible participants were required to complete blood draws, anthropometric measurements, personal health and a demographic questionnaire as well as informations about alcohol consumption. For practical reasons, the secondary screening was performed on three consecutive days because of the possibility to test a limited number of subjects. A total of 80 participants were randomized using a random number generator in Excel software (Microsoft, Seattle, WA) to receive oral administration of BPF (300 mg/daily plus 300 mg excipients; n = 20), CyC (300 mg/daily plus 300 mg excipients; n = 20) or a formulation 50/50% of both extracts (Bergacyn; 300 mg/daily plus 300 mg excipients; n = 20) or placebo (n = 20), as previously described.31 Each participant was required to maintain their habitual behavior, including diet, physical activity, and alcohol consumption, during the study. The number of supplements not consumed and log reviews were used to assess compliance. Participants returned at 16 weeks to provide a fasting blood sample and undergo liver ultrasound examination.
2.2. Preparation of BPF, CyC or formulation of BPF + CyC
The specification sheet with the most relevant active ingredients of BPF and CyC are displayed in the supplementary material (Supplementary Figs. 1 and 2). Briefly, bergamot juice (BJ) was obtained from peeled-off fruits by industrial pressing and squeezing as previously described.18 The juice was oil fraction-depleted by stripping, clarified by ultra-filtration and loaded on suitable polystyrene resin columns absorbing polyphenol compounds of MW between 300 and 600 Da (Mitsubishi). Polyphenol fraction was eluted by a mild KOH solution. Moreover, the phytocomplex was then neutralized by filtration on cationic resin at acidic pH. Finally, it was vacuum dried and minced to the desired particle size to obtain a powder. In particular, powder was micronized and co-grinded with bergamot albedo fibers when given alone or in combination. BPF powder was analysed by HPLC for flavonoid and other polyphenol content. In addition, toxicological analyses were performed including heavy metal, pesticide, phthalate and sinephrine content which revealed the absence of known toxic compounds. Standard microbiological tests detected no mycotoxins and bacteria. The same procedure was used for the production of CyC extract. Fibers obtained by bergamot albedo were used micronized and co-grinded with plant extracts as excipients for final formulations. All materials were provided by HEAD srl (Bianco, Italy.)
Finally, to obtain a formulation containing both extracts, 150 mg of BPF powder were combined with 150 mg of CyC and were encapsulated in capsules containing 300 mg of excipients represented by albedo fibers micronized and co-grinded with plant extracts (Seris srl, Cuneo, Italy). The final formulation contained 5% of cynaropicrin. Capsules containing 600 mg maltodextrin were prepared for placebo studies.
All capsules were put into bottles containing 56 capsules (for 4 week) and packaged in color-coded plastic bags containing 4 bottles (for 16 weeks) for distribution to participants. An unblinded researcher prepared and masked the samples. Participants were instructed to consume 2 capsules daily 10 min before breakfast and dinner. All procedures have been performed according to the European Community Guidelines concerning dietary supplements.
2.3. Anthropometric measurements
Height was measured using a calibrated stadiometer (Gima, Italy) with 1-mm precision. Body weight was measured on a digital scale (Gima, Italy) in light indoor clothes without shoes. BMI was derived from height and weight measurements.
2.4. Diagnostic criteria of NAFLD
Following exclusion of subjects with excessive alcohol consumption and viral or autoimmune liver disease, NAFLD was diagnosed by abdominal ultrasound, which is a widely accessible imaging technique with high diagnostic accuracy and reliability for the detection of fatty liver. An ultrasonographic examination was performed by an experienced radiologist using a real-time scanner (3.5 MHz; Mod. Aplio, Toshiba, Japan) equipped with a convex-array probe. All subjects were evaluated in the left lateral recumbent position of 15°-20° to see the liver parenchyma and the right kidney cortex was seen contemporaneously.
The brightness of both zones was examined. The liver was recorded from the intercostal space, posing the region of interest (of 1.5 cm Å∼ 1.5 cm) in the mid or anterior axillary line (seventh or eighth intercostal space).
