Abstract
Background & Aims
Autoimmune hepatitis (AIH) is a disease of unknown aetiology with a favourable response to immunosuppression. However, in the clinic, it appears that <50% of patients achieve complete response on standard treatment. Serum B cell-activating factor (BAFF) levels are elevated in patients with AIH and are likely to contribute to disease pathogenesis. Given that belimumab, a BAFF inhibitor, has been shown to be effective in other autoimmune diseases, we investigated its use as a third-line add-on treatment option in patients with advanced AIH who did not respond to conventional treatment.
Methods
Herein, we report for the first time two patients, a 27-year-old female and a 58-year-old male, both with AIH-related compensated cirrhosis at diagnosis, who were refractory to standard immunosuppressive therapies and received add-on third-line therapy with belimumab.
Results
Both patients achieved a complete response and remained in remission while receiving low-dose corticosteroids. No adverse events related to belimumab and/or disease decompensation were observed.
Conclusions
These preliminary findings indicate belimumab as a promising treatment option for patients with AIH and refractory and advanced liver-related fibrosis.
Lay summary
A small proportion of patients with autoimmune hepatitis (AIH) are refractory to standard treatments; these patients bear the highest probability of developing decompensated cirrhosis and hepatocellular carcinoma because third-line treatment options are not well established. In this case study, we showed that third-line add-on therapy with belimumab, a B cell-activating factor inhibitor, could be an alternative and promising treatment option in patients with advanced AIH who did not respond to conventional treatment.
Keywords: Autoimmune hepatitis, Belimumab, BAFF, B cells, Treatment
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ANA, antinuclear antibody; AST, aminotransferase; AZA, azathioprine; BAFF, B cell-activating factor; CR, complete response; DCs, dendritic cells; EBV, Epstein–Barr virus; GGT, gamma-glutamyl transferase; LSM, liver stiffness measurements; MMF, mycophenolate mofetil; SLE, systemic lupus erythematosus; TNF, tumour necrosis factor; Tregs, regulatory T cells; ULN, upper limit of normal
Highlights
-
•
Belimumab is a B cell-activating factor inhibitor that has been proposed for the treatment of autoimmune hepatitis.
-
•
In 2 patients with autoimmune hepatitis, belimumab led to complete response and remission.
-
•
No adverse events related to belimumab and/or disease decompensation were observed.
Introduction
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown aetiology that affects all ages and races and is characterised by female predominance, hypergammaglobulinaemia, circulating autoantibodies, interface hepatitis, and a favourable response to immunosuppression.1 AIH has been considered a T cell-mediated disease with defective immunoregulatory control by regulatory T cells (Tregs).2 However, B lymphocytes also appear to be implicated in disease immunopathology, contributing to necroinflammatory activity and having a crucial role in the development of fibrosis.3
The standard of care for AIH treatment is immunosuppression with corticosteroids either alone or more frequently in combination with azathioprine (AZA)1 although, in Greece, recent guidelines also suggest mycophenolate mofetil (MMF) as potential first-line treatment instead of azathioprine.4 In addition, Liberal et al.5 showed that the second-line agent most widely used in the clinic for patients with difficult-to-treat AIH was MMF, followed by tacrolimus. Response rates to standard treatment vary widely from 25% to 80%, whereas, in a recent large study with real-world data from UK, the on-treatment remission rates were between 55% and 62%.6 However, there is a small proportion of patients with refractory disease who, at the same time, are those with the highest probability of developing long-term complications, such as decompensated cirrhosis and hepatocellular carcinoma. For these patients, alternative third-line treatment regimens have not yet been established and, thus, are urgently warranted.7
B cell-activating factor (BAFF), expressed by T lymphocytes and dendritic cells (DCs), is a cytokine belonging to the tumour necrosis factor (TNF) family and is crucial for the development and differentiation of B cells. In this context, serum BAFF levels have already been shown to be associated with liver inflammatory activity and improved in patients with AIH in response to corticosteroid therapy.8 Thus, inhibition of BAFF could be a pathogenetically justified third-line treatment option for refractory AIH because its successful use has been reported for other autoimmune diseases, such as systemic lupus erythematosus (SLE).9 Indeed, belimumab, a human monoclonal antibody targeting BAFF, has been approved for the treatment of SLE.9
Accordingly, we report here case studies of two patients with cirrhosis and well-established AIH-type 1, who were refractory to standard treatments but achieved complete response (CR) under third-line add-on therapy with belimumab.
