Abstract
Nutrition is a major factor influencing many aspects of Drosophila melanogaster physiology. However, a wide range of diets, many of which are termed “standard” in the literature, are utilized for D. melanogaster research, leading to inconsistencies in reporting of nutrition-dependent phenotypes across the field. This is especially evident in microbiome studies, as diet has a pivotal role in microbiome composition and resulting host-microbe interactions. Here, we performed a meta-analysis of diets used in fly microbiome research and provide a web-based tool for researchers to determine the nutritional content of diets of interest. While our meta-analysis primarily focuses on microbiome studies, our goal in developing these resources is to aid the broader community in contextualizing past and future studies across the scope of D. melanogaster research to better understand how individual lab diets can contribute to observed phenotypes.
Keywords: Drosophila melanogaster, artificial diets, gut microbiota, nutritional analysis, host-microbe interactions
In the laboratory, the typical Drosophila melanogaster diet is composed of agar, yeast, a sugar source, and cornmeal. However, in reality dietary compositions vary greatly across laboratories, making it difficult to clearly define the composition of a “standard” fly diet. Commonly used “standard” diets exist, such as the Bloomington Standard or CalTech diets that originated at early hubs of D. melanogaster research. While many lab groups base their diets on these recipes, the vast majority of groups maintain flies on diets unique to their laboratory. Differences between these diets, despite their general suitability for fly rearing, can make it challenging to contextualize studies within the scope of D. melanogaster research, as nutrition is a critical factor influencing many aspects of physiology including metabolism (Piper et al. 2005; Brookheart and Duncan 2016), behavior (Edgecomb et al. 1994; Ormerod et al. 2017; Davies et al. 2018), development (Ormerod et al. 2017; Grangeteau et al. 2018), longevity (Piper et al. 2005; Ormerod et al. 2017; Stefana et al. 2017), sexual dimorphism (Rideout et al. 2015; Duxbury et al. 2017; Shingleton et al. 2017), and microbiome composition and function (Wong et al. 2014; Obadia et al. 2018; Erkosar et al. 2018). The relationship between nutrition and the gut microbiome is particularly important, as altering one will likely impact the other with physiologic consequences. Diet plays a pivotal role in shaping microbiome composition and affects interactions between microbiota and host, and the microbiome itself impacts the fly’s nutritional environment, both as a direct source of nourishment and via production and/or utilization of nutrients (Storelli et al. 2011; Shin et al. 2011; Wong et al. 2014; Yamada et al. 2015; Huang and Douglas 2015; Broderick 2016; Fischer et al. 2017; Keebaugh et al. 2018; Erkosar et al. 2018; Keebaugh et al. 2019). Together, dietary nutrition and the microbiome act in concert with one another to dictate nutritional physiology (Figure 1).
Figure 1.
Dietary nutrition and the microbiome are inextricably linked. Dietary nutritional content impacts the diversity and abundance of microbiome members, can influence microbe-microbe interactions, and affects metabolites produced by the microbiome. At the same time, the microbiome itself contributes to overall nutrition via production of metabolites, which are then utilized by the host, catabolism of carbohydrates, and by serving as a direct source of protein to the fly. Together, dietary nutrition and the microbiome interact to play a significant role in host physiology.
In an effort to aid in the contextualization of studies focused on the D. melanogaster microbiome, we performed a meta-analysis of diets used across the field. We analyzed the nutrition values of diet recipes, focusing on protein and carbohydrate content of diets to visualize how widely “standard” laboratory diets vary across D. melanogaster microbiome studies. Additionally, we have provided a web-based tool for use by the broader community that we’ve named the Drosophila Dietary Composition Calculator (DDCC, https://www.brodericklab.com/DDCC.php), which can be used to rapidly determine the macronutrient content of diets of interest simply by inputting amounts of each diet component for a given diet. It is our hope that this meta-analysis and the DDCC can be used to better understand dietary influences on previously observed phenotypes and serve as a resource for experimental design of future studies involving fly nutrition.
Methods
Nutritional information for dietary components
Values for calories, fiber, sugars, protein, fat, and carbohydrates were determined for each dietary component using nutritional labels for specific food products, information directly from manufacturers, or from NutritionData.com, a database of food nutritional values obtained from the United States Department of Agriculture’s National Nutrient Database for Standard Reference. The sources for each dietary component are provided in the Supplemental Files. The carbohydrate and protein information for raw fruits was determined using NutritionData.com.
