Abstract
Characterizing the distribution of fitness effects (DFE) for new mutations is central in evolutionary genetics. Analysis of molecular data under the McDonald-Kreitman test has suggested that adaptive substitutions make a substantial contribution to between-species divergence. Methods have been proposed to estimate the parameters of the distribution of fitness effects for positively selected mutations from the unfolded site frequency spectrum (uSFS). Such methods perform well when beneficial mutations are mildly selected and frequent. However, when beneficial mutations are strongly selected and rare, they may make little contribution to standing variation and will thus be difficult to detect from the uSFS. In this study, I analyze uSFS data from simulated populations subject to advantageous mutations with effects on fitness ranging from mildly to strongly beneficial. As expected, frequent, mildly beneficial mutations contribute substantially to standing genetic variation and parameters are accurately recovered from the uSFS. However, when advantageous mutations are strongly selected and rare, there are very few segregating in populations at any one time. Fitting the uSFS in such cases leads to underestimates of the strength of positive selection and may lead researchers to false conclusions regarding the relative contribution adaptive mutations make to molecular evolution. Fortunately, the parameters for the distribution of fitness effects for harmful mutations are estimated with high accuracy and precision. The results from this study suggest that the parameters of positively selected mutations obtained by analysis of the uSFS should be treated with caution and that variability at linked sites should be used in conjunction with standing variability to estimate parameters of the distribution of fitness effects in the future.
Keywords: adaptation, beneficial mutations, distribution of fitness effects, population genetics
Characterizing the distribution of fitness effects for beneficial mutations is central in evolutionary biology. The rate and fitness effects of advantageous mutations may determine important evolutionary processes such as how variation in quantitative traits is maintained (Hill 2010), the evolution of sex and recombination (Otto 2009) and the dynamics of evolutionary rescue in changing environments (Orr and Unckless 2014). However, despite its central role in evolution, relatively little is known about the distribution of fitness effects (DFE) for advantageous mutations in natural populations. The DFE for advantageous mutations can be estimated from data obtained via targeted mutation or from mutation accumulation experiments (e.g., Bank, Hietpas, Wong, Bolon, & Jensen 2014; Böndel et al., 2019; reviewed in Bailey & Bataillon 2016), but such efforts may be limited to laboratory systems. Alternatively, estimates of the DFE can be obtained for natural systems using population genetic methods.
When natural selection is effective, beneficial alleles are promoted to eventual fixation while deleterious variants are maintained at low frequencies. Migration, mutation, selection and genetic drift interact to shape the distribution of allele frequencies in a population (Wright 1937). Parameters of the DFE for both advantageous and deleterious mutations can be estimated by modeling population genomic data, specifically the site frequency spectrum (SFS). The SFS is the distribution of allele frequencies present in a sample of individuals drawn from a population. By contrasting the SFS for a class of sites expected to be subject to selection with that of a neutral comparator, one can estimate the parameters of the DFE if selected mutations are segregating in the population of interest (reviewed in Eyre-Walker & Keightley 2007). Typically, the DFE for nonsynonymous sites in protein coding genes is estimated using synonymous sites as the neutral comparator. Several methods have been proposed that estimate the DFE for deleterious mutations from the SFS under the assumption that beneficial mutations contribute little to standing genetic variation (e.g., Barton & Zeng 2018; Boyko et al., 2008; Keightley & Eyre-Walker 2007; Tataru, Mollion, Glemin, & Bataillon 2017).
The DFE for deleterious mutations can be used when estimating α, the proportion of between-species divergence attributable to adaptive evolution (Eyre-Walker and Keightley 2009). α can be estimated by rearranging the terms of the McDonald-Kreitman test (MK-test), which assesses the extent of positive selection. Under strong purifying selection, the ratio of divergence at nonsynonymous sites (dN) to that of synonymous sites (dS) should be exactly equal to the ratio of nucleotide diversity at nonsynonymous (πN) and synonymous sites (πS)(McDonald and Kreitman 1991). Adaptive evolution of protein sequences may contribute to dN such that dN/dS > πN/πS. Charlesworth (1994) suggested rearranging the terms of the MK-test to estimate the excess dN due to positive selection (α) as
Slightly deleterious alleles may contribute to both standing genetic variation and between-species divergence, estimates of α may therefore be refined by subtracting the contribution that deleterious alleles make to both polymorphism and divergence and this can be calculated using the DFE for harmful mutations (Eyre-Walker and Keightley 2009). Application of such methods to natural populations suggest that α is of the order of 0.5 in a large variety of animal taxa (Galtier 2016). However, if adaptive evolution is as frequent as MK-test analyses suggest, the assumption that advantageous alleles contribute little to standing variation may be violated and ignoring them could lead to biased estimates of the DFE (Tataru et al. 2017).
