Abstract
BACKGROUND:
The classification of diffuse malignant mesothelioma into epithelioid, biphasic, and sarcomatoid types is based on histologic patterns. The diagnosis is made on biopsies, and because of intratumoral heterogeneity, they may not be representative of the entire tumor. The number and volume of biopsies needed to reach diagnostic accuracy in diffuse malignant mesothelioma and their prognostic value remain unclear.
METHODS:
This study examined 759 consecutive patients with pleural diffuse malignant mesothelioma treated by pleurectomy/decortication or extrapleural pneumonectomy for the presence of epithelioid and/or sarcomatoid histology and classified both the presurgery biopsies (core-needle or thoracoscopic) and surgical resection specimens. The number and volume of biopsies were correlated with pre- and postsurgery histologies and overall survival.
RESULTS:
Diffuse malignant mesothelioma was classified as epithelioid (76%), biphasic (18%), sarcomatoid (5%), or indeterminate (1%) in biopsies and as epithelioid (64%), biphasic (32%), and sarcomatoid (4%) in surgical resection specimens (overall concordance, 80.6%). The positive likelihood ratios were 2.4, 13.6, and 90.1 for biopsies with epithelioid, biphasic, and sarcomatoid histologies, respectively. Concordant histologies between biopsies and resections were associated with a higher number of biopsies (median tissue blocks for concordant histologies vs discordant histologies, 3 vs 2; P < .002) but were less associated with a higher volume (median, 1.2 vs 1.1 cm3; P = .06). In a multivariate analysis, overall survival was independently predicted by histology in the resection specimen (P < .0001) but not in the biopsy (P = .09).
CONCLUSIONS:
In contrast to epithelioid histology, sarcomatoid histology in biopsies is highly accurate. Despite intratumoral heterogeneity, the accuracy of histologic classification increases with the number of tissue blocks examined, emphasizing the diagnostic value of extensive sampling by presurgery biopsies.
Keywords: extrapleural pneumonectomy, malignant mesothelioma, pathology
INTRODUCTION
Diffuse malignant mesothelioma is a rare but highly aggressive malignancy of serosal membranes.1–8 The initial diagnosis is made on biopsy specimens and usually requires a panel of confirmatory immunohistochemical markers.9 The classification of mesothelioma into epithelioid, sarcomatoid, and biphasic types is based on the presence of epithelioid histology, sarcomatoid histology, or both histologies in the tumor and has been shown to have prognostic value.3,10 Biphasic malignant mesothelioma has a mixture of at least 10% of both epithelioid and sarcomatoid histologies and often displays large degrees of intertumoral and intratumoral variability.11–14 In initial biopsies, a definitive diagnosis can be rendered only if sufficient tissue is present for histologic and immunohistochemical evaluation.15 Thoracoscopy is the preferred sampling method for the diagnosis of diffuse malignant mesothelioma when it is suspected on the basis of clinical and radiologic information and has reported high accuracy rates of more than 90%.16–19 Because of the considerable histologic heterogeneity of diffuse malignant mesothelioma, the final classification of the tumor would require thorough sampling and evaluation in surgical resections15,20,21 and may be expected to differ from the classification derived from initial biopsies.22 However, correlations between the diagnostic accuracy of the initial biopsies for classifying the types of diffuse malignant mesothelioma and the extent of the biopsy sampling remain unclear.
In this study, we evaluated the accuracy of the initial diagnostic biopsy specimens in determining the histologic type of diffuse malignant mesothelioma in a large series of 759 consecutive patients with diffuse pleural malignant mesothelioma treated by surgery (extrapleural pneumonectomy in 519 and pleurectomy/decortication in 240) at a single institution. We examined the epithelioid, biphasic, and sarcomatoid histologies in both presurgery biopsies and surgical resections, and we correlated concordant and discordant diagnoses with the number and volume of initial biopsies with postsurgery resection histologies. Finally, we compared the prognostic value of the histologies in the initial biopsies and those in subsequent surgical resections.