Case presentation
Case 1
A 27-year-old female was first referred to our tertiary centre at the age of 19 years for consultation because of unexplained elevated liver enzymes accompanied by hypergammaglobulinaemia. Her previous medical history revealed elevated aminotransferases since 11 years of age, when she was found to have SLE because of arthralgias and positive antinuclear (ANA) and anti-double-stranded (ds)DNA antibodies by ELISA. She had been treated with methylprednisolone and hydroxychloroquine and was being followed by paediatricians in another centre. Of note, from not only the first diagnosis of SLE, but also during follow-up and despite immunosuppression, her liver biochemistry was persistently abnormal with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) 2 to 3 times above the upper limit of normal (ULN: 40 U/L) and immunoglobulin G (IgG) >1.5×ULN.
On initial evaluation in our department, the laboratory work-up revealed (abnormal findings): AST, 90 U/L; ALT, 105 U/L; gamma-glutamyl transferase (γ-GT), 164 U/L (ULN: 40 U/L); and IgG, 2,870 mg/dL (ULN: 1,690 mg/dL). Serological testing for hepatitis viruses A, B, C, and E, Epstein–Barr virus (EBV), cytomegalovirus (CMV), and herpes simplex virus (HSV) proved negative. Investigations for Wilson's disease, coeliac disease, and haemochromatosis were also unrevealing. Liver autoimmune serology by indirect immunofluorescence showed high titres of ANA (1/1280), smooth muscle antibodies (SMA: 1/2560), and perinuclear antineutrophil cytoplasmic antibodies (pANCA: 1/320). Liver histology revealed active cirrhosis [staging score 6 by modified Hepatic Activity Index (mHAI) and grading score 10/18 as attested by interface hepatitis +4, portal inflammatory infiltration with lymphocytes and plasmacytes +4, confluent necrosis +1, and focal lytic necrosis +1], while hepatocyte rosetting and emperipolesis were also present.
Accordingly, a diagnosis of AIH-related compensated cirrhosis was established and treatment with prednisolone (1 mg/kg/day) and AZA (1.5 mg/kg/day) was initiated, according to the European and Hellenic guidelines for the management of AIH1,2 (Fig. 1A). Treatment was continued for 12 months, but with only a partial response; each attempt to reduce corticosteroids to <15 mg/day resulted in disease relapse. Given the failure to achieve CR and according to our experience, AZA was discontinued and treatment with MMF (2 g/day) was initiated.2,10 Treatment was continued for 54 months with a partial response and again multiple disease flares during any attempt to decrease corticosteroids to <10 mg/day.
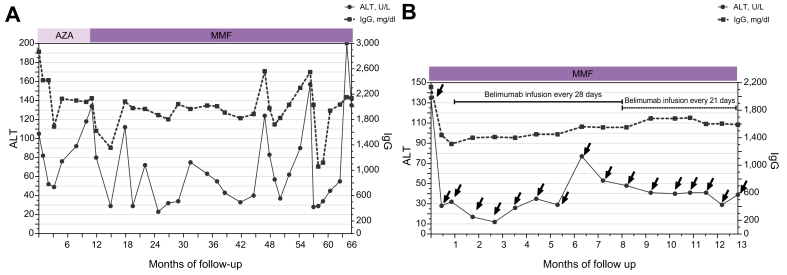
Fig. 1.
Patient 1.
(A) IgG and ALT serum levels during follow-up (66 months). Prednisolone 1 mg/kg/day was initiated at diagnosis followed by progressive tapering; every relapse of the disease determined by an increase in either ALT or IgG was managed with an increased dose of prednisolone (0.5–1 mg/kg/day). The patient also received AZA the first 12 months and then MMF. (B) ALT and IgG serum levels during belimumab infusions (arrows). The first three infusions were administered every 14 days. Initial prednisolone dose was 60 mg/day, with progressive tapering up to 5 mg/day along with MMF administration. ALT, alanine aminotransferase; AZA, azathioprine; IgG, immunoglobulin G; MMF, mycophenolate mofetil.
