Abstract
BACKGROUND
Mucinous cystic neoplasm (MCN) of the pancreas is characterized by mucin-producing columnar epithelium and dense ovarian-type stroma and at risk for malignant transformation. Early diagnosis and treatment of MCN are particularly important.
AIM
To investigate the clinical characteristics of and management strategies for pancreatic mucinous cystadenoma (MCA) and mucinous cystadenocarcinoma (MCC).
METHODS
The clinical and pathological data of 82 patients with pancreatic MCA and MCC who underwent surgical resection at our department between April 2015 and March 2019 were retrospectively analyzed.
RESULTS
Of the 82 patients included in this study, 70 had MCA and 12 had MCC. Tumor size of MCC was larger than that of MCA (P = 0.049). Age and serum levels of tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA12-5 were significantly higher in MCC than in MCA patients (P = 0.005, 0.026, and 0.037, respectively). MCA tumor size was positively correlated with serum CA19-9 levels (r = 0.389, P = 0.001). Compared with MCC, MCA had a higher minimally invasive surgery rate (P = 0.014). In the MCA group, the rate of major complications was 5.7% and that of clinically relevant pancreatic fistula was 8.6%; the corresponding rates in the MCC group were 16.7% and 16.7%, respectively.
CONCLUSION
Tumor size, age, and serum CEA, CA19-9, and CA12-5 levels may contribute to management of patients with MCN. Surgical resection is the primary treatment modality for MCC and MCA.
Keywords: Pancreatic neoplasms, Mucinous cystadenoma, Mucinous cystadenocarcinoma, Biochemical indexes, Diagnosis, Surgery
Core tips: In this study, we retrospectively analyzed the clinical and pathological records related with pancreatic mucinous cystadenoma (MCA) and mucinous cystadenocarcinoma (MCC). We found that the MCC tumor size was larger than that of MCA, and age, serum carcinoembryonic antigen, carbohydrate antigen (CA) 19-9, and CA12-5 levels were also higher in MCC patients. As the tumor size of MCA increased, the level of serum CA19-9 also increased. Surgical resection is the primary treatment for MCC and MCA.
INTRODUCTION
Mucinous cystic neoplasm (MCN) is a cyst-forming epithelial tumor composed of ovarian-type stroma and mucin-producing columnar epithelium[1]. It is a rare pancreatic disease that does not communicate with the pancreatic duct[2]. Currently, owing to the development of imaging and endoscopic techniques, as well as the increased understanding of the disease, the detection rate of MCN has been increasing every year. The biological characteristics of MCN can potentially lead to the development of malignant tumors, and atypical columnar cell hyperplasia can be observed on most cyst walls[3,4]. Mucinous cystadenocarcinoma (MCC) may be formed via the malignant transformation of MCN with the same origin. It is generally discovered when patients present at the clinic with obstructive jaundice and evident abdominal mass. MCC has a poor sensitivity to radiotherapy and chemotherapy, and surgical resection is the primary treatment modality for MCC[5]. Early diagnosis and treatment of MCN are particularly important because of the potentially malignant manifestations and the lack of specific clinical symptoms. Therefore, this study retrospectively analyzed the data of 82 patients with pancreatic MCN who underwent surgical resection at our department between April 2015 and March 2019.