Analysis of dietary differences across microbiome studies- fly microbiome diet database
Dietary compositions from over 50 articles (listed in Table S1) with a focus on the D. melanogaster microbiome were recorded in appropriate columns of the database (Columns A-AF). Calculations for calories per liter, grams of fiber per liter, sugars per liter, protein per liter, fat per liter, carbohydrates per liter, percent fiber, percent sugars, percent protein, percent fat, percent carbohydrates, and the ratio of protein to carbohydrates (P:C) (Columns AH-AT) were performed within the spreadsheet using the previously determined nutritional value for each dietary component. Nutritional information for the holidic fly diet (Piper et al. 2014) was determined by inputting the agar and sucrose amounts in the spreadsheet as normal and adding the calculated final mass of amino acids per liter to the formula in Column AL (grams of protein per liter). Similarly, for other diets containing one unique ingredient not otherwise represented in the database, calculations were performed as normal with the nutritional information for the unique ingredient added manually. In these cases, notes are made on the database to indicate special calculations. If it was not possible to calculate the nutritional information for an individual diet, it is noted in Columns AH-AM. Articles that did not readily provide dietary composition were documented for analytical purposes but excluded from the publicly available database. Ultimately, six “branded standard” diets and 71 explicitly reported diets from the literature were included in the database. An additional 14 studies examined did not provide their dietary composition.
The Drosophila Dietary Composition Calculator (DDCC)
Calculations used to obtain the nutrition facts for the Database were used to generate the calculator tool found at https://www.brodericklab.com/DDCC.php. The DDCC can be utilized to calculate nutritional information for diets not found in the Fly Microbiome Diet Database. We invite researchers to submit diet recipes using the provided web form in the DDCC for inclusion in the publicly available database.
Data availability
The source files for all nutritional information used to create the Fly Microbiome Diet Database and the DDCC are located at [https://doi.org/10.6084/m9.figshare.11920743.v1] as Files S1-S21. Each of these files corresponds to a different diet component, which is also detailed in the file name (for example, FileS1_YeastExtract_General.pdf, FileS7_Agar_Drosophila.pdf, FileS8_Molasses_Solids.pdf, FileS11_Molasses_General.pdf, etc). A downloadable version of the Fly Microbiome Diet Database is located at [https://doi.org/10.6084/m9.figshare.11920788.v2]. Supplemental tables are available at [https://doi.org/10.6084/m9.figshare.12241712.v1]. Table S1 details the studies used to compile the Fly Microbiome Diet Database including DOIs; Table S2 provides nutritional information and sources for raw fruits used to generate Figure 4.
Results and Discussion
Comparison of diets used across fly microbiome studies
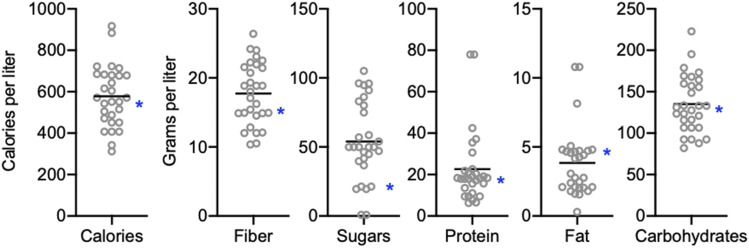
We analyzed the nutritional content of over 70 published diets used for D. melanogaster microbiome research based on the dietary components listed in the study methods. Dietary composition varies considerably both in the types of components used and the amounts of components, leading to a wide range of calories, protein, carbohydrate, fat, and fiber levels (Figure 2). Moreover, the type/source of a given ingredient can impact these values. For example, for a common ingredient like yeast, several different formulations are used including active, inactive, brewer’s, Lesaffre, and Springaline, all of which have unique nutritional compositions (e.g., protein content ranges from 38% in active dry yeast to 63% in Springaline yeast). Specific ingredients can also add unexpected components to diet. For example, Springaline yeast, used by a number of European fly immunity/microbiome labs contains 0.03 grams of the antioxidant glutathione per gram of yeast, meaning typical diets can range from 1.5-1.8 grams of added glutathione per liter of diet. This equates to a concentration of around 5 mM, a level used in some studies to block superoxide toxicity (Kim et al. 1997; Buchon et al. 2009).
