Abstract
Neurodegenerative diseases (NDD) are a range of debilitating conditions of the brain involving progressive loss of neurons, many of which are still currently incurable despite enormous efforts on drug discovery and development in the past decade. As NDD is closely linked to old age, the rapid worldwide growth in the aging population contributes to an increasing number of people with one of these incurable diseases and therefore it is considered a significant global health issue. There is an urgent need for novel effective treatments for NDD, and many new research strategies are centered on traditional medicine as an alternative or complementary solution. Several previous findings have suggested that glutamate toxicity drives neurodegeneration in many NDD, and the medicinal plants with anti-glutamate toxicity properties can be potentially used for their treatment. In order to obtain data relating to natural products against glutamate toxicity, six candidate plant species of Thailand were identified. Studies utilizing these herbs were searched for using the herb name (Latin and common names) along with the term “glutamate” in the following databases across all available years: PubMed, Scopus, and Google Scholar. This review emphasizes the importance of glutamate toxicity in NDD and summarizes individual plants and their active constituents with the mechanism of action against glutamate toxicity-mediated neuronal cell death that could be a promising resource for future NDD therapy.
Taxonomy (classification by evise)
Alzheimer’s disease, Neurodegenerative diseases, Cell culture, Molecular Biology, Traditional herbal medicine, Oxidative stress, Glutamate neurotransmitter.
Keywords: Glutamate, Neuroprotective, Neurotoxicity, Alzheimer’s disease, Oxidative stress, Plant extract, Herbal medicine, Traditional medicine
Graphical abstract
Highlights
-
•
This review provides updated evidence on Thai plants with anti-glutamate toxicity.
-
•
Six medicinal plants in this review can be promising resources for future NDD therapy.
-
•
ROS is a key player in both receptor-dependent and –independent glutamate toxicity.
List of abbreviations
- Aβ
Amyloid-beta
- AD
Alzheimer’s disease
- AIF
Apoptotic-inducing factor
- ALS
Amyotrophic lateral sclerosis
- CAT
Catalase
- CNS
Central nervous system
- CHOP
C/EBP homologous proteins
- EAAT
Excitatory amino acid transporter
- ER
Endoplasmic reticulum
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione
- GST
Glutathione S-transferase
- HD
Huntington’s disease
- iGluR
Ionotropic glutamate receptor
- mGluR
Metabotropic glutamate receptor
- MS
Multiple sclerosis
- NDD
Neurodegenerative diseases
- NMDA
N-methyl-d-aspartate
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- System Xc−
Cystine/Glutamate antiporter
- VGLUT
Vesicular glutamate transporter
1. Introduction
Neurodegenerative diseases (NDD) is a generic term used for a wide range of incurable and debilitating conditions, characterized clinically, by loss of neurological function (e.g., dementia, loss of movement control, paralysis), and pathologically, by progressive degeneration of nerve cells, particularly those in the central nervous system (CNS).1 The most common type of NDD is Alzheimer’s disease (AD), accounting for approximately two-thirds of all cases and typically found in people 65 years old and above.2 Other NDD include Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). As the world population ages, these age-dependent NDD are becoming one of the leading public health concerns with increasing prevalence worldwide, and there is currently no cure. At best, the available treatments are limited only for symptomatic management.3
The WHO, defines the term ‘medicinal plant’ as any plant, (including one or more of its organs) that contains substances that can be used directly for therapeutic purposes or indirectly as useful precursors for drug synthesis.4 Since ancient times, various types of medicinal plants have been utilized in traditional systems of herbal medicine by different cultures around the world, including Thai Traditional Medicine. Traditional Medicine still plays a crucial role in the health care of many rural communities, with an estimated 70–95% of populations relying on them.4 The global demand for herbal medicine is also growing popularity due to the rise in population, inadequate drug supplies, high cost of modern medicines and side effects of several synthetic drugs. Plants have become a significant source of new medicines since there are now approximately 391,000 plant species in the world, while only a small number has been explored for their medicinal properties. Furthermore, nearly 50% of modern drugs are derived from plants.5
Due to continued failure of NDD candidate drugs in clinical trials in the past decades, especially for AD,6 some researchers have gradually turned their attention to herbal medicine as an alternative or complimentary approach to NDD treatment. Naturally, a medicinal plant contains a large variety of several chemical constituents. The major classes of phytochemicals include phenolics, flavonoids, alkaloids, terpenoids, steroids, saponins, tannins, and glycosides. These phytochemicals have been shown to possess a broad range of biological activities. The multiple components of herbal medicines and the multi-target nature of NDD suggests that herbal medicines may achieve a more favorable clinical outcome by dealing with the complex nature of NDD etiology. Although the safety and toxicity of plants are still a paramount concern, they are generally considered to have mild toxic effects, and that only occurred in certain people.7
The development of effective medication for NDD is still challenging. Several recent efforts in searching for new treatments have been focused on glutamate-induced neurotoxicity, which has been implicated as a potential mechanism underlying neurodegeneration in several NDD.8 Therefore, targeting glutamate-mediated toxicity pathways along with other pathological mechanisms may provide better therapeutic benefits in the treatment of NDD. In this present review, we aimed to provide updated evidence to support the potential use of promising plants for NDD therapeutics with an emphasis on their protective activities against glutamate toxicity.