MATERIALS AND METHODS
Study population
Between April 2015 and March 2019, a total of 82 patients who underwent surgery at our department were included, of whom 70 had mucinous cystadenoma (MCA) and 12 had MCC as confirmed by postoperative pathology findings. The pancreatic MCN was defined as a pancreatic cystic tumor lined by columnar mucin-producing cells and overlying ovarian-type stroma. Carcinoma in situ and invasive carcinomas were considered malignant (MCC) and other MCN considered as MCA in this study. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Patient characteristics in the two treatment groups, n (%)
| Patient characteristic | MCA (n = 70) | MCC (n = 12) | P value |
| Age, yr, mean ± SD | 46.2 ± 13.1 | 56.8 ± 9.4 | 0.0081 |
| Sex (male:female) | 5:65 | 2:10 | 0.271 |
| Location, distal pancreas | 54 (77.1) | 7 (58.3) | 0.280 |
| Tumor size, cm, median (IQR) | 3.5 (2.5-6.1) | 5.8 (4.0-6.9) | 0.0491 |
| CEA (µg/L), median (IQR) | 1.4 (1.0-2.2) | 2.7 (1.6-5.5) | 0.0051 |
| > 5 µg/L | 2 | 3 | 0.0211 |
| CA19-9 (U/mL), median (IQR) | 14.2 (8.5-29.1) | 39.9 (13.0-71.0) | 0.0261 |
| > 37 U/mL | 13 | 6 | 0.0271 |
| CA12-5 (U/mL), median (IQR) | 12.1 (7.7-19.4) | 19.0 (10.8-36) | 0.0371 |
| > 35 U/mL | 3 | 3 | 0.0381 |
| Operative, minimally invasive | 66 (94.3) | 8 (66.7) | 0.0141 |
Values are statistically significant. MCA: Mucinous cystadenoma; MCC: Mucinous cystadenocarcinomas; IQR: Interquartile range; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; CA12-5: Carbohydrate antigen 12-5.
Preoperative evaluation and postoperative management
The surgical indications for MCA were based on the International Association of Pancreatology consensus guidelines[6-8]. Postoperative complication was defined as a complication occurring within 30 d after surgery or before discharge from the hospital. Clavien-Dindo grades II or less complications were categorized as moderate complications, and Clavien-Dindo grades III, IV, and V were considered major complications (graded by the Clavien-Dindo classification[9]). According to the 2016 update of the International Study Group on Pancreatic Surgery classification[10], fistulas of grades B and C were defined as clinically relevant pancreatic fistulas (CRPFs).
Study methods
Baseline patient characteristics, preoperative imaging results, preoperative laboratory parameters, intraoperative data, postoperative pathology, and postoperative complications were collected and analyzed.
Statistical analysis
Statistical analyses were performed using SPSS 22. Continuous variables are expressed either as the mean ± SD or median and interquartile range (IQR) depending on whether a normal distribution was verified. Specifically, data on age were normally distributed, and t test was used for comparisons; data on tumor size, serum carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, and CA19-9 did not follow a normal distribution, and Mann–Whitney U test was used for comparisons. Correlation testing was conducted using Spearman rank correlation test. Discrete data are represented as rates (%), and were compared using Fisher's exact test. A P value < 0.05 was considered statistically significant.
RESULTS
Pathology and symptoms
According to the pathology examination of the postoperative paraffin sections, there were 12 patients with MCC (including 3 cases of carcinoma in situ) and 70 patients with MCA.
The MCA tumor size was between 1.5 cm and 10 cm, with a median (IQR) of 3.5 cm (2.5-6.1 cm), and the MCC tumor size was between 2.5 and 10 cm, with a median (IQR) of 5.8 cm (4.0-6.9 cm). The tumor size of MCC was larger than that of MCA, and the difference was statistically significant (P = 0.049, Table 1).
Of the 70 patients with MCA, 22 had nonspecific upper abdominal bloating and abdominal pain, 11 had a palpable abdominal mass detected during physical examination, 4 had weight loss, 1 had jaundice, and 1 had gastrointestinal symptoms, such as nausea, vomiting, and fatigue. Of the 12 patients with MCC, 4 had a palpable abdominal mass, 4 had abdominal pain, and 2 had jaundice.
Tumor marker testing results
Chemiluminescent immunoassay was performed to detect serum CEA, CA19-9, and CA12-5.
