Abstract
Korean Red Ginseng (KRG) exerts chemopreventive effects on experimentally induced carcinogenesis through multiple mechanisms. In this study, we investigated effects of KRG on dextran sulfate sodium (DSS)-induced colitis and azoxymethane (AOM) plus DSS-induced colon carcinogenesis in mice. Male C57BL/6J mice were fed diet containing 1% KRG or a standard diet throughout the experiment. The mouse colitis was induced by administration of 3% DSS in drinking water for 1 week. DSS caused body weight loss, diarrhea, rectal bleeding and colon length shortening, and all these symptoms were ameliorated by KRG treatment. KRG inhibited DSS-induced expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) by suppressing activation of nuclear factor-kappa B (NF-κB) and signal transducer and activation of transcription 3 (STAT3). In another experiment, colon carcinogenesis was initiated by single intraperitoneal injection of AOM (10 mg/kg) and promoted by 2% DSS in drinking water. KRG administration relieved the symptoms of colitis and reduced the incidence, the multiplicity and the size of colon tumor. The up-regulation of COX-2, iNOS, c-Myc and Cyclin D1 by AOM plus DSS was attenuated in KRG fed mice which was associated with suppression of NF-κB and STAT3 activation. These results suggest that KRG is a potential candidate for chemoprevention of inflammation-associated cancer in the colon.
Keywords: Korean red ginseng, Colitis, Colon cancer, Cyclooxygenase-2, NF-κB, STAT3
Graphical abstract

1. Introduction
Inflammation arises from microbial infection or noninfective physical/chemical irritation. In general, acute (physiologic) inflammation is self-limiting as seen in wound healing. Failure in resolving the acute inflammation may cause chronic inflammation implicated in pathogenesis of many human disorders.1 Multiple lines of evidence from clinical, epidemiologic, genetic and pharmacological data support the association between inflammation and cancer.2, 3, 4, 5 It becomes increasingly evident that the tumor microenvironment is largely orchestrated by immune and inflammatory cells surrounding cancer cells, and inflammatory microenvironment is indispensable for the neoplastic process.3, 4, 5, 6, 7
The connection between inflammation and tumorigenesis has been well established in both heritable and sporadic forms of colorectal cancer (CRC). Inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis, is an important risk factor for the CRC.8 Thus, patients with long-standing IBD have an increased risk of developing CRC than general population.9,10 Distinct cytokines, chemokines and other proinflammatory mediators produced by immune cells are involved in virtually all steps of CRC development and progression.11
Cyclooxygenase-2 (COX-2), a rate-limiting enzyme in the arachidonic acid cascade, is consistently overexpressed in inflamed tissues. This enzyme converts arachidonic acid to prostaglandin H2 which is further converted to prostaglandin E2 (PGE2). NF-κB is a major transcription factor that regulates expression of prototypic pro-inflammatory enzymes, COX-2 and inducible nitric oxide synthase (iNOS). NF-κB is sequestered in the cytoplasm by IκBα in resting cells. Activation of NF-κB is dependent on degradation of IκBα through phosphorylation and ubiquitination. This liberates NF-κB in an active form, mainly as p65/p50 heterodimer, which migrates into the nucleus. p65 also undergoes phosphorylation which facilitates its nuclear translocation and recruitment of coactivators including p300/CBP, thereby regulating the expression of multiple target genes.12 STAT3 is another key transcription factor involved in inflammation and immunity.13 STAT3 is activated through phosphorylation at the tyrosine 705 (Tyr705) residue. The phosphorylated STAT3, in turn, dimerizes and translocates to the nucleus, where it directly regulates target gene expression.13 Persistent activation of NF-κB and STAT3 is implicated in inflammation-associated carcinogenesis. The interaction and cooperation between these two transcription factors play vital roles in controlling the communication between cancer cells and inflammatory cells. Understanding the molecular mechanisms of NF-κB and STAT3 cooperation in cancer will offer opportunities for the successful implementation of chemopreventive and chemotherapeutic approaches.14
Korean ginseng (Panax ginseng C.A. Meyer) has been used as a medicinal herb for thousands of years. One way to process raw ginseng is steaming and drying to generate red ginseng. Korean red ginseng (KRG) is known for its beneficial effects on immunity, improvement of cognitive function, amelioration of fatigue, cancer survival, etc. The active components of KRG include saponins (ginsenosides), polysaccharides, and fatty acids. Prolonged administration of KRG has been known to have cancer preventive effects.15 However, molecular mechanisms responsible for its chemopreventive effects remain still to be clarified.
