Abstract
Skin is the largest human organ that shields the inner body from contact with xenobiotic and genotoxic agents, and in this process, the skin’s cellular genome faces continuous stress due to direct exposure to these noxious factors. Accumulation of genetic stress results in genomic alterations leading to undesirable gene or protein alteration/expression in skin cells, which eventually causes the formation of non-melanoma skin cancers (NMSCs). Ultraviolet B (UVB) radiation from sun is the most prominent factor contributing to ∼5 million skin cancer cases (which are mostly NMSCs) in the United States (US) and western countries. UVB exposure causes aberrations in a range of biochemical and molecular pathways such as: thymine dimer formation, DNA damage, oxidative stress, inflammatory responses, altered cellular signaling, which ultimately contribute to the development of NMSCs. The focus of this review is to summarize the protective and preventive potential of silymarin and/or silibinin against UVB-induced NMSC in pre-clinical skin cancer studies. Over two decades of research has shown the strong potential of silibinin, a biologically active flavonolignan (crude form Silymarin) derived from milk thistle plant, against a wide range of cancers, including NMSCs. Silibinin protects against UVB-induced thymine dimer formation and in turn promotes DNA repair and/or initiates apoptosis in damaged cells via an increase in p53 levels. Additionally, silibinin has shown strong efficacy against NMSCs via its potential to target aberrant signaling pathways, and induction of anti-inflammatory responses. Overall, completed comprehensive studies suggest the potential use of silibinin to prevent and/or manage NMSCs in humans.
Keywords: Squamous cell carcinoma, Basal cell carcinoma, Photocarcinogenesis, Hedgehog pathway, Cyclobutane pyrimidine dimers
Graphical abstract
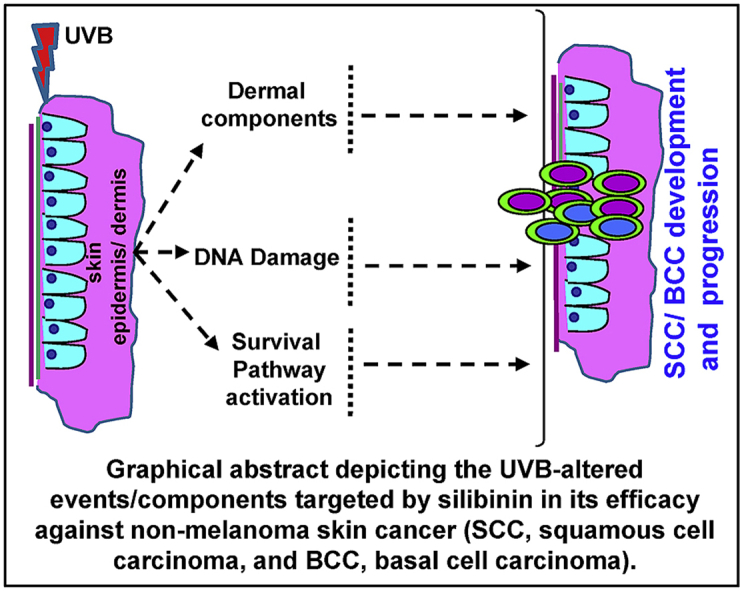
Highlights
-
•
Silibinin shows strong efficacy against all stages of photocarcinogenesis in NMSCs.
-
•
It also protects UVB-induced genomic instability, tumor growth and Progression.
-
•
Silibinin targets inflammatory and oxidative stress signaling to prevent BCC/SCC.
-
•
It induced apoptosis by targeting p53, MAPK, PI3K-Akt and other survival pathways.
List of abbreviations
- NMSCs
Non-melanoma skin cancers
- UVB
Ultraviolet B
- US
United States
- UVR
Ultraviolet Radiation
- BCC
Basal cell carcinoma
- SCC
squamous cell carcinoma
- DMBA
7, 12-Dimethylbenz[a]anthracene
- TPA
12-O-tetradecanoylphorbol-13-acetate
- APX1
Ascorbate peroxidase1
- WD
Well-differentiated
- MD
Moderately differentiated
- PD
Poorly differentiated
- AK
Actinic keratosis
- HPV
Human Papilloma virus
- EBV
Epstein-Barr virus
- CPDs
Cyclobutane pyrimidine dimers
- NER
Nucleotide excision repair
- GG-NER
Global genome nucleotide excision repair
- TC-NER
Transcription coupled nucleotide excision repair
- NHDFs
Normal human dermal fibroblasts
- DHS
2, 3-dehydrosilibinin
- COX
Cyclooxygenases
- iNOS
Inducible nitric oxide synthase
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- OA
Okadaic acid
- ROS
Reactive oxygen species
- BPO
Benzoyl peroxide
- SOD
Superoxide dismutase
- EGFR
Epidermal growth factor receptor
- Hh
Hedgehog
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- STAT3
Signal Transducer and Activator of Transcription 3
- PI3K
Phosphatidylinositol 3-kinase
1. Introduction
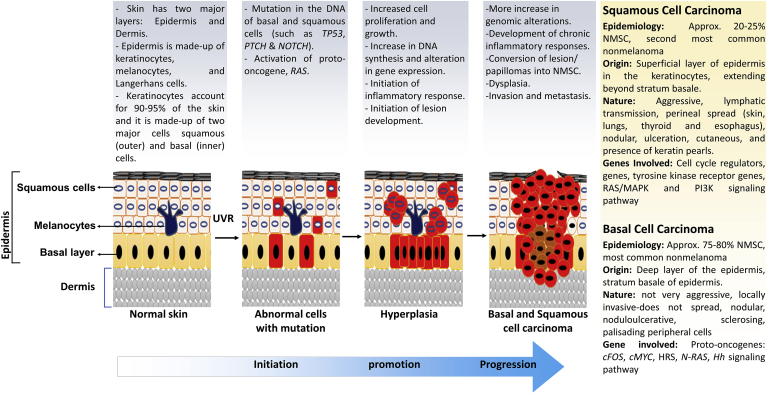
Skin is the largest human organ and works as the first line of defense against injuries and infection to the inner organs via external factors.1 Skin is always vulnerable to damage, as it is exposed to pathogens, solar ultraviolet radiation (UVR) and various noxious agents. These harmful factors create a range of molecular and biochemical stress, leading to the development of skin carcinogenesis.1 Transformation of normal skin cells into the malignant form is a multistage process, which involves initiation, promotion and progression events (Fig. 1). Carcinogens/mutagens primarily cause genomic alterations, which either lead to loss of tumor suppressor activity or gain of function of proto-oncogenes.2 Solar UVR is the most prominent factor for the initiation of skin carcinogenesis, mostly through direct DNA damage and also indirectly via inducing aberrant molecular signaling by oxidative stress and inflammation.3 UVR induced DNA damage is repaired by DNA repair mechanism; however, if DNA damage remains unrepaired, cells undergo irreversible/permanent DNA mutations.2 These genetic mutations lead to the loss of tumor suppressive activity of a critical protein p53 as well as gain of function mutations converting proto-oncogene into oncogenes (such as RAS), helping the skin cells to acquire the ability for autonomous growth.2 Finally, during progression stage, dividing cancer cells become more aggressive and start invading and migrating to local and distant tissue or organ sites.1,3 The epidermal layer manifests into skin cancer, and based on the involvement of cell type, skin cancer is categorized in two major groups, namely melanoma and non-melanoma skin cancers (NMSCs). NMSCs are further classified into two broad categories: basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Melanoma skin cancer is only ∼1% of total diagnosed skin cancers, but it causes majority of skin cancer-related deaths due to its high metastatic properties. Incidence of melanoma skin cancer increases in regions closer to the equator, with highest reported rates in Australia/New Zealand and in Caucasians/fair-skinned people.4 The remaining of the diagnosed skin cancers are NMSCs, out of which ∼80% are BCC and ∼20% are SCC. According to American Cancer Society estimates, about 5.4 million BCC and SCC cancers are diagnosed each year in the US in ∼3.3 million Americans (as some people have more than one lesion).5 The incidence of these cancers has been increasing for many years; more likely due to better skin cancer detection, increased sun exposure/tanning beds and longevity6; however, death from BCC and SCC is uncommon.5 NMSCs associated deaths (if any) are more likely in elderly patients, and immunosuppressed individuals. BCCs have extremely rare metastatic characteristics and show metastasis associated mortality incidence of 1 case per 14,000,000 patients. However, SCCs are relatively more aggressive and show a higher metastatic rate of 0.1–9.9%.4
Fig. 1.