2. Glutamate neurotransmission in CNS
Glutamate is one of the most abundant amino acids in the human body. It is the anionic form of glutamic acid, which can be both synthesized within the cells and derived from dietary sources such as plants and animals. Glutamate acts as a cellular “multi-tool” which is essential for a wide range of cellular functions, including serving as a building block in the biosynthesis of proteins, an essential compound in cellular metabolism, an intermediate in the body’s disposal of excess nitrogen, and a precursor of glutathione (GSH).9
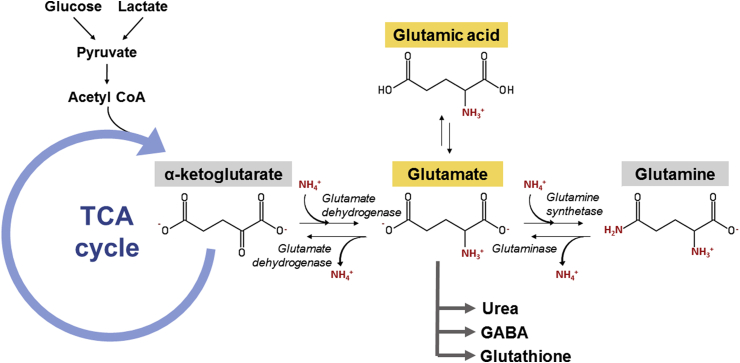
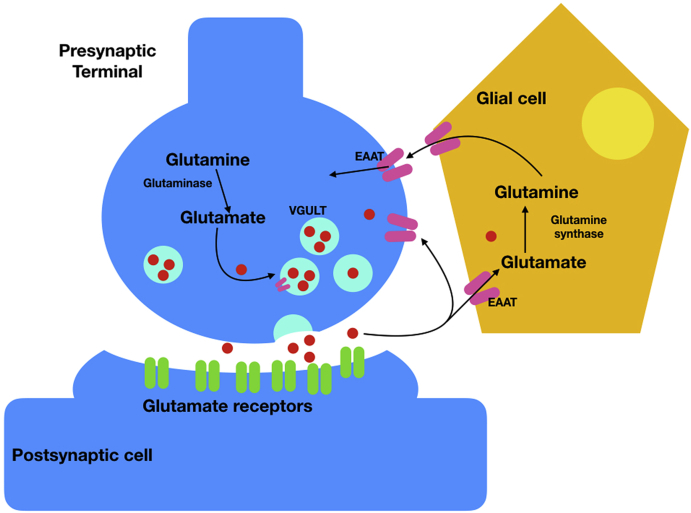
Glutamate also plays a crucial role in the CNS as a principal excitatory neurotransmitter and as a precursor to an inhibitory neurotransmitter, gamma-aminobutyric acid (GABA). Glutamate is known to be involved in a variety of normal brain functions, including cognition, memory, and learning.10 Its common biosynthetic pathway in astrocytes and neuronal cells was shown in Fig. 1. In the normal process of neurotransmission,11 after being synthesized from glutamine, glutamate is transported into synaptic vesicles via vesicular glutamate transporters (VGLUTs). Upon stimulation of the presynaptic neuron, the VGLUTs move to, and fuse with, the plasma membrane, releasing their package of glutamate into the synaptic cleft, where they can interact with glutamate receptors on the postsynaptic cells. In order for signals to be repeated, glutamate must be removed from the synaptic cleft. As there is no known synaptic enzyme that can degrade glutamate, removal of glutamate from the extracellular space is mainly achieved via cellular uptake by high-affinity glutamate transporters (excitatory amino acid transporters; EAATs) on surrounding glial cells (EAAT1/GLAST and EAAT2/GLT1) and to a lesser extent by transporters on pre-and postsynaptic membranes (EAAT3/EAAC1 and EAAT4). Once inside glial cells, glutamate is transformed into glutamine, and released again to the synapse where it is taken up by the presynaptic terminal and finally converted back into glutamate before repackaging into vesicles. This process is known as the glutamate-glutamine cycle (Fig. 2).
Fig. 1.
The biosynthetic pathway of glutamate synthesis and degradation in the human brain.
Fig. 2.
The glutamate-glutamine cycle. Diagram of glutamatergic neuron, showing glutamate enclosed in synaptic vesicles, before release and activation of glutamate receptors, and recycling through glial cells.
In mammals, there are two classes of glutamate receptors found in neurons, the ionotropic (iGluRs) and metabotropic glutamate receptors (mGluRs).12 The iGluRs form ligand-gated ion channels, which in the presence of glutamate (or its analog) allow ions such as Na2+, K+ or Ca2+ to pass through the membrane resulting in depolarization and action potentials which are what propagate signals along neurons. By contrast, the mGluRs act in part of second messenger signaling pathways coupled with G-proteins. The iGluRs are, therefore, associated with fast excitatory signaling, whereas the mGluRs mediate slower responses, thought to be involved in the neuromodulatory action of glutamate.13 The iGluRs are divided further into three subtypes depending on their pharmacological responses to selective agonists; N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (KA). The NMDA subtype is particularly more permeable Ca2+ than Na2+ or K+, whereas the AMPA and KA subtypes are less permeable to Ca2+ and equally permeable to Na2+ and K+. The mGluRs are separated into three groups, which are further divided into eight subgroups depending on sequence homology, second-messenger signaling mechanism and pharmacological ligand selectivity. Group I (mGluR1 & 5) is generally coupled to phospholipase C and function to regulate intracellular Ca2+ signaling to increase neuronal excitability, whereas group II (GluR2 & 3) and group III (mGluR4, 6, 7 & 8) are coupled to adenylyl cyclase, which functions to regulate neurotransmitter release negatively (inhibiting signaling).