In this study, we have investigated the effects of KRG on dextran sulfated sodium (DSS)-induced colitis and azoxymethane (AOM) plus DSS-induced colon carcinogenesis in C57BL/6J mice which mimic human IBD and inflammation-associated CRC, respectively.
2. Materials and methods
2.1. Materials
DSS with an average molecular weight of 36,000–50,000 was purchased from MP Biomedicals, LLC (Solon, OH, USA). AOM was obtained from Sigma-Aldrich (St Louis, MO, USA). Standardized KRG powder was supplied by Korea Ginseng Corporation (Seoul, South Korea). COX-2 (murine) polyclonal antibody produced from rabbit was supplied by Cayman Chemical (Ann Arbor, MI, USA). Polyclonal rabbit anti-iNOS antibody was provided by BD Biosciences (Franklin Lakes, NJ, USA). Primary antibodies against Cyclin D1, STAT3, p-STAT3 (Tyr705), p65 and p-IκBα (Ser32) were offered by Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against p-p65 (Ser536) and IκBα were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibody against lamin B1 was obtained from Invitrogen Corporation (Camarillo, CA, USA). Antibodies against actin and α-tubulin were bought from AbClon, Inc. (Seoul, South Korea). Horseradish peroxidase-conjugated anti-mouse and rabbit secondary antibodies were obtained from Zymed lavoratories (San Fransico, CA, USA). An NF-κB oligonucleotide probe containing the consensus sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′, 3′-TCA ACT CCC CTG AAA GGG TCC G-5′) was purchased from Promega (Madison, WI, USA). EPD and pico EPD Western blot detection kit were products of ELPISBIOTECH, Inc. (Daejeon, South Korea). All other chemicals used in our experiments were of the purest grade available from regular commercial sources.
2.2. Animal treatment
All the animal experiments were performed according to the approved guidelines of the Seoul National University (SNU-120629-1). Four-week-old male C57BL/6J mice were obtained from Central Lab Animal (Seoul, South Korea) and maintained on conventional housing conditions. After an acclimation for 7 days, mice were divided into groups as illustrated in Supplementary Fig. 1 and fed control or experimental diet throughout the experiment. Composition of experimental diet and content of ginsenosides of KRG are shown in Supplementary Tables S1 and S2, respectively.
For a short-term experiment to induce colitis, mice in the control group and the DSS group received the control diet. Mice in the DSS plus KRG group and the KRG alone group received control diet supplemented with 1% (w/w) KRG powder during the entire period of the experiment. DSS (3%, w/v) in drinking water was given for 1 week. To induce CRC formation, mice were given single intraperitoneal (i.p.) injection of AOM (10 mg/kg body weight) followed by one week later exposure to 2% DSS in drinking water for 7 days, and then kept without any further treatment for 14 weeks. Mice in the control group and the AOM plus DSS group received control diet, and mice in the AOM + DSS + KRG group received control diet supplemented with 1% KRG powder.
2.3. Macroscopic assessment
During 7 days of DSS treatment, the body weight of mice was measured every day. Rectal bleeding and stool consistency were monitored and scored from 0 to 3 in a modified design depending on the severity of blood and diarrhea. Disease activity index (DAI) was determined as the sum of scores of rectal bleeding and stool consistency. In the long-term tumor experiment, collected colon tissues were cut longitudinally and the tumors were identified. After the measurement of the number and the size of tumors, they were excised, collected and weighed.
2.4. Histological examination
Specimens of distal parts of the colon were fixed with 10% phosphate buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (H&E).