Description of sequential steps in carcinogenesis process during non-melanoma skin cancer (SCC and BCC) development and progression after UVR exposure.
Skin cancer prevention programs are making efforts to reduce skin carcinogenesis through public awareness about exposure to risk factors-particularly minimizing sun light exposure and use of sunscreens.7 However, increased incidences of skin cancer show that these strategies have not been very effective.3 As an alternative approach, the use of phytochemicals against many skin cancer cell lines and animal models shows their promising impact in skin cancer intervention.1 These phytochemicals are isolated from fruit, seed, root, flower and other parts of the plants; few examples mostly focusing on the studies done in our research program include silymarin/silibinin, grape seed extract, resveratrol, genistein, green tea and its catechins, etc.1, 2, 3 Whereas this review focuses mainly on the efficacy of silymarin/silibinin on UVR-induced NMSCs, over the last twenty-years, several studies have shown the chemopreventive effect of silymarin/silibinin in other cancers also.3,8 Agarwal and colleagues first reported the anti-cancer effect of silymarin in 7, 12-Dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin tumorigenesis model.9 Silymarin treatment inhibited the skin tumor growth by attenuating the expression and activity of epidermal ornithine decarboxylase.9 Several other studies have also shown the anti-cancer effect of silymarin/silibinin through targeting cell cycle regulators, tumor suppressor (p53), inflammatory pathways (TNFα, IL-1α and COX-2), angiogenic molecules (VEGF), and mitogenic and survival signaling (PI3K-Akt, MAPK and Survivin) pathways, suggesting the potential of silymarin/silibinin as pleotropic cancer chemopreventive as well as therapeutic agent against skin cancer and other epithelial malignancies.2,3
2. Natural occurrence and characterization of silymarin and silibinin
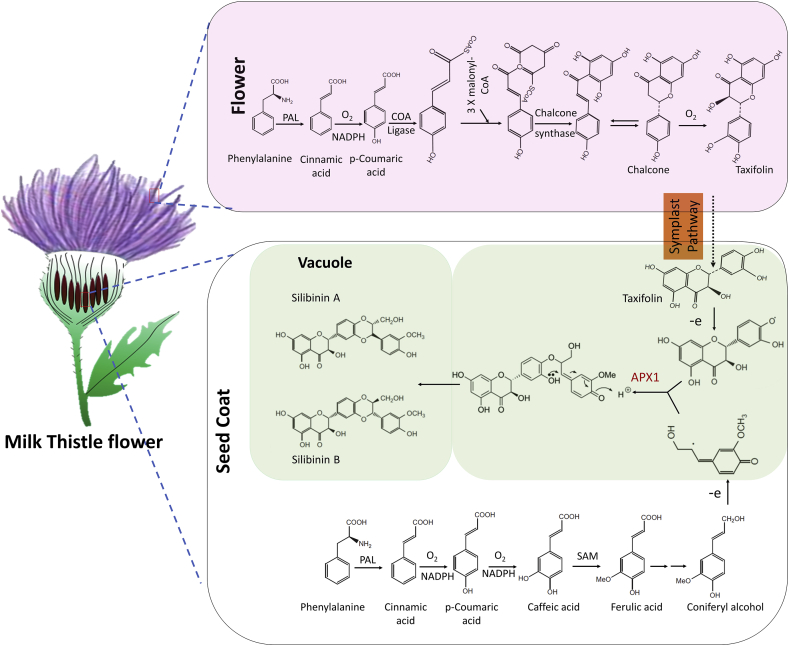
Silymarin is isolated from the seeds of milk thistle (Silybum marianum, Asteraceae family), which is an annual or biennial herb native of Mediterranean region (Fig. 2).10 Its fruit has been used as a herbal remedy to treat liver associated illness for over centuries and is currently being clinically used (Legalon® SIL) to treat hepatic-toxicity due to mushroom poisoning.11 Silymarin content and composition depend on Silybum marianum varieties, geographical region and genetic makeup.12 In the 1st century, Dioscorides, a Greek physician gave the name Silybum to this plant.10 Its medicinal use as liver protective herbal has been dated in the initial Greek references. Roman physician Pliny the Elder in A.D. 23-79 had written about the use of this plant as vegetable and juice mixed with honey to be good for “carrying off bile.” In 18th century, Culpepper mentioned that it is effective against liver and spleen obstructions, thereby is good for jaundice.13 During 20th century, medicinal use of milk thistle was generalized for the treatment of hepatitis, jaundice, cirrhosis, liver poisoning and drugs or alcohol abuse.13 Milk thistle seed extract contains flavonolignans, fatty acids (mainly linoleic, linolenic, myristic, oleic, palmitic and stearic acid), proteins, tocopherol, sterol and flavonoids.13 In 1968, Wagner et al. successfully isolated the active compound of milk thistle, which was designated as silymarin and later described as a mixture of chemicals known as flavonolignans.13 Further, constituents of silymarin were isolated and structurally characterized by Wagner and Seligmann, which include silybin (silibinin), silydianin and silychristin.14 Subsequent studies revealed additional flavonolignans including; isosilychristin, silandrin, silyhermin, silymonin and neosilyhermins.15 During isolation and structural characterization of silybin (silibinin), its structural isomer isosilybin (Isosilibinin) was discovered.16 Flavonolignans silibinin, isosilibinin and silychristin exit in two diastereoisomeric forms, namely A and B.17
Fig. 2.
