Abstract
The virtual crossmatch (VXM) is gaining acceptance as an alternative approach to assess donor:recipient compatibility prior to transplantation. In contrast to a physical crossmatch, the virtual crossmatch does not require viable donor cells but rather relies on complete HLA typing of the donor and current antibody assessment of the recipient. Thus, the VXM can be performed in minutes which allows for faster transplant decisions thereby increasing the likelihood that organs can be shipped across significant distances yet safely transplanted. Here, we present a brief review of the past 50 years of histocompatibility testing; from the original complement-dependent cytotoxicity crossmatch in 1969 to the new era of molecular HLA typing, solid-phase antibody testing and virtual crossmatching. These advancements have shaped a paradigm shift in our approach to transplantation. That is, foregoing a prospective physical crossmatch in favor of a VXM. In this review, we undertake an in-depth analysis of the pros- and cons- of physical and virtual crossmatching. Finally, we provide objective data on the selected use of the VXM which demonstrate the value of a VXM in lieu of the traditional physical crossmatch for safe and efficient organ transplantation.
Keywords: crossmatch, histocompatibility, HLA antibody, single antigen bead, virtual crossmatch
1 |. INTRODUCTION
2019 marks the 50th anniversary of the landmark paper from Patel and Terasaki, “Significance of the positive crossmatch test in kidney transplantation.”1 Their work established the concept that preformed allogeneic antibodies, later confirmed as anti-HLA antibodies, detected in transplant recipients via a complement-dependent cytotoxicity crossmatch (CDC-XM) were associated with hyperacute rejection of transplanted renal allografts. This seminal publication in transplant immunology demonstrated that a pretransplant laboratory test could help prevent early graft loss and, as such, is generally recognized as the birth of clinical histocompatibility testing.
Following its acceptance as a bonafide pretransplant crossmatch test to detect deleterious donor-specific antibody, the CDC-XM evolved into a pretransplant screening assay to gauge the overall level of sensitization in candidates awaiting transplantation. Using panels of lymphocytes from volunteers, the cytotoxic screening assay was used to define HLA antibody specificities, characterize new HLA antigens, and calculate the percent panel reactive antibodies (%PRA) (reviewed in Reference2). Subsequently, with advances in technology and molecular biology, new methods replaced serology for the identification of HLA antigens (ie, RFLP, PCR-SSP, PCR-SSOP, RT-PCR and NGS).3–5 In fact, Patel and Terasaki’s observation in 1969 was the impetus that launched the field of clinical human histocompatibility testing and set the stage for the era of the virtual crossmatch. In this review, we will review the utility of the physical crossmatch given the current methodological advances and assess the virtual crossmatch as its replacement in determining pretransplant donor:recipient compatibility for solid organ transplants.
2 |. CELL-BASED METHODS FOR HLA ANTIBODY IDENTIFICATION AND CROSSMATCHING
The CDC assay utilizes donor lymphocytes isolated from spleen, lymph node, or peripheral blood that are incubated with recipient serum and complement. A positive reaction indicates that antibodies in the serum have bound to antigens on the target cells which then activate complement leading to cell death. Although the advent of the CDC-XM reduced the risk of hyperacute rejection, more sensitive assays were warranted as some CDC-XM negative cases still resulted in early antibody-mediated rejection. To increase the sensitivity of the CDC-XM, a number of modifications were introduced. Such improvements included extended incubation times, increased number of wash steps, and the use of anti-human globulin (AHG). The AHG-CDC-XM improved the detection of both complement-fixing and non-complement-fixing anti-HLA antibodies and improved graft survival outcomes over that of conventional CDC-XM.6,7 Although the CDC-XM had a positive impact on improving transplant outcomes, an important issue arose as our understanding of the human MHC advanced. Studies revealed that there were two distinct classes of HLA molecules: Class I and Class II.8 Unfortunately, the CDC-XM, which used unfractionated lymphocytes, was ineffective in detecting antibodies against Class II HLA antigens. Although B cell-enriched CDC-XMs became an alternative to assess Class II antibodies, the test lacked specificity because B cells also express Class I HLA. Moreover, due to low B cell numbers, the sensitivity of the assay suffered9 (reviewed in Reference10).
Initially reported by Garovoy in 1983,11 the flow cytometric crossmatch (FCXM) gained popularity as an alternative to the AHG-CDC-XM but with greater sensitivity and increased ability to assess Class II HLA antibodies.12 The FCXM is similar to the CDC-XM in that recipient serum is incubated with donor cells, but instead of complement activation and cell death as the readout in the CDC-XM, the readout was antibody bound to the donor cells, detected by a fluorescent anti-human IgG antibody. This method increased the sensitivity of the crossmatch by enabling the direct detection of donor-specific antibodies (DSA) based solely on binding to donor cells.13–15 Given the multi-parameter capability of flow cytometry, it was also possible to simultaneously evaluate T and B cells in the crossmatch.16 This assay could also be used to determine a PRA using pooled target cells in an effort to determine the level of sensitization in recipients prior to transplantation.17 Due to its greater sensitivity, many centers have chosen the FCXM as their primary method of physical crossmatching.2
3 |. SOLID PHASE METHODS FOR HLA ANTIBODY IDENTIFICATION
An early alternative to the CDC-XM was developed that consisted of capturing HLA molecules from donor lymphocytes and adhering them to a microtiter plate. A patient’s serum could then be flushed over the solid phase, and bound antibody could be detected with anti-IgG, similar to an enzyme-linked immunosorbent assay (ELISA).18 However, this assay lacked both sensitivity and specificity as there was no way to verify that a negative test was truly negative or if the HLA antigen was not captured. A modification of the assay was also used for HLA antibody screening using random platelet donors to coat solid phase plates with purified HLA antigens. This assay had greater sensitivity and was more cost effective than the CDC-XM but lacked specificity.19,20 In addition, compared to the FCXM it was less sensitive. Thus, ELISA for detection of HLA antibody never reached significant usage worldwide.2
As of the past few decades, technological advances have made possible the isolation and purification of HLA antigens, either as a Class I or II phenotype (A, B, C or DR, DQ, DP), and their subsequent adherence to solid matrices. The most common approach has employed small plastic particles as the carrier of HLA proteins and these particles can be assessed either on a standard flow cytometer or the newer Luminex platform. Use of this technology has led to the development and implementation of a multitude of new assays. One such assay utilizes either Class I or Class II phenotypes adhered to beads and is called the FlowPRA, which is similar in concept to a PRA but does not require the use of live lymphocytes and is far more sensitive.21,22 FlowPRA allows for the simultaneous assessment of Class I and Class II antibodies and is done to monitor for sensitization. However, one major limitation is that it cannot define the individual specificities and cannot be used to report a percent PRA.