2.5. Western blot analysis
Mouse colon parts were cut longitudinally, and washed with phosphate-buffered saline (PBS), and stored at −70 °C until use. Colon tissue was homogenized in the lysis buffer [1 mM phenylmethylsulfonylfluoride (PMSF) and EDTA-free protease inhibitor cocktail tablet] followed by periodical vortex mixing for 2 h. Lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. Supernatants were collected and stored at −70 °C. The total protein concentration was quantified using a bicinchoninic acid protein assay kit (Pierce Biotechnology). After mixing and heating with sodium dodecyl sulfate (SDS) buffer, 20–30 μg of whole protein lysate was separated by SDS-PAGE and transferred to polyvinyliden difluoride membrane at 300 mA for 3 h. The blots were blocked in 5% skim milk in TBST (Tris-buffered saline with 0.1% Tween-20) for 1 h at room temperature and incubated with primary antibodies in TBST at 4 °C overnight. Blots were then washed with TBST for 30 min and incubated in horseradish peroxidase-conjugated secondary antibody in TBST for 1 h at room temperature. Blots were washed again three times, and transferred proteins were visualized with an enhanced chemiluminescence detection kit and the LAS-4000 image reader according to the manufacturer’s instructions.
2.6. Fractionation of nuclear and cytoplasmic extracts
Nuclear and cytoplasmic extracts of colonic tissues were prepared according to the standard method. Briefly, colon tissue was homogenized in hypotonic buffer A [10 mM HEPES (pH 7.8), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.2 mM PMSF] and incubated for 1 h on ice, and 0.1% NP-40 was added right before centrifugation. After centrifugation at 13,000 rpm for 15 min at 4 °C, the supernatants containing the cytoplasmic extract were collected and stored at −70 °C. Precipitated pellets were washed with buffer A for 2 times to remove remaining cytoplasmic components. Then pellets were re-suspended in buffer C [20 mM HEPES (pH 7.8), 20% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM DTT, 0.2 mM PMSF] and incubated on ice for 1 h with vortex mixing at 5 min interval. After centrifugation at 13,000 rpm for 15 min at 4 °C, the supernatants (the nuclear extract) were collected and stored at −70 °C.
2.7. Electrophoresis mobility shift assay (EMSA)
The DNA binding activity of NF-κB was measured with EMSA using a DNA binding detection kit according to manufacturer’s protocol (Gibco BRL; Grand island, NY, USA). T4 polynucleotide kinase catalyzes the transfer and exchange of the phosphate group from the γ-position of ATP to the 5′–hydroxyl terminus of the NF-κB oligonucleotide. After purification with a G-50 micro column. The [γ-32P]-labeled probe was mixed with 10 μg of nuclear extracts and incubation buffer [10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 4% glycerol and 0.1 mg/ml sonicated salmon sperm DNA]. All the samples were mixed with 2 μl 0.1% bromophenol blue loading dye after 50-min incubation and separated on 6% non-denatured polyacrylamide gel in a cold room. Finally, gels were dried and exposed to X-ray films.
2.8. Statistics
All values were expressed as the mean ± SD or the mean ± SE according to data type. Statistical significance was determined by the Student’s t-test and p < 0.05 was considered to be statistically significant.
3. Results
3.1. Effects of KRG on DSS-induced colitis in mice
DSS is a high molecular weight sulfated polysaccharide commonly used in animal models for inducing acute and chronic colitis.16 DSS increases the colonic mucosal permeability and activates inflammatory signaling pathways.17 From the 4th day of 3% DSS exposure, the body weight of mice became significantly decreased compared to the control group. KRG treatment inhibited body weight loss caused by DSS administration (Fig. 1A). DAI was scored according to the severity of bleeding and stool consistency. The DAI score of mice in the DSS plus KRG group was significantly lower than that of mice in the DSS only group (Fig. 1B). Moreover, DSS exposure for 7 days shortened the colon length of mice, and KRG treatment partially restored it (Fig. 1C). H&E staining of distal colon revealed that DSS administration resulted in colitis exhibiting epithelial degeneration, crypt loss and inflammatory cell infiltration. Dietary administration of KRG attenuated DSS-induced colonic mucosal damage (Fig. 1D).
Fig. 1.
Effects of KRG on experimentally induced colitis. Effects of KRG on the body weight change (A), DAI (B) and the colon length (C) in mice exposed to 3% DSS in drinking water for 1 week. (D) Microscopic examination of H&E stained colonic mucosa from control mice and those treated with 3% DSS alone for 1 week, 3% DSS plus 1% dietary KRG and KRG alone. Results are presented as means ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.2. Effects of KRG on DSS-induced inflammatory signaling
COX-2 and iNOS are prototypic pro-inflammatory enzymes which are often overexpressed in inflammatory conditions. The Western blot analysis of colon revealed that DSS induced overexpression of COX-2 and iNOS, which was significantly reduced by KRG (Fig. 2).