As a result of this insufficient response to second-line treatment in a young patient with cirrhosis and increased risk of corticosteroid-related adverse effects because of an inability to maintain a low corticosteroid dose, third-line add-on treatment with belimumab (10 mg/kg) was considered on month 66 of follow-up. After receiving the patient's consent and approval by the Greek National Organization for Medicines (GNOM) and Greek National Health Service Organization (GNHSO), belimumab was administered intravenously on Days 0, 14, and 28, and every 28 days thereafter based on previous publications on SLE. Standard treatment with prednisolone and MMF was maintained.
CR was achieved after the first infusion and remission was maintained for 6 months of follow-up along with a low dose of prednisolone (5 mg/day). On month 6 of CR, a mild elevation of liver enzymes (<2×ULN) was observed; therefore, prednisolone was increased to 10 mg/day and belimumab infusion intervals were shortened to 21-day intervals. Liver enzymes and IgG were normalised again, and remission was maintained for the next 6 months of follow-up (Fig. 1B). In addition, sequential liver stiffness measurements (LSM) by transient elastography (Echosens, Paris, France) were improved, from 14.6 kPa at the beginning of belimumab to 9 kPa at the end of follow-up, whereas a second liver biopsy at the end of follow-up showed significant improvement with minimal inflammatory activity and moderate fibrosis. In fact, the new liver histology revealed mHAI staging score of 3/6, while grading was decreased to 2/18, as attested by minimal portal inflammatory infiltration +1, no interface hepatitis or confluent necrosis, and focal lytic necrosis +1; of note neither emperipolesis nor rosetting was present. Therefore, belimumab was stopped and the patient is currently in CR under prednisolone (5 mg/day) and MMF 1.5 g/day. So far, no adverse events related to belimumab or any episode of decompensation of liver disease have been observed.
Case 2
A 58-year-old male was admitted to our hospital 6 years ago (52 years old at the time of admission) because of an episode of acute severe hepatitis. His previous medical history revealed another 2 episodes of acute severe icteric hepatitis during the previous 3 years, which were attributed by his physicians to acute alcoholic hepatitis (although the patient denied any alcohol misuse) and drug-induced liver injury (DILI) related to non-steroid anti-inflammatory drugs, respectively. His laboratory work-up at the first initial evaluation in our department revealed (abnormal findings): AST, 1,184 U/L; ALT, 1,617 U/L; γ-GT, 140 U/L; INR, 1.9; total bilirubin, 14.5 mg/dL; and IgG, 1,760 mg/dL (<1,690 mg/dL). Apart from jaundice, the physical examination was unrevealing.
After an extensive investigation for other causes of acute severe hepatitis (viral hepatitis because of hepatitis viruses A, B, C, and E, CMV, EBV, HSV, leptospirosis, acute Wilson's disease, acute alcoholic hepatitis, and DILI), the patient was found to have acute severe AIH based on positive liver autoimmune serology and typical liver histology.11 In brief, serology showed high titres of ANA (1/640), SMA (1/320), and anti-Ro52 antibodies (67 units; ULN, 20 units), whereas liver histology revealed advanced liver fibrosis with progression to cirrhosis (mHAI staging 5/6), along with 9/18 grading as shown by remarkable interface hepatitis (+4), portal inflammation (+4), and focal lytic necrosis (+1), in parallel with obvious emperipolesis and hepatocyte rosette formation.
The patient was treated with intravenous corticosteroids (methylprednisolone 1 g/day for 3 days), followed by prednisolone 1 mg/kg/day according to our experience11 and EASL guidelines.1 Combination treatment with AZA was initiated for remission maintenance; however, it was discontinued after a short period of time because of severe myelotoxicity. Therefore, treatment with MMF (2 g/day) along with progressive tapering of corticosteroids was initiated and maintained for 46 months (Fig. 2A). During this time, LSM values improved from 51.4 kPa at diagnosis to 21.8 kPa at 30-month follow-up in parallel with a decrease in ALT (from 1,617 to 50 U/L).
Fig. 2.
Patient 2.
(A) IgG and ALT serum levels during follow-up (47 months). Prednisolone 1 mg/kg/day was initiated at diagnosis, followed by progressive tapering; every relapse of the disease determined by increase in either ALT or IgG was managed with an increased dose of prednisolone (0.5–1 mg/kg/day). The patient was also receiving MMF. (B) ALT and IgG serum levels during belimumab infusions (arrows). The initial prednisolone dose was 60 mg/day with progressive tapering up to 7.5 mg/day along with MMF administration until the seventh belimumab infusion. ALT, alanine aminotransferase; IgG, immunoglobulin G; MMF, mycophenolate mofetil.