Another such assay that was developed resolved that conundrum through the use of Luminex technology, which permitted the assessment of individual HLA proteins (Class I or Class II) and is referred to as single antigen bead (SAB) testing. Use of this technology allows for individual HLA molecules to be attached to unique beads for the precise identification of anti-HLA antibodies. Although ELISA- based assays were the first to use captured HLA proteins, comparing ELISA to Luminex revealed that the Luminex SAB assay was more sensitive, more specific, and had fewer false positives, especially for Class II antibodies. Although more expensive than the ELISA assay, in the long run, Luminex-based assays were more cost effective.23 Comparing the Luminex vs CDC-XM and FCXM revealed that the Luminex was superior particularly in sensitized patients in defining both unacceptable and acceptable specificities.24 Moreover, this assay could also be scaled up by adding additional beads as new HLA alleles were identified. Newer Luminex technological advances including the introduction of the Flexmap-3D instrument permit the simultaneous assessment of up to 500 analytes. Because of the increased sensitivity and specificity that the SAB assay afforded, it has quickly become the gold standard both for pre- and post-transplant assessment of HLA antibody formation.
4 |. VIRTUAL CROSSMATCHING
As a result of the enhanced HLA antibody detection capability of the SAB Luminex approach, the virtual crossmatch (VXM) is gaining acceptance. The VXM is defined as “An assessment of immunologic compatibility based on the patient’s alloantibody profile compared to the donor’s histocompatibility antigens.”25 The VXM represents a very specific assessment for donor-specific anti-HLA antibody sensitization that combines molecular HLA typing of the donor with the detailed antibody profile of the recipient to render a determination of HLA compatibility.26 Because of the improved ability to identify donor-specific HLA antibodies, VXM has a significant advantage over physical crossmatching (CDC-XM or FCXM). In many instances, a VXM may be the only pretransplant “crossmatch” necessary. The following two quotations are statements related to the clinical utility of improved HLA antibody assessments:
This approach to donor selection reduces unnecessary crossmatching and increases the likelihood of finding crossmatch-compatible donors for highly reactive patients.27
We conclude that the approach of detailed antibody analysis can result in an improvement for successful transplantation of more dialysis patients who are highly sensitized to HLA allo-antigens.28
The interesting aspect of these two apparently timely quotations is that they are from 1983 and 1986, respectively. At the time, these quotations were referring to the power of better antibody assessments as determined by enhanced cytotoxicity testing. Similarly, in 1998, Kerman et al29 suggested forgoing a prospective physical crossmatch for non-sensitized candidates based only on AHG-CDC testing. Thus, it was apparent that the field had long considered the possibility of virtual crossmatching, but only recently did it gain a greater acceptance with the advent of solid-phase antibody testing.
5 |. FUTURE OF CROSSMATCHING
Historically, a physical crossmatch was a prerequisite for solid organ transplantation due to its ability to prevent hyperacute rejection. In part, this practice was predicated on the fact that our understanding of the HLA system, its alleles and antibodies, was woefully incomplete. Currently, we have efficient and accurate molecular-based HLA typing methods, solid-phase HLA antibody detection and a greater understanding of the complexities of the MHC. As a result, our ability to identify HLA antibodies in advance has vastly improved, calling into question the utility of requiring a physical crossmatch as a final proof of compatibility.
Since the implementation of the new Kidney Allocation Scheme in the United States in 2014, there has been increased sharing of organs across greater distances. Moreover, newer allocation schemes currently under discussion will also increase organ sharing across greater distances as the geographical location of a candidate becomes less of a factor. With these considerations in mind, it is important to reevaluate the importance of requiring a physical crossmatch prior to transplantation and understand if/when it plays a role in organ allocation. Studies have shown that VXM and FCXM have similar accuracy, specificity, and sensitivity in detecting DSA.30 Therefore, many programs worldwide have adopted the VXM as the only assessment performed prior to transplantation for selected candidates.31–36 We have recently published our VXM data both pre- and post-KAS implementation.37 During the first 9 months post-KAS, 129 deceased donor transplants were performed wherein 43 (33.3%) were candidates with CPRA of 99% to 100%. Note that the US national average for this time period was ~15%. For these recipients, 30(70%) were transplanted without a prospective physical crossmatch. There were no instances of hyperacute or accelerated antibody-mediated rejection during a 12-month follow-up period. We observed similar results pre-KAS with a 5-year follow-up study among 492 recipients showing no significant difference in outcomes as a function of PRA values.26 Subsequently, we published a 10-year follow-up data showing no significant differences in long-term patient or graft survival among these groups.37 Most recently, Roll et al38 published data showing that the use of the VXM is safe for highly sensitized candidates and facilitated organ sharing over long distances. In their study, 84.6% of import donor renal transplants were conducted without a prospective physical crossmatch. Among this group, 42% had a CPRA of 100%. There were no instances of hyperacute rejection and only infrequent acute rejection during the first year posttransplant. Taken together, current data from many centers support the utility of virtual crossmatching.
There are several factors that have moved the field in this direction. First, we now have rapid methods for accurately identifying the HLA antigens of donors. Second, solid-phase HLA antibody assessments provide the most detailed definition of a candidate’s HLA antibody reactivity. Third, the FCXM and VXM compare favorably in sensitivity when DSA is present with the exception that the VXM, based on solid-phase assay data, may identify DSAs at a level too low to produce a positive physical crossmatch. Fourth, studies have also shown that the integrity of crossmatch material, from deceased donors in particular, may not be optimum, jeopardizing the validity of the physical crossmatch result.39 Lastly, the FCXM and VXM differ greatly in timeliness. Since the VXM requires only donor HLA typing, which is necessary for allocation, and recipient antibody data already in existence, there is no need to wait for (or ship) donor tissue to perform a physical crossmatch. In many instances, due to increased organ sharing, samples for a physical crossmatch may need to travel great distances thereby increasing cold ischemia time or, more worrisome, leading to unnecessary organ refusals due to time constrains.