Fig. 2.
Inhibition of DSS-induced expression COX-2 and iNOS by KRG in mouse colon. All mice were killed after 7 days of DSS exposure, and colon tissue was collected. Colon was cut longitudinally and divided equally. Expression of COX-2 and iNOS was measured by Western blot analysis as described in Materials and methods. Results are presented as means ± SE. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
NF-κB is a principal transcription factor responsible for regulating the expression of many signaling molecules involved in inflammation and cell survival. DSS administration activated NF-κB signaling through blockage of phosphorylation (Fig. 3A) and degradation (Fig. 3B) of IκBα, an inhibitory protein that sequesters NF-κB in the cytoplasm. p65 is a functionally active subunit of NF-κB. Phosphorylation of p65 facilitates its nuclear localization and interaction with the co-activator, p300/CBP. Phosphorylation (Fig. 4A) and nuclear accumulation (Fig. 4B) of p65 were markedly increased in the colon of DSS-treated mice, and both events were abolished by KRG supplementation in the diet (Fig. 4A and B). Further, we found that DSS-induced colonic DNA binding activity of NF-κB measured by the gel-shift assay was significantly reduced in the KRG-fed group (Fig. 4C).
Fig. 3.
Inhibitory effects of KRG on DSS-induced IκBα phosphorylation and degradation. (A) Effects of KRG on DSS-induced IκBα phosphorylation were determined by Western blot analysis using cytoplasmic extracts. (B) Effects of KRG on DSS-induced IκBα degradation were determined by Western blot analysis. Results are presented as means ± SE. ∗p < 0.05 and ∗∗∗p < 0.001.
Fig. 4.
Inhibitory effects of KRG on DSS-induced phosphorylation, nuclear accumulation and DNA binding of NF-κB/p65. Phosphorylation (A) and nuclear accumulation (B) of NF-κB p65 were determined by Western blot analysis. NF-κB-DNA binding activity (C) was determined by EMSA. Incubation conditions and other experimental details are described in Materials and methods. Results are presented as means ± SE. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
3.3. Effects of KRG on AOM plus and DSS-induced colitis and colon carcinogenesis
DSS-induced colitis is necessary, but not sufficient to induce tumor formation in mouse colon. In order to determine whether KRG could prevent the colitis-associated carcinogenesis, we conducted a long-term experiment in which mice were given a single i.p. administration of the carcinogen, AOM followed by 2% DSS treatment for 1 week. During 7 days of DSS exposure, the body weight and the DAI of mice in each group were checked. Dietary supplementation of KRG ameliorated the severity of colitis as well as well as body weight loss caused by DSS (Supplementary Fig. 2). Combining the systemic administration of AOM with DSS in drinking water facilitates the formation of multiple colon tumors.18 Mice were sacrificed 16 weeks after the AOM injection. The colonic mucosa of mice given AOM and DSS showed epithelial thickening, hyperplasia and dysplasia (Fig. 5A), and these changes were ameliorated by KRG supplementation in the diet. All mice in the AOM plus DSS group developed colon tumors (Fig. 5B). However, mice fed KRG containing diet had a much lower incidence (Fig. 5C) and multiplicity (Fig. 5D) as well as the notably reduced volume (Fig. 5E) of colonic tumors.
Fig. 5.
Effects of KRG on AOM plus DSS-induced colonic tumorigenesis in mice. Mice were fed standard or 1% KRG diet for 17 weeks, starting 1 week before the single i.p. injection of AOM (10 mg/kg) followed by exposure to 2% DSS for 7 days. (A) H&E stained colonic tissues of control mouse and those treated with AOM and DSS with and without dietary KRG supplementation. (B) Macroscopic examination of mouse colon. Dietary administration of KRG inhibited colitis-induced carcinogenesis. The incidence (C), the number (D) and the total weight (E) of colon tumor were assessed. Results are presented as means ± SD. ∗p < 0.05.