The right kidney was evaluated, highlighting the region of interest (0.5 cm Å∼ 0.5 cm) in the cortical zone. Hepato- Renal Index Difference (HRI-diff: Echo Levels in the Liver–Echo Levels in the Kidney) was estimated using built-in software on the scanner enabling local measurement of attenuation in dB. Mild steatosis was diagnosed for hyper-echogenic liver tissue (compared with the kidney cortex) when the sonographic index results were between 1 and 2. Values between 2 and 2.5 were indicative for moderate liver steatosis. Finally, hepatic steatosis was diagnosed as severe when the hepatorenal ratio was >2.5. In each case, the calculation of the hepatorenal index was repeated at least twice.
2.5. Blood measurements
At the baseline and after 16 weeks of the experimental protocol, a 12-h fasting morning blood sample was collected, processed and stored at −80 °C. All serum marker concentrations or activities were measured using classical methods and commercial assay kits, according to the manufacturers’ instructions. Assay kits for alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), superoxide dismutase (SOD), glutathione peroxidase (GPx) and malondialdehyde (MDA) were purchased from Novamedical, Italy. An ELISA kit for Tumor necrosis factor-α (TNF-α) was purchased from Thermo Fisher Scientific (Milan). Chemiluminescence assay kits for serum liver fibrosis markers: hyaluronic acid (HA), type III precollagen (PC III), and type IV collagen (IV–C), were purchased from Sysmex (HQ: Kobe, Japan). Absorbance was measured using a Bio-Rad 680 microplate reader (Bio-Rad Co., Hercules, CA, USA). Chemiluminescence intensity was monitored on a luminescence reader (FlexStation 3, Molecular Devices, Sunnyvale, CA, USA).
All the laboratory tests were performed in a blinded manner in respect to the assigned treatment.
2.6. Assessment of endothelial function
Endothelial function was assessed using the EndoPAT 2000 technique, which measures PAT using the reactive hyperaemia index (RHI, arbitrary units). Briefly, after 20 min of rest in a 45° angled inclined chair, at room temperature, the blood pressure cuff was placed on the non-dominant upper arm (study arm), while the other arm was used as the control. The hands were placed on armchair supports with the palm side down, as the fingers hung freely.
The EndoPAT probes were then placed on the tip of each index finger of both hands. The probes were prevented from touching any other finger or object and then were electronically inflated. The PAT signal was continuously recorded on a personal computer during the test. Baseline pulse amplitude was measured from each fingertip for 5 min. After baseline recording, (5 min on each arm), arterial flow was then interrupted in the experimental arm by rapidly inflating the cuff to occlusion pressure of 200 mmHg or 60 mmHg plus systolic blood pressure. After a 5 min occlusion, the cuff pressure was rapidly deflated, and post-occlusion was recorded for other 5 min in the experimental arm as well as the control arm. Pulse amplitude response to hyperaemia was automatically calculated from the hyperaemia in the finger of the experimental arm as a ratio of post-deflation (average pulse finger) to obtain the RH–PAT ratio or PAT ratio. The EndoPAT 2000 not only measured endothelial function with the RHI but also assessed arterial stiffness by measuring the peripheral augmentation index (PAIx) from the radial pulse wave analysis. PAIx automatically calculated as the ratio of the difference between the early and late systolic peaks of the waveform, relative to the early and late systolic peak of the waveform related to the systolic peak, expressed as percentage.
2.7. Statistical analysis
All data in this study were expressed as mean ± standard deviation (M±SD) or %. Data distribution of continuous variables was analysed using the Kolmogorov - Smirnov normality test. The baseline characteristics of participants in the two groups were compared using independent sample t-tests and chi-squared (χ 2) or Fisher exact test. The changes in outcomes of the participants after the initiation of therapy and end of the trial were compared by paired sample t-tests. Analysis of covariance (ANCOVA) was used to identify any differences between the two groups after intervention. Data analyses were conducted using SPSS software (version 18.0).
3. Results
Baseline characteristics of the subjects are displayed in Table 1.
Table 1.
Baseline characteristics of the subjects.
| Parameters | Placebo (n = 20) | BPF (n = 20) | CyC (n = 20) | BPF + CyC (n = 20) |
|---|---|---|---|---|
| Age (years) | 51 ± 10 | 51.0 ± 9 | 50 ± 9 | 51 ± 8 |
| Weight (kg) | 77.8 ± 1.7 | 79.2 ± 1.8 | 79.5 ± 1.5 | 79 ± 1.1 |
| BMI (kg/m2) | 25.3 ± 0.4 | 27.1 ± 0.5 | 26.1 ± 0.8 | 25.3 ± 0.6 |
| Cigarette smoker (%) | 0 | 0 | 0 | 0 |
| Alcohol consumption (>20 g/day) | 0 | 0 | 0 | 0 |
BMI, body mass index.