Because of these issues, the VXM is gaining in popularity as an effective approach for many candidates. The VXM can reduce the time to transplantation and decrease cold ischemia time without impacting allograft survival, patient survival, or early rejection rates.40,41 Judicious application of the VXM can then allow for grafts to be shared over extended distances without incident even in highly sensitized recipients.42,43 Furthermore, eliminating a prospective physical crossmatch has been shown to decrease both time on waiting list and wait list mortality for lung and cardiac transplant recipients.44,45 With the known benefits of the VXM, is there still a role for the physical XM? If so, under what circumstances would it be useful? Below we will discuss some example scenarios that highlight the complex interpretations when differences occur between the FCXM and VXM results (Table 1). In Table 1, Scenarios 1 and 2 are straightforward wherein both the FCXM and VXM are concordant. However, Scenarios 3 and 4 represent are more complex and warrant further investigation. Our goal with this discussion is 2-fold: (a) to illustrate the utility of the VXM and (b) to define the conditions wherein a physical crossmatch may still be required. Below are defined scenarios that illustrate these points.
TABLE 1.
The four possible scenarios expected based on the results of the physical and virtual crossmatches
| Scenario | FCXM | VXM | Interpretation (proportion) |
|---|---|---|---|
| 1 | − | − | Concordant (~75%) |
| 2 | + | + | Concordant (~75%) |
| 3 | + | − | Discordant: (~20%)
|
| 4 | − | + | Discordant: (~5%)
|
Note: Scenarios 1 to 4 are ranked as concordant or discordant. Abbreviations: FCXM, flow cytometric crossmatch; MFI, XX; SAB, single antigen bead; VXM, virtual crossmatch.
6 |. COMPLEX SCENARIOS
6.1 |. Scenario 3: FCXM positive/VXM negative
Reports of this particular scenario in which the FCXM is positive in the absence of donor-specific HLA antibody occur with significant frequency. In work published by Sullivan et al,46 the rate for this discordant result, among living donors, was reported to be 20% (20 out of 100 cases reviewed). The majority (90%) of the positive FCXMs were B-cell only and were moderate to weak in strength. The average CPRA for this group was <10% and no candidates possessed Class II DSA. Furthermore, 8 of 20 candidates in the group were successfully transplanted. The remaining candidates were not transplanted for other reasons. Additional studies also report transplanting across this type of discordant result with no deleterious outcomes associated.46 Collectively, the clinical findings support the contention that such positive FCXMs were due to a non-HLA antibody and should not be considered a contraindication for transplantation.47–49
Moreover, false positive B-cell FCXMs can occur when immunoglobulins or immune complexes bind Fc or complement receptors on B cells. The implementation of pronase pretreatment of lymphocytes in the FCXM has been shown to decrease the false positive rate50 as well as increase sensitivity of the B-cell crossmatch without disrupting MHC expression.51 Interestingly, however, cases of false positives B-cell and T-cell crossmatches following pronase treatment have been reported52,53 and are likely due to non-HLA antibodies, autoantibodies that recognize exposed epitopes upon pronase treatment, or an artifact resulting from the use of extremely high concentrations of pronase.
In the scenario outlined above in which the FCXM is positive and VXM is negative, transplantation does not represent an increased risk to the patient. Hence, halting transplantation in these cases would inappropriately be denying a patient from receiving a transplant.
A second possibility that could lead to this discordance is when the physical crossmatch is positive due to HLA antibody but the VXM is deemed negative based on the threshold used to assign antibody specificity in the bead-based assays. Unfortunately, there is no agreed upon standard MFI value for assigning a positive result with SAB testing. Threshold MFI values range from ~500 to 10 000 MFI depending on the class, locus, and even specific antigen.54 For example, due to the lower expression of C, DP, and DQ some labs have increased their MFI threshold to account for lower levels of antigen on the surface of cells. Moreover, a recent study has analyzed whether crossing DP mismatches with a positive XM is clinically significant, and surprisingly they show that anti-DP DSAs are of no consequence to ABMR or ACR rates,55 although this remains a heavily controversial topic. In addition to MFI thresholds varying depending on the class, locus, and antigen, antibodies against shared epitopes that are expressed across many SABs may produce low MFI values that underrepresent the total amount of antibody present. For example, low level antibody (<1000 MFI) against the common epitopes of Bw4 or Bw6 may show low staining across a larger number of beads. However, when tested against a cell expressing Bw4 or Bw6, the crossmatch is “unexpectedly” positive. We call this phenomenon the “peanut butter” effect.56 That is, when a small amount of antibody tested against a single bead may be detected but when spread across many beads may yield a signal that is below the set threshold, analogous to a scoop of peanut butter being spread over one slice of bread as opposed to being distributed across all slices in an entire loaf.
Yet another explanation for the VXM to be deemed false-negative is the presence of serum components that can interfere with the detection of anti-HLA antibodies, sometimes incorrectly termed the “Prozone effect.” Seminal work by Schnaidt et al57 showed that serum treatment with EDTA specifically abrogates complement-mediated interference in the SAB assay. Their work showed that while heat-inactivation and DTT were also effective, EDTA was superior in its ability to minimize the prozone effect due to serum components. Additionally, work by our group has shown that the use of a long-linker, biotin-conjugated secondary antibody, followed by addition of phycoerythrin-conjugated streptavidin appears to overcome the inhibition as well in the absence of EDTA.58 Taken together, these data show that without the pretreatment of sera to abrogate interference, the VXM may be false negative and be in contradiction to the physical XM. It is important for laboratories to recognize this potentially serious complication and ensure proper steps are in place to rule out assay interference.
The final possibility for the above discrepancy would require that a patient make a private HLA antibody against an extremely rare allele/antigen not covered by the beads or that an antibody was present against a common epitope present on what was considered an unrelated HLA antigen. Hence, while a majority of the cases within this scenario are likely due to false positive FCXMs, we cannot exclude the possibility that true HLA antibody, under certain conditions, could be the underlying cause. Importantly, these possibilities are quite rare and can be resolved by performing a detailed antibody assessment to fully understand a candidate’s repertoire of HLA antibodies. To this end, additional testing such as surrogate crossmatches or supplemental bead products can be of value.