3. Mechanisms of cellular glutamate toxicity
When glutamate is acting as a mediator of excitatory signals, its concentration at the synapse must be tightly controlled in order to prevent neurotoxicity from persistent activation of the GluRs on postsynaptic neurons.12 Glutamate is usually sequestered inside the cells by the EAATs and VGULTs, resulting in intracellular glutamate levels several thousand times greater than outside.14 However, when the glutamate concentration regulatory mechanisms go awry, with excess glutamate release or ineffective glutamate removal, glutamate can contribute to neuronal cell death. Neurotoxicity related to glutamate has been implicated in a range of NDD, including AD, PD, HD, MS, and ALS.8,15,16
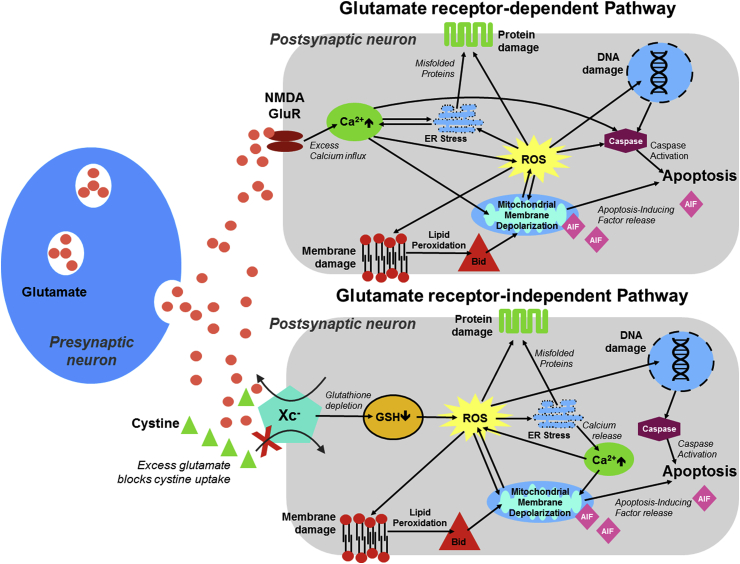
Neurotoxicity mediated by glutamate occurs through two separate pathways; The glutamate receptor-dependent and -independent pathways17 (Fig. 3). Notably, reactive oxygen species (ROS) are a critical factor in neuronal cell death in both pathways. The “classical” receptor-mediated pathway, known as excitotoxicity, occurs when there is excessive activation of the glutamate receptors, especially with the NMDA subtype. Overstimulation of these receptors leads to an excess of Ca2+ influx into the neurons, triggering a cascade of signaling events that can result in cell death. These downstream effects include the promotion of cellular-structure degrading agents (e.g. nucleases, proteases, and lipases), cell death signaling molecules (e.g. caspases), endoplasmic reticulum (ER) stress, mitochondrial depolarization and the production of ROS.18 The other glutamate toxicity pathway is independent of the glutamate receptor, which involves oxidative glutamate toxicity or oxytosis.19 In this toxicity pathway, the extracellular glutamate build-up saturates the cystine-glutamate antiporter (system Xc−), thus preventing cellular uptake of cystine. Cystine is the rate-limiting molecule in the production of GSH, which results in a shortage of GSH and thereby an increase in ROS and cellular oxidative stress. Excessive ROS has a detrimental effect on mitochondria’s structure and function that facilitates programmed cell death, where mitochondrial apoptosis-inducing factor (AIF) signaling, rather than caspase activation, is the underlying mechanism.20
Fig. 3.
Mechanisms of glutamate toxicity in neuronal cells.
4. Glutamate impairment in neurodegenerative diseases
In recent years, glutamate has become seen as a critical molecule underlying the pathogenesis of NDD, which a dramatic rise in the extracellular concentration of glutamate can eventually contribute to neuronal death.8 The glutamatergic hypothesis of neurodegeneration in NDD has emerged out of the observations of disturbance of normal glutamate neurotransmission in AD, PD, HD, MS, and ALS, supporting the involvement of glutamate in their pathophysiology. AD is characterized by progressive deterioration of neuronal cells and loss of cognitive function such as learning and memory. The activity of glutamine synthetase and the glutamate transporters were found to decrease in the brains of individuals with AD, particularly the hippocampal and neocortex regions.21 As both enzyme and transporter activities are required for clearance of glutamate from the synapse, it could be implicated that there was likely an excessive accumulation of glutamate outside of the neurons, that subsequently lead to neurotoxicity. Additionally, prolonged exposure of high glutamate levels to the cell was found to increase the production of toxic amyloid beta (Aβ), a hallmark of AD, via regulation of amyloidogenic processing. On the other hand, Aβ could also influence the concentration of glutamate in the synaptic cleft via the inhibition of glutamate uptake.22 There is more evidence of altered glutamate homeostasis in other neurodegenerative diseases. Impaired glutamate reuptake and over-activation of glutamate receptors were implicated in the degeneration of dopaminergic neurons in the substantia nigra, which is a hallmark of PD.16,23 Inherited NDD like HD was found involved with reduced EAAT2/GLT1 expression and enhanced receptor NMDA activation by the action of mutant Huntingtin, and result in neurodegeneration.23 Elevated glutamate levels and impaired glutamate clearance were found in the brains of patients suffering from MS, a chronic inflammatory demyelinating disease of the CNS.15 The increase of glutamate in cerebrospinal fluid along with the decreases in protein expression and functional activity of glutamate transporters were reported in patients who have ALS, a fatal disease caused by irreversible degeneration of motor neurons.24
5. Potential Thai medicinal plants with anti-glutamate toxicity
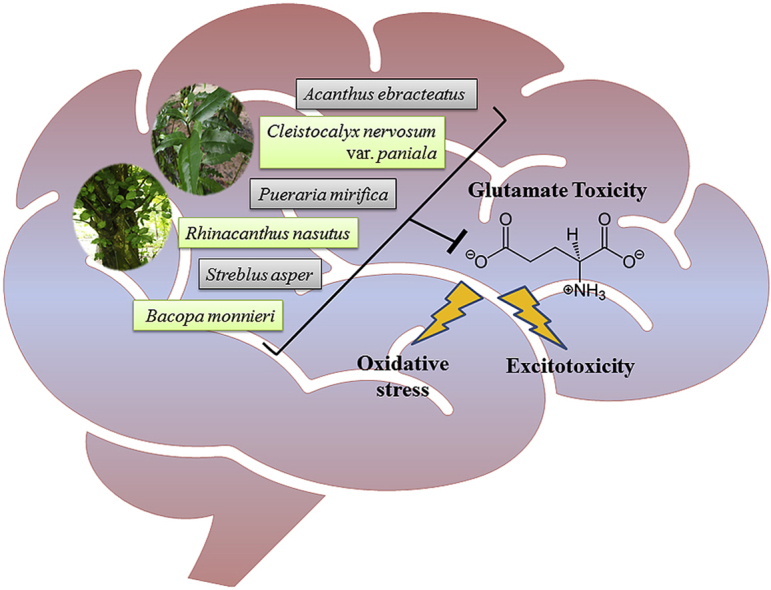
In the pursuit of herbal medicines for NDD therapy, various neuronal cell lines have been employed as a model of glutamate-mediated neurodegeneration for investigating medicinal plants with anti-glutamate toxicity.17 Among them, the HT-22 murine hippocampal neuronal cell line is the most widely used model for studying glutamate toxicity and researching the plants’ activities counteracting glutamate-induced neuronal damage. The HT-22 cells are devoid of NMDA-type iGluRs, making it a suitable model of glutamate toxicity via non-receptor dependent pathway. In addition, the hippocampus is the AD-associated brain region where a significant loss of neurons occurs. Here six promising plants for NDD treatment based on their neuroprotective properties against glutamate toxicity, majorly in HT-22 cells, are summarized.