The effects of BPF, CyC or BPF + CyC on liver injury markers are shown in Table 2. At week 16, supplementation with BPF + CyC decreased serum ALT and AST, whereas patients who received placebo showed no significant changes. In addition, treatment of patients with BPF + CyC improved other liver biomarkers, including GGT and ALP as well as the three serum markers of liver fibrosis: HA, PC III and IV-C.
Table 2.
The effects of BPF, CyC or BPF + CyC on liver injury parameters.
| Parameters | Placebo (n = 20) | BPF (n = 20) | CyC (n = 20) | BPF + CyC (n = 20) |
|---|---|---|---|---|
| ALP (U/L) | ||||
| Baseline | 64 ± 3.5 | 63 ± 5.1 | 64.5 ± 4.3 | 65.4 ± 5.6 |
| Δ16 weeks | −1.2 ± 0.7 | −12.3 ± 1.1 | - 15.2 ± 1,2 | −19.2 ± 1.85§ |
| GGT (U/L) | ||||
| Baseline | 66 + 3.7 | 66.2 ± 4.2 | 67.3 ± 3,8 | 68.3 + 4.3 |
| Δ16 weeks | - 2.73 ± 1.03 | - 15.2 ± 2,1 | - 15 ± 2.2 | −20.76 ± 2.4§ |
| ALT (U/L) | ||||
| Baseline | 56.4 + 4.6 | 55.3 ± 3.9 | 54 ± 4.4 | 54.8 + 4.7 |
| Δ16 weeks | −0.3 ± 0.6 | - 9 ± 1.1 | - 12 ± 1.8 | - 15.41 ± 2.6§ |
| AST (U/L) | ||||
| Baseline | 45.7 + 3.2 | 44.5 ± 3.8 | 43.2 ± 4,2 | 44.58 + 4.2 |
| 16 weeks | - 0.4 ± 0.69 | - 7 ± 1.9 | - 8 ± 1.8 | −18.23 ± 3.48§ |
| HA (ng/mL) | ||||
| Baseline | 86 + 6.8 | 87.4 ± 8,2 | 88.3 ± 6.8 | 82.6 ± 7.6 |
| Δ16 weeks | - 2.78 ± 3.21 | −16,6 ± 5,5 | −16.8 ± 4.1 | 21.6 ± 4.2§ |
| PC III (ng/mL) | ||||
| Baseline | 75.2 + 8.4 | 74.4 ± 6.4 | 76.2 ± 6.8 | 75.84 + 7.8 |
| Δ16 weeks | −1.9 ± 1.4 | −14.5 ± 2 | −16.5 ± 3.1 | −18.7 ± 3.4§ |
| IV-C (ng/mL) | ||||
| Baseline | 57.2 + 5.1 | 56.7 ± 5.1 | 57.6 ± 6.2 | 58.6 + 6.1 |
| Δ16 weeks | −1.57 ± 1.2 | −10 ± 3.1 | −13,1 ± 2,1 | −18.23 ± 3.48§ |
| Hepatorenal Index | ||||
| Baseline | 3.3 + 0.4 | 3.2 ± 0.6 | 3.3 ± 0.5 | 3.1 + 0.4 |
| Δ16 weeks | −0.3 + 0.1 | −1.1 ± 0.2 | 1.2 ± 0.3 | −1.6 + 0.3§ |
TB, total bilirubin; ALP, alkaline phosphatase; TP, total protein; GGT, gamma-glutamyltransferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HA, hyaluronic acid; PC-III, type III precollagen; IV-C, type IV collagen; Data are expressed as mean ± SD for each value; §P value of <0.05 between values at 16 week treatment with BPF + CyC compared to placebo group was taken as significant.
