Abstract
Eating habits and lifestyle directly impact general health. Consumption of fat- and sugar-rich foods and alcohol increase the risk of developing fatty liver disease. The prevalence of fatty liver disease is markedly critical, and its pathogenesis and progression are complicated. Chinese herbal medicine has been used to treat and prevent human diseases through-out history, and is a rich source of biologically active substances with unique curative properties. More recently, Chinese herbs and their extracts have been identified as a novel source of potential therapeutic agents in the prevention and treatment of fatty liver disease. Beneficial effects of these herbal medicines mean that they can be classified as novel candidates for the treatment and prevention of both alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD), in place of conventional allopathic treatments. In this review, we explore the current literature related to herbal medicines used for the treatment of or protection against fatty liver diseases and describe their mechanisms of action.
Keywords: Fatty liver disease, Alcoholic fatty liver disease, Non-alcoholic fatty liver disease, AFLD, NAFLD, High-fat diet, High fructose, Lipogenesis, Oxidative stress, Inflammation
Graphical abstract
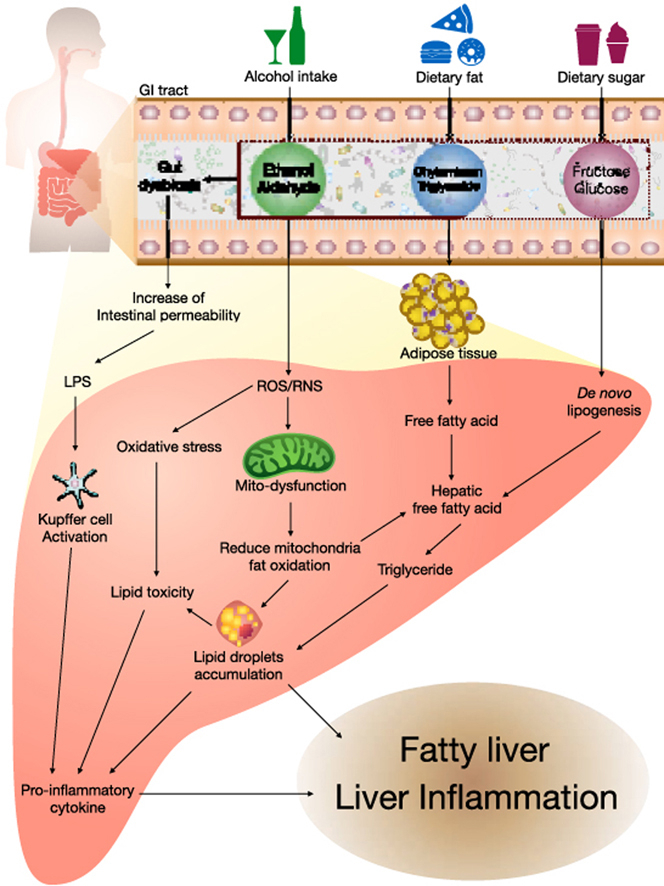
Highlights
-
•
Fatty liver is caused by many factors, for example, frequent consuming of high-calorie food, alcohol intake, as well as some other chronic disease.
-
•
Chinese herbs and their extracts have attracted interest as potential therapeutic agents to counter fatty liver disease.
-
•
Herbal medicine can effectively be used as a novel candidate for fatty liver treatment and prevention of both alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD), in place of conventional allopathic treatment.
Abbreviations
- ACC
acetyl coenzyme A carboxylase
- AFLD
alcoholic fatty liver disease
- ALT
alanine transaminase
- AST
aspartate transaminase
- CAT
catalase
- ChREBP
carbohydrate-responsive element-binding protein
- CPT-1
mitochondrial carnitine palmitoyltransferase-1
- CYP2E1
microsomal protein cytochrome P4502E1
- FAS
fatty acid synthase
- FFA
free fatty acids
- GSH
glutathione
- GPx
glutathione peroxidase
- GRd
glutathione reductase
- HMGCR
3-hydroxy-3-methyl-glutaryl-CoA reductase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- LPS
lipopolysaccharides
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic sateatohepatitis
- Nrf2
nuclear factor erythroid 2-related factor 2
- PPAR-α
peroxisome proliferator-activated receptor-α
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SREBP-1
sterol regulatory element-binding protein-1
- SOD
superoxide dismutase
- TC
total cholesterol
- TG
total triglyceride
- TNF-α
tumor necrosis factor α
- WHO
World Health Organization
1. Introduction
Eating habits and lifestyle directly impact human health and disease pathogenesis. Chronic diseases, including fatty liver disease, are a common problem for people worldwide. Fatty liver disease can be caused by many factors, including the frequent consumption of high-calorie foods or alcohol, and the development of other chronic diseases. Fatty liver is the initiating state for the development of steatohepatitis (NASH), cirrhosis, and eventually hepatocellular carcinoma.1,2 Typically, fatty liver disease presents as asymptomatic, and can be divided into two categories: (1) alcoholic fatty liver disease (AFLD): hepatic fat builds up as a result of high alcohol intake, and (2) non-alcoholic fatty liver disease (NAFLD): fat accumulation in the liver is not related to alcohol consumption.3 Usually, both AFLD and NAFLD do not cause serious problems, and the conditions can be reversed by changes in eating habits or, in severe cases, clinical intervention. However, both conditions may lead to more serious liver diseases including cirrhosis and hepatocellular carcinoma.4
Numerous Chinese herbs have been shown to exert a medicinal effect on fatty liver disease as well as preventing the accumulation of fat in the liver in the first place. As in all conditions prevention is better than cure, which means that if fatty liver disease can be prevented by the use of hepatoprotective herbs or diet it may help to reduce the burden of this chronic disease on the global health care system. In this review, we explore the current literature pertaining to the prevalence of fatty liver disease, the effect of diet on its development and its risk factors and pathogenesis, as well as reviewing the Chinese herbs that exhibit hepatoprotective effects against AFLD and NAFLD.
