Abstract
Since the COVID-19 pandemic began, many individuals have reported acute loss of smell and taste. In order to better characterize all patients with these symptoms, a longitudinal national survey was created. Since April 10, 2020, 549 completed the initial survey, with 295 completing 14-day, and 202 completing 1-month follow up surveys. At 1-month follow-up, 71.8% reported a return to “very good” or “good” smell, and 84.2% reported a return to “very good” or “good” taste. Chemosensory changes are a cardinal sign of COVID-19. Fortunately, our data, representing a large longitudinal study of patients experiencing smell and taste losses during the COVID-19 pandemic, indicates that the majority appear to recover within a month.
Keywords: Smell, Taste, COVID-19, Coronavirus, Epidemiology, Recovery
1. Introduction
Since the emergence of the COVID-19 pandemic, reports from across the globe suggest that chemosensory loss is among the cardinal symptoms of active infection [[1], [2], [3], [4]]. These findings led major health organizations worldwide to include acute loss of smell and taste as symptoms sufficiently indicative of COVID-19 infection to warrant evaluation. However, little is known about the natural course of these deficits including their potential for recovery. Given significant quality of life and safety impacts of such deficits, prognostic information would benefit patients and their healthcare providers [5]. We present one-month follow-up data from a large, prospectively collected nationwide patient cohort with smell and taste losses during the COVID-19 pandemic.
2. Methods
A web-based survey was opened to patients nationwide with sudden changes in smell and taste since January 2020. Following consent, patient demographics, associated symptoms, comorbidities, testing status, treatment, and recovery status were collected and managed using REDCap electronic data capture tool [6,7]. Recruitment was performed through online social media platforms beginning April 10, 2020, with follow-up surveys at 14 days, 1 month, 3 months, and 6 months later. Inclusion criteria were age ≥ 18 years and smell or taste changes since January 1, 2020; those with documented negative COVID-19 tests were excluded. Respondents rated smell and taste as “Very Good”, “Good”, “Poor”, “Very Poor”, or “Absent”. This study was approved by Virginia Commonwealth University Institutional Review Board (HM20019186).
3. Results
Through June 7, 2020, 549 individuals meeting inclusion criteria completed the initial survey, 295 (53.7%) completed the 14-day follow-up, and 202 (36.8%) completed the 1-month follow-up. Table 1 details demographic information. Among the 549 respondents, 260 (47.4%) were COVID-19-positive (C+) by testing or diagnosed by a medical professional, and 289 (52.6%) not tested/diagnosed. Within the C+ cohort, 19.2% completed survey within 1–6 days of diagnosis, 51.2% within 1–4 weeks, 26.9% within 1–2 months, and 2.7% within 3–6 months. Thus over 70% of respondents completed the survey within 1 month of diagnosis.
Table 1.
Subject demographics (N = 549).