6.2 |. Scenario 4: FCXM negative/VXM positive
In this scenario, the VXM reveals DSA in the potential recipient, but the FCXM is negative. Several different factors must be considered when interpreting these cases: (a) Recent evidence suggests that a proportion of VXM +/FCXM− cases are likely the result of weak anti-HLA antibody that cannot elicit a positive FCXM.46 Sullivan et al showed that this scenario represented ~5% (5/100) of cases, and, based on the FlowPRA screening assay, the positive VXM was the result of true, albeit weak, anti-HLA antibody. (b) The quality of donor material used in the crossmatch can greatly affect the outcome of the crossmatch. Living and deceased donor material and PBMC, spleen, and lymph node cells are all quite different with respect to HLA expression.39 The VXM is scored as positive predicated on low-level anti-HLA antibody, but low or weak HLA expression on target cells may yield a negative physical crossmatch. (c) Drugs including statins and steroids can downregulate the surface expression of HLA adding additional complexities to the interpretation of the FCXM.59,60 Due to the possibility that HLA surface expression may be compromised resulting in a false negative FCXM, one must reconsider the utility of a physical crossmatch when there exists low-level DSAs. The belief that a “negative” physical crossmatch confers a lower risk may not be valid in these instances. (d) Some apparently “non-HLA antibodies” can bind to HLA antigens on the surface of the beads. This is likely due to the expression of denatured, non-native conformation of the HLA protein or possibly peptides carried by HLA proteins expressed on the bead surface. Such reactivities would then lead to a false positive VXM result.61–63 Further confounding the interpretation are data by Visentin et al64 showing that denatured anti-HLA antibodies can bind to native HLA antigens/alleles, and native anti-HLA antibodies can bind denatured HLA antigens/alleles. Thus, in the presence of such data it may be difficult to tease out true HLA reactivity. It is interesting to note that data published by Santos et al65 showed that during T-cell activation, denatured HLA Class I molecules do exist on the cell surface as evidenced by HC-10 reactivity. Such expression may be a normal occurrence during T-cell activation.
A recent meta-analysis of these types of situations was performed and the culmination of the data showed no significant difference in acute rejection, graft failure, or patient mortality between patients with a negative FCXM and either a positive or negative VXM.66 While this is an interesting observation, there were some issues with this review. First, there was no clear indication that denatured antigens, nonexposed epitopes, or bead abnormalities were excluded. As stated above, this can lead to false positive VXM assessments. Second, the MFI cutoff for positivity was not consistent or even not reported among the studies included and could have led to false positive VXM reports. These “false positive” VXM cases would affect the statistics and dilute the clinical impact of true, weak HLA DSAs.
An alternative assay to determine if antibodies identified via the VXM are true is the use of epitope alignment as was described in a recent case report.67 This group first ensured the VXM was positive with two sets of SABs (OneLambda and Immucor) and then performed an epitope analysis. This analysis revealed the anti-HLA antibody was against a nonexposed epitope and was likely reacting to the SAB in the VXM because the HLA molecule was denatured. In this instance, the purported DSA was labeled as a false positive, and the patient was successfully transplanted without incident. Identifying the “false positive” VXM results in this manner would subsequently allow the true positive, albeit weak DSA to be acknowledged despite a negative FCXM. The danger in assuming all VXM+/FCXM− scenarios are false positives lies in the fact that in a small frequency of cases highly sensitized patients may have antibodies against both native and denatured antigens or when the positive VXM is actually the result of a weak HLA DSAs. In these instances, we cannot be certain that the VXM was false positive due to denatured antigens or the FCXM false negative due to poor quality donor material or low antigen expression, for example, C, DQ, and DRB3,4,5 loci.
This scenario in which the VXM is positive and the FCXM is negative has typically led to much debate about whether or not to proceed with the transplant. In most cases when confirming a true “false positive” due to denatured or cryptic antigens, transplantation is likely safe. However, in situations wherein the DSA identified by the SAB is a low titer HLA antibody, these situations can pose an increased risk for early graft rejection.68–70 It is important to discriminate between these two scenarios so that educated transplant decisions can be made and appropriate post-transplant therapies can be utilized.
7 |. DISCUSSION
The goal of this paper was to review objective data on the value of a physical crossmatch in the current era of solid-phase HLA antibody testing. Fortunately, for the majority of cases, a negative VXM correlates with a negative physical crossmatch and high titer DSAs correlate with a positive FCXM. However, there are instances where the two methods disagree. Taking a closer look at Scenarios 3 and 4 of Table 1 wherein there is discordance between the VXM and FCXM, the ability to understand the causes of false positive/negative results is essential. Although not complete, the knowledge base for understanding these false results is greater for the VXM than the FCXM. In the case of false positive VXMs (DSA+/physical XM−), centers are able to (and should) identify denatured or cryptic antigen bead patterns and implement epitope/eplet alignment to document the presence/absence of true HLA antibody. In the case of false positive FCXMs (DSA−/FCXM+), the reasons are not so easily identifiable and resolvable. Tissue expression of non-HLA antigens can be variable and impossible to predict and can lead to spurious false positive reactions. Alternatively, there is the remote possibility that a patient has HLA antibody against a unique epitope or allele not represented on the current bead panels.
One of the important benefits of the VXM is the ability to detect low titer donor-specific HLA antibodies that may not yield a positive physical XM. As mentioned above, relying on a physical XM to validate the VXM in such cases may not be reasonable. Thus, via an increased level of sensitivity, the VXM can further risk-stratify physical XM negative transplants. Although the physical XM relies on the VXM to confirm DSA, the reverse is not necessarily true. Hence, we must ask the question, does a positive physical XM impart a greater level of risk than a negative physical crossmatch in the face of low-level DSA? Conversely, in the presence of low-level DSA, does a negative physical XM signify a low-risk transplant? For this scenario, the evidence indicates that, for low-level DSA found via the VXM, there is no additional safety gained with a negative physical XM. Given its high frequency and the inability to resolve discordant results, a positive physical XM in the face of a negative VXM may result in greater patient harm if a transplant is unnecessarily canceled.
Nonetheless, there are certain scenarios in which a physical XM may be useful. In instances where there is HLA antibody present, but the exact specificities are unknown, a physical XM may provide relevant information. However, even here the physical XM might become obsolete as epitope and eplet discovery advance. Also, for centers that perform desensitization pretransplant, a negative physical crossmatch may be the threshold for a safe transplant.
Considering all we have discussed, we propose that via advances in technology and the evolution of the VXM, the clinical paradigm for transplantation is shifting (Figure 1). For low risk scenarios, that is, absence of a DSA, a physical crossmatch does not add useful information and may even result in needless cancelation. However, the level of sensitivity of the testing used for identifying DSA may impact the overall risk. For example, a negative physical crossmatch does not guarantee the absence of a DSA if solid-phase testing was not performed. For moderate risk transplants, the presence of weak DSA may still identify a patient at increased risk for early antibody-mediated rejection independent of a physical crossmatch result. Lastly, with the advent of desensitization protocols for high-risk candidates, a positive physical XM is not always a contraindication for transplantation. Furthermore, because of issues with false results in the physical XM, the VXM should be considered as a primary and, for selected candidates, the solitary HLA compatibility assessment prior to transplantation.
FIGURE 1.