2. Prevalence of fatty liver disease
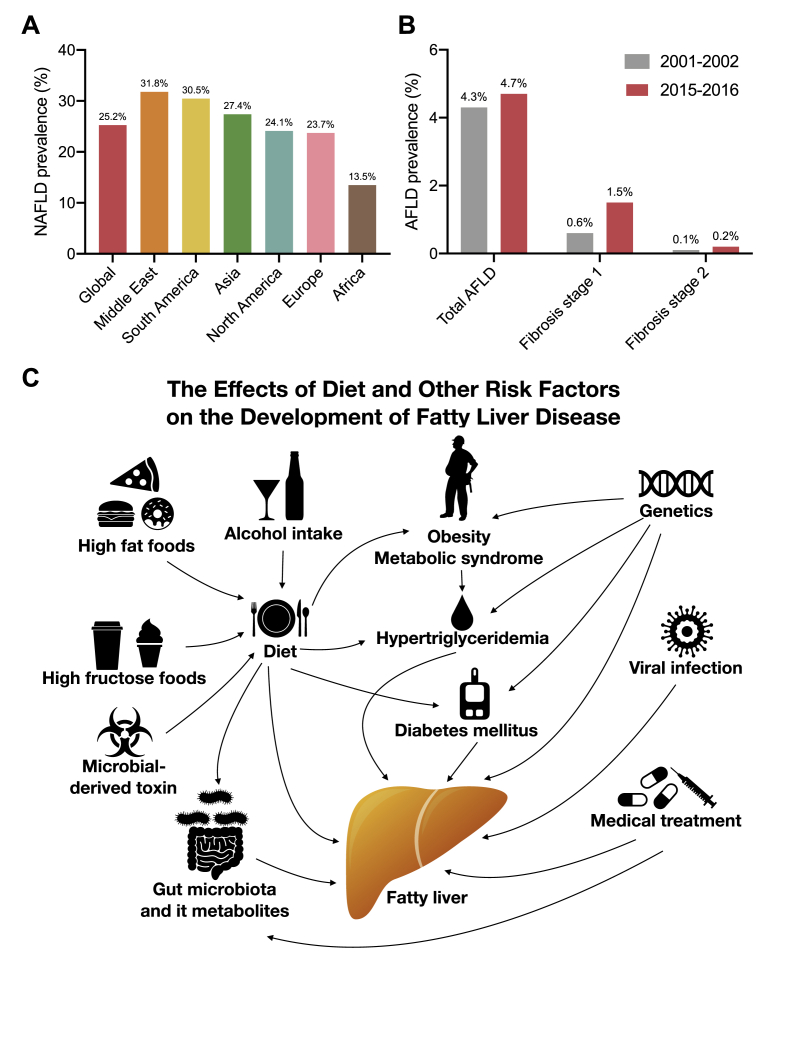
The prevalence of AFLD and NAFLD are different. In 2016, the global prevalence of NAFLD was approximately 25%. The Middle East was found to have the highest NAFLD prevalence (31.8%), followed by South America (30.5%) and Asia (27.4%), respectively. The lowest NAFLD prevalence was observed in Africa (13.5%) (Fig. 1A).5 The global prevalence of NAFLD is increasing proportionately with obesity and type 2 diabetes mellitus.6 The prevalence of alcohol associated fatty liver disease is very different from NAFLD. The World Health Organization (WHO) reported that the estimate average global alcohol consumption figure is around 6.4 L of alcohol per person and around three million deaths occur annually as a result of the harmful use of alcohol, which accounts for 5.3% of all global deaths. Moreover, alcohol intake can result in more than 200 diseases and injuries, including AFLD.7 In the USA, the AFLD prevalence rate was estimated to be 4.3% between 2001 and 2002 and remained stable at 4.7% in 2015–2016. In addition, in patients with stage II or higher AFLD fibrosis increased by 2-fold (Fig. 1B).8 In some regions of China, the prevalence of fatty liver disease was 5.32%, and was significantly higher in males than females, with this figure rising to 10% in alcohol users.9 NAFLD prevalence was higher than AFLD but NAFLD prevalence experienced incremental increases with alcohol consumption.
Fig. 1.
(A) The prevalence of non-alcoholic fatty liver disease; (B) the prevalence of alcoholic fatty liver disease; and (C) the effects of diet and other risk factors on the development of fatty liver disease.