Figure 2.
Nutritional content of “standard” D. melanogaster diets. Calories, grams of fiber, grams of sugars, grams of protein, grams of fat, and grams of carbohydrates per liter of food of laboratory diets reported as “standard” in the literature. Each point represents a different diet. The minimum and maximum values for each parameter as are follows: Calories- 311.97 and 917.13, Fiber- 10.36 and 26.38, Sugars- 0.80 and 105.00, Protein- 6.33 and 77.93, Fat- 0.30 and 10.80, Carbohydrates- 81.90 and 222.71. Line represents mean. For comparison purposes, blue asterisks indicate value of each parameter for the Bloomington Standard diet. n = 29 diets referred to as “standard” out of 71 diets.
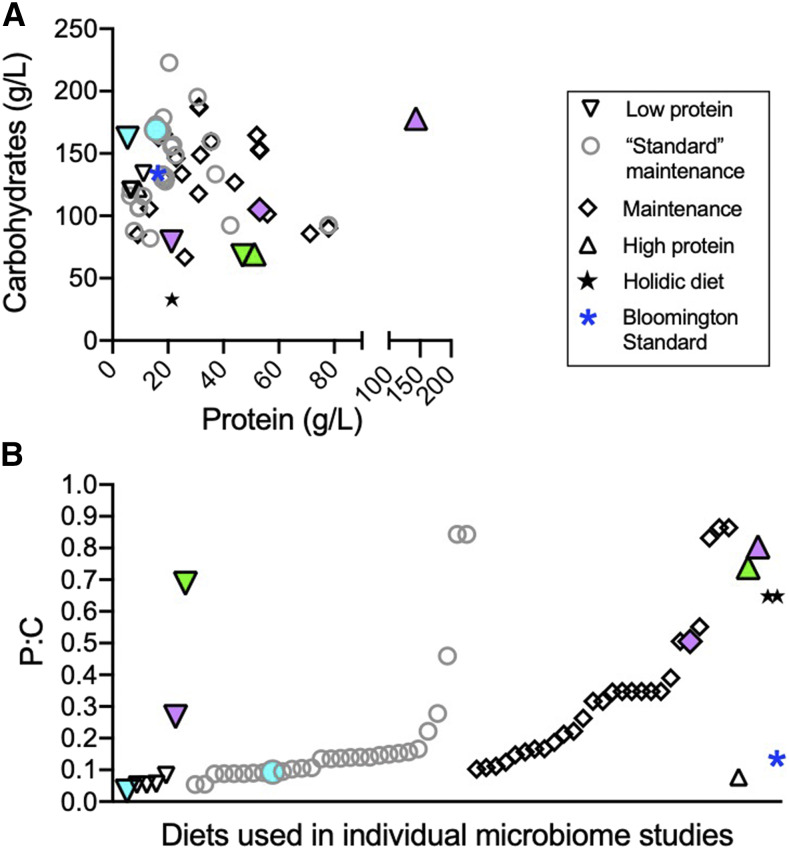
To get a better sense for nutritional differences across the diets, we focused on protein and carbohydrate content (Figure 3A). While some overlap was seen, particularly for diets based on the Bloomington Standard diet (indicated by blue asterisks) or multiple studies from the same laboratory, the overall spread of protein and carbohydrate content was large. Dietary protein to carbohydrate (P:C) ratio is known to be an important factor influencing life history traits (Lee et al. 2008; Jang and Lee 2018), so we next compared P:C of each diet and identified a range of maintenance diets (i.e., not experimental diets with altered diet components) with P:C’s from 0.05 to 0.86 (Figure 3B). We additionally noted that a range of P:C’s existed for diets considered “rich” or “poor” with regard to protein content. “Poor” diet P:C’s were between 0.03 and 0.69 with “rich” diets ranging from 0.05 to 0.8 (Figure 3B).
Figure 3.