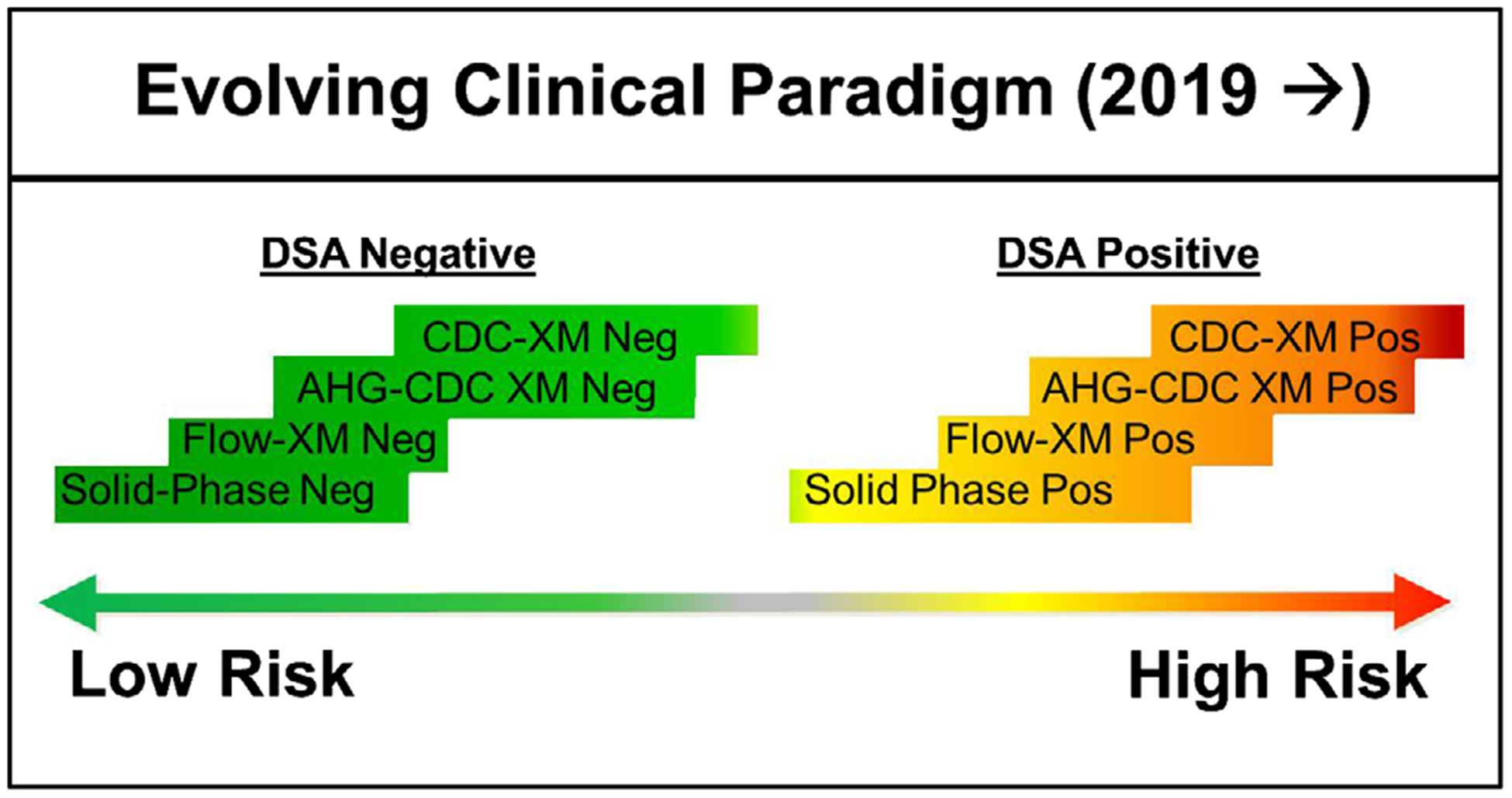
The evolving paradigm of clinical testing and risk stratification in which a solid-phase HLA antibody testing may provide information beyond that of the physical crossmatch
At our center, we utilize the FlowPRA screening assay along with the SAB assays to determine risk stratification and whether there exists a contraindication to transplantation. Figure 2 shows our algorithm for risk stratification and indicates what type of patient scenarios would likely benefit from a physical XM. In this approach, two different bead assays are used, allowing for better delineation of false positives/negatives due to denatured antigens therefore permitting a more reliable confirmation of DSA. Note that DSA designation is given without indicating specific MFI thresholds. This was done purposefully because of the complex nature of MFI interpretation.71 MFI thresholds are, for the most part, center-specific. As such, variability in MFI thresholds can be attributed to a center’s testing protocols, technologist’s experience, and local clinical practice.
FIGURE 2.

Emory’s strategy for risk stratification based on the outcome of the FlowPRA screen and the SAB Luminex assay
In summary, maintaining reliance on a physical crossmatch is, in many ways, redundant, and eliminating the physical XM in selected transplant scenarios will decrease ischemia time and facilitate organ sharing over greater distances. We recognize that this position will not be universally embraced. Reasons for such reticence may include: local resources, comfort level with interpretation of solid-phase testing, or a general unwillingness for change. Nonetheless, since many transplant programs worldwide are adopting this practice, it is apparent that the widespread utilization of the VXM is inevitable. Thus, we advocate for continued education of HLA laboratories and support global standardization of HLA practices. Increased utility of the VXM combined with judicious use of a physical crossmatch is, in our opinion, the new standard.
AUTHOR BIOGRAPHIES
Anna B. Morris, B.S. Anna Morris is currently finishing her PhD in T cell transplant immunology at Emory University. Her PhD work is on regulating CD8+ T cell co-signaling to prevent allograft rejection mediated by T cells. During the last year of her dissertation work, she has completed an HLA laboratory rotation and will pursue a career as an director training in the field of histocompatibility and immunogenetics following the completion of her PhD.
H. Cliff Sullivan MD. Dr. Sullivan completed his undergraduate and medical school training at Emory University in Atlanta, Georgia. During his anatomic and clinical pathology residency, he gravitated towards laboratory medicine, which lead him to pursue fellowships in Transfusion Medicine/Blood Banking and Histocompatibility and Immunogentics. After training, he stayed on as an assistant professor at Emory, where he serves as the laboratory director of the Cellular Therapy Laboratory and associate director of the HLA laboratory. His research interests focus on test utilization, quality improvement, medical education, and immunohematology. He has also made it a personal goal to be an ambassador for pathology in order to promote the field and highlight the value of laboratory medicine as a foundation of medicine. To this end, he serves as pathology representative to the American Medical Association as well as volunteers with the American Society of Pathology.
