Abstracts
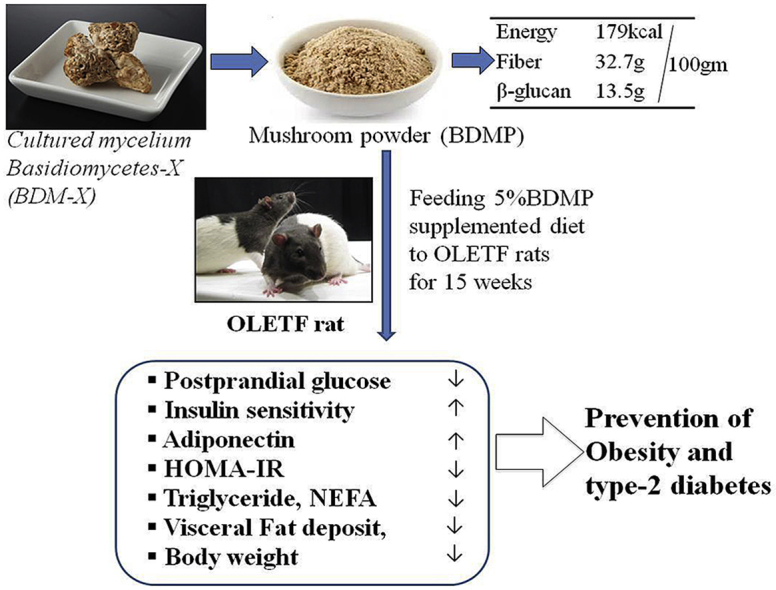
Echigoshirayukidake is an edible mushroom found in Uonuma, Japan in 1994. It was assigned to a new species of Basidiomycetes (BDM-X) but is uniquely defect of forming bashidium. The high antioxidant activity and β-glucan content of BDM-X suggest possible functions preventing type 2 diabetes. In the present study, anti-obesity and insulin resistance preventive functions of BDM-X were examined using genetically defined obese model rat, OLETF (Otsuka Long Evans Tokushima Fatty) by feeding regular diet with and without supplementation of 5% dried BDM-X powder (BDMP) for 15 weeks. BDMP supplementation to the diet significantly (p < 0.01) suppressed the body weight gain and also visceral fat accumulation during the feeding period compared to control diet. Simultaneously, the insulin resistance and the plasma levels of adiponectin and triglycerides were significantly (p = 0.003) ameliorated in the BDMP supplemented diet group. A statistical multivariate analysis showed the weight of three types of adipose tissue (epididymal, retroperirenal, and mesenteric fat) positively correlated with HOMA-IR (Homeostasis Model Assessment of Insulin Resistance), and negatively correlated with plasma adiponectin. These results indicate BDM-X is a new resource applicable to the functional foods or the complementary biomedicines to prevent metabolic syndromes leading to type 2 diabetes.
Keywords: Echigoshirayukidake (BDM-X), Obesity, Type 2 diabetes, Insulin resistance, Adiponectin
Abbreviations: BDM-X, Basidiomycetes-X; BDMP, BDM-X powder; OLETF, Otsuka Long Evans Tokushima Fatty rat; LETO, Long-Evans Tokushima Otsuka rat; OGTT, Oral Glucose Tolerance Test; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; NEFA, Non-Esterified Free fatty Acid
Graphical abstract
Highlights
-
•
A new mushroom, Echigoshirayukidake (BDM-X), ameliorates postprandial sugar and insulin spike enhancing insulin sensitivity.
-
•
BDM-X prevented body weight gain, hyperlipidemia, NEFA, and visceral fat deposition.
-
•
HOMA-IR was improved by BDM-X.
-
•
Anti-metabolic syndrome effect of BDM-X could be related to increase of adiponectin level.
1. Introduction
It is estimated that approximately, a quarter of the world’s adult population suffer from the metabolic syndromes being a major public health issue worldwide.1 Type 2 diabetes is one of the most common chronic diseases led by obesity related metabolic syndromes including insulin resistance and high blood sugar, and is the fourth or fifth leading causes of death in developed countries.2,3 Although lifestyle management or medication can control the obesity that is the risk factors of type 2 diabetes, it is hard to achieve long-term body weight control. Several drugs such as thiazolidinediones have been developed to improve insulin sensitivity and are used for treating diabetic condition, but are reported to have adverse effects such as hepatotoxicity and cardiovascular risk.4 As an alternate approach to fight against the lifestyle related disorders including obesity, much attention is paid on the herbs and natural products. Several plant resources have traditionally been used for treating diabetes and related conditions in folk medicine5 such as Morus alba and Salasia oblongs that contain 1-deoxynojirimycin and salasinol, respectively, as the active ingredient.6,7 Edible mushrooms are another targets of study because they have also been used as folk medicine to treat lifestyle related diseases in many countries.8
Echigoshirayukidake is the mushroom that was originally found in the mountain region of Uonuma area in Niigata, Japan, which does not form basidia but forms sclerotia similar to Truffle. Later, it was identified as Basidiomycetes-X (BDM-X) and registered to the database of the NPO organization for International Patent Organism Depositing (IPOD) in the Industrial Technology Institute of Japan (PCT/JP2004/006418). As many Basidiomycetes mushrooms are reported their variety of biological functions including anti-tumor activity,9,10 the health beneficial functions BDM-X have also been recognized and used locally as a folk medicine or tea for the purpose of nourishment or treating cancer. BDM-X as the fungal mycelium mass (sclerotia) is now produced by artificial culture and available in a market. BDM-X is a low calorie cuisine material and is rich in dietary fiber such as β-glucan (Table S1). The β-glucan is a well-known functional ingredient being effective for treating obesity related metabolic disorders like diabetes, cardiovascular diseases, liver lipotoxicity, and others.11,12 It is also reported that BDM-X has several times higher antioxidant potential than Agaricus Blazei Murill which is well-known antitumor mushroom also rich in β-glucans.13 It is evident that excess reactive oxygen species as well as oxidative stress-activated cellular signals are the mediators of insulin resistance and pancreatic β-cell dysfunction, and thus contribute to glucolipotoxicity in diabetes.14,15
The present study therefore attempts to examine the effects of this novel mushroom on obesity and insulin resistance using OLETF (Otsuka Long Evans Tokushima Fatty) rat as the model of type 2 diabetes.