Comparisons of diets used across microbiome research. A) Protein and carbohydrate content of individual diets as determined using the microbiome database. B) Protein-to-carbohydrate ratio (protein divided by carbohydrates) of individual diets. Each point represents a different diet reported in fly microbiome literature: inverted triangles represent diets designated as “poor” or low protein; gray circles represent maintenance diets that are described as “standard” in the literature; black diamonds represent diets used for normal maintenance of fly lines; triangles represent diets specifically defined as “rich” or high protein; stars represent the holidic fly diet; blue asterisk represents the Bloomington Standard diet. In (B), turquoise points are examples of two diets used in the same study that represent both a normal and low protein diet (Shin et al. 2011); green points similarly represent another study utilizing a high and low protein (Storelli et al. 2011); lavender points represent a third study using multiple diets (Erkosar et al. 2018). n = 71 diets (14 diets were not provided); all raw data can be found in the Fly Microbiome Diet Database.
Using this visualization of dietary composition, we observed an interesting comparison between two studies that each demonstrated a role for the microbiome in normal larval development in protein poor conditions (achieved through reduced yeast levels; Storelli et al. 2011 and Shin et al. 2011). Shin et al. used two diets that are relatively low in protein (turquoise points) and only differed in P:C by 0.06. Storelli et al. also used two diets that differed in P:C by a similar level (0.05), however compared to Shin et al. these diets were relatively protein rich (green points). Both studies show that the microbiome enhanced fly development on their respective low protein diets, but not on the higher protein version. Our comparative analysis indicates that small shifts in protein, even if not evident from P:C values, can be sufficient to reveal biologically important phenotypic effects of diet. However, while the observed phenotypes were similar in these studies, different mechanisms behind the observed developmental effects were reported, including being attributed to different microbiome members- Acetobacter pomorum in Shin et al. and Lactobacillus plantarum in Storelli et al. Our analysis shows that the overall diets differ significantly in both protein and carbohydrates levels (Figure 3), which could explain the different microbes and mechanisms, as macromolecule concentrations could greatly impact microbiome composition, microbe and/or host physiology, and/or the resulting interaction. This is supported by recent work by Erkosar et al. who showed that flies reared on diets containing significantly different concentrations of yeast (Figure 3, lavender points) had distinct shifts in microbial community composition (Erkosar et al. 2018). These examples highlight the importance of contextualizing studies based on dietary composition and how such comparisons can influence interpretation and subsequent studies.
The “standard diet” fallacy
At the time of writing, 16% of articles examined (14 of 85) gave no clearly defined diet composition and of this group, 71% (10 of 14) described their diet as “standard.” Overall, 46% of diets from all articles (39 of 85) were referred to as “standard,” yet both the range of diet components and total nutritional values of these diets are large (Figure 2 and shown as open gray circles in Figure 3). It is clear from the ranges we observed that no true “standard” diet exists, highlighting the problematic, but common, phrasing of “standard fly diet” in the literature, which is compounded when the diet recipe is not provided. Our analysis only looked at fly microbiome studies, but we expect this is a wide-spread problem and that other areas of D. melanogaster research have a similarly wide range of “standard” diets (whether explicitly reported or not). For example, a recent study by Ormerod et al. (2017) revealed significant differences in larval development and fly lifespan/aging, among other traits, even when using two commercially available “standard” diets (Equation 4-24 and Jazz-Mix). Considering the reported discrepancies in fly life history between just two “standard” diets in Ormerod et al,, it becomes apparent how the use of any number of other “standard” or non-standard diets can result in, and likely has resulted in, inconsistent observations between laboratories, particularly in the fields of development and aging, which are both so heavily dependent on nutrition (Piper et al. 2005; Lee et al. 2008; Stefana et al. 2017; Ormerod et al. 2017; Grangeteau et al. 2018).