3. Effect of diet and other risk factors in the development of fatty liver disease
Fig. 1C illustrates the effects of diet and other risk factors for fatty liver disease. The phrase “You are what you eat” reminds us that our eating habits influence our health.10 Western diets are associated with obesity and NAFLD,11,12 hypercaloric diets high in fats, sugars, or both, increase hepatic fat content.13 Soft drinks and desserts containing a large amount of sugar, including fructose, are central to these eating habits and are major contributors to diet induced NAFLD.14,15 Moderate alcohol intake can reduce the risk of cardiovascular disease,16,17 however, excessive alcohol intake can result in AFLD which may develop into hepatosteatosis, cirrhosis, and hepatocellular carcinoma.18, 19, 20, 21 Some foods may contain microbial toxins including aflatoxin, produced by mold, which can accumulate and damage the liver.22,23 Dietary nutrients are important for human health but they are also essential for the gut microbiota that reside in our intestines.24 Many studies have shown that our diet influences the gut microbiota and their metabolites, and these metabolites subsequently translocate to the liver via the portal vein and may affect liver function, for example, lipopolysaccharides (LPS) can result in hepatic inflammation.25, 26, 27, 28 In fact our diets may not only cause fatty liver disease but may also induce a number of other diseases.5 Many diseases have been reported as risk factors for the development of fatty liver disease including obesity,1 type 2 diabetes,29,30 metabolic syndrome,31 some genetic conditions,32,33 viral infections,34,35 and adverse drug reactions.36,37 In China, the risk of fatty liver disease is significantly increased for alcoholic patients, as well as in patients with hypertension, diabetes, and coronary heart disease.9 Understanding the genetic and environmental risk factors for fatty liver disease and their distribution in the global population is crucial in the development of treatment and prevention strategies, the implementation of public health policy and the recognition of this important chronic liver disease.38
4. Fatty liver disease pathogenesis
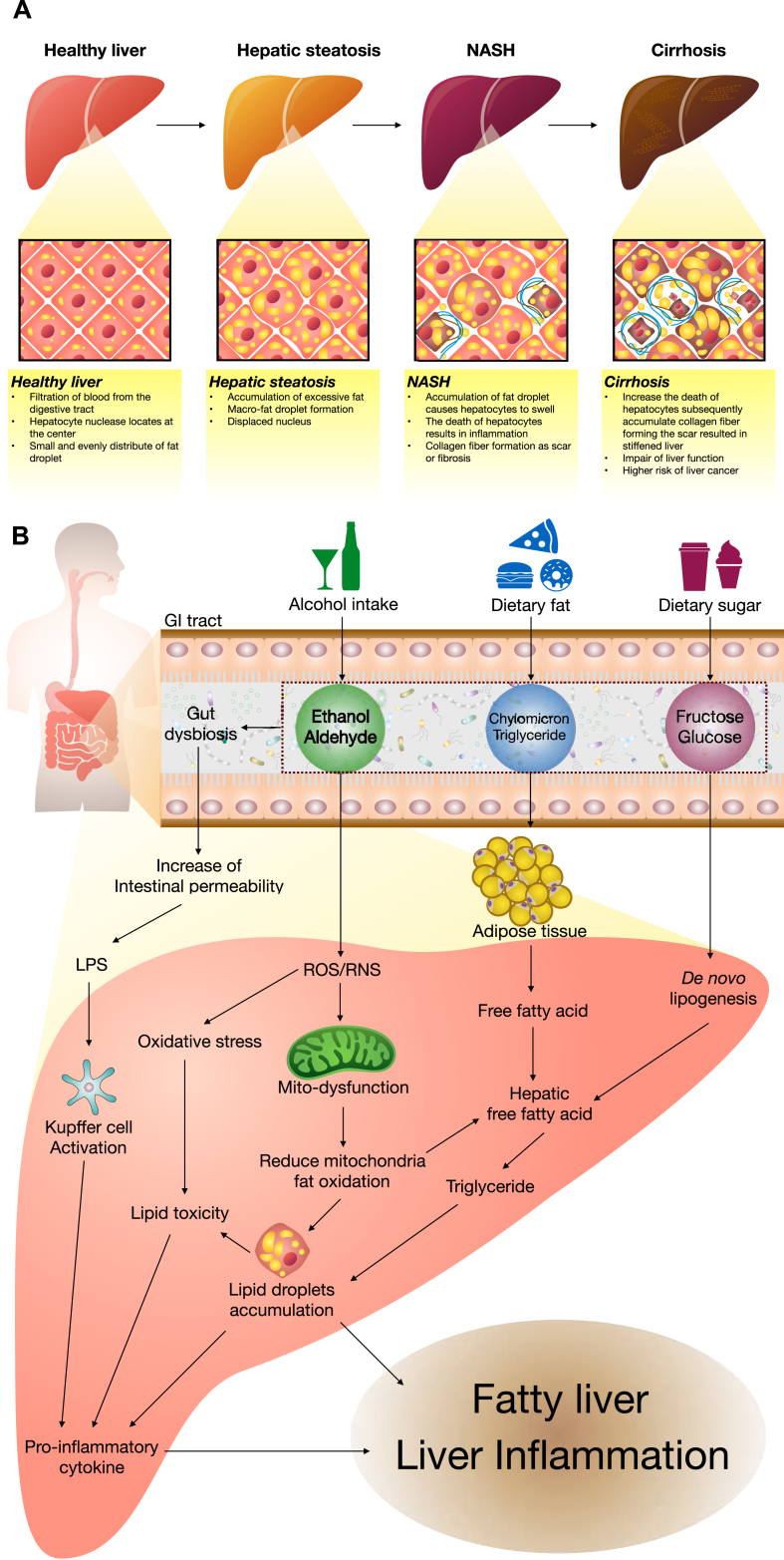
Fatty liver disease is defined as the accumulation of more than 5% fat tissue in the liver.39 As mentioned in the introduction, fatty liver can be split into two categories, AFLD and NAFLD. Fatty liver disease is not a serious problem but it can result in the development of steatohepatitis (NASH), cirrhosis, and eventually hepatocellular carcinoma.1,2 Liver diseases comprise a broad spectrum of diseases ranked from asymptomatic fatty liver disease to steatohepatitis to cirrhosis40,41 (Fig. 2A). The induction of fatty liver usually includes alcohol, high-fat food, or high sugar food intake (Fig. 2B), with each experiencing a different pathogenic mechanism. In this review, we will focus on the pathogenesis of the early stages of fatty liver disease.
Fig. 2.
(A) The spectrum of liver diseases; (B) the pathogenesis of fatty liver disease caused by alcohol intake, dietary fat, and dietary sugar.