5.1. Acanthus ebracteatus
Acanthus ebracteatus Vahl. is a medicinal mangrove plant belonging to the Acanthaceae family. This plant is widely cultivated in Southeast Asia, as well as India, Australia, and the western Pacific islands. It is called by various common names, including Sea Holly, Holly-leaved Mangrove, and “Nguak Pla Mo” (Thai). Several parts of A. ebracteatus have been historically used for a variety of medicinal purposes, such as hair root nourishment, blood purification, reduction of cough and fever, relief of rheumatoid arthritis pain and inflammation, treatment of hypertension, cancer, skin diseases such as rash, chronic wounds, and snakebites.25 In Thai traditional medicine, this plant is used as an essential ingredient in rejuvenating and neuro-tonic remedies for improving brain and body functions.
There are several groups of phytochemical compounds found in A. ebracteatus, including phenylethanol glycosides (e.g., verbascoside), megastigmane glycosides (e.g., ebracteatoside A), aliphatic glycosides (e.g., ebracteatosides B-D), flavonoids (e.g., apigenin 7-O-β-D-glucuronide), alkaloids (e.g., benzoxazin-3-one glucosides) and terpenoids (e.g., lupeol).26 Among these compounds, verbascoside (or acteoside) is a molecule of interest for neuroprotection as it showed protective function in various neuronal cell models.27
The usefulness of A. ebracteatus as a neuroprotective agent was highlighted due to the pharmacological significance one of the main bioactive components; verbascoside. It has been shown that this compound exerts an effect on memory and cognitive function.28 Verbascoside possesses antioxidant activity via direct free radical scavenging and up-regulation of the endogenous antioxidant defense system.29 It also showed protective activity against neuronal cell death induced by different neurotoxins such as 1-methyl-4-phenylpyridinium ion (MPP+),28 Aβ,28 lipopolysaccharide (LPS)/interferon (IFN)-γ30 as well as glutamate.31 A recent study from our laboratory showed the crude ethanolic extract of A. ebracteatus leaves provides protection against glutamate toxicity, in which 50 μg/mL of the extract could completely restore the viability of HT-22 hippocampal neuronal cells treated with 5 mM glutamate.32 This neuroprotective action was likely due to the activation of Nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant system, leading to attenuation of ROS accumulation and, thereby, inhibition of the AIF-mediated apoptotic pathway. Another study on anti-glutamate toxicity of verbascoside in primary rat cortical cells has demonstrated that the protective mechanisms of A. ebracteatus could also be related to the alleviation of glutamate excitotoxicity (100 μM) through reduction of Ca2+ influx, nitric oxide (NO) and ROS levels as well as enhancing the expression of antioxidant enzymes (e.g., GSH, SOD, glutathione reductase (GR), glutathione peroxidase (GPx)).31 Moreover, the study using a mouse model of AD induced by a combination of d-galactose and AlCl3 has shown the memory enhancing effects of verbascoside.33
5.2. Cleistocalyx nervosum var. paniala
Cleistocalyx nervosum var. paniala (the Myrtaceae family) is a native plant cultivated in the northern provinces of Thailand, commonly known as “Ma Kiang” in Thai. Another scientific name of this plant is Syzygium nervosum A. Cunn. ex DC. The fruit of C. nervosum, when it is ripe, is edible and tastes sweet and sour like a berry. It is locally consumed as fresh fruit or processed products (tea, juice, wine or jam). Previous studies on the effects of C. nervosum revealed the safety and many health benefits of this plant. The administration of aqueous fruit extract in the dose of 5,000 mg/kg did not cause any appearance of acute toxicity in rats.34 The C. nervosum extract was shown to have potent in vitro antioxidant properties35 and in vivo protective activity against chemical-induced oxidative damage.36,37 In addition, the anti-genotoxicity,34 the immune stimulating,38 and the tyrosinase inhibitory effects35 of this plant have been identified.