MATERIALS AND METHODS
Patient Characteristics
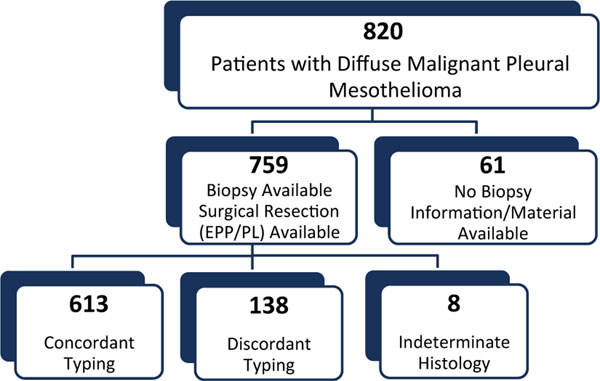
The initial study group included 820 consecutive patients with diffuse pleural malignant mesothelioma who had undergone pretreatment biopsy and pleurectomy/decortication or extrapleural pneumonectomy from 1988 to 2006 at Brigham and Women’s Hospital in Boston, Massachusetts (Fig. 1). Sixty-one patients were excluded because the pretreatment biopsy information was unavailable (the initial diagnosis was made via cytology preparations for 34 patients, and no biopsy information was available for 27 patients). This resulted in a study group of 759 consecutive patients who had both the initial diagnostic biopsies and surgical resection specimens available. The included and excluded patients had similar clinicopathologic characteristics (Supporting Table 1). The clinical stage was determined according to published American Joint Committee on Cancer guidelines (7th edition).23 The study was approved by the institutional review board at our institution.
Figure 1.
Diagram illustrating the assessment and selection of patients included in the study. Of the 820 consecutive patients diagnosed with diffuse malignant mesothelioma who underwent pleurectomy/decortication or extrapleural pneumonectomy, 61 had no pretreatment biopsy information and were excluded. The study group thus comprised 759 consecutive patients with both initial diagnostic biopsies and subsequent surgical resections. With the exclusion of the 8 mesothelioma cases with the indeterminate subtype in the initial biopsies, concordant histologic subtyping of mesothelioma between initial biopsies and subsequent resections was noted in 613 patients, whereas discordant subtyping was noted in 138 patients. Eight patients diagnosed with malignant mesothelioma had the indeterminate histologic type on biopsies. EPP indicates extrapleural pneumonectomy; PL, pleurectomy/decortication.
Assessment of Malignant Mesothelioma
All biopsy material had been previously reviewed according to intradepartmental guidelines at the Department of Pathology, Brigham and Women’s Hospital before patients were considered for surgery. Hematoxylin and eosin–stained slides were reviewed by 2 of 3 pathologists (W.C.F., M.D.H., and L.R.C.) with no knowledge of the prior diagnosis or clinical outcome; discrepant cases were resolved by common review and consensus with L.R.C. Tumors were classified as epithelioid, sarcomatoid, or biphasic (at least 10% of both epithelioid and sarcomatoid components) according to World Health Organization classification criteria.12 The total number of tissue blocks was retrieved from the gross description pathology reports, and the total volume of initial biopsies was computed from the specimen dimensions recorded in the gross description of each pathology report.
Statistical Analysis
Chi-square or Fisher exact tests were used to compare categorical data. Overall survival was calculated from the time of surgery to the time of death from any cause or to the time of last follow-up, at which point the data were censored. Patients who died in the perioperative period were excluded from the survival analysis. Overall survival curves were constructed with the Kaplan-Meier method, and a log-rank test was used to evaluate for statistical significance.
The prognostic significance of clinical and pathologic characteristics was determined with a univariate Cox regression analysis. Cox proportional hazards models were fitted for a multivariate analysis.
The sensitivity, specificity, positive predictive value, and positive likelihood ratio for each histologic type of mesothelioma were calculated with GraphPad InStat (version 3.10 for Windows; GraphPad Software, San Diego, California; https://www.graphpad.com/). Kaplan-Meier survival curves were drawn with GraphPad Prism (version 7.02 for Windows; GraphPad Software). A 2-sided significance level of .05 was used for all statistical analyses.
RESULTS
Characteristics of Patients With Diffuse Malignant Pleural Mesothelioma
Patient characteristics are summarized in Table 1. The cohort consisted of 759 patients—593 men (78%) and 166 women (22%)—with a median age of 61 years (range, 18–86 years). The initial pathologic diagnosis of diffuse pleural malignant mesothelioma was made on thoracoscopic biopsies in 728 patients and on core-needle biopsies in 31 patients. After the diagnosis by initial biopsy, 240 patients (32%) were treated with pleurectomy/decortication, and 519 patients (68%) were treated with extrapleural pneumonectomy. Of the 520 patients with available clinical staging, 14 (3%) were stage I, 50 (10%) were stage II, 344 (66%) were stage III, and 112 (21%) were stage IV.