| N | % | ||
|---|---|---|---|
| Sex | Female | 421 | 76.7 |
| Male | 127 | 23.1 | |
| Prefer to self-describe | 1 | 0.2 | |
| Ethnicity | White | 432 | 78.7 |
| Hispanic or Latino | 41 | 7.5 | |
| Black or African American | 38 | 6.9 | |
| More than one | 17 | 3.1 | |
| Asian | 15 | 2.7 | |
| American Indian/Alaska Native | 4 | 0.7 | |
| Native Hawaiian/Other Pacific Islander | 1 | 0.2 | |
| Unknown/not reported | 1 | 0.2 | |
| Tobacco use | Never used | 393 | 71.6 |
| Quit before Jan 1, 2020 | 113 | 20.6 | |
| Currently use/quit after Jan 1, 2020 | 43 | 7.8 | |
| Vaping use | Never used | 500 | 91.1 |
| Quit before Jan 1, 2020 | 27 | 4.9 | |
| Currently use/quit after Jan 1, 2020 | 22 | 4.0 |
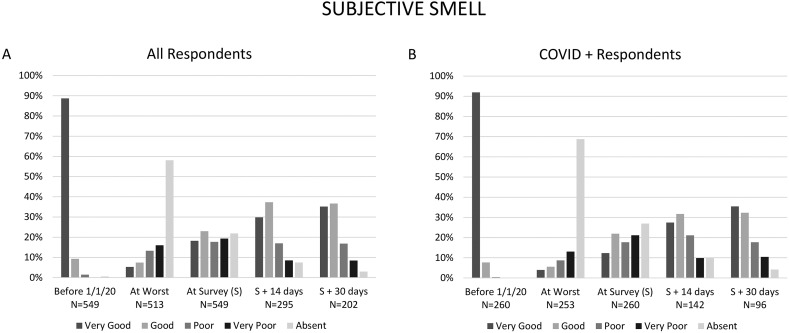
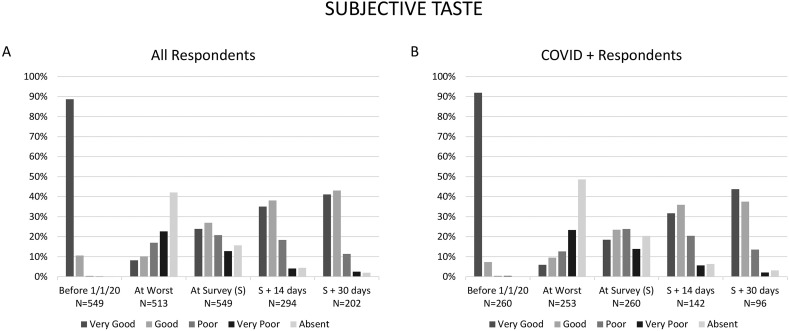
Fig. 1 shows the level of smell function over time for the entire cohort and for C+ respondents. At 14-day follow-up, 67.1% of respondents reported return of smell function to “very good” or “good,” increasing to 71.8% by 30-day follow-up. Fig. 2 similarly show taste function over time for the entire cohort and for C+ respondents. At 14-day follow-up, 73.1% of respondents reported return of taste function to “very good” or “good,” increasing to 84.2% by 1 month follow-up.
Fig. 1.
Self-reported sense of smell over time for all respondents (A), and only those respondents diagnosed with COVID-19 by medical professional or test (B).
Fig. 2.
Self-reported sense of taste over time for all respondents (A), and only those respondents diagnosed with COVID-19 by medical professional or test (B).
4. Discussion
Since onset of the COVID-19 pandemic, researchers sought to further our understanding of smell and taste changes associated with the disease. Initial work established acute loss of smell and taste as indicators of disease, with reported rates of chemosensory dysfunction among COVID-19 patients ranging from 30 to 85% [[8], [9], [10]]. Reported incidences reflect substantial variation due to differences in patient disease severity, timing of enrollment, study location, and assessment methods. Most studies determined degree of olfactory deficit as severe to complete anosmia in the majority of patients by self-report [1,9], or objective testing [4]. Despite this, early reports suggest rapid recovery. Our data suggest that within 1 month of diagnosis approximately one third of patients recover to normal or near normal smell and taste. After an additional month, over two thirds had recovered to this level, while less than 5% reported their sense of smell or taste as absent.
Initial publications from Europe and Asia report higher rates of recovery. A French study of PCR-tested patients showed 98% experiencing a complete subjective recovery within 28 days, with mean duration of anosmia near 9 days [11]. Workers in the United Kingdom repeated surveys 1 week after initial survey, revealing that 80% had experienced some recovery, while only 17% remained anosmic [12]. They also noted a “plateau” in recovery after approximately 3 weeks, with a 70% recovery rate for those with anosmia of 3 or more weeks duration. Similarly, a study from Korea using daily phone surveys of almost 500 newly diagnosed COVID-19 patients showed median duration of anosmia or ageusia of 7 days, and almost all recovering within 3 weeks [13].