2. Materials and methods
2.1. Animals and diets
OLETF is a genetically defined diabetes model rat that develops obesity, hyperinsulinemia, insulin resistance, hypertriglyceridemia, and hyperglycemia after 18 weeks of age.16 Age-matched male Long-Evans Tokushima Otsuka rat (LETO) served as the normal genetic control. OLETF and LETO rats were purchased from Tokushima Research Institute, Otsuka Pharmaceutical Co Ltd, Tokushima, Japan. Echigoshirayukidake mushroom (BDM-X) was cultured by Micology Techno Co., Ltd. (Niigata, Japan) and the dried powder (BDMP) was generously provided by the company for the experiments. The nutrient composition of BDMP was determined by Japan Food Research Laboratories (Tokyo, Japan) and is given in Table SI. The test diets for the present experiment were prepared in the laboratory based on the nutritional composition of regular standard chow (AIN-93 M, Zeigler, USA). The nutritional compositions of both regular standard chow and 5% BDMP supplemented chow are given in Table 1. Total protein contents and energy (calorie/100 g) of the BDM-X supplemented chow were adjusted to those of regular chow based on the analysis data of major nutrients in BDM-X (Table SI).
Table 1.
Dietary composition (% by weight) of BDMP containing and reference diets for feeding experiment.
| Components (g/100 g) | Control Diet (regular chow) | BDMP Diet (regular chow with 5% BDMP) |
|---|---|---|
| Casein lactic | 14.00 | 13.20 |
| Corn Sugar | 46.75 | 46.55 |
| Dextrin | 15.50 | 15.50 |
| Granular sugar | 5.00 | 5.00 |
| Cellulose | 3.50 | 1.87 |
| Soy oil | 1.00 | 0.905 |
| AIN-93 Vitamin mix | 1.00 | 1.00 |
| AIN-93 Mineral mix | 0.30 | 0.30 |
| l-Cysteine | 0.25 | 0.25 |
| Choline Bitartrate | 7.00 | 7.00 |
| BDMP | 0.00 | 5.00 |
2.2. Animal study
Male OLETF and LETO rats at 4 weeks age were acclimated for 7 days in the institutional animal facility and then randomly divided into three groups (n = 6): group 1; LETO with regular chow (LETO-normal), group 2; OLETF with regular chow (OLETF-control), and group 3; OLETF with BDMP supplemented (5% w/w) chow (OLETF-BDMP). All the rats were housed in three separate cages under controlled temperature (23 ± 2 °C), humidity (55 ± 5%) and artificial light cycle (12hr dark and 12hr light) condition. Rats were fed the respective chow for 15 weeks from the 6th week of age under the condition keeping free access to tap water and chow.
The animal study was conducted in accordance with the ethical guideline for the use and care of laboratory animals approved by the Niigata University of Pharmacy and Applied Life Sciences, Japan. The food intake of each rat was measured daily and the body weights were measured weekly using an electronic balance.
2.3. Oral glucose tolerance test (OGTT), blood and tissue sampling
At the end of feeding trial, all the animals were operated by arterial catheterization17 for a sequential sampling of arterial blood in the following oral-glucose-tolerance test (OGTT) that was done after an overnight fast. Arterial blood was sampled (833 mg/kg body weight, equivalent to 50 g glucose/60 kg adult) at 0, 15, 30, 60, 80, 100, 120, and 180 min after oral administration of glucose for the measurement of plasma glucose concentrations. The blood samples were centrifuged at 5000 rpm for 10 min at 4 °C using a refrigerated micro centrifuge model 3520 (Kubota Co. Ltd, Japan) to obtain blood plasma. After OGTT, the rats were sacrificed and the organ weights were determined. Visceral fat (epididymal, retroperirenal, and mesenteric fat) was dissected and weighed immediately. The relative visceral fat weight was calculated in relative to the body weight.18
2.4. Biochemical analysis of plasma markers
The plasma concentration of glucose, triglycerides (TG), and non-esterified fatty acids (NEFA) were measured using commercially available assay kits (Wako Pure Chemical Co. Ltd., Osaka, Japan). Insulin and adiponectin levels in the plasma were determined also using commercially available rat insulin ELISA Kit and mouse/rat HMW adiponectin ELISA kit (Shibayagi Co. Ltd., Gunma, Japan), respectively.