When advantageous alleles contribute to standing variation, parameters of the DFE for both deleterious and beneficial mutations can be estimated from the SFS (Schneider et al., 2011; Tataru et al., 2017). When data from an outgroup species are available, variable sites within a focal species can be polarized as either ancestral or derived and the unfolded SFS (uSFS) can be obtained. Inference of ancestral/derived states is, however, potentially error-prone (Keightley & Jackson 2018). The uSFS is a vector of length 2n, where n is the number of diploid genome copies sampled. The ith entry of the uSFS is the count of derived alleles observed at a frequency i in the sample. Note that when outgroup data are not available, alleles cannot be polarized and the distribution of minor allele frequencies (known as the folded SFS) is analyzed. There is limited power to detect positive selection from the SFS, so the DFE for beneficial mutations is often modeled as a discrete class of mutational effects, with one parameter specifying the fitness effects of beneficial mutations, γa = 2Nesa where Ne is the effective population size and sa is the positive selection coefficient in homozygotes, and another specifying the proportion of new mutations that are advantageous, pa. Estimates of γa and pa for nonsynonymous sites have only been obtained a handful of species, and these are summarized in Table 1. The positive selection parameter estimates that have been obtained for mice and Drosophila are fairly similar (Table 1). Note that the estimates for humans obtained by Castellano et al., (2019) did not provide a significantly greater fit to the observed data than did a model with no positive selection. Furthermore, Castellano et al., (2019) estimated the parameters for numerous great ape species, the parameters shown for humans are representative of the estimates for all taxa they analyzed.
Table 1. Estimates of the parameters of positive selection obtained from the uSFS for nonsynonymous sites.
| Common name | Scientific name | γa | pa | Reference | Method useda |
|---|---|---|---|---|---|
| House mouse | Mus musculus castaneus | 14.5 | 0.0030 | Booker & Keightley, (2018) | DFE-alpha |
| Fruit fly | Drosophila melanogaster | 23.0 | 0.0045 | Keightley et al., (2016) | DFE-alpha |
| Humans | Homo sapiens | 0.0064b | 0.000025 | Castellano et al., (2019) | polyDFE |
DFE-alpha implements the analysis methods described by Schneider et al., (2011), polyDFE implements the methods described by Tataru et al., (2017)
Castellano et al., (2019) estimated the mean fitness effect for an exponential distribution of advantageous mutational effects.
Depending on the rate and fitness effects of beneficial mutations, different aspects of population genomic data may be more or less informative for estimating the parameters of positive selection. As beneficial mutations spread through populations, they may carry linked neutral variants to high frequency, causing selective sweeps (Barton 2000). On the other hand, if advantageous mutations have mild fitness effects, they may take a long time to reach fixation and make a substantial contribution to standing genetic variation. Because of this, uSFS data and polymorphism data at linked sites may both be informative for understanding the parameters of positive selection. For example, Campos et al., (2017) used a model of selective sweeps to analyze the negative correlation observed between dN and πS in Drosophila melanogaster and estimated γa = 250 and pa = 2.2 × 10−4, but this method assumes a constant population size. An analysis of the uSFS from the same dataset that modeled population size change yielded estimates of γa = 23 and pa = 0.0045 for nonsynonymous sites (Keightley et al., 2016). The sharp contrast between the two studies’ estimates of the positive selection parameters may due to different assumptions but could potentially be explained if the DFE for advantageous mutations in D. melanogaster is bimodal. If this were so, the different methods (i.e., sweep models vs. uSFS analysis) may be capturing distinct aspects of the DFE for advantageous mutations, or it could be that both models are highly unidentifiable. The handful of studies that have attempted to estimate γa and pa from the uSFS have yielded similar estimates of positive selection (Table 1), which may indicate commonalities in the DFE for beneficial mutations across taxa. On the other hand, uSFS analyses may have only found evidence for mildly beneficial mutations because the approach is only powered to detect weakly beneficial mutations. Indeed, verbal arguments have suggested that rare strongly selected advantageous mutations, which may contribute little to standing variation, will be undetectable by analysis of the uSFS (Booker & Keightley 2018; Campos et al., 2017).