Scott M. Krummey, M.D., Ph.D. Dr. Krummey obtained his PhD in Immunology and Molecular Pathogenesis and his MD degree from Emory University in 2016. His PhD work investigated co-stimulation pathways of CD4 T cells and the impact of TCR affinity on graft-specific CD8 T cell responses. He completed Clinical Pathology residency at Emory University and is currently a Histocompatibility and Immunogenetics fellow. His current research interests include uncovering critical mechanisms of alloreactive memory B and T cell differentiation. He has been awarded the Graduate Career Award, the Emory Department of Pathology Trainee Scholarship Award, and the Transplant Immunology Research Network Basic Science Research Grant.
Howard M. Gebel, Ph.D., D(ABHI). Dr. Gebel, has been Professor of Pathology and Co-Director of the Histocompatibility and Molecular Immunogenetics Laboratory at Emory University since 2001. He was awarded his Ph.D. in Immunology from the University of Missouri in 1977. He was trained in HLA as a post-doctoral fellow at Washington University Medical Center in St. Louis, MO. In 1979, Dr. Gebel was appointed to the Washington University faculty in the Department of Pathology and named Associate Director of the Histocompatibility and Clinical Immunology Laboratories at Barnes Hospital. In 1984, Dr. Gebel was recruited to Rush Medical College in Chicago IL as Director of the Histocompatibility and Immunogenetics Laboratory. In 1998, Dr. Gebel accepted the position of Director of the Histocompatibility Laboratory at Louisiana State University in Shreveport. He is currently a board member of the American Society of Transplantation (AST), a senior staff scientist for the Scientific Registry of Transplant Recipients and an editorial board member of the American Journal of Transplantation and Human Immunology. He was a founding member of the Transplant Diagnostics Community of Practice. Dr. Gebel is a frequent speaker at transplant forums including the American Society for Histocompatibility and Immunogenetics (ASHI), the American Transplant Congress (ATC) and the Cutting Edge of Transplantation (CEOT). In 2019, ASHI named Dr. Gebel as the recipient of the Bernard Amos Distinguished Scientist Award.
Robert A. Bray, PhD, D(ABHI), HCLD/CC(ABB). Robert A. Bray, PhD., is currently a Professor in the Department of Pathology and Laboratory Medicine, School of Medicine, at Emory University and Co-Director of the Histocompatibility & Molecular Immunogenetics Laboratory (1989-Current). Dr. Bray received his Ph.D. from Indiana University School of Medicine (1985 -Indianapolis, Indiana) and did a post-doctoral fellowship at Rush Medical Center in Chicago, Il. (1985–1987). Dr Bray is a past President of ASHI and has served on the UNOS Board of Directors. He has served on the UNOS Kidney committees and served as Chair of the UNOS Histocompatibility committee. In addition he has served on many other Committees within UNOS, ASHI, ABHI, NMDP and the AFDT (formerly SEOPF). His work has focused on understanding the role of HLA antibodies in transplantation. In 2014 he was the recipient of the Paul Terasaki, Clinical Scientist Award by the American Society for Histocompatibility and Immunogenetics.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicting interests.
REFERENCES
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735–739. [DOI] [PubMed] [Google Scholar]
- 2.Bray RA, Tarsitani C, Gebel HM, Lee JH. Clinical cytometry and progress in HLA antibody detection. Methods Cell Biol. 2011;103: 285–310. [DOI] [PubMed] [Google Scholar]
- 3.Bunce M, Young NT, Welsh KI. Molecular HLA typing—the brave new world. Transplantation. 1997;64(11):1505–1513. [DOI] [PubMed] [Google Scholar]
- 4.Cereb N, Kim HR, Ryu J, Yang SY. Advances in DNA sequencing technologies for high resolution HLA typing. Hum Immunol. 2015;76(12):923–927. [DOI] [PubMed] [Google Scholar]
- 5.Edgerly CH, Weimer ET. The past, present, and future of HLA typing in transplantation. Methods Mol Biol. 2018;1802:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Fuller TC, Fuller AA, Golden M, Rodey GE. HLA alloantibodies and the mechanism of the antiglobulin-augmented lymphocytotoxicity procedure. Hum Immunol. 1997;56(1–2):94–105. [DOI] [PubMed] [Google Scholar]
- 7.Kerman RH, Kimball PM, Van Buren CT, et al. AHG and DTE/-AHG procedure identification of crossmatch-appropriate donor-recipient pairings that result in improved graft survival. Transplantation. 1991;51(2):316–320. [DOI] [PubMed] [Google Scholar]
- 8.Albert ED, Götze D. The Major Histocompatibility System in Man and Animals. Berlin, New York: Springer-Verlag; 1977. [Google Scholar]
- 9.Ettenger RB, Terasaki PI, Ting A, et al. Anti-B lymphocytotoxins in renal-allograft rejection. N Engl J Med. 1976;295(6):305–309. [DOI] [PubMed] [Google Scholar]
- 10.South AM, Grimm PC. Transplant immuno-diagnostics: crossmatch and antigen detection. Pediatr Nephrol. 2016;31(6):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garovoy MR, Rheinschmidt MA, Bigos M, Perkins H, Columbe B, Feduska N. Flow cytometry analysis: a high technology cross-match technique facilitating transplantation. Transplant Proc. 1983;15:1939–1944. [Google Scholar]
- 12.Graff RJ, Buchanan PM, Dzebisashvili N, et al. The clinical importance of flow cytometry crossmatch in the context of CDC crossmatch results. Transplant Proc. 2010;42(9):3471–3474. [DOI] [PubMed] [Google Scholar]
- 13.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003;3(12):1488–1500. [DOI] [PubMed] [Google Scholar]
- 14.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000;69(7):1370–1374. [DOI] [PubMed] [Google Scholar]
- 15.O’Rourke RW, Osorio RW, Freise CE, et al. Flow cytometry crossmatching as a predictor of acute rejection in sensitized recipients of cadaveric renal transplants. Clin Transplant. 2000;14(2):167–173. [DOI] [PubMed] [Google Scholar]