2.5. Calculation of the area under the curve (AUC) of OGTT and HOMA-IR
The area under the curve (AUC) of plasma glucose and also insulin concentrations (0–120 min during OGTT) were calculated using the trapezoid formula.19 The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the following formula.20
2.6. Statistical analysis
All the results are expressed as the mean ± standard error (SE) (n = 6). Statistical analysis was performed by SPSS (version 20) for Windows (IBM SPSS, Chicago, IL). Differences between experimental groups were evaluated by One-way ANOVA test. A Post Hoc multiple comparisons (LSD significance level at p < 0.05) were done for evaluating the differences caused by BDMP diet in adipose tissue weight and also other parameters among three groups. Correlations between individual parameter were determined by Pearson’s correlations coefficient.
3. Results
3.1. Effect of BDMP on food consumption, body weight gain, and organ weight
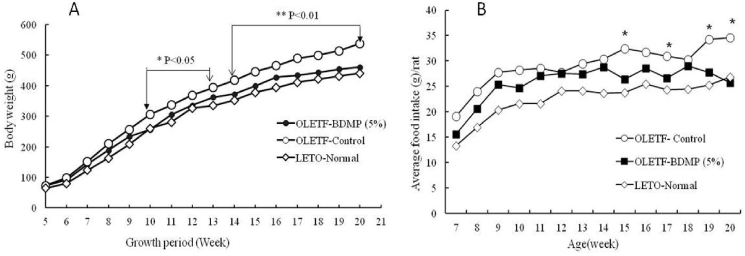
The body weight gain profiles of LETO control, obese OLETF control, and OLETF-BDMP groups during the feeding trial are shown in Fig. 1A. OLETF control gained the body weight significantly faster than LETO control (p < 0.05 and p < 0.01, respectively, after 5 and 9 weeks of feeding start). The body weight gain was markedly suppressed (p < 0.01) in OLETF-BDMP group compared to OLETF control, and the level was almost same as LETO control.
Fig. 1.
Effect of BDMP supplementation on Body weight changes (A) and summed weekly food consumption (B). Values represent mean ± SE (n = 6); ∗p < 0.05 and ∗∗p < 0.01indicate the significant difference between OLETF-BDMP and OLETF control groups.
When the daily food intake of each test group was compared, OLETF control was found to eat more than LETO normal group throughout the experimental period. BDMP supplementation suppressed the daily food intake of OLETF control, and the suppressive effect became obvious (p < 0.05) after 8 weeks of feeding start, although the daily intake level was yet higher than LETO control (Fig. 1B).
The average food intake and weight of several organs were compared for each experimental group at the end of 15 weeks feeding trial (Table S2). There was rather large difference in the averaged food intakes between LETO normal (22.37 g/day/rat) and OLETF control groups (29.05 ± 4.42 g/day/rat). The average food intake of OLETF-BDMP group (25.43 ± 4.60 g/day/rat) was also significantly low (p < 0.0003) compared to OLETF control. The organ weight determined in OLETF control rats was heavier than LETO control rats (p < 0.05) as expected from the body weight difference. The organ weights in OLETF-BDMP group were also low compared to OLETF control group but the differences were statistically insignificant, except spleen that showed significant difference.
3.2. Effect of BDMP supplemented diet on the glucose tolerance
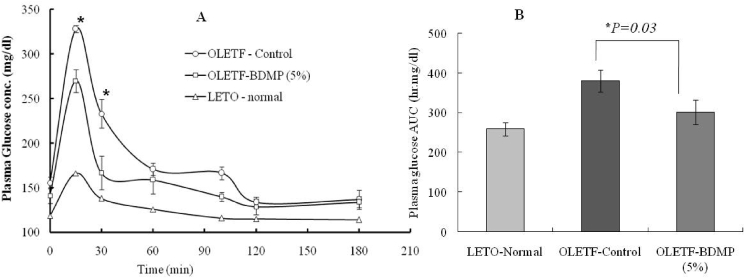
OGTT was carried out at the end of feeding trial. The plasma glucose concentration at 15 min after the oral glucose load was significantly higher (p < 0.05) in OLETF control group (328.13 mg/dl) than LETO control group (166.49 mg/dl). In OLETF-BDMP group, however, the blood glucose rise after oral glucose load was lower than OLETF control group, especially at 15 and 30 min (p < 0.05). The fasted glucose level of OLETF-BDMP determined before the oral glucose load (270.08 mg/dl) was also lower than OLETF-BDMP but still higher than LETO control group (Fig. 2A). Accordingly, the calculated AUC for 2 h plasma glucose showed a significant difference (p < 0.05) between OLETF control and OLETF- BDMP group (Fig. 2B).