TABLE 1.
Clinical Characteristics of the Study Group (n = 759)
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Male | 593 (78) |
| Female | 166 (22) |
| Age, y | |
| Mean | 60.9 |
| Median (range) | 61 (18–86) |
| Primary location, No. (%) | |
| Left pleura | 323 (43) |
| Right pleura | 436 (57) |
| Clinical stage, No. (%)a | |
| I | 14 (3) |
| II | 50 (10) |
| III | 344 (66) |
| IV | 112 (21) |
| Type of biopsy, No. (%) | |
| Core-needle | 31 (4) |
| Thoracoscopic | 728 (96) |
| Type of surgery, No. (%) | |
| Pleurectomy/decortication | 240 (32) |
| Extrapleural pneumonectomy | 519 (68) |
Two hundred thirty-nine patients in the study group did not have clinical staging information available because of incomplete staging studies.
Correlation Between Histologic Types in the Initial Biopsies and Surgical Resections
The relationship between histologic types in the initial biopsies and surgical resections is illustrated in Table 2 (see also Supporting Table 2 for thoracoscopic biopsies and Supporting Table 3 for core biopsies). In the initial biopsies, the 759 cases were classified as epithelioid (n = 575 [76%]), biphasic (n = 140 [18%]), sarcomatoid (n = 36 [5%]), and indeterminate (n = 8 [1%]). In sub-sequent surgical resections, the same cases were classified as epithelioid (n = 483 [64%]), biphasic (n = 243 [32%]), and sarcomatoid (n = 33 [4%]). The overall diagnostic concordance for histologic typing between initial biopsies and subsequent surgical resections was 81.6%: 80.5% (463 of 575) for epithelioid mesotheliomas, 86.4% (121 of 140) for biphasic mesotheliomas, and 80.6% (29 of 36) for sarcomatoid mesotheliomas. There was no statistically significant difference in the concordant diagnoses between thoracoscopic biopsies and core-needle biopsies (81.9% vs 71.0%; P = .12).
TABLE 2.
Relationship Between Histologic Types in the Initial Biopsies and Surgical Resections (n = 759)
| Biopsy Histology | Resection Specimen Histology, No. (% of Initial Biopsy)a | Total, No. | ||
|---|---|---|---|---|
| Epithelioid | Biphasic | Sarcomatoid | ||
| Epithelioid | 463 (81) | 112 (19) | 0 (0) | 575 |
| Biphasic | 15 (11) | 121 (86) | 4 (3) | 140 |
| Sarcomatoid | 0 (0) | 7 (19) | 29 (81) | 36 |
| Indeterminate | 5 (63) | 3 (38) | 0 (0) | 8 |
| Total | 483 (64) | 243 (32) | 33 (4) | 759 |
The P value is <.0001 (chi-square test) for the comparison of the initial biopsy histology and the final histology in the surgical resection. Eight patients with a diagnosis of malignant mesothelioma on the initial biopsy but with an indeterminate histologic type were excluded from the statistical analysis.
Because of rounding, not all the percentages total to 100.
The discordant diagnoses included 112 patients (19%) initially diagnosed with epithelioid mesothelioma who were subsequently found to have an additional sarcomatoid component of more than 10% in the surgical resection specimen and were thus classified as having biphasic malignant mesothelioma (Table 2). In addition, of the 140 patients initially diagnosed with biphasic malignant mesothelioma by biopsies, 15 (11%) were diagnosed with epithelioid mesothelioma and 4 (3%) were diagnosed with sarcomatoid mesothelioma in the surgical resections. Although each of these 19 cases showed both epithelioid and sarcomatoid patterns in the initial biopsy, they were reclassified because of the presence of a low proportion of 1 pattern in the resection (less than the diagnostic cutoff of at least 10% required for the biphasic type). Furthermore, 7 of the 36 patients (19%) with an initial biopsy diagnosis of sarcomatoid mesothelioma had a change in their diagnosis to the biphasic type in the surgical resection.