4.1. Alcoholic fatty liver disease (AFLD)
The mechanisms of alcohol-induced fatty liver injury are complicated and not completely understood. Typically, alcohol metabolites are degraded by alcohol dehydrogenase (ADH) forming acetaldehyde, which is toxic to the cells. This is then, metabolized to acetate by aldehyde dehydrogenase.42 Reduction of ADH activity can be caused by alcohol intake.43 In addition, acetate can be used in fatty acid and cholesterol biosynthesis resulting in the development of a fatty liver.44 Alcohol promotes the generation of free radicals like reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can lead to mitochondrial dysfunction and subsequently reduce the mitochondrial fat oxidation raising hepatic fat droplet accumulation.45 ROS and RNS also elevate oxidative stress increasing lipid toxicity that releases the pro-inflammatory cytokines.46 Alcohol consumption also induces microsomal protein cytochrome P4502E1 (CYP2E1) expression and activates the Kupffer cells generating pro-inflammatory cytokines, proinflammatory tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, which lead to liver inflammation.44,47,48 The sterol regulatory element-binding protein-1 (SREBP-1) and fatty acid synthase (FAS) are key factors regulating lipid metabolism, and the inhibition of SREBP-1 and FAS has been shown to prevent AFLD.49,50 Alcohol exposure also activates the peroxisome proliferator-activated receptor- α (PPAR-α), and subsequently accelerates synthesis of various fatty acids, resulting in AFLD.51 While activation of mitochondrial carnitine palmitoyltransferase-1 (CPT-1) retards the transport of fatty acids into the mitochondria for oxidation.44 Alcohol intake leads to the dysbiosis of the gut and increases intestinal permeability, resulting in increased translocation of LPS to the liver. Activating the Kupffer cells leading to liver inflammation.44,52
4.2. Non-alcoholic fatty liver disease (NAFLD)
NAFLD progression is different from AFLD. In the case of high-fat diets there are several factors at play in the progression of fatty liver disease. However, the mechanisms involved in NAFLD are not fully understood. High-calorie diets can elevate the storage of excessive fat in adipose tissues, causing adipocyte hypertrophy as well as adipose tissue expansion, leading to obesity. Free fatty acids (FFA), from adipose tissues, can be released into the liver stimulating the expression of key factors controlling cholesterol synthesis, including 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR), as well as dysregulating critical fatty acid synthesis factors SREBP1-c, acetyl coenzyme A carboxylase (ACC), and FAS.53 These enzymes increase the synthesis of liver lipids, like cholesterol and triglycerides. While the inhibition of PPAR-α and CPT-1 can result in decreases in fatty acid metabolism and β-oxidation, leading to the development of hepatic steatosis.53 Similar to in AFLD, CYP2E1 is a regulator of oxidative stress and inflammatory cytokines in NAFLD progression.54 Gut-derived LPS is also a factor in the pathogenesis of NASH.28
A high-fat diet is not the only cause of NAFLD, high-fructose diets have also been shown to result in NAFLD. After fructose intake, it is rapidly absorbed into the bloodstream and liver.55 The hepatic metabolism of fructose increases de novo lipogenesis and inhibits β-oxidation, allowing fat accumulation in the liver.56 ACC and FAS are important enzymes involved in de novo lipogenesis when carbohydrates are metabolized to fatty acids.57,58 Changes in the carbohydrate-responsive element-binding protein (ChREBP) and SREBP-1 are associated with hepatic lipogenesis induced by fructose intake and the development of NAFLD.59,60 In addition, CPT-1 which is involved in the metabolism of long-chain fatty acids and β-oxidation is also dysregulated.61 Fructose consumption is known to alter transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) expression, and this transcription factor is also known to participate in hepatic fatty acid metabolism and the promotion of hepatosteatosis.62 A recent study has found that the cause of fat accumulation in the liver following fructose consumption is closely associated with fructokinase C, ATP consumption, nucleotide turnover and the production of uric acid.63 Both high fat and high fructose diets cause hepatic inflammation via an increase in pro-inflammatory cytokines which in turn damages the liver and may result in more serious pathogenesis following prolonged exposure.