Sequential steps in the biosynthesis of silymarin components including silibinin from Milk Thistle plant (Silybum marianum).
Biosynthesis of constituents of silymarin (Fig. 2) is carried out by oxidative coupling reaction between phenylpropanoid (usually coniferyl alcohol) and taxifolin (flavonoid). Coniferyl alcohol is one of the prominent monolignols in the dicot angiosperm.12 It is distributed throughout the milk thistle plant, and its distribution correlated with the expression of related genes.12 Coniferyl alcohol biosynthesis mapped in milk thistle seed coat. It is produced through the caffeoyl-CoA and p-coumaroyl-CoA pathways by catalysis of series of enzymes.12 Another substrate of flavonolignans synthesis, Taxifolin is largely accumulated in the seed coat and flower of milk thistle. The level of taxifolin in the seed coat is much higher than that in the flower. However, genes associated with the biosynthesis of taxifolin are overexpressed in the flower because transcription of these genes is light dependent. Taxifolin is produced by series of enzymatic catalysis, including; coumarate-CoA ligase, cinnamate-4-hydroxylase, chalcone synthase, chalcone isomerase, flavanone 3-hydroxylase, and flavonoid 3′-monooxygenase. Chalcone synthase and chalcone isomerase catalyzed the addition of three malonyl-CoA to a cinnamoyl-CoA molecule and subsequent cyclization produced the chalcones.12 Chalcone is the precursor for most of the flavonoids found in plants. Synthesized flavonoid (taxifolin) is translocated from flower to seed coat through symplastic pathway.12 Enzyme ascorbate peroxidase1 (APX1) is located on the internal cell membrane of the seed coat, oxidized and produced the taxifolin and coniferyl alcohol free radicals. Phenoxy radical of taxifolin coupled with quinone methide radical of coniferyl alcohol to produce an adduct.10,12 Further intramolecular nucleophilic attack of hydroxyl group on the quinone methide ring produces silibinin and isosilibinin (Fig. 2).12
3. Non-melanoma skin cancers and their risk factors
Skin is made up of several layers, out of which, epidermis and dermis are the major ones.1 Epidermis consists of three kinds of cells, squamous cells (top layer with thin and flat cells), basal cells (below squamous cells with round cells) and melanocytes (lower part of epidermis, cells that make melanin).3 Skin cancer is the most common human malignancy, which begins in the epidermis.7 Based on the origin of cells, skin cancer is divided into two main types: melanoma and NMSCs. Nomenclature itself clarifies that melanoma is due to the result of malignant transformation of melanocytes and is more likely to invade nearby tissues and spread to other parts of the body18; while, NMSCs are formed by neoplastic transformation of all other types of skin cells. Melanoma is less common compared to NMSCs; the latter includes cutaneous lymphoma, adnexal tumors, sarcoma, Merkel-cell carcinoma, and the most common ones BCC and SCC.19 Based on our research focus on investigating the efficacy of silymarin/silibinin against SCC and BCC, the characteristics of these subtypes of NMSCs are further detailed in the next section (Fig. 1).
3.1. Squamous cell carcinoma (SCC)
SCC is the second most common NMSCs and more than 1 million cases of SCC are diagnosed in the US each year.20 It develops from recognized precursor lesions that contained hyperkeratotic nodules, which can be aggressive and metastasize. Invasive SCC is divided into three different stages based on histological characteristics: 1) well-differentiated (WD) SCCs have low malignant potential and are eosinophilic, show striking similarity to normal keratinocytes, and display a pattern of layers more-like normal squamous epithelium and produce large amounts of keratin (observed as extracellular keratin pearls); 2) moderately differentiated (MD) SCC is less like normal squamous epithelium and the typical architecture of epithelial layers is less defined; and 3) poorly differentiated (PD) SCC are more aggressive, lack epithelial architecture and tumor cells show a high degree of morphological atypia and keratin production is markedly reduced.21 SCC arise from cutaneous areas bearing multiple precursor lesions known as actinic keratosis (AK). AK usually appears as asymptomatic red, scaly papules on sun-exposed areas, with histological evidence of epidermal dysplasia, and are more common among Caucasians of advanced age, and multiple AK in an area of the skin (often chronically sun-exposed) is called ‘field cancerization’.22 Field cancerization can often be a relatively broad area with no obvious macroscopic changes in which pro-neoplastic mutations are already present, and thus represents the pre-neoplastic stage of SCC development.23 Although the precise mechanism of field cancer development is not entirely clarified, molecular data acquired from other tumors indicate that a single genetic event occurs in individual cells, which due to clonal expansion spread laterally and replaces normal epithelium and creates pre-neoplastic fields.22
3.2. Basal cell carcinoma (BCC)
BCC is the most common skin malignancy in humans also known as basalioma, basal cell epithelioma, rodent ulcer and Jocobs ulcer, affecting millions of people worldwide.20 It was first described by Jocobs in 1824; BCCs are so named because of their resemblance to basal keratinocytes of hair follicles and interfollicular epidermis.24 It is a slow growing, locally destructive, painless, skin tumor of epidermis.19 BCC is non-malignant tumor and it rarely metastasizes.19 25 Commonly, BCC occurs in sun light exposed areas of skin, although anatomical distribution varies between different types of BCC. Average lifetime risk of developing a BCC is approximately 30% in Caucasians and represents a significant public health issue.26