The studies describing the two most recently proposed methods for estimating the DFE for beneficial mutations from the uSFS (Schneider et al. 2011; Tataru et al. 2017) performed extensive simulations and their analysis methods worked well for parameter ranges tested. However, neither study tested the case of rare advantageous mutations with strong effects on fitness. Testing this case is important, as studies that have analyzed patterns of putatively neutral genetic diversity across the genome have indicated that the DFE for advantageous mutations contains strongly beneficial mutations in a variety of taxa (Booker & Keightley 2018; Campos et al., 2017; Elyashiv et al., 2016; Nam et al., 2017; Uricchio et al., 2019). Note that Tataru et al., (2017) did simulate a population subject to frequent strongly beneficial mutations (γa = 800 and pa = 0.02), but the parameter combination they tested may not be biologically relevant as the proportion of adaptive substitutions it yielded was far higher than is typically estimated from real data (α = 0.99). The limited parameter ranges tested in the simulations performed by Schneider et al., (2011) and Tataru et al., (2017) leave a critical gap in our knowledge as to how uSFS based methods perform when advantageous mutations are strongly selected and infrequent.
In this study, I use simulated datasets to fill this gap and examine how uSFS-based analyses perform when beneficial mutations are strongly selected and rare. I simulate populations subject to a range of positive selection parameters, including cases similar to those modeled by Tataru et al., (2017) and cases where beneficial mutations are strongly selected but infrequent. It has been pointed out that estimating selection parameters by modeling within species polymorphism along with between-species divergence makes the assumption that the DFE has remained invariant since the ingroup and outgroup began to diverge (Tataru et al. 2017). By analyzing only the polymorphism data, one can potentially avoid that problematic assumption. Using the state-of-the-art package polyDFE v2.0 (Tataru and Bataillon 2019), I analyze the uSFS data and estimate selection parameters for all simulated datasets with or without divergence. The results from this study suggest that, when beneficial mutations are strongly selected and rare, analysis of the uSFS results in spurious parameter estimates and the proportion of adaptive substitutions may be poorly estimated.
Methods
Population genomic simulations
I tested the hypothesis that the parameters of infrequent, strongly beneficial mutations are difficult to estimate by analysis of the uSFS using simulated datasets. Wright-Fisher populations of Ne = 10,000 diploid individuals were simulated using the forward-in-time package SLiM (v3.2; Haller & Messer 2019). Simulated chromosomes consisted of seven gene models, each separated by 8,100bp of neutrally evolving sequence. The gene models consisted of five 300bp exons separated by 100bp neutrally evolving introns. The gene models were based on those used by Campos & Charlesworth, (2019), but unlike that study, I did not model the untranslated regions of genes. Nonsynonymous sites were modeled by drawing the fitness effects for 2/3rds of mutations in exons from a distribution of fitness effects (DFE), while the remaining 1/3 were strictly neutral and used to model synonymous sites. The fitness effects of nonsynonymous mutations were beneficial with probability pa or deleterious with probability 1 – pa. Beneficial mutations had a fixed selection coefficient of γa = 2Nesa. The fitness effects of deleterious mutations were drawn from a gamma distribution with a mean of γd = 2Nesd = -2,000 and a shape parameter of β = 0.3 (sd being the negative selection coefficient in homozygotes). The gamma distribution of deleterious mutational effects was used for all simulated datasets and was based on results for nonsynonymous sites in Drosophila melanogaster (Loewe and Charlesworth 2006). Uniform rates of mutation (μ) and recombination (r) were set to 2.5 × 10−7 (giving 4Ner = 4Neµ = 0.01). Note that μ and r are far higher than is biologically realistic for most eukaryotes, I scaled up these rates to model a population with a large Ne using simulations of 10,000 individuals. Across simulations I varied the γa and pa parameters and performed 2,000 replicates for each combination of parameters. Thus, I simulated a dataset of 21Mbp of coding sequence for each combination of γa and pa tested.