5. Chinese herbs and fatty liver disease
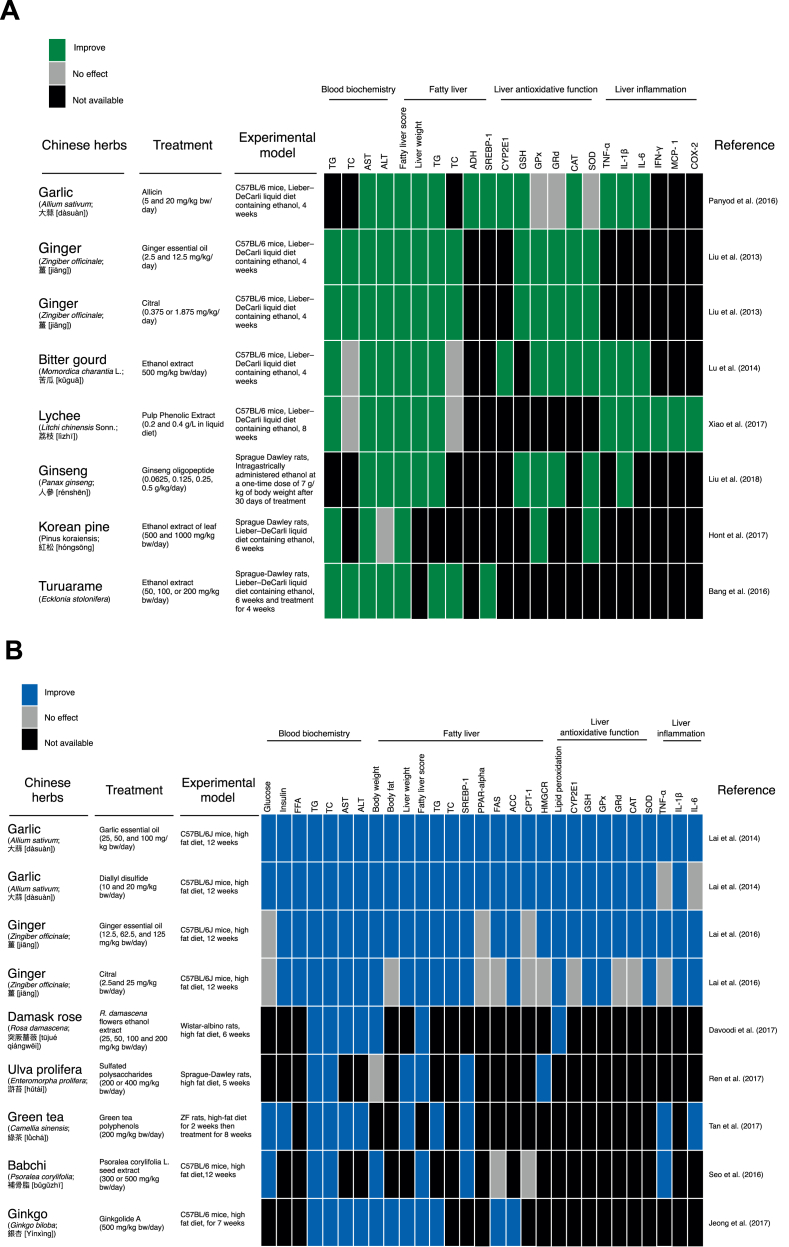
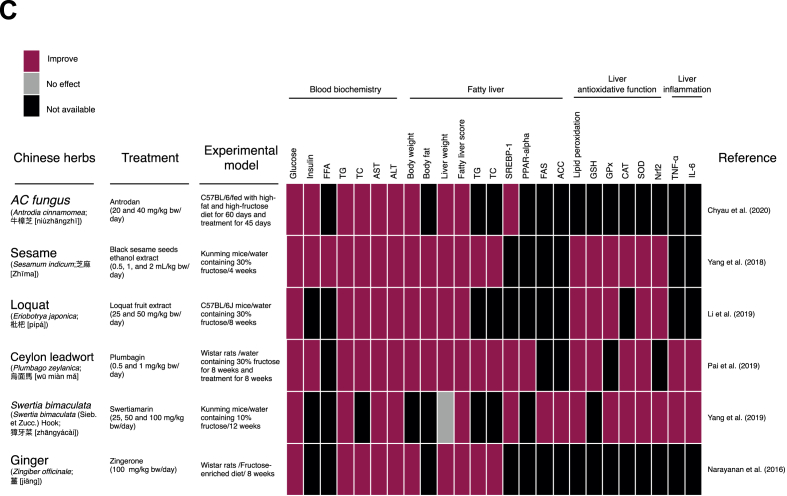
Various traditional herbal medicines including traditional Chinese Medicine, Ayurveda, Japanese, and Kampo medicine have been used in the treatment of fatty liver disease.64 Several animal studies have shown that Chinese herbs can protect against fatty liver disease. In this review, we evaluated studies using three kinds of fatty liver disease animal models: (1) alcohol induced fatty liver disease, (2) high-fat diet induced fatty liver disease, and (3) high fructose diet induced fatty liver disease. These studies demonstrate that Chinese herbs protect against fatty liver disease via different mechanisms based on their animal model (Fig. 3).
Fig. 3.
Chinese herbal medicines shown to protect the liver from (A) alcohol, (B) high-fat diet-, and (C) high fructose-induced fatty liver disease.
5.1. Chinese herbs and AFLD
Various Chinese herbs exhibit a hepatoprotective effect against AFLD. Garlic, ginger, bitter gourd, lychee, ginseng, Korean pine, and Turuarame extracts or their compounds attenuate AFLD by improving AST, ALT, hepatic triglyceride and fat accumulation in the liver (Fig. 3A).65, 66, 67, 68, 69, 70, 71 Allicin, the compound found in fresh garlic blends, improves alcohol dehydrogenase activity in AFLD mice. Most of the Chinese herbs that exhibit an attenuation effect on AFLD act as anti-oxidative or anti-inflammatory agents. Allicin and turuarame ethanol extracts downregulated SREBP-1 expression. Allicin, garlic essential oil, citral, and ginseng oligosaccharides increase glutathione concentrations in the liver, indicating that the oxidative stress response is improved by these compounds. Among these Chinese herbs, only bitter gourd and allicin have been reported to reduce hepatic CYP2E1 protein expression. Allicin, ethanol extracts of bitter gourd, and lychee exhibited anti-inflammatory effects against AFLD. In addition, allicin72 and lychee have been shown to suppress the LPS-CD14-toll-like receptor 4 pathway, and modulate intestinal microbiota dysbiosis and intestinal barrier dysfunction.73
5.2. Chinese herbs and NAFLD
The mechanisms underlying the protective effects of Chinese herbs against NAFLD are quite similar to those described for AFLD with most preventing lipogenesis, oxidative stress, and inflammation. However, there are some variations in the pathways used by these Chinese herbs to reduce lipogenesis in NAFLD. Fig. 3B illustrates the Chinese herbs and their anti-NAFLD effect in high-fat diet-induced NAFLD.74, 75, 76, 77, 78, 79, 80 Garlic, ginger and green tea improve blood glucose and insulin levels, while garlic essential oil, with a high proportion of diallyl disulfide, and ginger (citral) can reduce free fatty acid content in the blood stream reducing translocation to the liver thus reducing NAFLD. Garlic essential oil, diallyl disulfide, ginger, citral, Damask rose flowers ethanol extract, green tea polyphenols, and ginkgolide A reduce the concentration of triglycerides, cholesterol, AST and ALT in the blood stream. All the Chinese herbs in Fig. 3B exhibit hepatoprotective effects against high fat-diet induced NAFLD. Some of the Chinese herbs reduce liver weight, fatty liver score, liver cholesterol, and triglycerides. They prevent lipogenesis by suppressing hepatic lipogenesis through inhibition of SREBP-1, FAS, ACC, and HMGCR, as well as increasing β-fatty acid fat oxidation associated with PPARα and CPT-1 expression. In addition, garlic and ginger improved oxidative function preventing NAFLD by reducing lipid peroxidation, and CYP2E1 expression and improving glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GRd), catalase (CAT), and superoxide dismutase (SOD) activity. Moreover, they reduced hepatic inflammation by reducing TNF-α, interleukin IL-1β, and IL-6 expression.