Ionizing radiation has been implicated in the development of BCC and SCC.25,27 Originally, it was suggested that exposure to therapeutic radiation is associated with the development of BCC, but not SCC. Nonetheless, it was subsequently demonstrated that the risk of SCC may be increased with radiotherapy.19 Overall, risk factors for the development of NMSC are the combination of environmental, genetic and phenotypical factors. UVR is the most prominent skin carcinogen; UVB (280–315 nm) creates direct DNA damage, with a typical C-T substitution, resulting in the dimerization of pyrimidines, especially in individuals prone to sunburn.28 Infectious agents like Human Papilloma virus (HPV) and Epstein-Barr virus (EBV) are also reported to be correlated with increased risk of NMSC malignancy.29 These viruses create proto-oncogenic environment in the host cells by the expression of pathogen associated oncogenes, which inactivate the host tumor suppressor gene or induce chronic inflammation and reduce immunosurveillance.30 Studies also suggest that HPV may be contributing indirectly in the cancer pathogenesis, potentially by facilitating UV-related carcinogenesis by prevention of UV-induced apoptosis or impairing DNA repair.19 NMSCs is also the most common post-transplant malignancy in Caucasian organ transplant recipients.30 SCC occurs more frequently in organ transplant recipients compared to BCC, while in non-transplant population, the ratio of BCC and SCC is reversed.30 Some other risk factors, such as, smoking, alcohol abuse, exposure to arsenic, coal tar, insecticides, herbicides and petroleum products also contribute to the development of NMSC.31
4. Antitumor properties and mechanism of action of silymarin/silibinin against NMSCs
4.1. Protective effect against UVB induced-photocarcinogenesis during SCC and BCC development
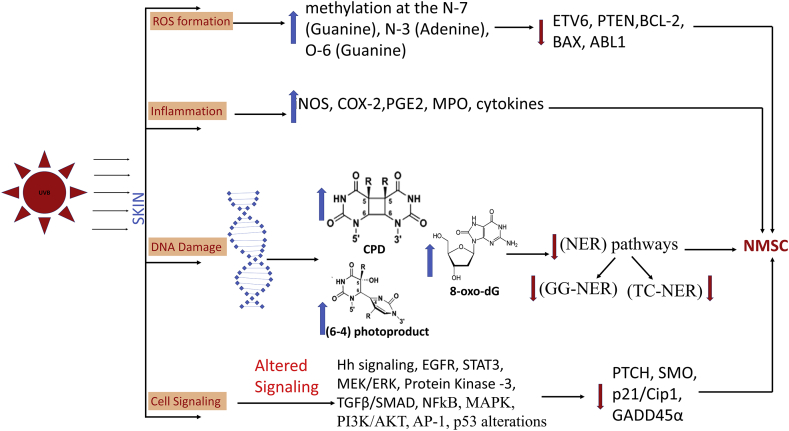
Exposure to solar radiation is the most important etiological risk factor for skin cancers. Given that higher doses (including duration of exposure) are needed for UVA-caused photocarcinogenesis and tumor development (in rodent studies) compared to UVB.32 Therefore, UVB exposure is considered the most clinically relevant form of UVR for the development of skin cancers. UVR exposure is known to create many photoproducts including; thymine dimers, cytosine photohydrates, DNA-DNA or DNA-protein cross link and DNA breaks.33 Among the different spectra of solar radiation, UVB radiation is most closely associated with photocarcinogenesis. DNA is a natural chromophore of UVB, and absorption of radiation leads to formation of thymine dimers and DNA damage. UVR exposure also leads to generation of reactive oxygen species, and longer exposure induces oxidative damage to DNA, proteins and lipids (Fig. 3).33 DNA damage leads to formation of cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts [6,4-photoproducts, (6,4-PPs)] in skin epidermis, and UVR induced oxidative stress indirectly causes DNA damage via 8-oxo-2′-deoxyguanosine (8-oxo-dG) formation.3 Oxidation of proteins, due to free radicals, changes the structure and function of proteins, which in turn alter or upregulate various signaling pathways playing important role in tumorigenesis.34 These free radicals also cause lipid peroxidation leading to cell membrane damage and apoptosis.35 In general, if nucleotide excision repair (NER) pathways, global genome nucleotide excision repair (GG-NER) and transcription coupled nucleotide excision repair (TC-NER) fail to repair DNA damage (CPDs, 6-4PPs and 8-oxo-dG), it leads to irreversible mutations in genes which manifest a 2,000-10,000 fold increase in initiation of skin carcinogenesis (Fig. 3).36
Fig. 3.
Description of various DNA damage events during solar-UVB radiation induced epidermal skin DNA damage/photocarcinogenesis, eventually leading to skin tumor (NMSC) development via inducing gene/protein alterations, and activation of aberrant cellular signaling pathways.
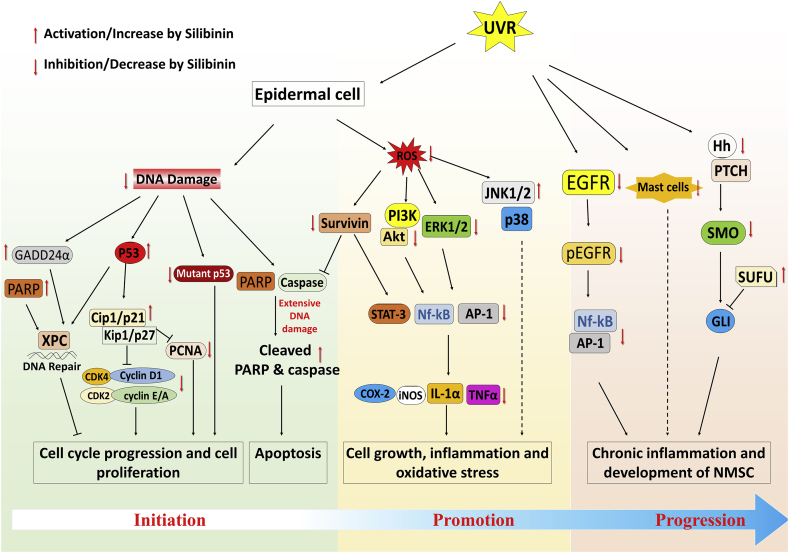
In this regard, several studies by us and others have shown that topical (9 mg in 200 μl acetone) as well as dietary treatment (50 mg/kg body weight; dose) with silibinin protects against UVB-induced DNA damage and photocarcinogenesis (Fig. 4).27,37 Mechanistic studies in cell culture indicated the possible role of p53 in the observed silibinin (Concentration 25–100 μM) efficacy.38 SKH-1 mice (p53+/+) exposed to UVB develop pre-malignant papilloma-III, MISCC-II and MISCC-III lesions.27 Upon pre-treatment with silibinin (9 mg in 200 μl acetone, before UV exposure), severity of the skin lesions was reduced drastically where only pre-malignant papillomas grade I, and II were formed. Thus, to address whether protective effects of silibinin are mediated via p53, we generated p53 heterozygous (p53+/-) and p53 knockout (p53−/−) mice on SKH-1 hairless background and assessed silibinin effects in both short- and long-term UVB exposure studies. While, silibinin accelerated the removal of UVB-induced DNA damage products CPDs and significantly reduced the tumor number, multiplicity, and volume in p53+/+ mice, its protective efficacy was compromised in p53 deficient mice.27
Fig. 4.
Scheme of different aberrant cellular signaling pathways associated with survival, apoptosis, inflammation, and oxidative stress (induced by UVR-exposure) which are targeted (increased or decreased) by silibinin treatment during prevention/intervention of skin NMSC growth and progression.