Testing Characteristics of Initial Biopsies in Determining the Histologic Type of Diffuse Malignant Pleural Mesothelioma
The testing characteristics for histology in initial biopsies for each type of mesothelioma are illustrated in Table 3 (see also Supporting Table 4 for thoracoscopic biopsies and Supporting Table 5 for core biopsies). The positive predictive value of the epithelioid type in the initial biopsy was 80.5% with a high sensitivity (96.9%) but a low specificity (59.0%). The positive predictive value of the biphasic type in the initial biopsies was 86.4% with a high specificity (96.3%) but a low sensitivity (50.4%). Finally, the diagnosis of sarcomatoid mesothelioma in the initial biopsies had a positive predictive value of 80.6% with a sensitivity of 87.9% and a specificity of 99.0%. The positive likelihood ratios were 2.4, 13.6, and 90.1 for epithelioid, biphasic, and sarcomatoid biopsies, respectively.
TABLE 3.
Comparison of the Positive Predictive Values, Sensitivities, Specificities, and Test Likelihood Ratios of Histology in the Initial Biopsy for Each Type of Mesothelioma (n = 751)
| Biopsy Histology | Positive Predictive Value, % | Sensitivity, % | Specificity, % | Positive Likelihood Ratio |
|---|---|---|---|---|
| Epithelioid | 80.5 | 96.9 | 59.0 | 2.4 |
| Biphasic | 86.4 | 50.4 | 96.3 | 13.6 |
| Sarcomatoid | 80.6 | 87.9 | 99.0 | 90.1 |
Eight patients with a diagnosis of malignant mesothelioma on the initial biopsy but with an indeterminate histologic type were excluded from the statistical analysis.
Number and Volume of Initial Biopsies as Predictors of the Histologic Type in Patients With Diffuse Malignant Pleural Mesothelioma
The relationship between the types of initial biopsies (core-needle vs thoracoscopic) and concordant or discordant diagnoses in the surgical resections is summarized in Table 4 and illustrated in Supporting Figure 1. In the 613 mesotheliomas with concordant histologies between the initial biopsies and surgical resections, the mean number of tissue blocks in the initial biopsies was 3.4 (median, 3; range, 1–20), whereas in the 138 mesotheliomas with discordant histologies, the mean number of tissue blocks in the initial biopsies was 2.7 (median, 2; range, 1–9; n = 751; P < .002; Table 4). Similarly, the number of tissue blocks examined was higher in concordant histologies than discordant histologies for thoracoscopic biopsies (n = 720; P < .005) but not for core-needle biopsies (n = 31; P = .56).
TABLE 4.
Comparison of Sampling Block Numbers in Initial Biopsies With Histology Concordant or Discordant With Surgical Resections in Diffuse Malignant Pleural Mesothelioma
| Initial Biopsy Type | No. of Tissue Blocks, Median (Range) | P | |
|---|---|---|---|
| Concordant With Surgical Resections | Discordant With Surgical Resections | ||
| All | 3 (1–20) | 2 (1–9) | <.002 |
| Thoracoscopic biopsies | 3 (1–20) | 2 (1–9) | <.005 |
| Core biopsies | 1 (1–3) | 1 (1–4) | .56 |
The accuracy of the histologic classification of mesothelioma gradually increased with the number of tissue blocks examined in biopsies (Supporting Fig. 2). Once the number of tissue blocks sampled in the biopsies was higher than 9 (10 or higher for 23 patients), the concordance rate reached 100%.
We also calculated the volumes of the initial thoracoscopic biopsies for 134 cases with available tissue dimensions recorded in the pathology reports, and we found that although there was a trend, there was no statistically significant difference between the volumes of concordant biopsies and discordant biopsies (median, 1.2 vs 1.1 cm3; mean, 2.9 vs 1.7 cm3; P = .06).
Prognostic Value of Histologic Types in the Initial Biopsies and Surgical Resections for Patients With Diffuse Malignant Pleural Mesothelioma
Univariate and multivariate analyses of the overall survival with respect to the clinicopathologic characteristics in this cohort are shown in Table 5. Clinical follow-up was available for 684 of the 759 patients (90%; 529 with epithelioid histology, 117 with biphasic histology, 31 with sarcomatoid histology, and 7 with indeterminate histology classified with the initial biopsies and 447 with epithelioid histology, 208 with biphasic histology, and 29 with sarcomatoid histology classified with the surgical resections). The median follow-up time of the overall group was 13.7 months (mean, 22.7 months; 95% confidence interval [CI], 20.8–24.6 months). The median potential follow-up time with censored data for the same group of patients was 26.8 months (mean, 44.0 months; 95% CI, 32.0–56.1 months). Univariate Cox regression analysis showed that sex, age, clinical stage, type of surgery, and histologic type in both the initial biopsies and the final resection specimens were prognostic indicators for overall survival (Table 5). No statistically significant differences in the overall survival were noted for patients with left-sided malignant pleural mesothelioma versus patients with right-sided malignant pleural mesothelioma.