The current study has limitations which may account for these differences. These include selection and recall biases inherent in any survey. Additionally, over half of subjects were not COVID-19 tested, but presumed positive given high prevalence of infection, and reports showing markedly higher rates of smell loss in COVID-19 positive than negative patients [10]. The similarity of time courses of COVID-19 diagnosed patients and the entire cohort further suggests that the vast majority of the untested subjects were in fact COVID-19 positive. Further, not all enrolled patients completed follow-up surveys. Recovered patients may have been less or more likely to complete follow-up surveys, thus potentially artificially increasing or decreasing observed recovery rates. Lastly insufficient data exists to quantify differences in virology or population susceptibility to effects of COVID-19 infection between studies undertaken in different countries.
It is noteworthy that our study and other reports suggest a rate of recovery from COVID-19-associated chemosensory deficits higher than those previously reported for post-viral anosmias, reported as low as 30% [14]. This may be due to intrinsic differences in the mechanisms by which COVID-19 impacts chemosensory systems. Alternately there may be differences in reporting. With the current pandemic causing heightened awareness, COVID-19 sufferers with milder deficits may be more likely to report than patients with other respiratory pathogens.
5. Conclusions
This study presents 1-month follow-up of patients with chemosensory changes participating in a large national longitudinal survey during the COVID-19 pandemic. Despite the severity of smell and taste loss experienced by most patients, nearly three-quarters will recover to normal or near-normal chemosensation within 2 months. The continued improvement noted between 14-day and 1-month surveys suggests that ongoing recovery is still in progress. Long term recovery and treatment effects will be a topic of further investigation. Clinicians who see patients with COVID-19-related chemosensory losses can reassure them that total or near-total recovery can be expected in the majority of instances.
Contributions
Conception: DHC, ZAK, RMC, ERR.
Design: DHC, ZAK, RMC, ERR.
Data Analysis: DHC, ZAK, RMC, ERR.
Manuscript Drafting: DHC, ZAK, RMC, ERR.
Final Approval: DHC, ZAK, RMC, ERR.
Funding
None.
Declaration of competing interest
None.
Acknowledgement
The use of the REDCap Database was partially funded by the National Center for Research Resources (NCRR), Award Number UL1TR002649.
References
- 1.Coelho D.H., Kons Z.A., Costanzo R.M., Reiter E.R. Subjective changes in smell and taste during the COVID-19 pandemic: a national survey-preliminary results. Otolaryngol–Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. May 19, 2020 doi: 10.1177/0194599820929957. Published online. 194599820929957. [DOI] [PubMed] [Google Scholar]
- 2.Gautier J.-F., Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obes Silver Spring Md. 2020;28(5):848. doi: 10.1002/oby.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.15% of COVID-19 patients lose sense of smell or taste: data. https://www.koreatimes.co.kr/www/nation/2020/03/119_286790.html
- 4.Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. April 17, 2020 doi: 10.1002/alr.22587. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pence T.S., Reiter E.R., DiNardo L.J., Costanzo R.M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–955. doi: 10.1001/jamaoto.2014.1675. [DOI] [PubMed] [Google Scholar]
- 6.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomelli A., Pezzati L., Conti F. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis Off Publ Infect Dis Soc Am. March 26, 2020 doi: 10.1093/cid/ciaa330. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. April 6, 2020 doi: 10.1007/s00405-020-05965-1. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. April 12, 2020 doi: 10.1002/alr.22579. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopfenstein T., Kadiane-Oussou N.J., Toko L. Features of anosmia in COVID-19. Med Mal Infect. April 16, 2020 doi: 10.1016/j.medmal.2020.04.006. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol - Head Neck Surg J Oto-Rhino-Laryngol Chir Cervico-Faciale. 2020;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reden J., Mueller A., Mueller C. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132(3):265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]