The beneficial effect of treating with BPF + CyC patients with T2DM associated to NAFLD was confirmed by data obtained when studying ultrasonografic pattern of NAFLD. Indeed, hepatorenal index was significantly (P < 0.05) reduced from 3.1 ± 0.4 to 1.8 ± 0.5 by the treatment with BPF + CyC, showing a reduction of liver brightness. This suggests that the treatment of patients, suffering from mild to severe NAFLD associated with T2DM, with BPF + CyC (300 mg twice a day for 16 weeks) leads to reduction of hepatic ultrosonographic pattern of NAFLD. Administration of BPF, CyC and BPF-CyC was accompanied by metabolic changes which are displayed in Supplementary Fig. 3.
3.1. Serum markers of oxidative stress and inflammation
The effects of BPF + CyC on oxidative stress markers SOD, GPx and MDA and inflammatory marker TNF-α are shown in Table 3. At week 16, the BPF + CyC group performed increased GPx (P < 0.001) and SOD (P < 0.001) levels and reduced MDA (P < 0.001) and TNF-α (P < 0.001) levels as compared to the placebo group.
Table 3.
The effect of treatments on GPx, SOD, MDA and TNF-α.
| Parameters | Placebo (n = 20) | BPF (n = 20) | CyC (n = 20) | BPF + CyC (n = 20) |
|---|---|---|---|---|
| GPx (U/mL) | ||||
| Baseline | 195.4 ± 3.6 | 190 .5 ± 4.3 | 192.1 ± 4.6 | 188.1 ± 4.2 |
| Δ16 weeks | 4.7 ± 2.6 | 18.6 ± 2.5 | 19.6 ± 3.8 | 25.9 ± 3.6§ |
| SOD (U/mL) | ||||
| Baseline | 33.7 ± 4.4 | 34.9 ± 4.4 | 35.3 ± 4.9 | 33.3 ± 3.6 |
| Δ16 weeks | 0.16 ± 0.7 | 6.2 ± 1.9 | 7.2 ± 1.3 | 9.8 ± 1.7§ |
| MDA (nmol/mL) | ||||
| Baseline | 4.8 ± 0.4 | 5.1 ± 0.8 | 4.8 ± 0.8 | 4.8 ± 0.6 |
| Δ16 weeks | −0.08 ± 0.05 | −0.9 ± 0.2 | −1.1 ± 0.4 | −1.41 ± 0.3§ |
| TNF-α (pg/mL) | ||||
| Baseline | 95.84 + 8.1 | 94.2 ± 6.8 | 95.3 ± 6.3 | 98.1 + 9.2 |
| Δ16 weeks | −4.6 ± 2.17 | −21 ± 3.1 | −25 ± 7.1 | −28.5 ± 2.2§ |
GPx, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde; TNF- tumor necrosis factor alpha; Data are expressed as mean ± SD for each value; §P value of <0.05 between values at 16 week treatment with BPF + CyC compared to placebo group was taken as significant.
3.2. The effect of BPF, CycC and formulation of BPF + CyC on endothelial dysfunction
BPF + CyC treatment of patients with NAFLD associated with hyperlipemia for 16 weeks improved endothelial dysfunction detected at baseline. Indeed, reactive hyperemia Index, Framingham’s Hyperemia Index and Augmentation Index scores, as detected via EndoPAT procedure were significantly improved by BPF + CyC compared to placebo group (Table 4).
Table 4.
Effect of BPF + CyC (16 weeks) compared to placebo in endothelial dysfunction of patients with hyperlipemia and NAFLD as detected by Endopat scores.
| Placebo (n = 20) | BPF (n = 20) | CyC (n = 20) | BPF + CYC (n = 20) | |
|---|---|---|---|---|
| Reactive hyperemia index (RHI) | ||||
| Baseline | 1.75 ± 0,3 | 1.75 ± 0.2 | 1.80 ± 0.4 | 1.78 ± 0.3 |
| Δ16 weeks | - 0.25 ± 0.09 | -.51 ± 0.06 | -.55 ± 0.08 | - 0.7 ± 0.11§ |
| Framingham Reactive Hyperemia Index (fRHI) | ||||
| Baseline | 0.24 ± 0.03 | 0.22 ± 0.02 | 0.24 ± 0.03 | 0.24 ± 0.05 |
| Δ16 weeks | −0.03 ± 0.02 | −0.9 ± 0.03 | −0.8 ± 0.04 | −1.3 ± 0.08§ |
| Augmentation Index (AI) | ||||
| Baseline | 8.4 ± 2.3 | 8.4 ± 2.5 | 8.3 ± 2.6 | −8.5 ± 3.2 |
| Δ16 weeks | 0.8 ± 0.4 | 7.2 ± 1.8 | 6.5 ± 2.1 | 8.5 ± 2.2§ |
Data are expressed as mean ± SD for each value; §P value of <0.05 between values at 16 week treatment with BPF + CyC compared to placebo group was taken as significant.