CR was achieved after 3 months of treatment. However, the patient had a new episode of acute AIH exacerbation when corticosteroid withdrawal was tried. Thereafter, he had multiple flares when corticosteroids were reduced to <15 mg/day. Therefore, on month 47 of follow-up, add-on treatment with belimumab (10 mg/kg) was considered. After receiving the patient's consent and approval by GNOM and GNHSO, belimumab was administered intravenously on days 0, 14, and 28, and every 28 days thereafter. Treatment with prednisolone and MMF (2 g/day) was maintained. After the third infusion, the patient was hospitalised because of a rectal fistula, which was managed efficiently with appropriate antibiotics. For this reason, the following fourth and fifth infusions were administered with a 45-day delay. CR was achieved after the fifth infusion and maintained for the next 8 months of follow-up, whereas prednisolone was tapered to 7.5 mg/day. After the seventh infusion, MMF was withdrawn because IgG was persistently declining (703 mg/dL). Following the tenth infusion, the patient is now receiving belimumab every 40 days with maintenance of CR (Fig. 2B), while LSM value has further improved (17.6 kPa at the end of follow-up in parallel with normal ALT, 35 U/L). Thus far, no severe adverse events related to belimumab or other episodes of acute exacerbation of AIH and/or any episode of decompensation of liver disease have been observed.
Discussion
CR to treatment is vital in AIH, because an insufficient response is associated with disease progression and poorer prognosis. Regardless, >50% of patients often do not achieve CR in the long-term by conventional therapy.1,7 However, second- and third-line therapies for AIH are not well established and are mainly based on centre experience and small series of patients.1,7 The major point arising from our case studies is that belimumab could be a promising option for difficult-to-treat patients, such as those with refractory AIH and advanced liver fibrosis, as seen in our case studies.
Several agents have been used as second-line treatments, but their effectiveness has not been examined in large clinical trials. MMF is the most widely used second-line agent, followed by tacrolimus, ciclosporin, and infliximab.6 However, although MMF appears to have high efficacy in patients with naïve AIH,10 the response rates when used as second-line treatment in difficult-to-treat patients vary from 32% to 82% among different cohorts, with the notion that it is more effective among those who fail AZA because of intolerance and/or the development of adverse effects.12,13 In addition, there is no consensus on the best time point for treating patients with third-line therapy in an attempt to achieve CR and tackle the disease progression. Therefore, the advent of the use of new drugs based on disease pathophysiology is a significant challenge in AIH.
Belimumab is a human monoclonal antibody that inhibits soluble BAFF, also known as B-lymphocyte stimulator, and is approved for SLE treatment.8 BAFF is vital for the proliferation, differentiation, and survival of B cells, and is also important for regulating class switch recombination as well as in the selection of autoreactive B cells. Three BAFF receptors have been identified: transmembrane activator and calcium-modulating and cyclophilin ligand interactor, BAFF-receptor (BAFF-R), and B cell maturation molecule. The binding and downstream signalling of BAFF and BAFF receptors are essential for B-cell survival and maturation. BAFF-R is the predominant BAFF receptor expressed not only on peripheral B cells, but also activated/memory T cells and, because BAFF costimulation drives T cell proliferation, BAFF might act as a survival factor of T cell activation as it does of B cells. Currently, the main indication of belimumab in SLE is as an add-on therapy in adults with active, autoantibody-positive SLE with a high degree of disease activity in the skin and/or musculoskeletal systems that remains active despite optimised standard immunosuppression. So far, the prolonged use of belimumab has been well tolerated and free of major adverse events.
Recently, further support of the importance of B-cell depletion therapies in AIH came from the use of a CD20-targeting treatment in difficult-to-manage AIH, which was shown to effectively induce remission of the disease.14 Under this context, belimumab could be an alternative add-on treatment for patients with AIH who are refractory to standard treatment regimens. Nevertheless, a multicentre Phase II/III trial (NCT03217422) is under way to estimate the efficacy of lanalumab (another BAFF receptor inhibitor) in patients with AIH non-responding to standard treatment.