It has been reported that the ripe fruit of C. nervosum is found to be rich in flavonoids and polyphenolic compounds, especially anthocyanins (majorly cyanidin-3-glucoside, cyanidin-5-glucoside, and cyanidin-3,5-glucoside),34,39 which are known to possess a wide range of biological activities including antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, anti-microbial, anti-cardiovascular and anti-obesity effects.40
The potential of C. nervosum extract as a neuroprotectant has been primarily documented through the study in a rat model of cerebral ischemic stroke. It was found that the water extract of C. nervosum fruit, administered daily at 500 mg/kg daily, markedly improved spatial cognitive function, cerebral infarction size, and neuronal densities in hippocampal regions.41 Moreover, in a recent study from our laboratory, C. nervosum fruit water extract showed antioxidant and anti-glutamate toxicity activities in the HT-22 hippocampal neuronal cell line.42 We found that crude aqueous extract of C. nervosum fruit (0.05–1 μg/mL) protects neuronal HT-22 cells from 5 mM glutamate-induced cell apoptosis through suppression of ROS generation and the ER stress pathway. The expression of ROS and several signaling molecules involved with ER stress-mediated apoptotic cell death including calpain, caspase-12 and C/EBP homologous proteins (CHOP) were decreased in parallel with the increase in the increase in mRNA expression of antioxidant-related genes (e.g., SOD, catalase (CAT), Glutathione S-transferase (GST), GPx) in response to the extract treatment. Additionally, chronic administration of cyanidin-3-glucoside has shown to alleviate learning and memory deficits in diabetic rat model.43
5.3. Pueraria mirifica
Pueraria candollei Graham ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham (commonly shortened to Pueraria mirifica) is an indigenous Thai medicinal plant with broad-spectrum pharmacological properties. P. mirifica, locally known as White “Kwao Krua,” belongs to the family Leguminosae and naturally grows in deciduous forests in the Northern and Western parts of Thailand. This plant has long been used as a natural dietary supplement for rejuvenation and enhancing vitality, particularly among older women. It has been extensively used to alleviate menopausal symptoms and osteoporosis, promote breast growth, and improve memory as well as skin/hair health.44
P. mirifica is well recognized as a great source of active phytoestrogens or plant-derived estrogen-like substances with a similar structure to 17-β-estradiol. A study on the chemical composition of this plant revealed that it contains three major groups of compounds with potent estrogenic activity, namely, isoflavonoids, coumestans, and chromenes.44 These compounds include ten isoflavonoids (e.g., genistein, daidzein, and puerarin), four coumestans (coumestrol, mirificoumestan, mirificoumestan glycol and mirificoumestan hydrate) and three chromenes (miroestrol, deoxymiroestrol and isomiroestrol).
The possible beneficial role of P. mirifica in neuroprotection seems to be attributed to the function of its phytoestrogen content. Since it has been well documented that memory deficits are linked to a decline in estrogen level,45 phytoestrogen, as an estrogen-like substance, has been claimed to enhance cognitive performance in older adults, menopausal women, and possibly in people with AD, for which menopause and oophorectomy are implicated as risk factors.46 Several previous findings from both in vitro and in vivo studies have strengthened the neuroprotective role of P. mirifica. It was reported that, through activation of the estrogen receptor, the isoflavonoid puerarin was able to protect against Aβ induced neuronal death and promote neurite growth.47 In addition, the ethyl acetate extract of P. mirifica tuberous roots was found to increase the level of synaptophysin, a presynaptic vesicle protein, in primary rat hippocampal neurons, which this effect is dependent on the activity of estrogen receptor.48 In the studies of ovariectomized rodents, the ethanol extract of this plant roots along with its active components, puerarin, and miroestrol, were shown to ameliorate cognitive impairment, at least via the downregulation of genes associated with Aβ production, hyperphosphorylated Tau, brain-derived neurotrophic factor (BDNF) and cyclic AMP-responsive element-binding protein (CREB).49,50 Furthermore, another study on the neuroprotective mechanism of P. mirifica revealed the involvement of anti-glutamate toxicity. The ethyl acetate-ethanol extract of P. mirifica root at concentrations of 10 and 50 μg/mL could protect HT-22 cells against excessive glutamate (3.5 mM), which was likely mediated through decreasing ROS accumulation. However, two active constituents of P. mirifica, daidzein and genistein, both individually and in combination, did not show any protection in this cell model.51
5.4. Rhinacanthus nasutus
Rhinacanthus nasutus (L.) Kurz is a member of the Acanthaceae family and is native to Southeast Asian countries, as well as India and China. This plant is more commonly known as Snake Jasmine, named from its flower shape and traditional use as an antidote against snake bites. Different parts of R. nasutus have been reported in traditional medicine for the treatment of a wide range of ailments such as pulmonary tuberculosis, diabetes, hypertension, hepatitis, and several types of skin diseases. Its root extract also has potential anti-cancer effects against various tumor cells.52
Several groups of phytochemical compounds have been identified in R. nasutus, which include flavonoids, steroids, terpenoids, naphthoquinones, anthraquinones, glycosides, and lignans.52 Among them, naphthoquinones are reported to be the primary active constituents of R. nasutus with several analogs (rhinacanthin-A to -Q and rhinacanthone) demonstrating different pharmacological activities.53
The neuroprotective properties of R. nasutus were first identified in experiments from our laboratory using cultured neuronal cell line.54 Pretreatment of its ethanolic root extract (1 and 10 μg/mL) could protect against the death of HT-22 cells caused by a hypoxia (18 h)/reoxygenation cycle (6 h). Subsequent study into the role of R. nasutus in neuroprotection revealed that the ethanol extracts from the root and the leaf parts were also capable of attenuating the HT-22 cell death induced by glutamate (5 mM) and Aβ toxicity (both of which are involved in the pathogenesis of AD) through decreasing ROS accumulation.55 This anti-glutamate toxicity activity of R. nasutus could be a cumulative or synergistic effect of the active compounds such as lupeol, stigmasterol, β-sitosterol, and β-amyrin.55 Whereas, rhinacanthin-C is a protective molecule for Aβ-mediated cellular toxicity and neurite degeneration.56 The goal of ongoing research is to elucidate the neuroprotective mechanisms of R. nasutus as well as its phytochemical components to ascertain their usefulness as therapeutic agent against AD.
5.5. Streblus asper
Streblus asper Lour. is a well-known medicinal plant in traditional Indian medicine (Ayurveda). This plant belongs to the Moraceae family. It is widely distributed in several Asian countries and generally called by various names such as Toothbrush tree, Siamese rough bush, and “Khoi” (Thailand). Almost every part of S. asper has been traditionally used for the treatment of different diseases, e.g., filariasis, syphilis, fever, diarrhea, toothache, epilepsy, heart disease, wounds, inflammatory swellings, and cancer.57 This plant is also one of the ingredients in a traditional Thai longevity formula used for health promotion and restoration.