Artificial vs. natural diets
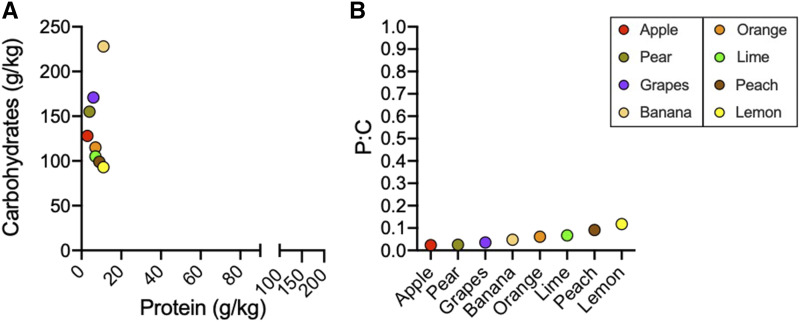
To understand how the range of laboratory diets compares to natural fruit diets that D. melanogaster encounters in the wild, we obtained protein and carbohydrate information (grams per kilogram) for apples, pears, grapes, bananas, oranges, limes, peaches, and lemons. Carbohydrates spanned from 93 g/kg to 228 g/kg and protein from 3 g/kg to 11 g/kg, resulting in a range of P:C’s from 0.02 to 0.11 (Figure 4). While many artificial diets fall within this range, protein content is typically much higher in laboratory conditions compared to natural diets, which may contribute to the lower diversity of microbes found in laboratory reared flies compared to wild-caught (Chandler et al. 2011; Erkosar et al. 2018). In either natural or artificial diets, however, the nutritional role of microbes must also be considered. In nature, D. melanogaster only associates with decomposing (ripe/over-ripe) fruit that support high densities of yeasts and bacteria, which consume carbohydrates within the substrate and can serve as a source of protein (Keebaugh et al. 2018; Huang and Douglas 2015). As such, we would expect the presence of microbes to lead to higher P:C’s in decaying fruits than represented in Figure 4, possibly approaching more those seen in artificial diets. However, the degree to which microbes alter nutrition of these natural substrates is unknown. Additionally, while artificial diets remove the requirement for microbes to break down complex plant material before consumption by the fly, microbes likely still impact nutrition in artificial diets, but the extent of this and its impacts on the fly in “standard” conditions has not been extensively explored.
Figure 4.
Comparison of protein and carbohydrate content of fruits. A) Protein and carbohydrates of raw fruits. B) Protein-to-carbohydrate ratio (protein divided by carbohydrates) of raw fruits. Each point represents nutritional information for a different fruit as provided by the United States Department of Agriculture (see Table S2 for raw data).
Does D. melanogaster need a standard diet?
It is clear that differences in fly diet have led to issues in reproducibility of results across the field (See Sharon et al. 2010, Obadia et al. 2018, and Leftwich et al. 2018 for one example; Douglas 2018 for commentary on another). One approach to combat such issues is the use of a fully defined diet such as the holidic diet (Piper et al. 2014). There are many advantages of using a chemically defined diet, as diet components are more strictly controlled, providing greater power to assess the role of individual nutrients on host physiology and microbiome-mediated impacts. However, chemically defined diets are costly and labor-intensive to make and are less representative of natural, complex dietary substrates (which include complex textures, different particle sizes, etc.) making this an unrealistic option for standardization of fly rearing and research across fields. We suggest that a manageable and reasonable approach to address dietary differences across studies is simply to require explicit reporting of diet composition at the time of publication. While having such data does not eliminate variability, it is invaluable for contextualizing results and phenotypes, provides potential explanations for observed differences, and testable hypotheses for follow-up in subsequent studies. We also expect that use of complex diet components is beneficial for discovery of physiologically relevant phenotypes that may otherwise be lost or artificially altered on more defined diets. For example, food particle size in animal gut ecosystems is known to impact digestion and bulk passage rate as well as microbiome composition through attachment and microcolony support (Cheng et al. 1981; Martz and Belyea 1986; Bjorndal et al. 1990; McAllister et al. 1994; Vermeulen et al. 2018; Kiarie and Mills 2019). Ultimately, what is important is that researchers understand the nutritional implications of the diets they use and look to nutritional information as a resource to aid in analysis of results and comparison across laboratories. While our study focused specifically on fly microbiome papers, diet has profound impacts on many aspects of animal physiology. We anticipate that the examples highlighted in this meta-analysis and the data provided by the DDCC will aid in a broader appreciation for the importance of dietary reporting, and help to contextualize observations across research studies using D. melanogaster.
Acknowledgments
The Drosophila Dietary Composition Calculator was created by Big Rose Web Design, LLC. This work was supported by the National Institutes of Health [R35GM128871] and the University of Connecticut.