Fig. 2.
Effect of BDMP supplemented diet to plasma glucose kinetics in OGTT (A) and the AUC (0–120min) (B). The ∗sign in (A) shows the significance level between the OLETF-BDMP and OLETF control groups.
3.3. Effect of BDMP supplemented diet to plasma insulin kinetics and HOMA-IR
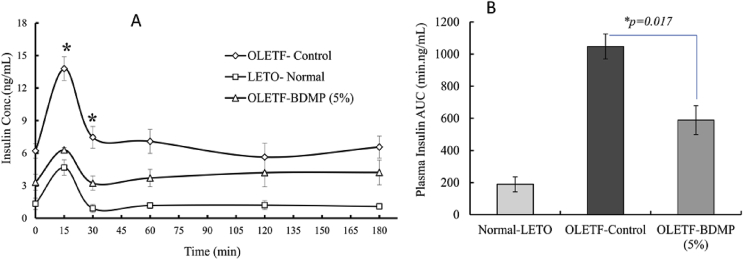
Fasting plasma insulin level was obviously higher in OLETF control group (6.21 ± 0.42 ng/mL) compared to LETO control groups (1.32 ± 0.38 ng/mL). The fasting plasma insulin level of OLETF-BDMP group (3.31 ± 0.4 ng/mL), however, was apparently low compared to OLETF control group, and was as low as the level of LETO control groups (1.32 ± 0.38 ng/mL) (Fig. 3A).
Fig. 3.
Effect of long term BDMP supplemented diet on plasma Insulin kinetics in OGTT (A) and insulin AUC (0–120min) (B). Values represent mean ± SE (n = 6); ∗p < 0.05 versus control group as determined by SPSS multivariate comparison.
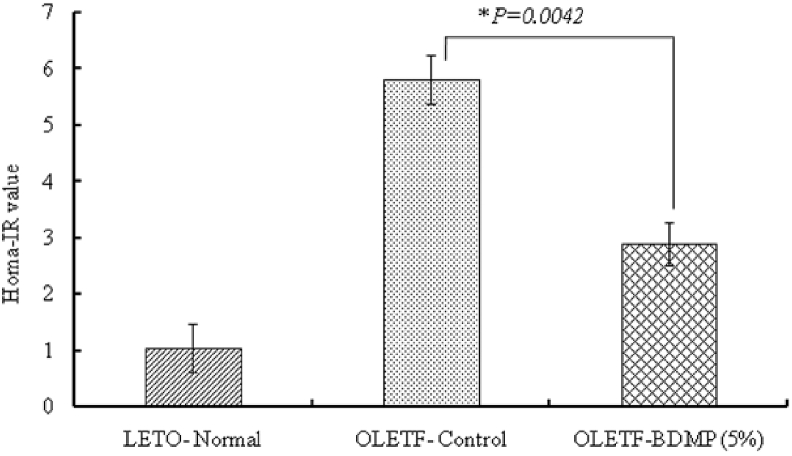
In OGTT, after oral glucose load, the rise of insulin level of OLETF control group such as at 15 and 30min was significantly (p < 0.01) higher than LETO control group. The initial rise of the plasma insulin level in OLETF control group was significantly decreased in OLETF-BDMP group, and the level was almost same as the level of LETO control group. The stationary level after 30 min of oral glucose load was also the highest in OLETF control group and then followed by OLETF-BDMP and LETO control group, respectively. AUC values calculated from the plasma insulin kinetic profiles are summarized in Fig. 3B. The HOMA-IR values calculated using these OGTT data are shown in Fig. 4. It was found that the supplementation of BDMP to the diet significantly decreased the high HORMA-IR value observed in OLETF control (p < 0.01), although the value was yet higher than LETO control.
Fig. 4.
Effect of BDMP supplemented diet on HOMA-IR value. Values represent mean ± SE (n = 6); ∗p < 0.05 shows significant difference versus OLETF control group.
3.4. Effect of BDMP supplemented diet on plasma lipids and triglyceride kinetics
The fasting plasma levels of triglycerides (TG), non-esterified fatty acids (NEFA) and postprandial cholesterol levels were determined after 15 weeks of feeding trial. They all were high in OLETF control group compared to LETO control group but their levels were significantly decreased (p < 0.05) in OLETF-BDMP group (Table S3).
The fasting plasma TG levels of both OLETF control and OLETF-BDMP groups were considerably high compared to LETO control group although the level of OLETF-BDMP was somewhat low compared to OLETF control group.