- 16.Bray RA, Lebeck LK, Gebel HM. The flow cytometric crossmatch. Dual-color analysis of T cell and B cell reactivities. Transplantation. 1989;48(5):834–840. [PubMed] [Google Scholar]
- 17.Cicciarelli J, Helstab K, Mendez R. Flow cytometry PRA, a new test that is highly correlated with graft survival. Clin Transplant. 1992;6(3, pt 1):159–164. [PubMed] [Google Scholar]
- 18.Buelow R, Chiang TR, Monteiro F, et al. Soluble HLA antigens and ELISA—a new technology for crossmatch testing. Transplantation. 1995;60(12):1594–1599. [DOI] [PubMed] [Google Scholar]
- 19.Kao KJ, Scornik JC, Small SJ. Enzyme-linked immunoassay for anti-HLA antibodies—an alternative to panel studies by lymphocytotoxicity. Transplantation. 1993;55(1):192–196. [DOI] [PubMed] [Google Scholar]
- 20.Moore SB, Ploeger NA, DeGoey SR. HLA antibody screening: comparison of a solid phase enzyme-linked immunoassay with antiglobulin-augmented lymphocytotoxicity. Transplantation. 1997;64(11):1617–1620. [DOI] [PubMed] [Google Scholar]
- 21.Rebibou JM, Chabod J, Bittencourt MC, et al. Flow-PRA evaluation for antibody screening in patients awaiting kidney transplantation. Transpl Immunol. 2000;8(2):125–128. [DOI] [PubMed] [Google Scholar]
- 22.Tambur AR, Bray RA, Takemoto SK, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000;70(7):1055–1059. [DOI] [PubMed] [Google Scholar]
- 23.Aung FM, Cano P, Fernandez-Vina M, Lichtiger B. Results of HLA antibody testing using ELISA vs the fluorescent bead method and retrospective review of data for recipients of packed RBCs and platelets from male HLA-immunized donors. Am J Clin Pathol. 2011;135(1):90–95. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CJ, Kosmoliaptsis V, Summers DM, Bradley JA. Back to the future: application of contemporary technology to longstanding questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol. 2009;70(8):563–568. [DOI] [PubMed] [Google Scholar]
- 25.Bray RA. The acceptability and application of virtual crossmatching in lieu of serologic crossmatching for transplantation. Virtual Crossmatch Workgroup Report, CDC 2014. [Google Scholar]
- 26.Bray RA, Nolen JD, Larsen C, et al. Transplanting the highly sensitized patient: the Emory algorithm. Am J Transplant. 2006;6(10): 2307–2315. [DOI] [PubMed] [Google Scholar]
- 27.Delmonico FL, Fuller A, Cosimi AB, et al. New approaches to donor crossmatching and successful transplantation of highly sensitized patients. Transplantation. 1983;36(6):629–633. [DOI] [PubMed] [Google Scholar]
- 28.Oldfather JW, Anderson CB, Phelan DL, Cross DE, Luger AM, Rodey GE. Prediction of crossmatch outcome in highly sensitized dialysis patients based on the identification of serum HLA antibodies. Transplantation. 1986;42(3):267–270. [DOI] [PubMed] [Google Scholar]
- 29.Kerman RH, Susskind B, Ruth J, Katz S, Van Buren CT, Kahan BD. Can an immunologically, nonreactive potential allograft recipient undergo transplantation without a donor-specific crossmatch? Transplantation. 1998;66(12):1833–1834. [DOI] [PubMed] [Google Scholar]
- 30.Perasaari JP, Jaatinen T, Merenmies J. Donor-specific HLA antibodies in predicting crossmatch outcome: comparison of three different laboratory techniques. Transpl Immunol. 2018;46:23–28. [DOI] [PubMed] [Google Scholar]
- 31.Bingaman AW, Murphey CL, Palma-Vargas J, Wright F. A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008;86(12):1864–1868. [DOI] [PubMed] [Google Scholar]
- 32.Bohmig GA, Fidler S, Christiansen FT, Fischer G, Ferrari P. Transnational validation of the Australian algorithm for virtual crossmatch allocation in kidney paired donation. Hum Immunol. 2013;74(5):500–505. [DOI] [PubMed] [Google Scholar]
- 33.Taylor CJ, Kosmoliaptsis V, Sharples LD, et al. Ten-year experience of selective omission of the pretransplant crossmatch test in deceased donor kidney transplantation. Transplantation. 2010;89(2):185–193. [DOI] [PubMed] [Google Scholar]
- 34.Bielmann D, Honger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7(3):626–632. [DOI] [PubMed] [Google Scholar]
- 35.Turner D, Battle R, Akbarzad-Yousefi A, Little AM. The omission of the “wet” pre-transplant crossmatch in renal transplant centres in Scotland. HLA. 2019;94(1):3–10. [DOI] [PubMed] [Google Scholar]
- 36.Worsley CM, Mayne ES, Suchard MS. Luminex-based virtual crossmatching for renal transplantation in South Africa. S Afr Med J. 2011;102(1):40–43. [PubMed] [Google Scholar]
- 37.Ruhil R, Talebagha S, Beus J, et al. HLA-DR mismatching is not associated with decreased graft survival in highly sensitized kidney recipients. Am J Transplant. 2013;13(suppl 5):420. [Google Scholar]
- 38.Roll GR, Webber AB, Gae DH, et al. A virtual crossmatch based strategy facilitates sharing of deceased donor kidneys for highly sensitized recipients. Transplantation. 2019;XX:XX–XX. [DOI] [PubMed] [Google Scholar]
- 39.Badders JL, Jones JA, Jeresano ME, Schillinger KP, Jackson AM. Variable HLA expression on deceased donor lymphocytes: not all crossmatches are created equal. Hum Immunol. 2015;76(11):795–800. [DOI] [PubMed] [Google Scholar]
- 40.Eby BC, Redfield RR, Ellis TM, Leverson GE, Schenian AR, Odorico JS. Virtual HLA crossmatching as a means to safely expedite transplantation of imported pancreata. Transplantation. 2016; 100(5):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson CP, Schiller JJ, Zhu YR, et al. Renal transplantation with final allocation based on the virtual crossmatch. Am J Transplant. 2016;16(5):1503–1515. [DOI] [PubMed] [Google Scholar]
- 42.Stehlik J, Islam N, Hurst D, et al. Utility of virtual crossmatch in sensitized patients awaiting heart transplantation. J Heart Lung Transplant. 2009;28(11):1129–1134. [DOI] [PubMed] [Google Scholar]
- 43.Bray R, Parsons R, Turgeon N, Gebel H. Virtual crossmatching under the new KAS: safely bypassing a physical crossmatch when allocating kidneys to highly sensitized candidates. Am J Transplant. 2016;16(suppl 3), 624. [Google Scholar]