Additionally, we found that silibinin enhances the repair of UVB-induced DNA damage by activating p53-dependent GADD45α in SKH-1 mouse skin and NER pathway in NER proficient normal human dermal fibroblasts (NHDFs), but its DNA damage repair efficacy is compromised in NER (xeroderma pigmentosum (XP) A, XPB) deficient dermal fibroblasts.39 Importantly, it was found that silibinin treatment post-UVB exposure not only increased the spatial-temporal presence of NER factors (XPA, XPG, XPF) in UVB exposed NHDFs but also upregulated transcriptional (mRNA) production of some of the NER factor-XPA and GADD45α involved in DNA damage repair.39 Taken together, these results strongly suggest that silibinin application could reduce UVB-induced DNA damage, probably through a faster NER response rather than a sunscreen effect.
Based on above strong effects of silibinin in mouse skin SCC model, in ongoing studies, we also assessed its effects against UVB-induced BCC. Indeed, silibinin decreased UVB-induced CPDs in Ptch1+/− mouse excised ear skin and inhibited UVB-induced microscopic BCC formation in Ptch1+/- mice (R. Agarwal unpublished data). Taken together above findings, it is clear that silibinin has strong efficacy against UVB-induced SCC via p53 involvement and that it is also efficacious against UVB-induced BCC formation; however, it is not known whether p53 is dispensable in silibinin efficacy against UVB-induced DNA damage and BCC formation in Ptch1+/− mice; a model where main pathway driving BCC is Hh.
4.2. Protective effect against UVB-induced genomic instability
UVB and other related causative factors for skin cancer lead to alteration in genetic material (Fig. 3), for example-TP53, CDKN2A, RAS, PIK3CA, BRAF, NOTCH, FAT1, MLL and CASP8 genes have been reported to undergo mutation in skin SCC.40 Specifically, skin SCC genome shows ∼ 5–15 fold higher mutation rate (nearly 50–60 mutations per megabase in genome) compared to normal sun exposed tissue.41 With regards to skin BCC, mutations occur predominantly in PTCH, TP53, MYC, RAS, SMO, SUFU and NOTCH genes.42 Overall, TP53 mutations are one of the prominent somatic mutation identified in both SCC and BCC. UVB exposure-induced skin carcinogenesis is associated with increase in DNA abnormality due to pyrimidine dimer formation in epithelial cells. If left unrepaired, the DNA damage introduces wrong bases during replication, which leads to mutation or inappropriate expression of downstream molecules.19 In our studies, silibinin treatment showed a strong inhibitory effect on these UVB-induced genetic alterations through inhibition of thymine dimer formation and initiating DNA damage repair.3 In the presence of silibinin, DNA damage repair-response triggers the expression of p53, which enhances the levels of CDK inhibitor Cip1/p21 and stops the cell cycle progression and DNA replication.39 In UVB-induced SCC in SKH-1 mice, silibinin was found to increase the cellular levels of cell cycle arrest-associated molecules such as Cip1/p21, Kip1/p27 and p53 and cause concomitant reduction in the cellular levels of CDK2, CDK4 and cyclins A, E, and D1 (essential molecules for cell cycle progression).43 This process allows more time to repair genomic damage of the cell. Together, our studies show that silibinin treatment increases p53 levels in UVB exposed mice2 and in turn prevents cell cycle progression of cells with damaged or mutated DNA (Fig. 4).43,44
4.3. Inhibitory effect against skin SCC/BCC growth and proliferation
Treatment with silymarin/silibinin (oral/dietary administration or topical application) has been shown to strongly inhibit UVB-induced skin cancer (SCC type), including chemically induced skin carcinogenesis, in various in vitro and in vivo pre-clinical models (Fig. 4). For example, DMBA/TPA induced skin tumor in SENCAR mice, silibinin treatment not only caused a reduction in tumor growth but also caused regression of developed tumor.2 In another skin cancer cell line A431, silibinin significantly decreased cell proliferation by reducing the phosphorylation of ERK1/2, and increasing the activation of stress activated protein kinase/JUN–NH2–terminal kinase ½ (SAPK/JNK ½) and p38 kinase protein, which further increased the cleaved caspase-3 levels.45
Though, our lab has extensively studied and reported the protective role of silibinin in SCC for decades,3 the first evidence of potential efficacy of silibinin against BCC cells in culture and animal models was highlighted by us only a few years back.3,38 Our studies have shown that treatment with silibinin or its oxidized form 2, 3-dehydrosilibinin (DHS) significantly reduced the cell proliferation, cancer stem cell spheroid formation, and clonogenicity of both p53 null and p53 mutated- BCC cells (BSZ and ASZ cells, respectively) in time and dose dependent manner.38 Mouse allograft studies using these agents confirmed the strong efficacy of silibinin on BCC tumor growth.25 These silibinin effects were associated with modulation of EGFR/AKT, MAPK/ERK1/2, NF-κB and AP1 pathways.25
4.4. Induction of apoptotic cell death of skin epidermal cells after UVB exposure
Previous studies have shown that at lower dose/acute exposure of UVB, silymarin/silibinin promoted cell cycle arrest and initiated DNA repair mechanism in HaCaT cells and SKH-1 mice.46 However, silibinin treatment induced apoptosis in chronic UVB exposed SKH-1 mice SCC tumors; also, in vitro cell culture experiments using JB6 cells showed that silibinin protective effects were mediated by induction of p53 levels by stabilizing its phosphorylation at serine 15 site, which further promoted apoptotic cell death.47 Thus, during UVB exposure, if DNA damage and associated cellular stress are mild then silibinin increases p53 levels and promotes cell cycle arrest, and when there is severe DNA damage and stress then apoptosis is induced.39 Silibinin treatment increased cleaved caspase-3 and PARP levels and also decreased anti-apoptotic Bcl2 level in these cells.43 PIK3-Akt signaling in response to the growth factors is another pathway that promotes the cell survivability in skin cancer. P53 plays an important role in regulation of this cellular signaling axis through PTEN molecule by inhibiting the phosphorylation of Akt.48 UVB induced skin cancer also showed increased expression of phosphorylated Akt (Ser 473) levels, while silibinin treatment was found to cause complete inhibition of Akt phosphorylation leading to activation of apoptotic signal.43 Survivin is another important anti-apoptotic molecule, which interacts with caspases and is reported to promote skin cancer progression.2 Silibinin treatment has been shown to reduce the cellular levels of survivin in UVB-induced skin tumors. Overall, these findings suggest that silibinin works as a chemopreventive/intervention agent against skin cancer by targeting p53, MAPK, PI3K-Akt and other survival pathways (Fig. 4).