Previous studies on the phytochemical constituents of S. asper showed that this plant contains a large number of cardiac glycosides such as strebloside, mansonin, and asperoside.57 Other components identified include lignans (e.g., strebluslignanol), flavonoids (e.g., myricetin), terpenoids (e.g., β-sitosterol) and alkaloids (e.g., flazine).58
Apart from the effects related to its traditional usage mentioned above, S. asper was first demonstrated for its neuroprotective property in 2015 by Singsai et al.59 The administration of S. asper leaf water extract (200 mg/kg) was able to reverse not only motor but also social recognition deficits in Parkinson’s mice model induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Moreover, this plant was recently reported to have anti-glutamate toxicity capacity on neuronal cells.60 Crude ethanolic extract of S. asper leaves was shown to prevent glutamate-induced apoptotic cell death in the HT-22 neuronal cell line. The 50 μg/mL of the extract could completely restore the viability of HT-22 cells that was declined by 5 mM glutamate treatment. This protective mechanism was mediated through the attenuation of ROS production, the inhibition of nuclear translocation of AIF, and the up-regulation of antioxidant-related genes under the control of Nrf2. Subsequent fractionation of crude ethanolic leaf extract showed that neutral fraction possessed the most potent anti-glutamate toxicity activity. It is noteworthy that the anti-cholinesterase activity was also exhibited in this fraction.61 These dual actions suggest the potential therapeutic effect of S. asper leaf for AD.
5.6. Bacopa monnieri
Bacopa monnieri (Linn.) Wettst is a medicinal herb native to South and Southeast Asia, and is known for its memory-enhancing properties. There have been many clinical studies investigating the memory-enhancing properties of this plant in healthy volunteers of varying ages, as well as some studies in patients suffering from memory loss.62,63 Behavioral studies in mice have shown B. monnieri’s neuroprotective effects with potential effects on the glutamatergic and cholinergic systems.64 The neuroprotective mechanisms of B. monnieri in AD have been widely studied, with many pathways thought to be affected, including reducing beta-amyloid deposition in the brains of mice,65 and the reduction of beta-amyloid toxicity in primary cortical neurons.66 Studies from our laboratory have shown that the hexane and dichloromethane extracts of B. monnieri can protect against glutamate toxicity (5 mM) in HT-22 cells, and improve the lifespan as well as the “health-span” of C. elegans.67 Our study indicated that B. monnieri provided protection against endoplasmic reticulum stress, and oxidative stress caused by mitochondrial damage.
Furthermore, there is much interest in B. monneri, for its neuroprotective effects, that are associated with other neurodegenerative diseases than AD with regard to glutamate toxicity. In a rat model of PD, where the disease was induced by intraperitoneal injection with rotenone, causing an increase in glutaminase activity and decreased glutamate dehydrogenase, and glutamine synthase, whereas treatment with B. monnieri reversed these effects, in a similar manner to that of levodopa.68
6. Conclusions
This review highlights the significance of glutamate toxicity on neurodegeneration in NDD. Since NDD is incurable, understanding the underlying mechanisms of glutamate-induced toxicity that leads to the discovery of herbal neuroprotectants against glutamate toxicity may benefit for improving NDD therapy. This work summarizes the promising findings based on the mechanistic studies of plant extracts from six species and their chemical constituents. Their potential as anti-glutamate toxicity evaluated by the healthspan and lifespan of neuronal cells has shed light on preventing and curing NDD, yielding encouraging results for future research and applications.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was partially supported by Grants for Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University, Thailand. JMB was supported by the Rachadapisek Sompote Fund for Postdoctoral Fellowship, Chulalongkorn University, Thailand.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Anchalee Prasansuklab, Email: anchalee.pr@chula.ac.th.
James M. Brimson, Email: james.b@chula.ac.th.
Tewin Tencomnao, Email: tewin.t@chula.ac.th.
References
- 1.Sheikh S., Safia, Haque E., Mir S.S. Neurodegenerative diseases: multifactorial conformational diseases and their therapeutic interventions. J Neurodegener Dis. 2013;2013:563481. doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain R., Zubair H., Pursell S., Shahab M. Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018;8(9) doi: 10.3390/brainsci8090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duraes F., Pinto M., Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals. 2018;11(2) doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson M.M., Zhang X. World Health Organization; Geneva: 2011. The World Medicines Situation 2011, Traditional Medicines: Global Situation, Issues and Challenges; pp. 1–12. [Google Scholar]
- 5.De Luca V., Salim V., Atsumi S.M., Yu F. Mining the biodiversity of plants: a revolution in the making. Science. 2012;336(6089):1658–1661. doi: 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- 6.Mehta D., Jackson R., Paul G., Shi J., Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expet Opin Invest Drugs. 2017;26(6):735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George P. Concerns regarding the safety and toxicity of medicinal plants-An overview. J Appl Pharmaceut Sci. 2011;1(6):40–44. [Google Scholar]
- 8.Lewerenz J., Maher P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newsholme P., Procopio J., Lima M.M., Pithon-Curi T.C., Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct. 2003;21(1):1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 10.Meldrum B.S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl l) doi: 10.1093/jn/130.4.1007S. p. 1007s-15s. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y., Danbolt N.C. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm. 2014;121(8):799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niciu M.J., Kelmendi B., Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100(4):656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard M., Takano T., Hansen A.J. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3(9):748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 15.Macrez R., Stys P.K., Vivien D., Lipton S.A., Docagne F. Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol. 2016;15(10):1089–1102. doi: 10.1016/S1474-4422(16)30165-X. [DOI] [PubMed] [Google Scholar]