Mann-Whitney U test showed that the serum levels of all the three markers (CEA, CA19-9, and CA12-5) were significantly higher in MCC than in MCA patients (P = 0.005, 0.026, and 0.037, respectively), while the percentages of patients with CEA > 5 µg/L, CA19-9 > 37 U/mL, or CA12-5 > 35 U/mL were higher in MCC patients than in MCA patients (P = 0.021, 0.027, and 0.038, respectively; Table 1). Furthermore, the MCA tumor size was positively correlated with serum CA19-9 levels (r = 0.389, P = 0.001).
Imaging results
Imaging results showed that MCA tumors were located in the head of the pancreas in 13 (18.6%) patients, in the neck of the pancreas in 3 (4.3%), and in distal pancreas (the body and tail of the pancreas) in 54 (77.1%). MCC tumors were located in the head of the pancreas in 5 (41.7%) patients and in the body and tail of the pancreas in 7 (58.3%).
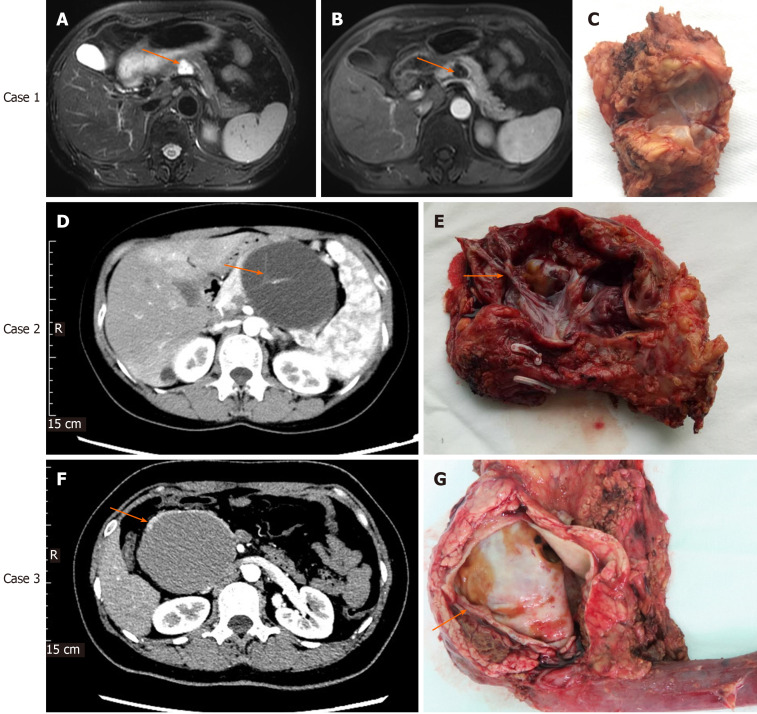
MCA usually appeared as oligocystic or macrocystic lesions with < 6 cysts, and the inner cyst diameter was generally larger than 2 cm. MCA often occurred in the body and tail of the pancreas. If the possibility of pancreatic pseudocyst was ruled out, the diagnosis of MCA should be considered for oligocystic lesions that occurred in the body and tail of the pancreas in middle-aged women (Figure 1). The risk of malignant transformation should be considered when the diameter of the cyst was too large (Figure 1).
Figure 1.
Imaging and histological characteristics of pancreatic mucinous cystadenoma and mucinous cystadenocarcinomas. Case 1: Contrast-enhanced magnetic resonance imaging (MRI) for pancreatic mucinous cystadenoma (MCA). A: Pronounced cystic lesion approximately 2 cm in length in the body of the pancreas (arrow) as seen on a T2W axial MRI image; B: Cyst wall and internal septations enhancement in the portal phase; C: Cut surface of the tumor with MCA pathology; Case 2: Contrast-enhanced computed tomography (CT) for pancreatic MCA. D: Cystic lesion in the body of the pancreas observed in the arterial phase of CT, with prominently enhanced internal septations (arrow); E: Cut surface of the tumor, with visible and pronounced internal septations (arrow) and MCA pathology; Case 3: Contrast-enhanced CT for pancreatic mucinous cystadenocarcinoma (MCC). F: Cystic lesion in the head of the pancreas observed in the arterial phase of CT. The cyst wall was thickened, but no internal septations were seen; G: Cut surface of the tumor. No internal septations can be seen. The thickness of the cyst wall measured approximately 3.5 mm (arrow); pathology testing showed features of MCC.