The anti-NAFLD effects of Chinese herbs in dietary sugar induced-NAFLD are based on decreasing de novo lipogenesis, increasing β-oxidation, improving antioxidant activity and suppressing inflammation (Fig. 3C).81, 82, 83, 84, 85, 86 All the Chinese medicines in Fig. 3C reduced blood glucose levels. Sesame, loquat, Ceylon leadwort, and Antrodia cinnamomea reduced insulin concentrations in fructose induced NAFLD rodent models while most of the Chinese herbs reduced blood TG, TC, AST, ALT, and fatty liver values. Chinese herbs also inhibited SREBP-1, FAS, ACC, and improved PPAR-α expression. Swertimarin, sesame, and loquat reduced lipid peroxidation and improved liver antioxidative enzyme activity while inhibiting Nrf2 expression. Only swertimarin and plumbagin have been reported to have anti-inflammatory effects reducing TNF- α and IL-6 concentrations.
Many drugs have been developed to treat NAFLD but there are huge challenges that need to be overcome before these drugs can progress to clinical trial.87 In addition, the differences between Chinese Herb and western allotherapy interventions in AFLD/NAFLD still need to be evaluated. Changing dietary patterns, including shifting to a Mediterranean diet, can reduce fatty liver disease.88,89 To prevent NAFLD, controlling diet and maintaining an exercise routine is vital. Collectively, the major mechanisms of Chinese herbs in ameliorating fatty liver disease (both AFLD and NAFLD) are the inhibition of lipogenesis, increasing fat oxidation, reducing oxidative stress, and suppressing inflammation in the liver. Thus, the use of Chinese herbs as a supplement is an alternative way to prevent the progression of fatty liver disease and may provide potential agents for the treatment of fatty liver disease in the future.
Declaration of competing interest
None of the authors has any conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.02.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Qian Y., Fan J.G. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4(2):173–177. [PubMed] [Google Scholar]
- 2.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 3.Brunt E.M. Pathology of fatty liver disease. Mod Pathol. 2007;20:S40–S48. doi: 10.1038/modpathol.3800680. [DOI] [PubMed] [Google Scholar]
- 4.Perdomo C.M., Fruhbeck G., Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3) doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Organization W.H. Alcohol. 2018. https://www.who.int/news-room/fact-sheets/detail/alcohol
- 8.Wong T., Dang K., Ladhani S., Singal A.K., Wong R.J. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001-2016. JAMA, J Am Med Assoc. 2019;321(17):1723–1725. doi: 10.1001/jama.2019.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu S., Yu M., Chen X. Prevalence and metabolic abnormalities of fatty liver disease among adults in mianyang city, sichuan province. Acta Acad Med Sin. 2019;41(3):323–330. doi: 10.3881/j.issn.1000-503X.10720. [DOI] [PubMed] [Google Scholar]
- 10.Dietz W.H. You are what you eat - what you eat is what you are. J Adolesc Health. 1990;11(1):76–81. doi: 10.1016/0197-0070(90)90133-m. [DOI] [PubMed] [Google Scholar]
- 11.Naja F., Hwalla N., Itani L., Karam S., Sibai A.M., Nasreddine L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13-19 years): a cross-sectional study. Br J Nutr. 2015;114(11):1909–1919. doi: 10.1017/S0007114515003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trovato F.M., Martines G.F., Catalano D. Addressing Western dietary pattern in obesity and NAFLD. Nutrire. 2018;43(11) [Google Scholar]
- 13.Parry S.A., Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Invest Med. 2017;65(8):1102–1115. doi: 10.1136/jim-2017-000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelmalek M.F., Suzuki A., Guy C. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik V.S., Popkin B.M., Bray G.A., Despres J.P., Hu F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel R.A. Alcohol, heart disease, and mortality: a review. Rev Cardiovasc Med. 2002;3(1):7–13. [PubMed] [Google Scholar]
- 17.Rimm E.B., Klatsky A., Grobbee D., Stampfer M.J. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits? Br Med J. 1996;312(7033):731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath E., Jones A., Field M. Acute stress increases ad-libitum alcohol consumption in heavy drinkers, but not through impaired inhibitory control. Psychopharmacology (Berlin) 2016;233(7):1227–1234. doi: 10.1007/s00213-016-4205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smedile A., Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9(5):291–293. [PubMed] [Google Scholar]
- 20.Lieber C.S. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Savolainen V.T., Liesto K., Mannikko A., Penttila A., Karhunen P.J. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res. 1993;17(5):1112–1117. doi: 10.1111/j.1530-0277.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton P.B., Garlich J.D. Aflatoxin as a possible cause of fatty liver syndrome in laying hens. Poultry Sci. 1971;50(3):800–&. doi: 10.3382/ps.0500800. [DOI] [PubMed] [Google Scholar]
- 23.Eraslan G., Sarica Z.S., Bayram L.C., Tekeli M.Y., Kanbur M., Karabacak M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int. 2017;24(36):27931–27941. doi: 10.1007/s11356-017-0232-7. [DOI] [PubMed] [Google Scholar]