3.4. Effects of KRG on the inflammatory and proliferative signaling in the colon of mice treated with AOM and DSS
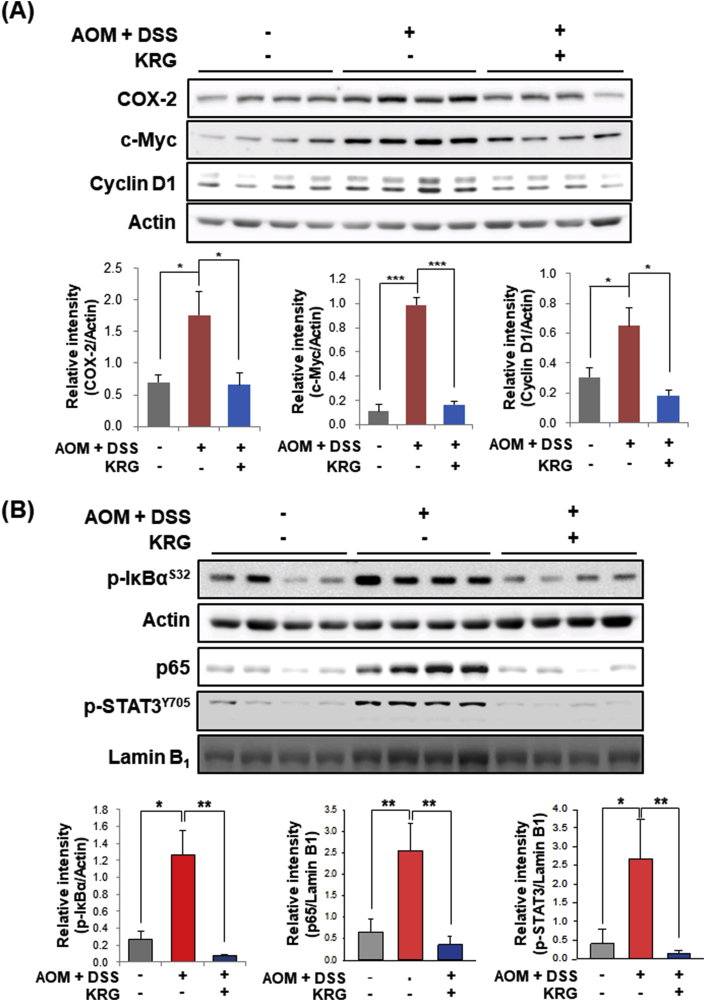
Aberrant upregulation of COX-2 is known to promote CRC. The expression of COX-2 was elevated in the colon of the mice treated with AOM and DSS, and this was abolished by KRG supplementation (Fig. 6A). c-Myc and Cyclin D1 are representative oncogenic proteins that stimulate cell proliferation. In tumor-free region of colon in the AOM plus DSS group, expression of these proteins was significantly up-regulated compared to the control group, which was suppressed by KRG (Fig. 6A).
Fig. 6.
Inhibitory effects of KRG on AOM plus DSS-induced up-regulation of and inflammation and proliferation marker proteins and their regulators. Whole colons were collected. After excision of all visible tumors, remaining colon tissue was subject to Western blot analysis for the measurement of inflammatory and proliferative protein markers (A) and their upstream regulators (B). Animal treatment and other experimental details are described in Materials and methods. Results are presented as means ± SE. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
NF-κB and STAT3 play a vital role in inflammation-associated carcinogenesis by upregulating the proinflammatory gene transcription. Colons of the mice in the AOM plus DSS group showed persistent activation of NF-κB and STAT3 as evidenced by phosphorylation of IκBα (Fig. 6B) with subsequent nuclear accumulation of NF-κB/p65 and nuclear accumulation of p-STAT3 (Fig. 6B), respectively. Dietary administration of KRG inhibited all these events.
4. Discussion
Chronic inflammation is closely related to the pathogenesis of many different types of malignancies.1, 2, 3, 4, 5, 6, 7 Inflammatory cells, including macrophages and neutrophils, secrete various signaling molecules such as chemokines and cytokines that activate NF-κB and STAT3 in epithelial cells. Chronically inflamed colonic tissues have constitutive activation of these two key proinflammatory transcription factors which upregulate expression of oncogenic proteins, such as COX-2 and c-Myc, respectively. This creates inflammatory microenvironment favourable for tumor development and progression.19 Therefore, inhibition of chronic inflammation is considered to be a practical way to prevent cancer.