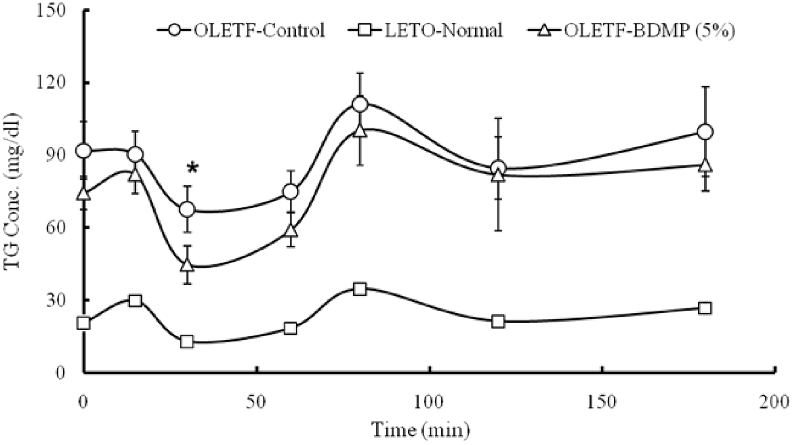
In OGTT experiment, the plasma level of TG after oral glucose load was maintained high in both OLETF control and OLETF-BDMP groups at all time-points measured compared to LETO control, although OLETF-BDMP group showed rather lower TG level than OLETF control group (the difference was statistically significant (p < 0.05) only at 30 min after oral glucose load). The kinetic profiles were similar to each other (Fig. 5).
Fig. 5.
Effect of BDMP supplemented diet on fasting plasma TG kinetics during OGTT. Values represent mean ± SE (n = 6); ∗p < 0.05 indicates significant difference versus control group.
3.5. Effect of BDMP supplemented diet on body fat accumulation and fat index
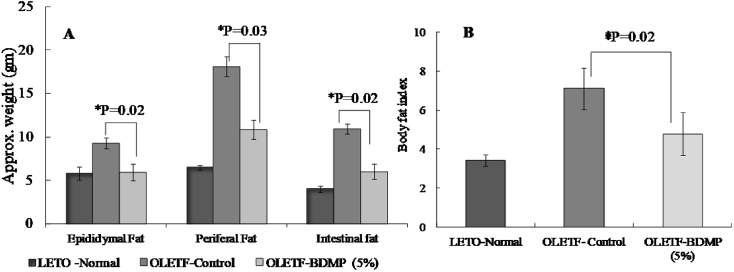
The visceral fat deposition was measured and the results showed marked differences among the three rat groups examined. OLETF control group showed significantly high deposition (p < 0.05) compared to LETO control group after 15 weeks feeding trial (Fig. 6A). However, in OLETF-BDMP, the fat accumulations were significantly decreased in all visceral tissues examined such as in epididymal (P = 0.02), mesenteric (P = 0.03), and retroperitoneal fat (p = 0.02), respectively. The body fatness was also compared by the body fat index that is the amount of visceral fat deposited in epididymal, mesenteric and retroperitoneal tissues per 100 g of body weight in Fig. 6B. OLETF control group showed obviously high value compared to LETO control group but the fat index was significantly decreased in OLETF-BDMP group to the level of LETO control group.
Fig. 6.
Effect of long term BDMP feeding on body fat distribution (A) and Body fat index (B). Values represent mean ± SE (n = 6); ∗p < 0.05 versus OLETF control group.
3.6. Effect of BDMP feeding on plasma adiponectin
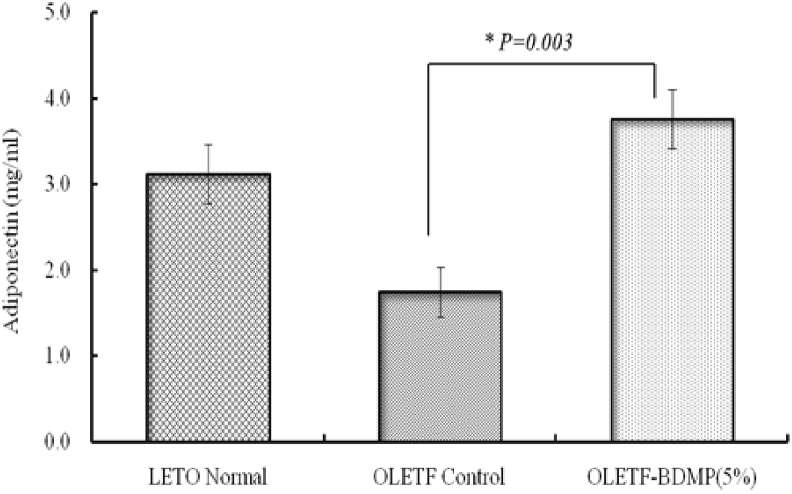
Plasma adiponectin level was measured for the three groups of rats after 15 weeks feeding trial, because adiponectin is a key modulator of sugar and fat metabolism.21 The adiponectin level was significantly decreased (p < 0.01) in OLETF control compared to LETO control group. However, in OLETF-BDMP group, the adiponectin level was significantly (p < 0.01) recovered and was even higher than LETO control group (Fig. 7).
Fig. 7.
Effect of BDMP feeding on plasma adiponectin level. Values represent mean ± SE (n = 6); ∗p < 0.05 versus OLETF control group.