Surgery and postoperative complications
Among the 70 patients with MCA, 4 underwent open surgery, 7 underwent laparoscopic surgery, and 59 underwent robotic surgery. The rate of minimally-invasive surgery was 94.3%. Among the 12 patients with MCC, 4 underwent open surgery and 8 underwent robotic surgery. The rate of minimally-invasive surgery was 66.7%. Minimally invasive surgery was significantly more frequent in patients with MCA compared with those with MCC (Table 1).
For patients with MCA, the rate of major complications was 5.7% and that of CRPF was 8.6%. The median postoperative hospital stay was 6.5 d. Postoperative complications are shown in Table 2. For patients with MCC, the rate of major complications was 16.7% and that of CRPF was 16.7%. The median postoperative hospital stay was 9 d. Postoperative complications are shown in Table 3.
Table 2.
Postoperative complications of pancreatic mucinous cystadenoma (n = 70)
| Feature | n (%) |
| Pancreaticoduodenectomy | 9 (12.9) |
| Distal pancreatectomy | 46 (65.7) |
| Central pancreatectomy | 3 (4.3) |
| Enucleation | 12 (17.1) |
| Major complications (Clavien-Dindo ≥ 3) | 4 (5.7) |
| CRPF | 6 (8.6) |
| Grade B | 6 (8.6) |
| Grade C | 0 (0) |
| No CRPF | 64 (91.4) |
| Biochemical Leak | 36 (51.4) |
| Normal enzyme level | 28 (40.0) |
| Postoperative haemorrhage | 2 (2.9) |
| Delayed gastric emptying | 2 (2.9) |
| 90-d mortality | 0(0) |
| Postoperative hospital stay, days, median (IQR) | 6.5 (5.0-8.0) |
MCA: Mucinous cystadenoma; CRPF: Clinically relevant pancreatic fistula; IQR: Interquartile range.
Table 3.
Postoperative complications of mucinous cystadenocarcinoma (n = 12)
| Feature | n (%) |
| Pancreaticoduodenectomy | 5 (41.7) |
| Distal pancreatectomy | 7 (58.3) |
| Major complications (Clavien-Dindo ≥ 3) | 2 (16.7) |
| CRPF | 2 (16.7) |
| Grade B | 2 (16.7) |
| Grade C | 0 (0) |
| No CRPF | 10 (83.3) |
| Biochemical leak | 6 (50.0) |
| Normal enzyme level | 4 (33.3) |
| Postoperative haemorrhage | 2 (16.7) |
| Delayed gastric emptying | 2 (16.7) |
| 90-d mortality | 0 (0) |
| Postoperative hospital stay, days, median (IQR) | 9.0 (7.3-13.5) |
MCC: Mucinous cystadenocarcinomas; CRPF: Clinically relevant pancreatic fistula; IQR: Interquartile range.
DISCUSSION
Approximately 90% of MCNs occur in middle-aged premenopausal women[11]. MCNs accounts for approximately 10% of pancreatic cystic lesions, most of which are solitary cystic lesions typically located in distal pancreas[12] and possess the potential to become MCC. In this study, MCAs were primarily located in distal pancreas (77.1%), whereas 58.3% of MCCs were found in distal pancreas.