KRG has been known for its preventive effects on development of various cancers.20 However, molecular mechanisms underlying its chemopreventive effects have not been elucidated well. In this study, we investigated effects of KRG on colitis and colitis-associated colon carcinogenesis in mice. It has been reported that American ginseng has anti-inflammatory effects on experimentally induced mouse colitis which is associated with suppression of key inflammatory markers such as COX-2 and iNOS.21,22 Our results showed that KRG ameliorated DSS-induced colitis through down-regulation of COX-2 and iNOS and blockage of NF-κB signaling responsible for regulating these proinflammatory enzymes.
There appears to be a close interrelationship between COX-2 and iNOS and their products in inducing colitis and colitis-induced cancer. PGE2, a major catalytic product of COX-2, affects the biosynthesis of iNOS by modulating the expression/catalytic activity of iNOS, whereas nitric oxide (NO) synthesized by iNOS influences COX-2 expression/activity andconsequently, the synthesis of PGE2.23 Therefore, combined blockage of COX-2 and iNOS would provide a more efficient strategy than suppression of each enzyme alone in dietary or pharmacological intervention of IBD and inflammation-associated CRC. It will be of interest to examine the profile of PGE2 and NO following intake of KRG in the context of its impact on coordinated regulation of NF-κB-COX-2/PGE2-iNOS/NO axis.
Of the numerous ingredients of KRG, saponins (ginsenosides) are potent candidates that exert anti-inflammatory effects.24, 25, 26 A panaxadiol-type ginsenoside, Rg3 inhibited expression of inflammatory mediators in mouse skin challenged with the tumor promoter, phorbol ester27 and LPS/IFN-γ-stimulated BV-2 cells.28 Another type of ginsenosides, 20(S)-protopanaxatriol, inhibited the expression of COX-2 and iNOS through inactivation of NF-κB in LPS-stimulated macrophages.29 Based on these findings, ginsenosides are most likely be the main components of KRG responsible for the inhibition of mouse colitis. Ginsenosides have structural similarity to steroids.30 Thus, ginsenosides contained in KRG could bind to and consequently activate a specific steroid receptor, which may account for the preventive effects of KRG on DSS-induced colitis.
Besides ginsenosides, other ingredients of red ginseng have health beneficial effects. Red ginseng oil (RGO) containing linoleic acid and β-sitosterol as main bioactive components has been shown to inhibit AOM plus DSS-induced colitis.31 Oral administration of RGO reduced the plasma NO concentration and inhibited the production of proinflammatory factors such as COX-2, iNOS, interleukin-1β, interleukin-6, and tumor necrosis factor-α in the mouse colitis tissue. Phosphorylation of p65 and IκB in the colon of AOM and DSS treated mice was also attenuated by RGO administration.31 In line with this notion, the hexane fraction of American ginseng that consists mainly of fatty acids and polyacetylenes suppressed DSS-induced murine colitis and AOM-initiated and DSS-promoted intestinal carcinogenesis.22
Colon is exposed to a vast variety of environmental factors that can influence the intestinal microbiome. IBD is considered to result from an exaggerated immune response, triggered by environmental insults towards the altered gut microbiota.32 Thus, imbalance of enterobacteria provokes host immune responses33 and induces colitis in rats and mice.34 DSS-induced colitis begins with the penetration of luminal bacteria into colon epithelium.35
Recently, probiotics have attracted public interest because of their effective capability of promoting the balance of gut microbiota as well as protecting the intestinal mucosa and strengthening the intestinal barrier. It has been reported that supplement of probiotics ameliorates experimental colitis by restoring/maintaining a balance in the microbial environment in mouse colon.36 Dietary ginseng has been reported to alter the colonic microbial diversity.37 Thus, extracts of Panax ginseng inhibited the growth of the harmful bacteria, clostria while enhancing the growth of the Bifidobactierum spp., a prototypic probiotic organism in vitro.38 Results from animal experiments suggest that saponins can suppress intestinal inflammation, promote intestinal barrier repair, maintain the diversity of the intestinal flora, and decrease the incidence rate of inflammation-associated colon cancer.39 After treatment with AOM and DSS, gut microbiota and metabolomic profiles were obviously changed. Ginseng inhibited these changes, which may account for its protection against colitis-associated carcinogenesis.40 Therefore, the beneficial effects of KRG on the intestinal microflora may also contribute to mitigation of DSS-induced colonic inflammation processes and colitis-associated CRC.