Footnotes
Communicating editor: J. Tennessen
Supplemental material available at https://doi.org/10.6084/m9.figshare.12241712
Literature Cited
- Bjorndal K. A., Bolten A. B., and Moore J. E., 1990. Digestive Fermentation in Herbivores: Effect of Food Particle Size. Physiol. Zool. 63: 710–721. 10.1086/physzool.63.4.30158172 [DOI] [Google Scholar]
- Broderick N. A., 2016. Friend, foe or food? Recognition and the role of antimicrobial peptides in gut immunity and Drosophila-microbe interactions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371: 20150295 10.1098/rstb.2015.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookheart R. T., and Duncan J. G., 2016. Modeling dietary influences on offspring metabolic programming in Drosophila melanogaster. Reproduction 152: R79–R90. 10.1530/REP-15-0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Chakrabarti S., and Lemaitre B., 2009. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23: 2333–2344. 10.1101/gad.1827009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. A., Lang J. M., Bhatnagar S., Eisen J. A., and Kopp A., 2011. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 7: e1002272 10.1371/journal.pgen.1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Fay J. P., Coleman R. N., Milligan L. P., and Costerton J. W., 1981. Formation of bacterial microcolonies on feed particles in the rumen. Appl. Environ. Microbiol. 41: 298–305. 10.1128/AEM.41.1.298-305.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L. R., Schou M. F., Kristensen T. N., and Loeschcke V., 2018. Linking developmental diet to adult foraging choice in Drosophila melanogaster. J. Exp. Biol. 221: jeb175554 10.1242/jeb.175554 [DOI] [PubMed] [Google Scholar]
- Douglas A. E., 2018. Contradictory Results in Microbiome Science Exemplified by Recent Drosophila Research. MBio 9: e01758 10.1128/mBio.01758-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury E. M. L., Rostant W. G., and Chapman T., 2017. Manipulation of feeding regime alters sexual dimorphism for lifespan and reduces sexual conflict in Drosophila melanogaster. Proc. Biol. Sci. 284: 20170391 10.1098/rspb.2017.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgecomb R. S., Harth C. E., and Schneiderman A. M., 1994. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J. Exp. Biol. 197: 215–235. [DOI] [PubMed] [Google Scholar]
- Erkosar B., Yashiro E., Zajitschek F., Friberg U., Maklakov A. A. et al. , 2018. Host diet mediates a negative relationship between abundance and diversity of Drosophila gut microbiota. Ecol. Evol. 12: 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C. N., Trautman E. P., Crawford J. M., Stabb E. V., Handelsman J. et al. , 2017. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. eLife 6: e18855 10.7554/eLife.18855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeteau C., Yahou F., Everaerts C., Dupont S., Farine J.-P. et al. , 2018. Yeast quality in juvenile diet affects Drosophila melanogaster adult life traits. Sci. Rep. 8: 13070–13079. 10.1038/s41598-018-31561-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.-H., and Douglas A. E., 2015. Consumption of dietary sugar by gut bacteria determines Drosophila lipid content. Biol. Lett. 11: 20150469 10.1098/rsbl.2015.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang T., and Lee K. P., 2018. Comparing the impacts of macronutrients on life-history traits in larval and adult Drosophila melanogaster: the use of nutritional geometry and chemically defined diets. J. Exp. Biol. 221: jeb181115 10.1242/jeb.181115 [DOI] [PubMed] [Google Scholar]
- Keebaugh E. S., Yamada R., and Ja W. W., 2019. The Nutritional Environment Influences the Impact of Microbes on Drosophila melanogaster Life Span. MBio 10: e00885 10.1128/mBio.00885-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh E. S., Yamada R., Obadia B., Ludington W. B., and Ja W. W., 2018. Microbial Quantity Impacts Drosophila Nutrition, Development, and Lifespan. iScience 4: 247–259. 10.1016/j.isci.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E. G., and Mills A., 2019. Role of Feed Processing on Gut Health and Function in Pigs and Poultry: Conundrum of Optimal Particle Size and Hydrothermal Regimens. Front. Vet. Sci. 6: 19 10.3389/fvets.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Han D., Ahn B. H., and Rhee J. S., 1997. Effect of glutathione, catechin, and epicatechin on the survival of Drosophila melanogaster under paraquat treatment. Biosci. Biotechnol. Biochem. 61: 225–229. 10.1271/bbb.61.225 [DOI] [PubMed] [Google Scholar]
- Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O. et al. , 2008. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 105: 2498–2503. 10.1073/pnas.0710787105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leftwich P. T., Clarke N. V. E., Hutchings M. I., and Chapman T., 2018. Reply to Obadia et al.: Effect of methyl paraben on host-microbiota interactions in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 115: E4549–E4550. 10.1073/pnas.1805499115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz F. A., and Belyea R. L., 1986. Role of particle size and forage quality in digestion and passage by cattle and sheep. J. Dairy Sci. 69: 1996–2008. 10.3168/jds.S0022-0302(86)80626-9 [DOI] [PubMed] [Google Scholar]
- McAllister T. A., Bae H. D., Jones G. A., and Cheng K. J., 1994. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 72: 3004–3018. 10.2527/1994.72113004x [DOI] [PubMed] [Google Scholar]
- Obadia B., Keebaugh E. S., Yamada R., Ludington W. B., and Ja W. W., 2018. Diet influences host-microbiota associations in Drosophila. Proc. Natl. Acad. Sci. USA 115: E4547–E4548. 10.1073/pnas.1804948115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod K. G., LePine O. K., Abbineni P. S., Bridgeman J. M., Coorssen J. R. et al. , 2017. Drosophila development, physiology, behavior, and lifespan are influenced by altered dietary composition. Fly (Austin) 11: 153–170. 10.1080/19336934.2017.1304331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. D. W., Blanc E., Leitão-Gonçalves R., Yang M., He X. et al. , 2014. A holidic medium for Drosophila melanogaster. Nat. Methods 11: 100–105. 10.1038/nmeth.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. D. W., Skorupa D., and Partridge L., 2005. Diet, metabolism and lifespan in Drosophila. Exp. Gerontol. 40: 857–862. 10.1016/j.exger.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Rideout E. J., Narsaiya M. S., and Grewal S. S., 2015. The Sex Determination Gene transformer Regulates Male-Female Differences in Drosophila Body Size. PLoS Genet. 11: e1005683 10.1371/journal.pgen.1005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G., Segal D., Ringo J. M., Hefetz A., Zilber-Rosenberg I. et al. , 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 107: 20051–20056. 10.1073/pnas.1009906107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. C., Kim S.-H., You H., Kim B., Kim A. C. et al. , 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334: 670–674. 10.1126/science.1212782 [DOI] [PubMed] [Google Scholar]
- Shingleton A. W., Masandika J. R., Thorsen L. S., Zhu Y., and Mirth C. K., 2017. The sex-specific effects of diet quality vs. quantity on morphology in Drosophila melanogaster. R. Soc. Open Sci. 4: 170375 10.1098/rsos.170375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefana M. I., Driscoll P. C., Obata F., Pengelly A. R., Newell C. L. et al. , 2017. Developmental diet regulates Drosophila lifespan via lipid autotoxins. Nat. Commun. 8: 1384 10.1038/s41467-017-01740-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J. et al. , 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 14: 403–414. 10.1016/j.cmet.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Vermeulen K., Verspreet J., Courtin C. M., Haesebrouck F., Baeyen S. et al. , 2018. Reduced-Particle-Size Wheat Bran Is Efficiently Colonized by a Lactic Acid-Producing Community and Reduces Levels of Enterobacteriaceae in the Cecal Microbiota of Broilers. Appl. Environ. Microbiol. 84: e01343 10.1128/AEM.01343-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. C.-N., Dobson A. J., and Douglas A. E., 2014. Gut microbiota dictates the metabolic response of Drosophila to diet. J. Exp. Biol. 217: 1894–1901. 10.1242/jeb.101725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R., Deshpande S. A., Bruce K. D., Mak E. M., and Ja W. W., 2015. Microbes Promote Amino Acid Harvest to Rescue Undernutrition in Drosophila. Cell Rep. 10: 865–872. 10.1016/j.celrep.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source files for all nutritional information used to create the Fly Microbiome Diet Database and the DDCC are located at [https://doi.org/10.6084/m9.figshare.11920743.v1] as Files S1-S21. Each of these files corresponds to a different diet component, which is also detailed in the file name (for example, FileS1_YeastExtract_General.pdf, FileS7_Agar_Drosophila.pdf, FileS8_Molasses_Solids.pdf, FileS11_Molasses_General.pdf, etc). A downloadable version of the Fly Microbiome Diet Database is located at [https://doi.org/10.6084/m9.figshare.11920788.v2]. Supplemental tables are available at [https://doi.org/10.6084/m9.figshare.12241712.v1]. Table S1 details the studies used to compile the Fly Microbiome Diet Database including DOIs; Table S2 provides nutritional information and sources for raw fruits used to generate Figure 4.