MCA is generally unilocular or multilocular, with a cyst diameter > 2 cm, and the internal fibrous septations are more apparent after enhancement[13,14]. Studies have drawn different conclusions regarding the specific threshold value of cyst diameter over which the risk of malignancy is increased. It is generally believed that the cyst wall diameter in malignant MCN is usually > 4 cm[15], or that a diameter of ≥ 6 cm is a risk factor for malignant tumors[11,16,17]. In addition, other manifestations suggestive of malignant MCA include peripheral calcification, irregularly contoured cyst walls, thickening of internal septations, increased papillary projections, intracystic nodules, local organ invasion, and vascular obstruction and compression. Di Paola et al[16] studied 65 patients with MCNs who underwent magnetic resonance imaging and found that there may be a risk of malignant transformation if the diameter is greater than 7 cm, septa and wall thickness was > 3 mm, and there were nodules. In this study, the median diameter of MCA was 3.5 cm and that of MCC was 5.8 cm. The MCC size was larger than that of MCA. Because malignant MCN less than 4 cm is rare (0.03%[18]), European Guidelines use this as a cut-off size for surveillance without resection[19]. However, one (8.3% of MCCs) patient in the current study with a tumor of 2.5 cm had invasive carcinoma. The cut-off value of tumor size might be reconsidered in the future revisions of guidelines.
Recently, a large multicenter study[1] on MCN showed that older age, high levels of serum CEA or CA19-9, large tumor size, and the presence of mural nodules were risk factors for MCC. Similar results were also observed in the current study. Age and serum levels of tumor markers CEA, CA19-9, and CA12-5 were significantly higher in MCC than in MCA patients. In addition, our study showed that the MCA tumor size was positively correlated with the level of serum CA19-9.
Given the challenges in the diagnosis of pancreatic cystic diseases, as well as the high malignant potential of MCN, the International Association of Pancreatology consensus guidelines recommended surgical resection. However, conventional laparotomy is associated with several issues, such as an overly large incision, delayed recovery, and significant psychological burden on the patients. With the development of minimally invasive technology, the use of laparoscopy and robotics has successfully eliminated the above-mentioned problems. Especially for younger patients, there is an urgent need for aesthetics of the wound and high quality of life after operation. Compared with laparoscopy, robotic surgery has distinct technical advantages, including the high-definition three-dimensional stereoscopic visualization, the flexible biomimetic mechanical wrist, and the stable tremor-free arm[20,21]. These advantages allow for the precise dissection and fine suturing required in pancreatic surgery[22,23]. In this study, the minimally invasive operation rate in the MCA group was 94.3%, which was higher than that (66.7%) of the MCC group. In minimally invasive surgery, robotic procedures accounted for the majority. Among patients with MCA included in the present study, 65.7% underwent distal pancreatectomy, 12.9% underwent pancreaticoduodenectomy, 4.3% underwent central pancreatectomy, and 17.1% underwent enucleation. For patients with MCC, 58.3% underwent distal pancreatectomy and 41.7% underwent pancreatico-duodenectomy. Distal pancreatectomy is a common surgery for MCN and the spleen should be preserved as much as possible for patients with MCA. In the MCA group, the rate of major complications was 5.7% and that of grade B pancreatic fistula was 8.6% with no grade C, which were slightly lower than other reports on pancreatectomy available in the literature[24,25].
This study had several shortcomings. First, the number of patients included is small, and as a single-center study, there may be statistical bias. Second, this study is retrospective; thus selection bias cannot be eliminated. The conclusions of this study still need to be validated in multi-center large-scale studies in the future.
In summary, MCN is commonly found in middle-aged women and typically occurs in the body and tail of the pancreas. Most MCN are oligocystic or macrocystic lesions with malignant potential. There remain considerable challenges for a definite diagnosis prior to surgery. Older age, high levels of serum CEA, CA19-9, or CA12-5, large tumor size, and the presence of mural nodules were risk factors for MCC. Minimally invasive surgical resection is a safe and effective treatment modality for patients with MCC and MCA.