- 16.Dong X.X., Wang Y., Qin Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kritis A.A., Stamoula E.G., Paniskaki K.A., Vavilis T.D. Researching glutamate - induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci. 2015;9:91. doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Qin Z.H. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 19.Tan S., Schubert D., Maher P. Oxytosis: a novel form of programmed cell death. Curr Top Med Chem. 2001;1(6):497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- 20.Fukui M., Song J.H., Choi J., Choi H.J., Zhu B.T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617(1-3):1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield D.A., Pocernich C.B. The glutamatergic system and Alzheimer’s disease: therapeutic implications. CNS Drugs. 2003;17(9):641–652. doi: 10.2165/00023210-200317090-00004. [DOI] [PubMed] [Google Scholar]
- 22.Revett T.J., Baker G.B., Jhamandas J., Kar S. Glutamate system, amyloid ss peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38(1):6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheldon A.L., Robinson M.B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51(6-7):333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foran E., Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxidants Redox Signal. 2009;11(7):1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandaranayake W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 1998;2(3):133–148. [Google Scholar]
- 26.Kanchanapoom T., Kasai R., Picheansoonthon C., Yamasaki K. Megastigmane, aliphatic alcohol and benzoxazinoid glycosides from Acanthus ebracteatus. Phytochemistry. 2001;58(5):811–817. doi: 10.1016/s0031-9422(01)00306-5. [DOI] [PubMed] [Google Scholar]
- 27.Alipieva K., Korkina L., Orhan I.E., Georgiev M.I. Verbascoside--a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol Adv. 2014;32(6):1065–1076. doi: 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Shiao Y.J., Su M.H., Lin H.C., Wu C.R. Acteoside and isoacteoside protect amyloid beta peptide induced cytotoxicity, cognitive deficit and neurochemical disturbances in vitro and in vivo. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sgarbossa A., Dal Bosco M., Pressi G., Cuzzocrea S., Dal Toso R., Menegazzi M. Phenylpropanoid glycosides from plant cell cultures induce heme oxygenase 1 gene expression in a human keratinocyte cell line by affecting the balance of NRF2 and BACH1 transcription factors. Chem Biol Interact. 2012;199(2):87–95. doi: 10.1016/j.cbi.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Esposito E., Dal Toso R., Pressi G., Bramanti P., Meli R., Cuzzocrea S. Protective effect of verbascoside in activated C6 glioma cells: possible molecular mechanisms. Naunyn-Schmiedeberg’s Arch Pharmacol. 2010;381(1):93–105. doi: 10.1007/s00210-009-0466-0. [DOI] [PubMed] [Google Scholar]
- 31.Koo K.A., Kim S.H., Oh T.H., Kim Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006;79(7):709–716. doi: 10.1016/j.lfs.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Prasansuklab A., Tencomnao T. Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate. BMC Compl Alternative Med. 2018;18(1):278. doi: 10.1186/s12906-018-2340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao L., Peng X.M., Huo S.X., Liu X.M., Yan M. Memory enhancement of acteoside (verbascoside) in a senescent mice model induced by a combination of D-gal and AlCl3. Phytother Res. 2015;29(8):1131–1136. doi: 10.1002/ptr.5357. [DOI] [PubMed] [Google Scholar]
- 34.Charoensin S., Taya S., Wongpornchai S., Wongpoomchai R. Assessment of genotoxicity and antigenotoxicity of an aqueous extract of Cleistocalyx nervosum var. paniala in in vitro and in vivo models. Interdiscipl Toxicol. 2012;5(4):201–206. doi: 10.2478/v10102-012-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manosroi J., Chankhampan C., Kumguan K., Manosroi W., Manosroi A. In vitro anti-aging activities of extracts from leaves of Ma Kiang (Cleistocalyx nervosum var. paniala) Pharm Biol. 2015;53(6):862–869. doi: 10.3109/13880209.2014.946058. [DOI] [PubMed] [Google Scholar]
- 36.Taya S., Punvittayagul C., Inboot W., Fukushima S., Wongpoomchai R. Cleistocalyx nervosum extract ameliorates chemical-induced oxidative stress in early stages of rat hepatocarcinogenesis. Asian Pac J Cancer Prev APJCP. 2014;15(6):2825–2830. doi: 10.7314/apjcp.2014.15.6.2825. [DOI] [PubMed] [Google Scholar]
- 37.Prasanth M.I., Brimson J.M., Chuchawankul S., Sukprasansap M., Tencomnao T. Antiaging, stress resistance, and neuroprotective efficacies of cleistocalyx nervosum var. paniala fruit extracts using Caenorhabditis elegans model. Oxid Med Cell Longev. 2019;2019:7024785. doi: 10.1155/2019/7024785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriwanthana B., Treesangsri W., Boriboontrakul B., Niumsakul S., Chavalittumrong P. In vitro effects of Thai medicinal plants on human lymphocyte activity. In Vitro. 2007;29:1. [Google Scholar]
- 39.Chailungka A., Junpirom T., Pompimon W., Nuntasaen N., Meepowpan P. Two flavonoids first isolated from the seed of Syzygium nervosum and preliminary study of their anticancer and anti-HIV-1 reverse transcriptase activities. Maejo Int J Sci Technol. 2017;11(01):58–67. [Google Scholar]
- 40.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61(1):1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phachonpai W., Wattanathorn J. Neuroprotective effect of Cleistocalyx nervosum var. paniala extract in a rat model of ischemic stroke. Naresuan Phayao J. 2015;8(3):137–141. [Google Scholar]
- 42.Sukprasansap M., Chanvorachote P., Tencomnao T. Cleistocalyx nervosum var. paniala berry fruit protects neurotoxicity against endoplasmic reticulum stress-induced apoptosis. Food Chem Toxicol. 2017;103:279–288. doi: 10.1016/j.fct.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Nasri S., Roghani M., Baluchnejadmojarad T., Balvardi M., Rabani T. Chronic cyanidin-3-glucoside administration improves short-term spatial recognition memory but not passive avoidance learning and memory in streptozotocin-diabetic rats. Phytother Res. 2012;26(8):1205–1210. doi: 10.1002/ptr.3702. [DOI] [PubMed] [Google Scholar]