4. Discussion
Our data show, for the first time, that a combination of two extracts originating from bergamot juice and CyC (BPF and CyC respectively) is able to produce a significant improvement of biomarkers associated with NAFLD in patients with T2DM. The study is randomized, double blind and placebo-controlled. In addition, this effect is accompanied by the reduction of oxidative stress/inflammation biomarkers, being this effect mostly contributing to significant improvement of NO-mediated reactive vasodilation, as explored via EndoPAT analysis. On the other hand, the BPF + CyC combination acts synergistically, thus producing an effect which is significantly higher when compared to both extracts given separately.
These data, even if collected from a limited cohort of diabetic patients, are totally in accordance with previous data obtained in vitro and in vivo in which supplementation of patients with bergamot-derived antioxidants, and CyC extract led to a significant improvement of both glycemic and lipemic profile.18,24,31, 32, 33, 34 In addition, this effect was accompanied with an improved vascular reactivity (explored via EndoPAT methodology) and to a better profile of biomarkers of NAFLD (detected via ultrasound measurement of fatty accumulation in the liver as well as via quantification of serum biomarkers of liver injury). Moreover, both oxidative stress and inflammatory biomarkers have been found improved as a consequence of the treatment with BPF and CyC as previous described.20,35, 36, 37, 38, 39 The novelty of this study is represented by the synergistic response of all the clinical and laboratory parameters seen when both products were combined into an innovative formulation. Indeed, the extract originating from the combination of BPF and CyC, showed significant enhancement of overall serum, Echo and inflammatory markers of liver dysfunction associated to T2DM. In addition, a sustained enhancement of reactive vasodilatation occurred in subjects undergoing treatment with BPF and CyC formulation, suggesting that both extracts may influence significantly and synergistically NO release from endothelial cells, which is impaired in both T2DM and liver steatosis.40, 41, 42
The innovative formulation seems to contribute significantly to this effect. In fact, evidence has been collected that micronization and co-grinding of both drugs and nutraceuticals leads to a better absorption and tissue distribution of orally given compounds.43,44 On the other hand, the combination of BPF and CyC seems to represent more than a simple blend of two plant extracts, due to the synergistic response compared to single doses of both products, being the mechanism of synergism to be better elucidated.
Evidence has been collected that BPF may produce relevant antinflammatory response in severe liver dysfunction in a rat model of NAFLD. In particular, both reduction of oxidative stress and antinflammatory responses were found in orally BPF-treated mice, suggesting that polyphenols contained in bergamot extract may be associated to a significant improvement of liver function.22 These effects are implemented by CyC in a way which seems to potentiate the antinflammatory/antioxidant responses found with BPF alone, when both products are given in combination. In particular, CyC, due to the high concentration of both polyphenols and sesquiterpenes (such as cynaropicrin), leads to a better metabolic balance and better liver functionality in patients with T2DM, an effect which enhances BPF response.24,32,45 This effect seems also to better reverse the impaired NO release in patients with both T2DM and NAFLD. In particular, it is known that endothelial dysfunction occurs in patients with T2DM.40,41 On the other hand, both metabolic syndrome and NAFLD are accompanied by reduced NO release by endothelial cells.40,41,46 The reason of the impaired endothelial function in metabolic disorders is unknown. However, a clear association has been found to exist between increased oxidative stress and endothelial dysfunction.47 Moreover, the occurrence of inflammation in liver tissues leads to impaired fat metabolism which affects arginine availability and impairs the NO-generating machinery at multilevels.40,47 Thus, it is likely that the antioxidant properties of BPF are enhanced when sesquiterpenes and polyphenols of CyC are used in combination, due to the better metabolic profile of diabetic patients treated with combination of both extracts. This leads to substantial benefit for better liver protection and for restoring vascular reactivity which is essential for cardiometabolic risk prevention.