ARTICLE HIGHLIGHTS
Research background
Mucinous cystic neoplasm (MCN) of the pancreas is characterized by mucin-producing columnar epithelium and dense ovarian-type stroma and at risk for malignant transformation. Early diagnosis and treatment of MCN are particularly important.
Research motivation
We comprehensively evaluated the clinical and pathological characteristics of MCA and MCC and further explored effective treatment strategy.
Research objectives
In this study, the authors aimed to investigate the clinical characteristics of and management strategies for pancreatic mucinous cystadenoma (MCA) and mucinous cystadenocarcinomas (MCC).
Research methods
The clinical and pathological data of 82 patients with pancreatic MCA and MCC who underwent surgical resection at our department between April 2015 and March 2019 were retrospectively analyzed.
Research results
Of the 82 patients included in this study, 70 had MCA and 12 had MCC. Tumor size of MCC was larger than that of MCA. Age and serum levels of tumor markers carcinoembryonic antigen (CEA), CA19-9, and CA12-5 were significantly higher in MCC than in MCA patients. MCA tumor size was positively correlated with serum CA19-9 levels. Compared with MCC, MCA had a higher minimally invasive surgery rate. In the MCA group, the rate of major complications was 5.7% and that of clinically relevant pancreatic fistula was 8.6%; the corresponding rates in the MCC group were 16.7% and 16.7%.
Research conclusions
Tumor size, age, and serum CEA, CA19-9, and CA12-5 levels may contribute to management of patients with MCN. Surgical resection is the primary treatment modality for MCC and MCA.
Research perspectives
Age and serum CEA, CA19-9, and CA125 levels can be used as an effective tool to help clinicians quickly identify MCC and MCA. Minimally invasive surgical resection is an effective treatment for MCC and MCA.
Footnotes
Institutional review board statement: The study was approved by the Institutional Review Board of the Chinese People's Liberation Army General Hospital (S2016-098-02).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: April 30, 2020
First decision: May 15, 2020
Article in press: May 21, 2020
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chamberlain MC, Dueland S, Kressel A, Sumi K S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Qi LL
Contributor Information
Zhi-Ming Zhao, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Nan Jiang, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Yuan-Xing Gao, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Zhu-Zeng Yin, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Guo-Dong Zhao, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Xiang-Long Tan, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Yong Xu, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Rong Liu, Second Department of Hepatopancreatobiliary Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing 100853, China. liurong301@126.com.
Data sharing statement
No additional data are available.
References
- 1.Ohtsuka T, Nakamura M, Hijioka S, Shimizu Y, Unno M, Tanabe M, Nagakawa Y, Takaori K, Hirono S, Gotohda N, Kimura W, Ito K, Katanuma A, Sano T, Urata T, Kita E, Hanada K, Tada M, Aoki T, Serikawa M, Okamoto K, Isayama H, Gotoh Y, Ishigami K, Yamaguchi H, Yamao K, Sugiyama M, Okazaki K. Prediction of the Probability of Malignancy in Mucinous Cystic Neoplasm of the Pancreas With Ovarian-Type Stroma: A Nationwide Multicenter Study in Japan. Pancreas. 2020;49:181–186. doi: 10.1097/MPA.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 2.Kurita Y, Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Koda H, Tajika M, Shimizu Y, Nakajima A, Kubota K, Niwa Y. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci Rep. 2019;9:6893. doi: 10.1038/s41598-019-43314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375–388. doi: 10.3978/j.issn.2078-6891.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacevic B, Karstensen JG, Havre RF, Pham KD, Giovannini M, Dabizzi E, Arcidiacono P, Santo E, Sequeiros EV, Klausen P, Rift CV, Hasselby JP, Toxværd A, Kalaitzakis E, Hansen CP, Vilmann P. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video) Endosc Ultrasound. 2018;7:383–388. doi: 10.4103/eus.eus_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131–136. doi: 10.1016/j.pan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M International Study Group on Pancreatic Surgery (ISGPS) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Bauer F. Pancreatic Cystic Lesions: Diagnostic, Management and Indications for Operation. Part II. Chirurgia (Bucur) 2018;113:318–334. doi: 10.21614/chirurgia.113.3.318. [DOI] [PubMed] [Google Scholar]
- 12.van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676–689. doi: 10.1038/s41575-019-0195-x. [DOI] [PubMed] [Google Scholar]
- 13.Farrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut Liver. 2015;9:571–589. doi: 10.5009/gnl15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H, Ma S, Guo X, Zhang X, Wang X. Preoperative differentiation of pancreatic mucinous cystic neoplasm from macrocystic serous cystic adenoma using radiomics: Preliminary findings and comparison with radiological model. Eur J Radiol. 2020;122:108747. doi: 10.1016/j.ejrad.2019.108747. [DOI] [PubMed] [Google Scholar]
- 15.Gerry JM, Poultsides GA. Surgical Management of Pancreatic Cysts: A Shifting Paradigm Toward Selective Resection. Dig Dis Sci. 2017;62:1816–1826. doi: 10.1007/s10620-017-4570-6. [DOI] [PubMed] [Google Scholar]
- 16.Di Paola V, Manfredi R, Mehrabi S, Cardobi N, Demozzi E, Belluardo S, Pozzi Mucelli R. Pancreatic mucinous cystoadenomas and cystoadenocarcinomas: differential diagnosis by means of MRI. Br J Radiol. 2016;89:20150536. doi: 10.1259/bjr.20150536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceppa EP, De la Fuente SG, Reddy SK, Stinnett SS, Clary BM, Tyler DS, Pappas TN, White RR. Defining criteria for selective operative management of pancreatic cystic lesions: does size really matter? J Gastrointest Surg. 2010;14:236–244. doi: 10.1007/s11605-009-1078-1. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson LN, Keane MG, Shamali A, Millastre Bocos J, Marijinissen van Zanten M, Antila A, Verdejo Gil C, Del Chiaro M, Laukkarinen J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology. 2016;16:1028–1036. doi: 10.1016/j.pan.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 19.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troisi RI, Pegoraro F, Giglio MC, Rompianesi G, Berardi G, Tomassini F, De Simone G, Aprea G, Montalti R, De Palma GD. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints? Surg Oncol. 2019 doi: 10.1016/j.suronc.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Kamarajah SK, Sutandi N, Robinson SR, French JJ, White SA. Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB (Oxford) 2019;21:1107–1118. doi: 10.1016/j.hpb.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Liu Q, Zhao ZM, Tan XL, Gao YX, Zhao GD. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study. J Surg Oncol. 2017;116:461–469. doi: 10.1002/jso.24676. [DOI] [PubMed] [Google Scholar]
- 23.Jin JB, Qin K, Yang Y, Shi YS, Wu ZC, Deng XX, Chen H, Cheng DF, Shen BY, Peng CH. Robotic pancreatectomy for solid pseudopapillary tumors in the pancreatic head: A propensity score-matched comparison and analysis from a single center. Asian J Surg. 2020;43:354–361. doi: 10.1016/j.asjsur.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Memeo R, Sangiuolo F, de Blasi V, Tzedakis S, Mutter D, Marescaux J, Pessaux P. Robotic pancreaticoduodenectomy and distal pancreatectomy: State of the art. J Visc Surg. 2016;153:353–359. doi: 10.1016/j.jviscsurg.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Han Y, Byun Y, Kim H, Kwon W, Jang JY. Central Pancreatectomy Versus Distal Pancreatectomy and Pancreaticoduodenectomy for Benign and Low-Grade Malignant Neoplasms: A Retrospective and Propensity Score-Matched Study with Long-Term Functional Outcomes and Pancreas Volumetry. Ann Surg Oncol. 2020;27:1215–1224. doi: 10.1245/s10434-019-08095-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.