TABLE 5.
Univariate and Multivariate Analyses of Overall Survival With Respect to Clinical and Pathologic Characteristics
| Characteristic | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex | ||||||
| Male vs female | 1.37 | 1.5–1.64 | .0004 | 0.89 | 0.70–1.14 | .37 |
| Age | ||||||
| <61.45 vs ≥61.45 y | 0.70 | 0.59–0.82 | <.0001 | 1.31 | 1.07–1.60 | .01 |
| Primary location | ||||||
| Left pleura vs right pleura | 0.97 | 0.83–1.14 | .71 | 0.98 | 0.81–1.19 | .86 |
| Clinical stagea | ||||||
| I vs II vs III vs IV | 1.37 | 1.18–1.59 | <.0001 | 1.38 | 1.19–1.60 | <.0001 |
| Type of surgery | ||||||
| Pleurectomy/decortication vs extrapleural pneumonectomy | 1.18 | 1.00–1.41 | .05 | 0.70 | 0.17–2.81 | .61 |
| Histology in biopsy specimenb | ||||||
| Sarcomatoid vs biphasic vs epithelioid | 1.93 | 1.67–2.23 | <.0001 | 1.28 | 0.96–1.70 | .09 |
| Histology in resection specimen | ||||||
| Sarcomatoid vs biphasic vs epithelioid | 2.09 | 1.81–2.41 | <.0001 | 1.61 | 1.24–2.10 | <.0001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Clinical stage information was available for 469 patients with clinical follow-up.
Seven cases were classified as indeterminate on the initial diagnostic biopsies and were excluded from the survival analyses.
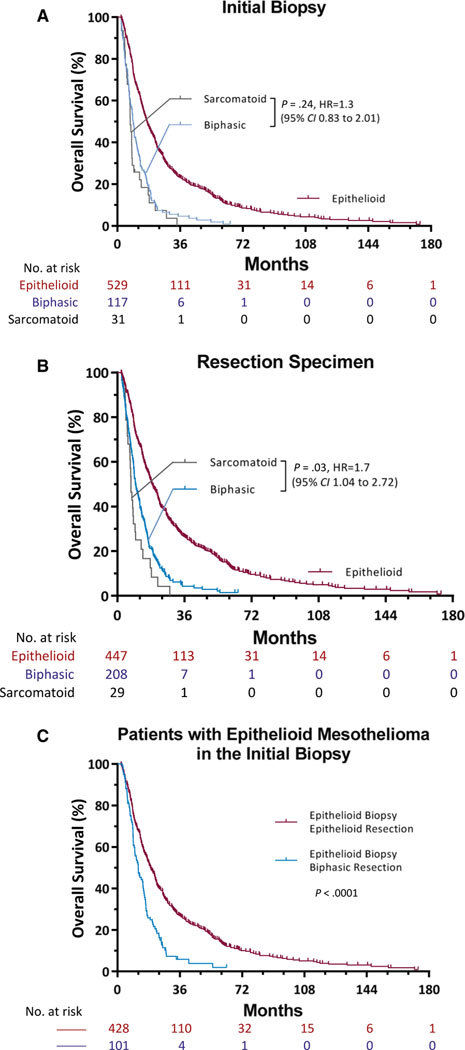
Based on the diagnosis from the initial biopsies, the median overall survival time was significantly better for patients with epithelioid histology (16.9 months) than patients with biphasic histology (9.1 months) or sarcomatoid histology (7.3 months; P < .0001; Table 5). The overall survival did not differ significantly between patients with biphasic mesothelioma and patients with sarcomatoid mesothelioma (95% CI, 0.83–2.01; P = .24; Fig. 2A).
Figure 2.