3.7. Correlation analysis
Multivariate correlation analysis clearly showed a significant positive correlation between individual adipose tissue stores and the HOMA-IR (Table S4). The correlation coefficient for the epididymal, retropritoneal and mesenteric adipose tissues are: r = 0.799, p = 0.006; r = 0.819, p = 0.004; r = 0.760, p = 0.011, respectively, meaning that a low adiposity is associated with the lower blood glucose and insulin level. Further, as expected, the adiponectin level is negatively correlated with the epididymal (r = −-0.660, p= 0.038), retropritoneal (r = −-0.0590, p = 0.072), and mesenteric (r = −-0.661, p = 0.038) type adipose tissues.
4. Discussion
Mushrooms are well known healthy cuisine material because of their nutritional characteristics such that they are low in fat but rich in numerical nutrients including dietary fiber, minerals, and vitamins. Furthermore, their medicinal functions have been attracting a great attention, especially in the fields of anti-carcinogenesis, immune modulation and anti-metabolic syndromes.22 Therefore, the searches for not only functionality but also new species of mushroom are currently accelerated. From this view, Echigoshirayukidake (BDM-X) is an attractive target to study the health beneficial functions since it is a newly found edible mushroom having high antioxidant activity and β-glucans content. In the present study, we focused our attention to the anti-obesity function of BDM-X because obesity is the causative factor of type 2 diabetes that is characterized by such abnormalities as insulin resistance, hyperglycemia, and hyperlipidemia.2,6,23,24
The outcome of the study clearly showed the anti-obesity function of BDM-X when the body weight gain was compared between OLETF control and OLETF-BDMP group after 15 weeks feeding trial. Throughout the feeding period, the body weight gain in OLETF control group was larger than LETO control group, but the body weight gain in OLETF-BDMP group was almost same as LETO control group. Consistently, abdominal and peripheral fat depositions were low in OLETF-BDMP group compared to OLETF control group.
After the long term feeding trial of 15 weeks, fasting plasma glucose level in OLETF control group was significantly higher than LETO control group. Likewise, OGTT study revealed postprandial glucose spike and AUC of plasma glucose remarkably increased in OLETF control group (AUC, 379.88 h mg/dl) indicating OLETF control group has developed diabetic condition as indicated elsewhere.25 Simultaneously, both fasted and postprandial insulin levels in OLETF control group showed higher level than LETO control group. However, the marked increases of plasma glucose and insulin observed in OLETF control group were significantly suppressed in the OLETF-BDMP group (insulin AUC from approx. 1047.6 min ng/mL to 588.9 min ng/mL). Since the elevation of fasting plasma insulin level is positively correlated to the increased insulin resistance,26 it is indicated that BDM-X prevented the development of insulin resistance in OLETF rats during the feeding trial, and thus prevented hyperinsulinemia which leads to metabolic syndromes including impaired glucose tolerance, hypertension, and dyslipidemia.23 The preventive function of BDM-X against insulin resistance was further indicated when HOMA-IR as the marker of insulin resistance was calculated based on above results. The HOMA-IR value was significantly decreased in OLETF-BDMP group even though the level was still higher than LETO control group.
Various studies indicate the association of fasting plasma triglycerides (TG) level with insulin resistance in different ethnic groups.27,28 Therefore, hypertriglyceridaemia implicated as the most common abnormality of lipid metabolism observed in insulin resistance. As the postprandial TG level in OLETF-BDMP group was significantly decreased compared to OLETF control group, although the level was still much higher than LETO control group, it is suggested that BDM-X might decrease the risks of metabolic disorders associated with insulin resistance, such as atherosclerosis, and oxidative stress-mediated endothelial dysfunction.29, 30, 31 Adipocytokines take part in energy homeostasis including glucose and lipid metabolism in skeletal muscle and liver32,33 through modulation of insulin-induced signal transduction to improves insulin sensitivity.34 It was found in the present study that the plasma adiponectin level was markedly decreased in OLETF control group compared to LETO control group. However, in OLETF-BDMP group, the adiponectin level was remarkably recovered (P < 0.01) even to the level higher than LETO control group. Since hypoadiponectinemia is associated with a variety of metabolic syndromes like insulin resistance, cardiovascular disease and hypertension,21 BDM-X is predicted to suppress the metabolic syndromes through modulating adiponectin level. Indeed, multivariate correlation analysis showed that adiponectin level was negatively correlated with lipid storage in epididymal, retroperitoneal and mesenteric adipose tissues. This is consistent with the human study conducted on total of 73 Japanese men having type-2 diabetes, where the serum adiponectin level was negatively correlated with subcutaneous and visceral fat areas.35
Further, we found that plasma non-esterified fatty acids (NEFA) was significantly high in OLETF-control group compared to LETO control group, but was decreased in OLETF-BDMP group. This is consistent with the report that the increase of plasma NEFA resulting from the increased adipose tissue mass in obesity suppresses insulin receptor tyrosin phosphorylation leading impairment of insulin signaling,36 and thus mediates many adverse metabolic effects including the development of insulin resistance.25 It is also reported that the increased plasma glucose and NEFA cause oxidative stress along with activation of stress-sensitive signaling pathway, which disturb the insulin signaling and causes insulin resistance.37,38 Therefore, high antioxidant potential of BDM-X13 might play critical role for preserving the insulin sensitivity in obese rats.