- 44.Appel JZ 3rd, Hartwig MG, Cantu E 3rd, Palmer SM, Reinsmoen NL, Davis RD. Role of flow cytometry to define unacceptable HLA antigens in lung transplant recipients with HLA-specific antibodies. Transplantation. 2006;81(7):1049–1057. [DOI] [PubMed] [Google Scholar]
- 45.Zangwill SD, Ellis TM, Zlotocha J, et al. The virtual crossmatch— a screening tool for sensitized pediatric heart transplant recipients. Pediatr Transplant. 2006;10(1):38–41. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan HC, Dean CL, Liwski RS, et al. (F)Utility of the physical crossmatch for living donor evaluations in the age of the virtual crossmatch. Hum Immunol. 2018;79(10):711–715. [DOI] [PubMed] [Google Scholar]
- 47.Yadav K, Cotterell A, Kimball P, et al. Antibody mediated rejection due to de-novo DSA causing venous thrombosis of pancreas allograft—a case report. Transpl Immunol. 2018;47:22–25. [DOI] [PubMed] [Google Scholar]
- 48.Eng HS, Bennett G, Tsiopelas E, et al. Anti-HLA donor-specific antibodies detected in positive B-cell crossmatches by Luminex predict late graft loss. Am J Transplant. 2008;8(11):2335–2342. [DOI] [PubMed] [Google Scholar]
- 49.Couzi L, Araujo C, Guidicelli G, et al. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 2011;91(5):527–535. [DOI] [PubMed] [Google Scholar]
- 50.Lobo PI, Spencer CE, Stevenson WC, McCullough C, Pruett TL. The use of pronase-digested human leukocytes to improve specificity of the flow cytometric crossmatch. Transpl Int. 1995;8(6): 472–480. [DOI] [PubMed] [Google Scholar]
- 51.Apithy MJ, Desoutter J, Gicquel A, et al. Pronase treatment improves flow cytometry crossmatching results. HLA 2017;90(3): 157–164. [DOI] [PubMed] [Google Scholar]
- 52.Hart JD, Lutz CT, Jennings CD, et al. Falsely incompatible B-cell flow cytometry crossmatch after pronase treatment: a case report. Transplant Proc. 2015;47(3):831–833. [DOI] [PubMed] [Google Scholar]
- 53.Park H, Lim YM, Han BY, Hyun J, Song EY, Park MH. Frequent false-positive reactions in pronase-treated T-cell flow cytometric cross-match tests. Transplant Proc. 2012;44(1):87–90. [DOI] [PubMed] [Google Scholar]
- 54.Reed EF, Rao P, Zhang Z, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA-drilling down on key sources of variation. Am J Transplant. 2013;13(11):3050–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajalingam R, Gae DD, Laszik ZG, et al. Successful kidney transplantation of highly sensitized candidates across positive cross match and strong donor-specific HLA-DP antibodies without desensitization [abstract]. Am J Transplant. 2019;19(suppl 3), 384. [Google Scholar]
- 56.Roberts-Wilson T, Tumer G, Gebel HM, Bray RA. Disconnects with solid-phase HLA antibody assays: re-connecting the dots. ASHI Quart. 2013;37(3):12–20. [Google Scholar]
- 57.Schnaidt M, Weinstock C, Jurisic M, Schmid-Horch B, Ender A, Wernet D. HLA antibody specification using single-antigen beads—a technical solution for the prozone effect. Transplantation. 2011;92(5):510–515. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan HC, Gebel HM, Bray RA. Understanding solid-phase HLA antibody assays and the value of MFI. Hum Immunol. 2017; 78(7–8):471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruers TJ, Leeuwenberg JF, Spronken EE, van der Linden CJ, Buurman WA. The effect of steroids on the regulation of major histocompatibility complex-class II expression on nonlymphoid tissue. Transplantation. 1989;47(3):492–499. [DOI] [PubMed] [Google Scholar]
- 60.Wu X, Schott M, Liu C, et al. Statins decrease the aberrant HLA-DR expression on thyrocytes from patients with Hashimoto’s thyroiditis. Horm Metab Res. 2008;40(12):838–841. [DOI] [PubMed] [Google Scholar]
- 61.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, Lee JH, El-Awar N, Alberu J. “Natural” human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86(8):1111–1115. [DOI] [PubMed] [Google Scholar]
- 62.Jacob EK, De Goey SR, Gandhi MJ. Positive virtual crossmatch with negative flow crossmatch results in two cases. Transpl Immunol. 2011;25(1):77–81. [DOI] [PubMed] [Google Scholar]
- 63.Grenzi PC, de Marco R, Silva RZ, Campos EF, Gerbase-DeLima M. Antibodies against denatured HLA class II molecules detected in Luminex-single antigen assay. Hum Immunol. 2013;74(10):1300–1303. [DOI] [PubMed] [Google Scholar]
- 64.Visentin J, Guidicelli G, Moreau JF, Lee JH, Taupin JL. Deciphering allogeneic antibody response against native and denatured HLA epitopes in organ transplantation. Eur J Immunol. 2015;45(7):2111–2121. [DOI] [PubMed] [Google Scholar]
- 65.Santos SG, Powis SJ, Arosa FA. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J Biol Chem. 2004;279(51): 53062–53070. [DOI] [PubMed] [Google Scholar]
- 66.Buttigieg J, Ali H, Sharma A, Halawa A. Positive Luminex and negative flow cytometry in kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018, 1–11. [DOI] [PubMed] [Google Scholar]
- 67.Pandey S, Harville TO. Epitope analysis aids in transplant decision making by determining the clinical relevance of apparent pre-transplant donor specific antibodies (DSA). Ann Clin Lab Sci 2019;49(1):50–56. [PubMed] [Google Scholar]
- 68.Kwon H, Kim YH, Choi JY, et al. Impact of pretransplant donor-specific antibodies on kidney allograft recipients with negative flow cytometry cross-matches. Clin Transplant. 2018;32(6):e13266. [DOI] [PubMed] [Google Scholar]
- 69.Amico P, Hirt-Minkowski P, Honger G, et al. Risk stratification by the virtual crossmatch: a prospective study in 233 renal transplantations. Transpl Int. 2011;24(6):560–569. [DOI] [PubMed] [Google Scholar]
- 70.Adebiyi OO, Gralla J, Klem P, et al. Clinical significance of pretransplant donor-specific antibodies in the setting of negative cell-based flow cytometry crossmatching in kidney transplant recipients. Am J Transplant. 2016;16(12):3458–3467. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan HC, Liwski RS, Bray RA, Gebel HM. The road to HLA antibody evaluation: do not rely on MFI. Am J Transplant. 2017; 17(6):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]