Kaplan-Meier estimates of overall survival among patients with diffuse malignant pleural mesothelioma according to the histology in (A) the initial biopsy and (B) the surgical resection. (A) In the initial biopsy, there was no difference in survival between the group with biphasic histology and the group with sarcomatoid histology (P = .24). (B) In the surgical resection, there was a difference in survival between the group with biphasic histology and he group with sarcomatoid histology (P = .03). (C) In their initial biopsies, both groups of patients were diagnosed with epithelioid malignant mesothelioma (n = 576). In 20% of these patients (n = 113), the diagnosis changed to a biphasic type in the resection specimen. The overall survival was longer for patients with epithelioid histology than patients with biphasic histology in the surgical resection (P < .0001).
Based on the final diagnosis determined from surgical resections, the median overall survival time was also significantly better for patients with epithelioid histology (18.7 months) than patients with biphasic histology (9.7 months) or sarcomatoid histology (7.3 months; P < .0001; Table 5). Furthermore, the overall survival based on surgical resections was significantly different between patients with biphasic mesothelioma and patients with sarcomatoid mesothelioma (hazard ratio, 1.7; 95% CI, 1.04–2.72; P = .03; Fig. 2B).
Finally, despite the initial biopsy diagnosis of epithelioid malignant mesothelioma, patients with epithelioid histology determined from the final surgical resections survived longer than patients with biphasic histology determined from the final surgical resections (median overall survival time, 19.2 vs 11.9 months; P < .0001; Fig. 2C). According to the multivariate analysis, age (P = .01), clinical stage (P < .0001), and histology in the resection specimen (P < .0001) were independent predictors of overall survival in diffuse malignant pleural mesothelioma. However, overall survival was not predicted by histology in the initial biopsy in the multivariate analysis (P = .09; Table 5).
DISCUSSION
A primary goal for the classification of diffuse malignant mesothelioma into epithelioid, biphasic, and sarcomatoid types in initial biopsies is to inform treatment decisions. Importantly, histologic classification is a powerful predictor of survival for patients with diffuse malignant mesothelioma. Several studies have evaluated survival differences among histologic types of diffuse malignant pleural mesothelioma.3,10,13,24–26 Until recently, studies on the accuracy of diagnostic biopsies have primarily focused on patients with pleural effusions or those undergoing pleural biopsy.27–30 A study comparing diagnostic techniques for malignant pleural mesothelioma in 83 patients found overall concordance rates of 83% for thoracotomy and 74% for thoracoscopic biopsy in comparison with extrapleural pneumonectomy.29 In our study, we evaluated the accuracy of the initial diagnostic biopsy specimens for determining the histologic type of diffuse malignant mesothelioma in a large series of 759 consecutive patients with diffuse malignant pleural mesothelioma treated by surgery at a single institution. We examined the epithelioid, biphasic, and sarcomatoid histologies in both presurgery biopsies and surgery resections, and we correlated concordant and discordant diagnoses with the number and volume of initial biopsies with postsurgery resection histologies. The overall discordant rate between histologies in initial biopsies and surgical resections was 18.4%, and this was similar to those previously reported for pleural biopsies in the diagnosis of malignant mesothelioma.15,19,29,31 We have demonstrated that histologic classification of malignant mesothelioma based on initial biopsies is both less accurate and less prognostic than histologic classification based on surgical resection. The evaluation of surgical resection specimens is the best modality for predicting survival for patients with diffuse malignant mesothelioma who are surgical candidates (Fig. 2).
Overall, the diagnostic accuracy was the highest for the sarcomatoid type, and this indicates that an initial diagnosis of sarcomatoid mesothelioma predicts with a high probability a final diagnosis of sarcomatoid malignant mesothelioma in the surgical resection (positive likelihood ratio, 90.1; Table 3). The presence of a biphasic pattern in the initial biopsy was less sensitive (50.4%) but highly specific (96.3%) for the diagnosis of biphasic malignant mesothelioma in the surgical resection. Finally, a diagnosis of epithelioid mesothelioma in the initial biopsy was not specific and was changed to the biphasic or sarcomatoid type in 19.5% of cases.