We preliminarily studied the effect of BDMP on postprandial glucose rise in plasma after a single shot gavage of white rice with and without BDMP supplementation (1% w/w) to LETO rats. In that, BDMP supplementation significantly suppressed postprandial plasma glucose rise (data not shown). These data indicate the inhibition of postprandial glucose spike is one of the mechanisms involved in the obesity preventive action of BDM-X. It is also interesting to note that the food intake was significantly small in OLETF-BDMP group compared to OLETF control group, especially, at the later stage of feeding period. This indicates suppression of overeating might be another factor involved in the obesity prevention function of BDM-X. Recent study39 suggests that eating mushroom-rich breakfast causes feeling of less hunger and greater fullness so as to help weight management. Indeed, the lower body fat index was achieved in OFETF-BDMP group.
Currently, it becomes apparent that several peptide hormones such as ghrelin excreted from stomach play critical role in controlling appetite.40 Wang et al. (2007) reported that dietary fiber supplemented (10%) diet rat group significantly reduces ghrelin gene expression in stomach compared to high fat diet control group.41 Therefore, inhibition of ghrelin excretion in stomach might also explain the lower food intake observed in OLETF-BDMP group. In addition, presently observed suppressive effect of BDM-X on body weight gain will not be due to the toxic effect of BDM-X because there were no observable appearance changes such as hair condition during the feeding period, and the toxicity evaluation carried out elsewhere proved the safety of this mushroom after 90 days gavage of 3000 mg/day BDM-X in rats (not reported).
We recently identified three pyrrolaldehyde homologues as the major antioxidant ingredients from BDM-X (unpublished data). One of them has been reported as the antioxidant ingredient of Lycium chinense fruits having liver protecting function.42 Further precise studies in molecular level are warranted to know how these ingredients play roles in the anti-obesity and insulin resistance manipulating functions of BDM-X and also in appetite control, although the integrated action of these antioxidant food factors and other factors like β-glucan is also a plausible explanation for the anti-obesity and anti-diabetic functions of BDM-X.
5. Conclusion
Feeding Echigoshirayukidake (BDM-X) mushroom supplemented chow (5% w/w) for 15 weeks resulted in successful prevention of body weight gain, hyperlipidemia, and visceral fat deposition in genetically defined obese model rat (OLETF). At the same time, both the insulin sensitivity and decreased plasma adiponectin level were significantly ameliorated. Consequently, OGTT revealed suppression of postprandial plasma glucose spike as the outcome of improved insulin sensitivity. It is thus concluded BDM-X is a health beneficial new resource for developing functional foods or nutraceuticals preventing metabolic syndromes. However, further studies are obviously required to understand the underlying molecular mechanisms of anti-obesity and type-2 diabetes preventive functions of BDM-X.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
We thank Micology Techno Co. Ltd, in Niigata Japan for generously providing cultured mycelium powder of Echigo Shirayukidake for the experiments. This study was carried out in the project named Development of Second generation Functional foods and Application, supported by Grant-in-Aid (Contract numbers: s1001030) from the Promotion and Mutual Aid Corporation for Private Schools of Japan directed by Professor Tetsuya Konishi. The authors also would like to acknowledge Kana Kawazura for her helpful advice in rat cannulation technique.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.03.004.
Contributor Information
Mst Afifa Khatun, Email: afifabaec@gmail.com.
Tetsuya Konishi, Email: t.konishi@bg.wakwak.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.International Diabetes Federation The IDF consensus worldwide definition of the metabolic syndrome. 2006. http://www.idf.org/metabolic-syndrome Available at:
- 2.Fonseca V.A. The metabolic syndrome, hyperlipidemia, and insulin resistance. Clin Cornerstone. 2005;7(2-3):61–72. doi: 10.1016/s1098-3597(05)80069-9. [DOI] [PubMed] [Google Scholar]
- 3.Stern M.P., Williams K., Gonzalez-Villalpando C., Hunt K.J., Haffner S.M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27(11):2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 4.Triggle C.R., Ding H. Cardiovascular impact of drugs used in the treatment of diabetes. Ther Adv Chronic Dis. 2014;5(6):245–268. doi: 10.1177/2040622314546125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H., Kalita P., dwivedi P., Das A.K., Namsa N.D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya northeast, India. J Ethnopharmacol. 2012;141(3):786–795. doi: 10.1016/j.jep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Kim G.N., Kwon Y.I., Jang H.D. Mulberry leaf extract reduces postprandial hyperglycemia with few side effects by inhibiting a-glucosidase in normal rats. J Med Food. 2011;14(7–8):712–717. doi: 10.1089/jmf.2010.1368. [DOI] [PubMed] [Google Scholar]
- 7.Benalla W., Bellahcen S., Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010;6(4):247–254. doi: 10.2174/157339910791658826. [DOI] [PubMed] [Google Scholar]
- 8.Wasser S.P. Medical mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 9.Vetchinkina E., Shirokov A., Bucharskaya A. Antitumor activity of extracts from medicinal basidiomycetes mushrooms. Int J Med Mushrooms. 2016;18(11):955–964. doi: 10.1615/IntJMedMushrooms.v18.i11.10. [DOI] [PubMed] [Google Scholar]