By contrast, the TNF-α pathway has also been implicated in the induction of AIH, suggesting that the use of TNF-α inhibitors would have a pathophysiological basis for AIH management.15 Accordingly, infliximab efficacy has already been shown in some small uncontrolled series of difficult-to-treat AIH, albeit with increased incidence of infections.16 However, it is already known from the use of TNF-α blockade treatments that these agents can also be immunogenic, with the development of either autoantibodies or true autoimmune diseases, making such management a ‘double-edged sword’.17 Under this context, it has already reported that TNF-α inhibition can sometimes result in exactly the opposite outcome.[17], [18], [19], [20], [21] Indeed, in a recent large international pharmacovigilance database including 389 patients with anti-TNF-α inhibitor-associated AIH, it was shown that infliximab was the most frequently involved drug, followed by adalimumab and etanercept.17 This ‘therapeutic paradox’ is mainly attributed to the disruption of the regulatory role of TNF-α signalling in the immune system. Thus, inhibition of the TNF-α pathway appears to be supported for the management of refractory AIH. However, because of the problematic prediction of ‘unforeseen serious complications’, such as the emergence of severe infections or, in particular, the development and/or deterioration of autoimmunity, we believe that safer agents are required to support the use of such an approach.
Based on the aforementioned data, we selected a BAFF inhibitor instead of a TNF-α blockade regimen as third-line treatment for 2 patients with difficult-to-manage AIH who had also advanced but well-compensated disease. Both patients achieved CR after belimumab administration, which was maintained for 6 and 8 months, respectively, in parallel with effective corticosteroid tapering to low doses and good tolerability. Furthermore, in the first patient, we were able to demonstrate not only the biochemical response, but also improvements at the histological level. Of note, LSM values decreased considerably in both patients in association with the achievement of CR. These findings are in accordance with our experience in a series of almost 200 patients with AIH having serial LSM evaluations (Dalekos et al., 2020, unpublished data) and a recent study that showed that complete remission was: (i) strongly linked to regression of LSM values; and (ii) the only independent predictor for histological fibrosis regression that can be monitored by transient elastography in patients with AIH.22
Concluding remarks
In conclusion, both case studies in the present report suggest the potential use of anti-BAFF therapy as an alternative and promising add-on third-line treatment in cases of refractory AIH with advanced fibrosis. However, multicentre randomised control trials are warranted to assess whether BAFF-target in addition to CD20-target therapies could have good efficacy and safety in the short- and long-term in patients with difficult-to-manage AIH.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contribution
PA treated the patients; collected, analysed and interpreted the data, wrote the first draft of the manuscript; and gave final approval of the version to be published. GG and SG treated the patients; collected, analysed. and interpreted the data; and gave final approval of the version to be published. KZ developed the concept and design of the work; treated the patients; analysed and interpreted the data; wrote the first draft of the manuscript; and gave final approval of the version to be published. GKK performed the histological evaluations; analysed and interpreted the data; and gave final approval of the version to be published. GND developed the concept and design of the work; treated the patients; analysed and interpreted the data; critically revised the manuscript; and gave final approval of the version to be published.
Conflicts of interest
The authors declare that they have no conflict of interest or financial support in relation to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100123.
Supplementary data
References
- 1.European Association for the Study of the Liver EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Longhi M.S., Ma Y., Mieli-Vergani G., Vergani D. Aetiopathogenesis of autoimmune hepatitis. J Autoimmun. 2010;34:7–14. doi: 10.1016/j.jaut.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S.A., Assis D.N., Mack C.L. The contribution of B cells in autoimmune liver diseases. Semin Liver Dis. 2019;39:422–431. doi: 10.1055/s-0039-1688751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalekos G.N., Koskinas J., Papatheodoridis G.V. Hellenic Association for the Study of the Liver clinical practice guidelines: autoimmune hepatitis. Ann Gastroenterol. 2019;32:1–23. doi: 10.20524/aog.2018.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberal R., de Boer Y.S., Andrade R.J., Bouma G., Dalekos G.N., Floreani A. Expert clinical management of autoimmune hepatitis in the real world. Aliment Pharmacol Ther. 2017;45:723–732. doi: 10.1111/apt.13907. [DOI] [PubMed] [Google Scholar]