To our knowledge, the relationship between diagnostic accuracy and sampling extent, expressed as either the number of biopsies or the volume of sampled tissue, in malignant mesothelioma has not been explored before this study. As expected, all discordant cases involved the biphasic category, with biphasic mesotheliomas being misclassified as epithelioid or sarcomatoid in initial biopsies or vice versa. Because the diagnosis of the biphasic type required the identification of at least 10% of both epithelioid and sarcomatoid components and a biopsy of approximately 1 cm3 was only approximately 0.1% to 1% of the tumor burden (estimated to be approximately 100–1000 cm3) in a patient with malignant mesothelioma, even large biopsies may nevertheless be limited in sampling all histologies. Accuracy in the histologic classification of mesothelioma was best achieved with techniques such as thoracoscopic biopsy or thoracotomy rather than core-needle biopsy as previously suggested29 (Table 4). Our data also suggest that the volume of initial thoracoscopic biopsies would not improve the degree of concordance with the histologic type in the surgical resection.
The correlation between diagnostic accuracy and histologic type or clinical outcome in malignant mesothelioma remains unclear. Overall, our study has demonstrated prolonged survival for mesothelioma patients with epithelioid histology in comparison with patients with nonepithelioid histology, and this is consistent with the study by Sugarbaker et al24 and subsequent studies.20,22,25,32–35 Interestingly, a study by Arrossi et al31 of 56 patients with mesothelioma found that the histologic type determined by diagnostic biopsy, but not the histologic evaluation of surgical resection specimens, was associated with disease-free survival. In our study, in a larger cohort of patients with mesothelioma, histologies from surgical resections appeared to be a better prognostic predictor than histologies from initial biopsies (Fig. 2A,B). The reason for this discrepancy between the study by Arrossi et al and our study is unclear, but the discrepancy is likely due to different sample sizes and methodologies (measurement of disease-specific survival vs overall survival). Nevertheless, the strong correlation between the histologic type determined from surgical resections and overall survival suggests that clinical trials and outcome studies of malignant mesothelioma that are based solely on initial biopsies alone should be interpreted with caution.
To our knowledge, this study is the first to address important questions about the number of tissue blocks and the volume of the specimen needed for accurate histologic typing of malignant mesotheliomas because currently there are no recommendations for specimen processing or sampling in any published practice guidelines or international classification of malignant mesothelioma. We showed that the initial thoracoscopic biopsies that had concordant diagnoses with surgical resections were sampled with a median of 3 tissue blocks. We also showed that the diagnostic accuracy rates gradually increased with the number of biopsies from 80% and reached 100% with more than 9 tissue blocks sampled. These findings could have valuable clinical practice implications for medical professionals involved in the evaluation, diagnosis, and treatment of patients with malignant mesothelioma.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This study was supported by the International Mesothelioma Program.
CONFLICT OF INTEREST DISCLOSURES
Lucian R. Chirieac undertakes medicolegal work related to mesothelioma. Paul A. VanderLaan reports personal fees from Gala Therapeutics and Foundation Medicine outside the submitted work. Raphael Bueno receives support from the National Cancer Institute, Verastem, Genentech-Roche, and Castle Biosciences via research grants to Brigham and Women’s Hospital and reports grants from Merck, Siemens, Epizyme, Gritstone, and HTH and other from Navigation Sciences. The financial disclosures do not apply to the current study, which is not associated with a specific source of funding. The other authors made no disclosures.