4.5. Targeting of inflammatory and oxidative stress signaling to prevent skin cancer
Inflammation is another important factor for the growth and progression of skin cancer.3 UV radiation or toxic chemicals caused skin injury promotes the infiltration of immune cells to the site, these cells secrete different kinds of cytokines, prostaglandins, chemokines and growth factors, and thus lead to the generation of chronic inflammation and UVB-induced skin carcinogenesis (Fig. 3).3
Exposure to UVB radiation causes cutaneous tissue injury, inflammation and immune suppression.49 UVB radiation promotes secretion of pro-inflammatory cytokines which over-all influence UVB induced inflammation.49 Studies have shown that in dermal cells, UVB exposure induced autophagy through the activation of IGFR1-PI3K-Akt signaling axis and prevents these cells from undergoing apoptosis.50 However, in epidermal cells UVB exposure induced apoptosis, which causes the secretion of chemokines and inflammatory cytokines to promote the movement of immune cells and leads to skin inflammation and cancer development.51 Several studies have shown that enzymes [cyclooxygenases (COX), inducible nitric oxide synthase (iNOS)], transcription factors (AP-1, NF-κB and STAT3), and chemical inducers or ligands such as (arachidonic acid, prostaglandins and TNF α) are responsible for initiation of skin inflammation.49 A population based case-control study has highlighted the beneficiary effect of nonsteroidal anti-inflammatory drugs (NSAIDs) in non-melanoma skin cancer.49 We have also observed the similar preventive effect of silibinin in UVB-induced skin inflammation in SKH-1 mice.52 Analysis of skin tissues from topical or dietary silibinin treated SHK-1 mice showed a remarkable decrease in UVB-induced COX-2 and iNOS levels; furthermore silibinin decreased the phosphorylation of STAT-3 (Tyr705) and NF-κB-p65 (Ser536) levels, which are the upstream transcription regulator for COX-2 and iNOS.52 Silibinin also reduced the microvessel density near UVB-induced skin injuries by inhibiting the levels of hypoxia-inducible factor 1α (HIF-1α) and VEGF.52 Also, in TPA or okadaic acid (OA)-caused skin tumors in SENCAR mice, which show high expression of inflammatory cytokines (IL-1α) and tumor necrosis factor α (TNFα), which are the central mediators of skin tumor progression, topical treatment of silymarin prior to the TPA exposure resulted in the complete inhibition of IL-1α and TNFα mRNA expression.53,54
In another study, our group showed that immune-modulatory potential of silibinin protected against DNA damage and apoptosis in skin epidermal cells (both in cell culture and murine model) by modulating IL-12 levels.55 Notably, in a more recent study, we have shown that silibinin treatment reduced the inflammatory response by inhibiting the UVB-induced recruitment of mast cells in UVB exposed skin of Ptch+/− mouse model of basal cell carcinoma.56
Furthermore, the strong efficacy of silymarin/silibinin against skin cancer growth and progression has been attributed to its strong antioxidant potential via reversing reactive oxygen species (ROS)-induced cellular damages. In this regard, we have observed the protective effect of silymarin, against skin tumor promoting agent benzoyl peroxide (BPO) -induced depletion of antioxidant enzymes such as superoxide dismutase (SOD), catalase and GPX activity in SENCAR mouse epidermis.57 Additionally, it has been observed that silibinin also has the potential to increase the skin cellular levels of other anti-oxidant enzymes (glutathione-S-transferase and quinine reductase) that play an essential role in the removal of cellular reactive species.37 All together, these findings indicate the inhibitory effect of silymarin/silibinin on molecular expression and activation of inflammatory molecules and oxidative pathways which play an essential role in skin tumorigenesis growth and progression (Fig. 4).
4.6. Inhibitory effect against drug-resistant BCC cells
Hh signaling plays a central role in basal cell survival, proliferation and progression and its activation is crucial for BCC development.58 Executer of Hh signaling, Gli, is activated via loss-of-function mutations in Ptch1 or via activating mutations in SMO.58 Therefore, several Hh pathway inhibitors have been evaluated against BCCs to reduce tumor progression58; to date two inhibitors [Vismodegib (GDC-0449) and Sonidegib (LDE-225)] have received FDA approval to treat BCC.59 Whereas toxicity of these agents is usually mild to moderate, it could be chronic and persistent, and is the main cause of therapy discontinuation.60 Also, the acquired resistance against Hh pathway inhibitors is a limitation that is linked to distinct mechanisms, e.g. mutations in SMO, transcription factor Gli amplification, and up-regulation of synergistic signals e.g. epidermal growth factor receptor (EGFR) and AKT.61 Importantly, in our most recent studies, a combination of silibinin and Hh pathway inhibitors (Sant-1 and GDC-0449) significantly increased the growth inhibitory effects of the drugs alone; silibinin also reversed BCC cells’ resistance to both these drugs via targeting EGFR/AKT and Hh pathway and induction of apoptosis.38 Specifically, we reported that silibinin can overcome this drug resistance in BCC cells via reducing the phosphorylation of EGFR (a key molecule responsible for Hh inhibitor resistance), and upregulation of SUFU (a tumor suppressor molecule of the Hh pathway.38
5. Conclusion and clinical usefulness of silibinin/silymarin formulations
Increasing evidence suggests the beneficial effect of phytochemicals in cancer prevention and intervention. Silymarin/silibinin are flavonolignans isolated from the milk thistle plant, which have shown strong efficacy against all stages of photocarcinogenesis during NMSC growth and progression, such as, a) protection from UVB-induced DNA damage, b) initiating DNA damage repair following UVB exposure, c) increase in tumor suppressor p53 levels, d) initiation of p53 mediated-cell cycle arrest or apoptosis based on acute/chronic exposure effects of UVB, e) modulation of survival and mitogenic signaling, and f) initiating anti-inflammatory and anti-oxidative responses (Fig. 4).