In conclusion, the present preliminary data show that combination of BPF and CyC leads to liver protection in subjects with T2DM and NAFLD. This effect is accompanied by consistent reduction of oxidative stress, compared to both extracts given alone, an effect which is accompanied by enhanced reactive vasodilatation thereby leading to better prevention of cardiometabolic risk in T2DM complicated by NAFLD.
Funding
This work was supported by Ministero dell’Istruzione, dell’Università e della Ricerca: Programma Operativo Nazionale (PON03PE_00078_1 and PON03PE_00078_2).
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.02.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.El Hadi H., Di Vincenzo A., Vettor R., Rossato M. Cardio-metabolic disorders in non-alcoholic fatty liver disease. Int J Mol Sci. 2019;20:2215. doi: 10.3390/ijms20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 5.Targher G., Marchesini G., Byrne C.D. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: causal association or epiphenomenon? Diabetes Metab. 2016;42:142–156. doi: 10.1016/j.diabet.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Loomba R., Abraham M., Unalp A. Bass the Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams C.D., Stengel J., Asike M.I. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 9.Ballestri S., Zona S., Targher G. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 10.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Hossain N., Afendy A., Stepanova M. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–1229. doi: 10.1016/j.cgh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Fracanzani A.L., Valenti L., Bugianesi E. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 13.Goh G.B., Pagadala M.R., Dasarathy J. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141–145. doi: 10.1016/j.bbacli.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bril F., Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 15.Mills E.P., Brown K.P.D., Smith J.D., Vang P.W., Trotta K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a review of e_cacy and safety. Ther Adv Endocrinol Metab. 2018;9:15–28. doi: 10.1177/2042018817741852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cusi K., Orsak B., Bril F. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 18.Mollace V., Sacco I., Janda E. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia. 2011;82:309–316. doi: 10.1016/j.fitote.2010.10.01. [DOI] [PubMed] [Google Scholar]
- 19.Parafati M., Lascala A., La Russa D. Bergamot polyphenols boost therapeutic effects of the diet on non-alcoholic steatohepatitis (NASH) induced by "Junk Food": evidence for anti-inflammatory activity. Nutrients. 2018;10:1604. doi: 10.3390/nu10111604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musolino V, Gliozzi M, Scarano F. Bergamot polyphenols improve dyslipidemia and pathophysiological features in a mouse model of non-alcoholic fatty liver disease. Sci Rep. 2020;10:2565. doi: 10.1038/s41598-020-59485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gliozzi M., Carresi C., Musolino V. The effect of bergamot-derived polyphenolic fraction on LDL small dense particles and non-alcoholic fatty liver disease in patients with MS. Adv Biol Chem. 2014;4:129–137. doi: 10.4236/abc.2014.42017. [DOI] [Google Scholar]
- 22.Carresi C., Musolino V., Gliozzi M. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J Mol Cell Cardiol. 2018;119:10–18. doi: 10.1016/j.yjmcc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Parafati M., Lascala A., Morittu V.M. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J Nutr Biochem. 2015;26:938–948. doi: 10.1016/j.jnutbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Rondanelli M., Opizzi A., Faliva M. Metabolic management in overweight subjects with naive impaired fasting glycaemia by means of a highly standardized extract from Cynara scolymus: a double-blind, placebo-controlled, randomized clinical trial. Phytother Res. 2014;28:33–41. doi: 10.1002/ptr.4950. [DOI] [PubMed] [Google Scholar]
- 25.Rondanelli M., Giacosa A., Opizzi A. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: a double-blind, randomized, placebo-controlled trial. Int J Food Sci Nutr. 2013;64:7–15. doi: 10.3109/09637486.2012.700920. [DOI] [PubMed] [Google Scholar]
- 26.Rondanelli M., Giacosa A., Orsini F., Opizzi A., Villani S. Appetite control and glycaemia reduction in overweight subjects treated with a combination of two highly standardized extracts from Phaseolus vulgaris and Cynara scolymus. Phytother Res. 2011;25:1275–1282. doi: 10.1002/ptr.3425. [DOI] [PubMed] [Google Scholar]