- 44.Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front Med. 2012;6(1):8–21. doi: 10.1007/s11684-012-0184-8. [DOI] [PubMed] [Google Scholar]
- 45.Luine V.N. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soni M., Rahardjo T.B., Soekardi R. Phytoestrogens and cognitive function: a review. Maturitas. 2014;77(3):209–220. doi: 10.1016/j.maturitas.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Li L., Xue Z., Chen L., Chen X., Wang H., Wang X. Puerarin suppression of Abeta1-42-induced primary cortical neuron death is largely dependent on ERbeta. Brain Res. 2017;1657:87–94. doi: 10.1016/j.brainres.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Chindewa R., Lapanantasin S., Sanvarinda Y., Chongthammakun S. Pueraria mirifica, phytoestrogen-induced change in synaptophysin expression via estrogen receptor in rat hippocampal neuron. J Med Assoc Thai. 2008;91(2):208–214. [PubMed] [Google Scholar]
- 49.Anukulthanakorn K., Parhar I.S., Jaroenporn S., Kitahashi T., Watanbe G., Malaivijitnond S. Neurotherapeutic effects of Pueraria mirifica extract in early- and late-stage cognitive impaired rats. Phytother Res. 2016;30(6):929–939. doi: 10.1002/ptr.5595. [DOI] [PubMed] [Google Scholar]
- 50.Monthakantirat O., Sukano W., Umehara K., Noguchi H., Chulikhit Y., Matsumoto K. Effect of miroestrol on ovariectomy-induced cognitive impairment and lipid peroxidation in mouse brain. Phytomedicine. 2014;21(11):1249–1255. doi: 10.1016/j.phymed.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Sucontphunt A., De-Eknamkul W., Nimmannit U., Dan Dimitrijevich S., Gracy R.W. Protection of HT22 neuronal cells against glutamate toxicity mediated by the antioxidant activity of Pueraria candollei var. mirifica extracts. J Nat Med. 2011;65(1):1–8. doi: 10.1007/s11418-010-0442-5. [DOI] [PubMed] [Google Scholar]
- 52.Bukke S., Raghu P., Sailaja G., Kedam T.R. The study on morphological, phytochemical and pharmacological aspects of Rhinacanthus nasutus (L.) kurz (A review) J Appl Pharmaceut Sci. 2011;1(8):26–32. [Google Scholar]
- 53.Brimson J.M., Tencomnao T. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. Elsevier; London: 2015. Rhinacanthus nasutus extract as a neuroprotectant; pp. 77–84. [Google Scholar]
- 54.Brimson J.M., Tencomnao T. Rhinacanthus nasutus protects cultured neuronal cells against hypoxia induced cell death. Molecules. 2011;16(8):6322–6338. doi: 10.3390/molecules16086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brimson J.M., Brimson S.J., Brimson C.A., Rakkhitawatthana V., Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-beta neurotoxicity in HT-22 mouse hippocampal cells: possible active compounds include lupeol, stigmasterol and beta-sitosterol. Int J Mol Sci. 2012;13(4):5074–5097. doi: 10.3390/ijms13045074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang K.-A., Li M.-H., Lin N.-H. Rhinacanthin C alleviates amyloid-β fibrils’ toxicity on neurons and attenuates neuroinflammation triggered by LPS, amyloid-β, and interferon-γ in glial cells. Oxidat Med Cellular Longev. 2017;2017 doi: 10.1155/2017/5414297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rastogi S., Kulshreshtha D.K., Rawat A.K. Streblus asper Lour. (Shakhotaka): a review of its chemical, pharmacological and ethnomedicinal properties. Evid Based Complement Alternat Med. 2006;3(2):217–222. doi: 10.1093/ecam/nel018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C., Huang C., Lu T. Tandem mass spectrometric fragmentation behavior of lignans, flavonoids and triterpenoids in Streblus asper. Rapid Commun Mass Spectrom. 2014;28(21):2363–2370. doi: 10.1002/rcm.7035. [DOI] [PubMed] [Google Scholar]
- 59.Singsai K., Akaravichien T., Kukongviriyapan V., Sattayasai J. Protective effects of Streblus asper leaf extract on H2O2-induced ROS in SK-N-sh cells and MPTP-induced Parkinson’s disease-like symptoms in C57bl/6 mouse. Evid base Compl Alternative Med. 2015;2015 doi: 10.1155/2015/970354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasansuklab A., Meemon K., Sobhon P., Tencomnao T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Compl Alternative Med. 2017;17(1):551. doi: 10.1186/s12906-017-2050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasansuklab A., Theerasri A., Payne M., Ung A.T., Tencomnao T. Acid-base fractions separated from Streblus asper leaf ethanolic extract exhibited antibacterial, antioxidant, anti-acetylcholinesterase, and neuroprotective activities. BMC Compl Alternative Med. 2018;18(1):223. doi: 10.1186/s12906-018-2288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goswami S., Kumar N., Thawani V., Tiwari M., Thawani M. Effect of Bacopa monnieri on cognitive functions in Alzheimer’s disease patients. Int J Collab Res Intern Med Public Health. 2011;3(4):285–293. [Google Scholar]
- 63.Kongkeaw C., Dilokthornsakul P., Thanarangsarit P., Limpeanchob N., Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2014;151(1):528–535. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Le X.T., Pham H.T., Do P.T. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem Res. 2013;38(10):2201–2215. doi: 10.1007/s11064-013-1129-6. [DOI] [PubMed] [Google Scholar]
- 65.Holcomb L.A., Dhanasekaran M., Hitt A.R., Young K.A., Riggs M., Manyam B.V. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimers Dis. 2006;9(3):243–251. doi: 10.3233/jad-2006-9303. [DOI] [PubMed] [Google Scholar]
- 66.Limpeanchob N., Jaipan S., Rattanakaruna S., Phrompittayarat W., Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120(1):112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 67.Brimson J.M., Prasanth M.I., Plaingam W., Tencomnao T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J Tradit Complement Med. 2019 doi: 10.1016/j.jtcme.2019.10.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swathi G., Visweswari G., Rajendra W. Evaluation of rotenone induced Parkinson’s disease on glutamate metabolism and protective strategies of Bacopa monnieri. Int J Plant Ani Environ Sci. 2013;3:62–67. [Google Scholar]