REFERENCES
- 1.Mazurek JM, Syamlal G, Wood JM, Hendricks SA, Weston A. Malignant mesothelioma mortality—United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2017;66:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: malignant pleural mesothelioma, version 3.2016. J Natl Compr Canc Netw. 2016;14:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano MT, Zhang H . Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol. 2010;5:1841–1848. [DOI] [PubMed] [Google Scholar]
- 4.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999;79:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39:576–588. [DOI] [PubMed] [Google Scholar]
- 6.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res. 2015;196:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boffetta P, Malvezzi M, Pira E, Negri E, La Vecchia C. International analysis of age-specific mortality rates from mesothelioma on the basis of the International Classification of Diseases, 10th Revision. J Glob Oncol. 2018;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647–667. [DOI] [PubMed] [Google Scholar]
- 10.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol. 2007;2:957–965. [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. [DOI] [PubMed] [Google Scholar]
- 12.Travis W, Brambilla A, Burke A, Marx A, Nicholson A. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2015:165–168. [Google Scholar]
- 13.Fusco V, Ardizzoni A, Merlo F, et al. Malignant pleural mesothelioma. Multivariate analysis of prognostic factors on 113 patients. Anticancer Res. 1993;13:683–689. [PubMed] [Google Scholar]
- 14.Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg. 1999;68:1799–1804. [DOI] [PubMed] [Google Scholar]
- 15.Bueno R, Reblando J, Glickman J, Jaklitsch MT, Lukanich JM, Sugarbaker DJ. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg. 2004;78:1774–1776. [DOI] [PubMed] [Google Scholar]
- 16.Boutin C, Rey F. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: diagnosis. Cancer. 1993;72:389–393. [DOI] [PubMed] [Google Scholar]
- 17.Astoul P Pleural mesothelioma. Curr Opin Pulm Med. 1999;5: 259–268. [DOI] [PubMed] [Google Scholar]
- 18.Xu LL, Yang Y, Wang Z, Wang XJ, Tong ZH, Shi HZ. Malignant pleural mesothelioma: diagnostic value of medical thoracoscopy and long-term prognostic analysis. BMC Pulm Med. 2018;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greillier L, Cavailles A, Fraticelli A, et al. Accuracy of pleural biopsy using thoracoscopy for the diagnosis of histologic subtype in patients with malignant pleural mesothelioma. Cancer. 2007;110:2248–2252. [DOI] [PubMed] [Google Scholar]
- 20.Johansson L, Linden CJ. Aspects of histopathologic subtype as a prognostic factor in 85 pleural mesotheliomas. Chest. 1996;109:109–114. [DOI] [PubMed] [Google Scholar]
- 21.van Gelder T, Hoogsteden HC, Vandenbroucke JP, van der Kwast TH, Planteydt HT. The influence of the diagnostic technique on the histopathological diagnosis in malignant mesothelioma. Virchows Arch A Pathol Anat Histopathol. 1991;418:315–317. [DOI] [PubMed] [Google Scholar]
- 22.Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women’s Hospital experience over two decades, 1965–1985. J Clin Oncol. 1988;6:147–153. [DOI] [PubMed] [Google Scholar]
- 23.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. New York, NY: Springer; 2002. [Google Scholar]
- 24.Sugarbaker DJ, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol. 1993;11:1172–1178. [DOI] [PubMed] [Google Scholar]
- 25.Van Gelder T, Damhuis RA, Hoogsteden HC. Prognostic factors and survival in malignant pleural mesothelioma. Eur Respir J. 1994;7:1035–1038. [PubMed] [Google Scholar]
- 26.Verma V, Ahern CA, Berlind CG, et al. Survival by histologic sub-type of malignant pleural mesothelioma and the impact of surgical resection on overall survival. Clin Lung Cancer. 2018;19:e901–e912. [DOI] [PubMed] [Google Scholar]
- 27.Attanoos RL, Gibbs AR. The comparative accuracy of different pleural biopsy techniques in the diagnosis of malignant mesothelioma. Histopathology. 2008;53:340–344. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp HD, Kundra NK, Aranson R, Chong F, MacDonnell KF. The role of closed pleural needle biopsy in the diagnosis of malignant mesothelioma of the pleura. Chest. 1992;102:1110–1112. [DOI] [PubMed] [Google Scholar]
- 29.Kao SC, Yan TD, Lee K, et al. Accuracy of diagnostic biopsy for the histological subtype of malignant pleural mesothelioma. J Thorac Oncol. 2011;6:602–605. [DOI] [PubMed] [Google Scholar]
- 30.Poe RH, Israel RH, Utell MJ, Hall WJ, Greenblatt DW, Kallay MC. Sensitivity, specificity, and predictive values of closed pleural biopsy. Arch Intern Med. 1984;144:325–328. [PubMed] [Google Scholar]
- 31.Arrossi AV, Lin E, Rice D, Moran CA. Histologic assessment and prognostic factors of malignant pleural mesothelioma treated with extrapleural pneumonectomy. Am J Clin Pathol. 2008;130: 754–764. [DOI] [PubMed] [Google Scholar]
- 32.Neragi-Miandoab S, Richards WG, Sugarbaker DJ. Morbidity, mortality, mean survival, and the impact of histology on survival after pleurectomy in 64 patients with malignant pleural mesothelioma. Int J Surg. 2008;6:293–297. [DOI] [PubMed] [Google Scholar]
- 33.Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996;111:815–825. [DOI] [PubMed] [Google Scholar]
- 34.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. [DOI] [PubMed] [Google Scholar]
- 35.Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg. 2011;40:298–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.