- 6.Dyson J.K., Wong L.L., Bigirumurame T., Hirschfield G.M., Kendrick S., Oo Y.H. Inequity of care provision and outcome disparity in autoimmune hepatitis in the United Kingdom. Aliment Pharmacol Ther. 2018;48:951–960. doi: 10.1111/apt.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyson J.K., De Martin E., Dalekos G.N., Drenth J.P.H., Herkel J., Hubscher S.G. Review article: unanswered clinical and research questions in autoimmune hepatitis-conclusions of the International Autoimmune Hepatitis Group Research Workshop. Aliment Pharmacol Ther. 2019;49:528–536. doi: 10.1111/apt.15111. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa H., Enomoto H., Iwata Y., Kishino K., Shimono Y., Hasegawa K. B-cell activating factor belonging to the tumor necrosis factor family and interferon-γ-inducible protein-10 in autoimmune hepatitis. Medicine (Baltimore) 2016;95:e3194. doi: 10.1097/MD.0000000000003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stohl W. Inhibition of B cell activating factor (BAFF) in the management of systemic lupus erythematosus (SLE) Expert Rev Clin Immunol. 2017;13:623–633. doi: 10.1080/1744666X.2017.1291343. [DOI] [PubMed] [Google Scholar]
- 10.Zachou K., Gatselis N., Arvaniti P., Gabeta S., Rigopoulou E.I., Koukoulis G.K. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis. Aliment Pharmacol Ther. 2016;43:1035–1047. doi: 10.1111/apt.13584. [DOI] [PubMed] [Google Scholar]
- 11.Zachou K., Arvaniti P., Azariadis K., Lygoura V., Gatselis N.K., Lyberopoulou A. Prompt initiation of high-dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol Res. 2019;49:96–104. doi: 10.1111/hepr.13252. [DOI] [PubMed] [Google Scholar]
- 12.Santiago P., Schwartz I., Tamariz L., Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:830–839. doi: 10.1111/apt.15157. [DOI] [PubMed] [Google Scholar]
- 13.Baven-Pronk A.M., Coenraad M.J., van Buuren H.R., de Man R.A., van Erpecum K.J., Lamers M.M. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34:335–343. doi: 10.1111/j.1365-2036.2011.04727.x. [DOI] [PubMed] [Google Scholar]
- 14.Than N.N., Hodson J., Schmidt-Martin D., Taubert R., Wawman R.E., Botter M. Efficacy of rituximab in difficult-to-manage autoimmune hepatitis: results from the International Autoimmune Hepatitis Group. JHEP Rep. 2019;1:437–444. doi: 10.1016/j.jhepr.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamoto S., Kido M., Aoki N., Nishiura H., Maruoka R., Ikeda A. TNF-α is essential in the induction of fatal autoimmune hepatitis in mice through upregulation of hepatic CCL20 expression. Clin Immunol. 2013;146:15–25. doi: 10.1016/j.clim.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Weiler-Normann C., Schramm C., Quaas A., Wiegard C., Glaubke C., Pannicke N. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529–534. doi: 10.1016/j.jhep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer O., Felten R., Mertz P., Lebrun-Vignes B., Salem J.E., Arnaud L. Characterization of auto-immune hepatitis associated with the use of anti-TNFα agents: an analysis of 389 cases in VigiBase. Autoimmun Rev. 2020;19:102460. doi: 10.1016/j.autrev.2020.102460. [DOI] [PubMed] [Google Scholar]
- 18.van Casteren-Messidoro C., Prins G., van Tilburg A., Zelinkova Z., Schouten J., de Man R. Autoimmune hepatitis following treatment with infliximab for inflammatory bowel disease. J Crohns Colitis. 2012;6:630–631. doi: 10.1016/j.crohns.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam K., Chitturi S., Brown M., Pavli P. Infliximab-induced autoimmune hepatitis in Crohn's disease treated with budesonide and mycophenolate. Inflamm Bowel Dis. 2011;17:E149–E150. doi: 10.1002/ibd.21880. [DOI] [PubMed] [Google Scholar]
- 20.Saitis A., Gatselis N., Zachou K., Dalekos G.N. Use of TNFα antagonists in refractory AIH: revealing the unforeseen. J Hepatol. 2013;59:197–198. doi: 10.1016/j.jhep.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Valgeirsson K.B., Hreinsson J.P., Björnsson E.S. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. 2019;39:2341–2349. doi: 10.1111/liv.14224. [DOI] [PubMed] [Google Scholar]
- 22.Hartl J., Ehlken H., Sebode M., Peiseler M., Krech T., Zenouzi R. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68:754–763. doi: 10.1016/j.jhep.2017.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.