- 27.Fantini N., Colombo G., Giori A. Evidence of glycemia-lowering effect by Cynara scolymus L. extract in normal and obese rats. Phytother Res. 2011;25:463–466. doi: 10.3390/molecules21050564. [DOI] [PubMed] [Google Scholar]
- 28.Heidarian E., Soofiniya Y. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J Med Plants Res. 2011;5:2717–2723. [Google Scholar]
- 29.Nazni P., Vijayakumar T.P., Alagianambi P., Amirthaveni M. Hypoglycemic and hypolipidemic effect of Cynara scolymus among selected type 2 diabetic individuals. Pakistan J Nutr. 2006;5:147–151. doi: 10.3923/pjn.2006.147.151. [DOI] [Google Scholar]
- 30.Glasl S., Tsendayush D., Batchimeg U. Choleretic effects of the Mongolian medicinal plant Saussurea amara in the isolated perfused rat liver. Planta Med. 2007;73:59–66. doi: 10.1055/s-2006-957063. [DOI] [PubMed] [Google Scholar]
- 31.Ferro Y., Montalcini T., Mazza E. Randomised clinical trial: bergamot citrus and wild cardoon reduce body weight and liver steatosis in non-diabetic individuals. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.00494. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 33.Janda E., Lascala A., Martino C. Molecular mechanisms of lipid- and glucose-lowering activities of bergamot flavonoids. PharmaNutrition. 2016;4:S8–S18. doi: 10.1186/s12944-019-1061-0. [DOI] [Google Scholar]
- 34.Tang X., Wei R., Deng A., Lei T. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients. 2017;9:1000. doi: 10.3390/nu9091000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Boshy M., Ashshi A., Gaith M. Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ Sci Pollut Res Int. 2017;24:12372–12383. doi: 10.1007/s11356-017-8876-x. [DOI] [PubMed] [Google Scholar]
- 36.Rangboo V., Noroozi M., Zavoshy R., Rezadoost S.A., Mohammadpoorasl A. The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in the patients with nonalcoholic steatohepatitis. Int J Hepatol. 2016;2016:4030476. doi: 10.1155/2016/4030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahboubi M. Cynara scolymus (artichoke) and its efficacy in management of obesity. Bull Fac Pharm Cairo Univ. 2018;56:115–120. [Google Scholar]
- 38.Trombetta D., Cimino F., Cristani M. In vitro protective effects of two extracts from bergamot peels on human endothelial cells exposed to tumor necrosis factor-alpha (TNF alpha) J Agric Food Chem. 2010;58:8430–8436. doi: 10.1021/jf1008605. [DOI] [PubMed] [Google Scholar]
- 39.Anavi S., Madar Z., Tirosh O. Non-alcoholic fatty liver disease, to struggle with the strangle: oxygen availability in fatty livers. Redox Biol. 2017;13:386–392. doi: 10.1016/j.redox.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eccleston H.B., Andringa K.K., Betancourt A.M. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxidants Redox Signal. 2011;15:447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollace V., Rosano G., Malara N. Aspirin wears smart. Eur Heart J Cardiovasc Pharmacother. 2017;3:185–188. doi: 10.1093/ehjcvp/pvx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollace V., Gliozzi M., Musolino V. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: role of oxidative stress and LOX-1 receptor expression. Int J Cardiol. 2015;184:152–158. doi: 10.1016/j.ijcard.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Pannu N., Bhatnagar A. Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed Pharmacother. 2019;109:2237–2251. doi: 10.1016/j.biopha.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 44.Rondanelli M., Monteferrario F., Perna S., Faliva M.A., Opizzi A. Health-promoting properties of artichoke in preventing cardiovascular disease by its lipidic and glycemic-reducing action. Monaldi Arch Chest Dis. 2013;80:17–26. doi: 10.4081/monaldi.2013.87. [DOI] [PubMed] [Google Scholar]
- 45.Persico M., Masarone M., Damato A. Non alcoholic fatty liver disease and eNOS dysfunction in humans. BMC Gastroenterol. 2017;17:35. doi: 10.1186/s12876-017-0592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gliozzi M., Scicchitano M., Bosco F. Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. Int J Mol Sci. 2019;20:E3294. doi: 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwakiri Y., Kim M.Y. Nitric oxide in liver diseases. Trends Pharmacol Sci. 2015;36:524–536. doi: 10.1016/j.tips.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