- 24.Gentile C.L., Weir T.L. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Shanab A., Quigley E.M.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 26.Schnabl B., Brenner D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceccarelli S., Panera N., Mina M. LPS-induced TNF-a factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget. 2015;6(39):41434–41452. doi: 10.18632/oncotarget.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compare D., Coccoli P., Rocco A. Gut-liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovas. 2012;22(6):471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Targher G., Bertolini L., Padovani R. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 30.Younossi Z.M., Gramlich T., Matteoni C.A., Boparai N., Mccullough A.J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2(3):262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 31.Marchesini G., Bugianesi E., Forlani G. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 32.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotman Y., Koh C., Zmuda J.M., Kleiner D.E., Liang T.J., Crn N. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52(3):894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altiparmak E., Koklu S., Yalinkilic M. Viral and host causes of fatty liver in chronic hepatitis B. World J Gastroenterol. 2005;11(20):3056–3059. doi: 10.3748/wjg.v11.i20.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong V.W.S., Wong G.L.H., Chu W.C.W. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56(3):533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Schwingel P.A., Cotrim H.P., Salles B.R. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011;31(3):348–353. doi: 10.1111/j.1478-3231.2010.02346.x. [DOI] [PubMed] [Google Scholar]
- 37.Oray M., Abu Samra K., Ebrahimiadib N., Meese H., Foster C.S. Long-term side effects of glucocorticoids. Expet Opin Drug Saf. 2016;15(4):457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 38.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q., Bengmark S., Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD) Lipids Health Dis. 2010;9 doi: 10.1186/1476-511X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy T., Oakley F., Anstee Q.M., Day C.P. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol-Mech. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 41.Altamirano J., Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8(9):491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 42.Zakhari S., Li T.K. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 43.Vidal F., Perez J., Morancho J., Pinto B., Richart C. Hepatic alcohol-dehydrogenase activity in alcoholic subjects with and without liver-disease. Gut. 1990;31(6):707–711. doi: 10.1136/gut.31.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purohit V., Gao B., Song B.J. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33(2):191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol Commun. 2018;2(12):1425–1439. doi: 10.1002/hep4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S., Hong M., Tan H.Y., Wang N., Feng Y.B. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid Med Cell Longev. 2016;201:4234061. doi: 10.1155/2016/4234061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dey A., Cederbaum A.I. Alcohol and oxidative liver injury. Hepatology. 2006;43(2):S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 48.Szabo G., Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16(11):1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donohue T.M. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13(37):4974–4978. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endo M., Masaki T., Seike M., Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element blinding protein-1c (SREBP-1c) Exp Biol Med. 2007;232(5):614–621. [PubMed] [Google Scholar]
- 51.Yu S.T., Rao S., Reddy J.K. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3(6):561–572. doi: 10.2174/1566524033479537. [DOI] [PubMed] [Google Scholar]
- 52.Miller A.M., Horiguchi N., Jeong W.I., Radaeva S., Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35(5):787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malaguarnera M., Di Rosa M., Nicoletti F., Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med. 2009;87(7):679–695. doi: 10.1007/s00109-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 54.Bell L.N., Molleston J.P., Morton M.J. Hepatic lipid peroxidation and cytochrome P-450 2E1 in pediatric nonalcoholic fatty liver disease and its subtypes. J Clin Gastroenterol. 2011;45(9):800–807. doi: 10.1097/MCG.0b013e31821377e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 57.Brownsey R.W., Boone A.N., Elliott J.E., Kulpa J.E., Lee W.M. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34(Pt 2):223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- 58.Dorn C., Riener M.O., Kirovski G. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2010;3(5):505–514. [PMC free article] [PubMed] [Google Scholar]
- 59.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Louet J.F., Chatelain F., Decaux J.F. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J. 2001;354(Pt 1):189–197. doi: 10.1042/0264-6021:3540189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu W., Shao L., Zhou C., Wang H., Guo J. Upregulation of Nrf2 expression in non-alcoholic fatty liver and steatohepatitis. Hepato-Gastroenterology. 2011;58(112):2077–2080. doi: 10.5754/hge10501. [DOI] [PubMed] [Google Scholar]
- 63.Jensen T., Abdelmalek M.F., Sullivan S. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jadeja R., Devkar R.V., Nammi S. Herbal medicines for the treatment of nonalcoholic steatohepatitis: current scenario and future prospects. Evid Based Complement Alternat Med. 2014;2014:648308. doi: 10.1155/2014/648308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panyod S., Wu W.K., Ho C.T. Diet supplementation with allicin protects against alcoholic fatty liver disease in mice by improving anti-inflammation and antioxidative functions. J Agric Food Chem. 2016;64(38):7104–7113. doi: 10.1021/acs.jafc.6b02763. [DOI] [PubMed] [Google Scholar]