Considering all these published reports and the fact that this natural agent is non-toxic and has other pleotropic health benefits, silibinin could be a novel preventive/intervention option to be tested in clinical trials against non-melanoma skin cancer to efficiently manage and reduce patient skin cancer burden. In this regard, there are important developments in recent years in support of clinical usefulness of silibinin/silymarin. This is an important step forward as most phytochemicals that have shown efficacy in pre-clinical cancer models do not move forward in clinic due to: a) safety and toxicity issues, and b) non-availability of a formulation that can be used clinically in human patients. Legalon® SIL is an FDA approved pharmaceutical formulation (Rottapharm/Madaus, Germany) of silibinin as water-soluble succinate form given intravenously.11 It is an anti-dote of choice in patients with acute hepatotoxicity from amatoxin poisoning due to ingestion of cytotoxic mushrooms (Amanita phalloides and related species) which often leads to immediate renal and hepatic failure and patient death.11 Importantly, based on clinical reports/trials (both published and unpublished), out of 1,491 Amanita-poisoned patients treated with Legalon® SIL, survival rate was 93%.11 Furthermore, silibinin as Legalon® SIL is also being evaluated in clinical trials in other diseases as it is generally well tolerated and makes the drug systemically available. For example, oral silibinin phosphatidylcholine combinations such as SiliphosR and SilipideR (alone or in combination with other chemopreventive agents) as well as silymarin-based topical cream LeviadermR are commercially available formulations, which have been clinically evaluated for their efficacy against prostate cancer, colorectal cancer, advanced hepatocellular carcinoma/diseases and radiation dermatitis in breast cancer patients.62 Notably, LeviadermR has been found to be an effective treatment for the prevention of acute skin lesions caused by radiotherapy of breast cancer patients.63 Difinsa53™ Barrier Repair topical cream of silibinin with vitamins B3, and E, and hyaluronic acid was also recently commercially marketed to protect against sun/environment induced skin damages. These topical formulations are also an important step towards providing an effective cure against human autosomal recessive hereditary disorders such as xeroderma pigmentosum (XP). This skin disorder is associated with defects in NER machinery, where the patients exhibit extreme sensitivity to sun exposure and a marked predisposition to skin cancer.64 Importantly, the National Institute of Health in a four decade follow up study found that in 65% of XP patients (under age 20), NMSCs risk was increased 10,000-fold and melanoma risk was increased 2000-fold.65 Thus, given the fact that silibinin accelerates the repair of CPDs caused by UVB radiation and promotes UVB-induced DNA damage repair via activating several components of the NER pathway, including up regulation of p53 and GADD45α levels, for UVB-induced DNA damage protection, it is very likely that this agent also has strong protective benefits against skin cancer initiation in XP patients.
Declaration of competing interest
The authors declare that there are no conflicts to disclose.
Acknowledgment
The original silibinin and skin cancer chemoprevention studies in our laboratory are supported by National Institute of Health (NIH)/National Cancer Institute (NCI), USA, Research grant R01-CA140368 awarded to RA.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.02.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ng C.Y., Yen H., Hsiao H.Y., Su S.C. Phytochemicals in skin cancer prevention and treatment: an updated review. Int J Mol Sci. 2018;19(4) doi: 10.3390/ijms19040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R.P., Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Canc. 2005;41(13):1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R., Deep G., Agarwal R. An overview of ultraviolet B radiation-induced skin cancer chemoprevention by silibinin. Curr Pharmacol Rep. 2015;1(3):206–215. doi: 10.1007/s40495-015-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Didona D., Paolino G., Bottoni U., Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):1–15. doi: 10.3390/biomedicines6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M., Qureshi A.A., Geller A.C., Frazier L., Hunter D.J., Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30(14):1588–1593. doi: 10.1200/JCO.2011.39.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson M., Holman D.M., Maguire-Eisen M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs. 2016;32(3):241–254. doi: 10.1016/j.soncn.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deep G., Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Canc Metastasis Rev. 2010;29(3):447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R., Katiyar S.K., Lundgren D.W., Mukhtar H. Inhibitory effect of silymarin, an anti-hepatotoxic flavonoid, on 12- O -tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in SENCAR mice. Carcinogenesis. 1994;15(6):1099–1103. doi: 10.1093/carcin/15.6.1099. [DOI] [PubMed] [Google Scholar]
- 10.Corchete P. Silybum marianum (L.) Gaertn: the source of silymarin. In: Ramawat K.G., Merillon J.M., editors. Bioactive Molecules and Medicinal Plants. Springer; Berlin, Heidelberg: 2008. pp. 123–148. [Google Scholar]
- 11.Mengs U., Torsten Pohl R., Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharmaceut Biotechnol. 2012;13(10):1964–1970. doi: 10.2174/138920112802273353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv Y., Gao S., Xu S., Du G., Zhou J., Chen J. Spatial organization of silybin biosynthesis in milk thistle [ Silybum marianum (L.) Gaertn] Plant J. 2017;92(6):995–1004. doi: 10.1111/tpj.13736. [DOI] [PubMed] [Google Scholar]
- 13.Wagner H., Hörhammer L., Münster R. On the chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.) Arzneimittelforschung. 1968;18(6):688–696. [PubMed] [Google Scholar]
- 14.Flora K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal M., Goldberg A.B.J. American Botanical Council; 2000. Herbal Medicines: Expanded Commission E Monographs. [Google Scholar]
- 16.Wagner H., Diesel P., Seitz M. The chemistry and analysis of silymarin from Silybum marianum Gaertn. Arzneimittelforschung. 1974;24(4):466–471. [PubMed] [Google Scholar]
- 17.Smith W.A., Lauren D.R., Burgess E.J., Perry N.B., Martin R.J. A silychristin isomer and variation of flavonolignan levels in milk thistle (Silybum marianum) fruits. Planta Med. 2005;71(9):877–880. doi: 10.1055/s-2005-864187. [DOI] [PubMed] [Google Scholar]
- 18.Apalla Z., Nashan D., Weller R.B., Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther. 2017;7:5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madan V., Lear J.T., Szeimies R.M. Non-melanoma skin cancer. Lancet. 2010;375(9715):673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 20.Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 21.Chernock R.D. Morphologic features of conventional squamous cell carcinoma of the oropharynx: “keratinizing” and “nonkeratinizing” histologic types as the basis for a consistent classification system. Head Neck Pathol. 2012;6(1):41–47. doi: 10.1007/s12105-012-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman B., Cockerell C.J., Zografos P. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Acad Dermatol. 2013;68(1):10–19. doi: 10.1016/j.jaad.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Rubin H. Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture. Bioessays. 2011;33(3):224–231. doi: 10.1002/bies.201000067. [DOI] [PubMed] [Google Scholar]