- 10.Lemieszek M., Rzeski W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp Oncol. 2012;16(4):285–289. doi: 10.5114/wo.2012.30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han S., Jiao J., Zhang W. Dietary fiber prevents obesity-related liver lipotoxicity by modulating sterol-regulatory element binding protein pathway in C57BL/6J mice fed a high-fat/cholesterol diet. Sci Rep. 2015;5 doi: 10.1038/srep15256. Article number: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury D.E., Cuda C., Luhovyy B.L., Anderson G.H. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutri Met. 2012 doi: 10.1155/2012/851362. Article ID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T., Nakajima Y., Konishi T. In vitro and in vivo anti-oxidant activity of hot water extract of basidiomycetes-X, newly identified edible fungus. Biol Pharm Bull. 2008;31(1):111–117. doi: 10.1248/bpb.31.111. [DOI] [PubMed] [Google Scholar]
- 14.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Newsholme P., Haber E.P., Hirabara S.M. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano K., Hirashima T., Mori S., Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24(Suppl):S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 17.Niswender K.D., Shiota M., Postic C., Cherrington A.D., Magnuson M.A. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J Biol Chem. 1997;272:22570–22575. doi: 10.1074/jbc.272.36.22570. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Storlien L.H., Huang X.F. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab. 2002;282:E1352–E1359. doi: 10.1152/ajpendo.00230.2001. [DOI] [PubMed] [Google Scholar]
- 19.Purves R.D. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC) J Pharmacokinet Biopharm. 1992;20:211–227. doi: 10.1007/BF01062525. [DOI] [PubMed] [Google Scholar]
- 20.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F. Homeostasis model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadler M. Nutritional properties of edible fungi. British Nutrition Foundation Nutrition Bulletin. 2003;28:305–308. [Google Scholar]
- 23.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 24.Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi K., Takeda K., Maeda M. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2016;7:53–58. doi: 10.1007/s13340-015-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olefsky J., Farquhar J.W., Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Diabetes. 1973;22:507–513. doi: 10.2337/diab.22.7.507. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman R.P. Increased fasting triglyceride levels are associated with hepatic insulin resistance in Caucasian but not African-American adolescents. Diabetes Care. 2006;29(6):1402–1404. doi: 10.2337/dc06-2460. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero-Romero F., Villalobos-Molina R., Jiménez-Flores J.R. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Vossen M., Todter K., Altenburg C., Beisiegel U., Scheja L. Plasma triglycerides after oral glucose load specifically associate with metabolic risk markers in healthy type 2 diabetes offspring. Atherosclerosis. 2011;217(1):214–219. doi: 10.1016/j.atherosclerosis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Teno S., Uto Y., Nagashima H. Association of post-prandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23:1401–1406. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- 31.Anderson R.A., Evans M.L., Ellis G.R. The relationships between post-prandial lipidemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154:475–483. doi: 10.1016/s0021-9150(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 32.Berg A.H., Combs T.P., Scherer P.E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trend Endocrinol metab. 2002;13(2):84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T., Kamon J., Minokoshi Y. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 34.Gulcelik N.E., Usman A., Gurlek A. Role of adipocytokines in predicting the development of diabetes and its late complications. Endocrine. 2009;36:397–403. doi: 10.1007/s12020-009-9234-7. [DOI] [PubMed] [Google Scholar]
- 35.Yatagai T., Nagasaka S., Taniguchi A. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52(10):1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 36.Noushmehr H., D’Amico E., Farilla L. Fatty acid translocase (FAT/CD36) is localized on insulin containing granules in human pancreatic β-cells and mediates fatty acid effects on insulin secretion. Diabetes. 2005;54(2):472–481. doi: 10.2337/diabetes.54.2.472. [DOI] [PubMed] [Google Scholar]
- 37.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 38.Bloch-Damti A., Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxidants Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 39.Hess J.M., Wang Q., Krafta C., Slavin J.L. Impact of Agaricus bisporus mushroom consumption on satiety and food intake. Appetite. 2017;117:179–185. doi: 10.1016/j.appet.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Yoshihara F., Kojima M., Hosoda H., Nakazato M., Kangawa K. Ghrelin. A novel peptide for growth hormone release and feeding regulation. Curr Opin Clin Nutr Metab Care. 2002 Jul;5(4):391–395. doi: 10.1097/00075197-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z.Q., Zuberi A., Zhang X.H. Effects of dietary fibers on weight gain, carbohydrate metabolism and gastric ghrelin gene expression in high fat diet fed mice. Metabolism. 2007 December;56(12):1635–1642. doi: 10.1016/j.metabol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin Y.W., Lim S.W., Kim S.H. Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorg Med Chem Lett. 2003;13(1):79–81. doi: 10.1016/s0960-894x(02)00846-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.