- 66.Liu C.T., Raghu R., Lin S.H. Metabolomics of ginger essential oil against alcoholic fatty liver in mice. J Agric Food Chem. 2013;61(46):11231–11240. doi: 10.1021/jf403523g. [DOI] [PubMed] [Google Scholar]
- 67.Lu K.H., Tseng H.C., Liu C.T., Huang C.J., Chyuan J.H., Sheen L.Y. Wild bitter gourd protects against alcoholic fatty liver in mice by attenuating oxidative stress and inflammatory responses. Food Funct. 2014;5(5):1027–1037. doi: 10.1039/c3fo60449g. [DOI] [PubMed] [Google Scholar]
- 68.Xiao J., Zhang R., Zhou Q. Lychee (litchi chinensis sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation. J Agric Food Chem. 2017;65(44):9675–9684. doi: 10.1021/acs.jafc.7b03791. [DOI] [PubMed] [Google Scholar]
- 69.Liu R., Chen Q.H., Ren J.W. Ginseng (panax ginseng meyer) oligopeptides protect against binge drinking-induced liver damage through inhibiting oxidative stress and inflammation in rats. Nutrients. 2018;10(11) doi: 10.3390/nu10111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong S.H., Lee H., Lee H.J. Ethanol extract of pinus koraiensis leaf ameliorates alcoholic fatty liver via the activation of LKB1-AMPK signaling in vitro and in vivo. Phytother Res. 2017;31(5):783–791. doi: 10.1002/ptr.5801. [DOI] [PubMed] [Google Scholar]
- 71.Bang C.Y., Byun J.H., Choi H.K., Choi J.S., Choung S.Y. Protective effects of ecklonia stolonifera extract on ethanol-induced fatty liver in rats. Biomol Ther (Seoul). 2016;24(6):650–658. doi: 10.4062/biomolther.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panyod S., Wu W.K., Lu K.H. Allicin modifies the composition and function of the gut microbiota in alcoholic hepatic steatosis mice. J Agric Food Chem. 2020;68(10):3088–3098. doi: 10.1021/acs.jafc.9b07555. [DOI] [PubMed] [Google Scholar]
- 73.Xiao J., Zhang R., Huang F. Lychee (litchi chinensis sonn.) pulp phenolic extract confers a protective activity against alcoholic liver disease in mice by alleviating mitochondrial dysfunction. J Agric Food Chem. 2017;65(24):5000–5009. doi: 10.1021/acs.jafc.7b01844. [DOI] [PubMed] [Google Scholar]
- 74.Lai Y.S., Chen W.C., Ho C.T. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. 2014;62(25):5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]
- 75.Lai Y.S., Lee W.C., Lin Y.E. Ginger essential oil ameliorates hepatic injury and lipid accumulation in high fat diet-induced nonalcoholic fatty liver disease. J Agric Food Chem. 2016;64(10):2062–2071. doi: 10.1021/acs.jafc.5b06159. [DOI] [PubMed] [Google Scholar]
- 76.Davoodi I., Rahimi R., Abdollahi M. Promising effect of Rosa damascena extract on high-fat diet-induced nonalcoholic fatty liver. J Tradit Complement Med. 2017;7(4):508–514. doi: 10.1016/j.jtcme.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren R., Gong J., Zhao Y., Zhuang X., Ye Y., Lin W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017;8(5):1899–1904. doi: 10.1039/c7fo00103g. [DOI] [PubMed] [Google Scholar]
- 78.Tan Y., Kim J., Cheng J. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol. 2017;23(21):3805–3814. doi: 10.3748/wjg.v23.i21.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seo E., Oh Y.S., Jun H.S. Psoralea corylifolia L. Seed extract attenuates nonalcoholic fatty liver disease in high-fat diet-induced obese mice. Nutrients. 2016;8(2):83. doi: 10.3390/nu8020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeong H.S., Kim K.H., Lee I.S. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed Pharmacother. 2017;88:625–634. doi: 10.1016/j.biopha.2017.01.114. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y., Li J., Wei C. Amelioration of nonalcoholic fatty liver disease by swertiamarin in fructose-fed mice. Phytomedicine. 2019;59:152782. doi: 10.1016/j.phymed.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Yang Y., Wang J., Zhang Y., Li J., Sun W. Black sesame seeds ethanol extract ameliorates hepatic lipid accumulation, oxidative stress, and insulin resistance in fructose-induced nonalcoholic fatty liver disease. J Agric Food Chem. 2018;66(40):10458–10469. doi: 10.1021/acs.jafc.8b04210. [DOI] [PubMed] [Google Scholar]
- 83.Li W., Yang H., Zhao Q., Wang X., Zhang J., Zhao X. Polyphenol-rich loquat fruit extract prevents fructose-induced nonalcoholic fatty liver disease by modulating glycometabolism, lipometabolism, oxidative stress, inflammation, intestinal barrier, and gut microbiota in mice. J Agric Food Chem. 2019;67(27):7726–7737. doi: 10.1021/acs.jafc.9b02523. [DOI] [PubMed] [Google Scholar]
- 84.Pai S.A., Munshi R.P., Panchal F.H. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed Pharmacother. 2019;111:686–694. doi: 10.1016/j.biopha.2018.12.139. [DOI] [PubMed] [Google Scholar]
- 85.Narayanan J.M., Jesudoss V.A. Hepatoprotective potential of zingerone against nonalcoholic fatty liver disease in rats fed with fructose-enriched diet. Gen Physiol Biophys. 2016;35(2):185–194. doi: 10.4149/gpb_2015041. [DOI] [PubMed] [Google Scholar]
- 86.Chyau C.-C., Wang H.-F., Zhang W.-J. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int J Mol Sci. 2020;21(360) doi: 10.3390/ijms21010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Konerman M.A., Jones J.C., Harrison S.A. Pharmacotherapy for NASH: current and emerging. J Hepatol. 2018;68(2):362–375. doi: 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 88.Romero-Gomez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Baratta F., Pastori D., Polimeni L. Adherence to mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol. 2017;112(12):1832–1839. doi: 10.1038/ajg.2017.371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.