- 24.Wang G.Y., Wang J., Mancianti M.L., Epstein E.H. Basal cell carcinomas arise from hair follicle stem cells in Ptch1+/- mice. Canc Cell. 2011;19(1):114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilley C., Deep G., Agarwal C. Silibinin and its 2,3-dehydro-derivative inhibit basal cell carcinoma growth via suppression of mitogenic signaling and transcription factors activation. Mol Carcinog. 2016;55(1):3–14. doi: 10.1002/mc.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samarasinghe V., Madan V., Lear J.T. Focus on basal cell carcinoma. J Skin Cancer. 2011;2011:1–5. doi: 10.1155/2011/328615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigby C.M., Roy S., Deep G. Role of p53 in silibinin-mediated inhibition of ultraviolet B radiation-induced DNA damage, inflammation and skin carcinogenesis. Carcinogenesis. 2017;38(1):40–50. doi: 10.1093/carcin/bgw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichter M.D., Karagas M.R., Mott L a, Spencer S.K., Stukel T a, Greenberg E.R. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. The New Hampshire Skin Cancer Study Group. Arch Dermatol. 2000;136(8):1007–1011. doi: 10.1001/archderm.136.8.1007. [DOI] [PubMed] [Google Scholar]
- 29.Harwood C.A.1, Surentheran T., McGregor J.M., Spink P.J., Leigh I.M., Breuer J.P.C. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;3(61):289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Collins L., Asfour L., Stephany M., Lear J.T., Stasko T. Management of non-melanoma skin cancer in transplant recipients. Clin Oncol. 2019;31(11):779–788. doi: 10.1016/j.clon.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Zanetti R., Rosso S., Martinez C. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Canc. 2006;94(5):743–751. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhtar H., Elmets C.A. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63(4):356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 33.De Gruijl F.R. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359–366. doi: 10.1016/s0076-6879(00)19035-4. [DOI] [PubMed] [Google Scholar]
- 34.Gruijl F.R., Rebel H. Early events in UV carcinogenesis—DNA damage, target cells and mutant p53 foci. Photochem Photobiol. 2008;84(2):382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 35.Godar D.E. UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol. 1999;112(1):3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 36.Petruseva I.O., Evdokimov A.N., Lavrik O.I. Molecular mechanism of global genome nucleotide excision repair. Acta Nat. 2014;6(20):23–34. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J., Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20(11):2101–2108. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 38.Dheeraj A., Rigby C.M., O’Bryant C.L. Silibinin treatment inhibits the growth of hedgehog inhibitor-resistant basal cell carcinoma cells via targeting EGFR-MAPK-akt and hedgehog signaling. Photochem Photobiol. 2017;93(4):999–1007. doi: 10.1111/php.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillermo-Lagae R., Deep G., Ting H., Agarwal C., Agarwal R. Silibinin enhances the repair of ultraviolet B-induced DNA damage by activating p53-dependent nucleotide excision repair mechanism in human dermal fibroblasts. Oncotarget. 2015;6(37):39594–39606. doi: 10.18632/oncotarget.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering C.R., Zhou J.H., Lee J.J. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Canc Res. 2014;20(24):6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dotto G.P., Rustgi A.K. Squamous cell cancers: a unified perspective on biology and genetics. Canc Cell. 2016;29(5):622–637. doi: 10.1016/j.ccell.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., Gutiérrez García-Rodrigo C., Fargnoli M.C. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci. 2017;18(11):1–16. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallikarjuna G., Dhanalakshmi S., Singh R.P., Agarwal C., Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64(17):6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 44.Dhanalakshmi S., Mallikarjuna G.U., Singh R.P., Agarwal R. Silibinin prevents ultraviolent radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25(8):1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 45.Singh R.P., Tyagi A.K., Zhao J., Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23(3):499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 46.Dhanalakshmi S., Mallikarjuna G.U., Singh R., Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25(1):99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 47.Dhanalakshmi S., Agarwal C., Singh R.P., Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280(21):20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 48.Singh B., Reddy P.G., Goberdhan A. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16(8):984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nichols J.A., Katiyar S.K. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W., Otkur W., Li L. Interference of silibinin with IGF-1R signalling pathways protects human epidermoid carcinoma A431 cells from UVB-induced apoptosis. Biochem Biophys Res Commun. 2013;432(2):314–319. doi: 10.1016/j.bbrc.2013.01.109. [DOI] [PubMed] [Google Scholar]
- 51.Wang Q., Ye Y., Liu W. Dual effects of silibinin treatment on autophagy-regulated dermal apoptosis retardation and epidermal apoptosis up-regulation in UVB-induced skin inflammation. J Asian Nat Prod Res. 2012;14(7):688–699. doi: 10.1080/10286020.2012.685725. [DOI] [PubMed] [Google Scholar]
- 52.Gu M., Singh R.P., Dhanalakshmi S., Agarwal C., Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67(7):3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J., Sharma Y., Agarwal R. Significant inhibition by the flavonoid antioxidant silymarin against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1? expression in SENCAR mouse epidermis: Implication. Mol Carcinog. 1999;26(4):321–333. [PubMed] [Google Scholar]
- 54.Zi X., Mukhtar H., Agarwal R. Novel cancer chemopreventive effects of a flavonoid antioxidant silymarin: inhibition of mRNA expression of an endogenous tumor promoter TNFα. Biochem Biophys Res Commun. 1997;239(1):334–339. doi: 10.1006/bbrc.1997.7375. [DOI] [PubMed] [Google Scholar]
- 55.Mcallister F., Kolls J.K. Th17 cytokines in non-melanoma skin cancer. Eur J Immunol. 2015;45(3):692–694. doi: 10.1002/eji.201545456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigby C., Deep G., Jain A., Orlicky D.J., Agarwal C., Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mast cells recruitment and bone morphogenetic protein 2 expression in the skin at early stages in Ptch(+/−) mouse model of basal cell carcinoma. Mol Carcinog. 2019;58(7):1260–1271. doi: 10.1002/mc.23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J., Lahiri-Chatterjee M., Sharma Y., Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21(4):811–816. doi: 10.1093/carcin/21.4.811. [DOI] [PubMed] [Google Scholar]
- 58.Mahindroo N., Punchihewa C., Fujii N. Hedgehog-Gli signaling pathway inhibitors as anticancer agents. J Med Chem. 2009;52(13):3829–3845. doi: 10.1021/jm801420y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proctor A.E., Thompson L.A., O’Bryant C.L. Vismodegib: an inhibitor of the hedgehog signaling pathway in the treatment of basal cell carcinoma. Ann Pharmacother. 2014;48(1):99–106. doi: 10.1177/1060028013506696. [DOI] [PubMed] [Google Scholar]
- 60.Sekulic A., Migden M.R., Oro A.E. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brechbiel J., Miller-Moslin K., Adjei A.A. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Canc Treat Rev. 2014;40(6):750–759. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Tamayo C., Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.) Integr Canc Ther. 2007;6(2):146–157. doi: 10.1177/1534735407301942. [DOI] [PubMed] [Google Scholar]
- 63.Becker-Schiebe M., Mengs U., Schaefer M., Bulitta M., Hoffmann W. Topical use of a silymarin-based preparation to prevent radiodermatitis: results of a prospective study in breast cancer patients. Strahlenther Onkol. 2011;187(8):485–491. doi: 10.1007/s00066-011-2204-z. [DOI] [PubMed] [Google Scholar]
- 64.Sugasawa K. Xeroderma pigmentosum genes: functions inside and outside DNA repair. Carcinogenesis. 2008;29(3):455–465. doi: 10.1093/carcin/bgm282. [DOI] [PubMed] [Google Scholar]
- 65.Bradford P.T., Goldstein A.M., Tamura D. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.