The saponin components of ginseng taken orally undergo metabolism by gut microflora, including those with probiotic characteristics (e.g., Bifidobacterium spp., Lactobacillus spp., and Saccharomyces spp.). Considering that the resulting metabolites exert the main pharmacological activities of ginseng, its efficacy after oral administration varies among individuals, depending on their microbiome status. KRG extract fermented with Bifidobacterium longum H-1 had anticolitic effects in two experimentally induced murine colitis models.41 Further, oral administration of KRG powder fermented with Lactobacillus plantarum alleviated DSS-induced colitis to a greater extent than that achieved with oral administration of ginseng powder or the probiotics alone.42
Compound K (20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol) is an enteric gut microbiome metabolite of Rb1. The treatment with the antibiotic metronidazole reduced the serum levels of Compound K following oral administration of ginseng.37 Compound K is more efficiently absorbed into the systemic circulation and possesses stronger cancer chemopreventive potential than its parent ginsenoside. Likewise, the gut microbial metabolite compound K showed significant anti-inflammatory effects even at low concentrations, compared to its parent ginsenoside Rb1. Intestinal microbial metabolites of American ginseng also significantly reduced chemically-induced colitis, possibly through inhibition of pro-inflammatory cytokine expression.43 Likewise, Compound K ameliorated DSS-induced colitis by modulating NF-κB-mediated inflammatory responses.44 Ginseng inhibited colonic inflammation and tumorigenesis promoted by Western diet, and Compound K appears to contribute to the chemopreventive effects of ginseng on colonic tumorigenesis.37
In summary, KRG prevented colitis-induced induced colon carcinogenesis by blocking activation of NF-κB and STAT3 and overexpression of their target proteins (Fig. 7). Anti-carcinogenic activity of KRG results from the inhibition of colitis which is a prerequisite for promotion of colon carcinogenesis. Therefore, KRG is a potential candidate for chemoprevention of inflammation-associated colon carcinogenesis.
Fig. 7.
Molecular mechanisms by which KRG inhibits experimentally induced colitis and CRC formation.
Funding
This work was supported by the grant from the Korean Society of Ginseng funded by the Korea Ginseng Corporation (2012–2013).
Declaration of competing interest
The authors declare no conflicts of interest in this work/
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.04.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Bondar T., Medzhitov R. The origins of tumor-promoting inflammation. Canc Cell. 2013;24:143–144. doi: 10.1016/j.ccr.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol Canc Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 4.Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 5.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S., Yang X., Wang L., Zhang C. Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncol Lett. 2018;16:679–686. doi: 10.3892/ol.2018.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comen E.A., Bowman R.L., Kleppe M. Underlying causes and therapeutic targeting of the inflammatory microenvironment. Front Cell Dev Biol. 2018;6 doi: 10.3389/fcell.2018.00056. Article 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 9.Stidham R.W., Higgins P.D.R. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31:168–178. doi: 10.1055/s-0037-1602237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes J.M., Campbell B.J. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–16. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 11.Terzić J., Grivennikov S., Karin E., Karin M. Inflammation and colon cancer. Gastroenterol. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi Koji, Karin Michael. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 13.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Canc. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y., Mao R., Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 16.Melgar S., Karlsson A., Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kitajima S., Takuma S., Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 18.Neufert C., Becker C., Neurath M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 19.Ji Z., He L., Regev A., Struhl K. Inflammatory regulatory network mediated by the joint action of NF-κB, STAT3, and AP-1 factors is involved in many human cancers. Proc Natl Acad Sci USA. 2019;116:9453–9462. doi: 10.1073/pnas.1821068116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun T.K., Zheng S., Choi S.Y. Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers. J Med Food. 2010;13:489–494. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y., Kotakadi V.S., Ying L. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–2359. doi: 10.1093/carcin/bgn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poudyal P., Le P.M., Davis T. A hexane fraction of American ginseng suppresses mouse colitis and associated colon cancer: anti-inflammatory and proapoptotic mechanisms. Canc Prev Res. 2012;5:685–696. doi: 10.1158/1940-6207.CAPR-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sklyarov A.Y.A., Panasyuk N.B., Fomenko I.S. Role of nitric oxide synthase and cyclooxygenase/lipoxygenase systems in development of experimental ulcerative colitis. J Physiol Pharmacol. 2011;62:65–73. [PubMed] [Google Scholar]
- 24.Baek K.S., Yi Y.S., Son Y.J. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J.H., Kang T.K., Oh J.H., Jeong J.U., Ko K.P., Kim S.T. The effect of Korean red ginseng on symptoms and inflammation in patients with allergic rhinitis. Ear Nose Throat J. 2020 Feb 19 doi: 10.1177/0145561320907172. [Online ahead of print]. PMID: 32070136. [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Ahn H., Han B.C. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158:143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Keum Y.-S., Han S.S., Chun K.-S. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-κB activation and tumor promotion. Mutat Res. 2003;523–524:75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 28.Bae E.A., Kim E.J., Park J.S., Kim H.S., Ryu J.H., Kim D.H. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta Med. 2006;72:627–633. doi: 10.1055/s-2006-931563. [DOI] [PubMed] [Google Scholar]
- 29.Oh G.S., Pae H.O., Choi B.M. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-κB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Canc Lett. 2004;205:23–29. doi: 10.1016/j.canlet.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 30.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 31.Truong V.L., Bak M.J., Jeong W.S. Chemopreventive activity of red ginseng oil in a mouse model of azoxymethane/dextran sulfate sodium-induced inflammation-associated colon carcinogenesis. J Med Food. 2019;22:578–586. doi: 10.1089/jmf.2018.4328. [DOI] [PubMed] [Google Scholar]
- 32.Khan I., Ullah N., Zha L. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathog. 2019;8:126. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sartor R.B. Microbial-host interactions in inflammatory bowel diseases and experimental colitis. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:121–132. doi: 10.1159/000235787. [DOI] [PubMed] [Google Scholar]
- 34.Rath H.C., Schultz M., Freitag R. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69:2277–2285. doi: 10.1128/IAI.69.4.2277-2285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson M.E., Gustafsson J.K., Sjöberg K.E. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PloS One. 2010;5(8) doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osman N., Adawi D., Ahrné S. Probiotics and blueberry attenuate the severity of dextran sulfate sodium (DSS)-induced colitis. Dig Dis Sci. 2008;53:2464–2473. doi: 10.1007/s10620-007-0174-x. [DOI] [PubMed] [Google Scholar]
- 37.Dougherty U., Mustafi R., Wang Y. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: role of EGFR. BMC Compl Alternative Med. 2011;11:111. doi: 10.1186/1472-6882-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsuoka T. Intestinal flora and aging. Nutr Rev. 1992;50:438–446. doi: 10.1111/j.1753-4887.1992.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 39.Dong J., Liang W., Wang T. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol Res. 2019;144:66–72. doi: 10.1016/j.phrs.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Wang C.J., Yu C., Wen X.D. American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Canc Prev Res. 2016;9:803–811. doi: 10.1158/1940-6207.CAPR-15-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae E.A., Yoo Y.I., Lee I.A., Han M.J., Kim D.H. The anti-inflammmatory effect of fermented red ginseng in experimental colitic mice. Food Agric Immunol. 2008;19:313–323. [Google Scholar]
- 42.Jang S.H., Park J., Kim S.H. Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chin J Nat Med. 2017;15:192–201. doi: 10.1016/S1875-5364(17)30035-3. [DOI] [PubMed] [Google Scholar]
- 43.Wang C.F., Yao H., Zhang C.F. American ginseng microbial metabolites attenuate DSS-induced colitis and abdominal pain. Int Immunopharm. 2018;64:246–251. doi: 10.1016/j.intimp.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Li Juan, Zhong W., Wang W. Ginsenoside metabolite Compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PloS One. 2014;9 doi: 10.1371/journal.pone.0087810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.