In this study, I assumed a discrete class of beneficial mutational effects, which is likely unrealistic for real organisms. Theoretical arguments have been proposed that the DFE for beneficial mutations that go to fixation should be exponential (Orr 2003). However, the studies that have estimated the DFE for beneficial mutations from population genetic data have often modeled discrete classes of effects (Campos et al., 2017; Elyashiv et al., 2016; Keightley et al., 2016; Uricchio et al., 2019). I chose to model discrete selection coefficients in the simulated datasets in order to better understand the limitations of the methods rather than to accurately model the DFE for beneficial mutations.
To model the accumulation of nucleotide substitutions after the split of a focal population with an outgroup, I recorded all substitutions that occurred in the simulations. Campos & Charlesworth, (2019) analyzed simulations very similar to those that I performed in this study and showed that populations subject to beneficial mutations with γa = 250 and pa = 0.0002 took 14Ne generations to reach mutation-selection-drift equilibrium. In this study I modeled a range of positive selection parameters, so to ensure that my simulations reached equilibrium I performed 85,000 (34Ne) generations of burn-in before substitutions were scored. The expected number of neutral nucleotide substitutions that accumulate per site in T generations is dNeutral = Tµ. The point mutation rate in my simulations was set to µ = 2.5 × 10−7per site per generation, so I ran the simulations for 200,000 generations beyond the end of the burn-in phase to model a neutral divergence of dNeutral = 0.05. All variants present in the population sampled at a frequency of 1.0 were also scored as substitutions.
Using the 2,000 simulated datasets, I constructed 100 bootstraps by sampling with replacement. From each bootstrap sample, I collated variants and constructed the uSFS for synonymous and nonsynonymous sites for 20 diploid individuals.
Analysis of simulation data
I calculated several summary statistics from the simulated datasets. First, I calculated pairwise nucleotide diversity at synonymous sites (πs) and expressed it relative to the neutral expectation of π0 = 4Neµ = 0.01. Second, divergence at nonsynonymous sites for both advantageous (dNa) and deleterious mutations (dNd) was used to calculate the observed proportion of adaptive substitutions, αObs = dNa/(dNa + dNd). Finally, I recorded the total number of beneficial mutations segregating in simulated populations, SAdv, as well as the total number of segregating nonsynonymous sites (S).
I estimated DFEs from simulated data by analysis of the uSFS using polyDFE (v2.0; Tataru & Bataillon 2019). polyDFE fits an expression for the uSFS expected under a full DFE to data from putatively neutral and selected classes of sites and estimates parameters by maximum likelihood. For each set of positive selection parameters, simulated uSFS data were analyzed under “Model B” in polyDFE (a gamma distribution of deleterious mutational effects plus a discrete class of advantageous mutations). Initial parameters for the maximization were calculated from the data using the ‘-e’ option and the uSFS was analyzed either with or without divergence using the “-w” option in polyDFE. Analyzing the uSFS without divergence causes the selection parameters to be inferred from polymorphism data alone. For each replicate, I tested whether the inclusion of beneficial mutations in the DFE improved model fit using likelihood ratio tests between the best-fitting model and a null model with pa set to 0.0. Setting pa = 0.0 means that positive selection does not influence the likelihood, so two fewer parameters are being estimated. Twice the difference in log-likelihood between the full DFE model and the null model with pa = 0.0 was tested against a χ2 distribution with 2 degrees of freedom. Likelihood surfaces were estimated by running polyDFE using a grid of fixed values for DFE parameters. Finally, positive selection parameters obtained under both the null model (pa = 0.0) and the best-fitting alternative model were combined using model averaging using weights determined from each model’s Aikeike Information Criteria (AIC). Model averaging was performed using the “postprocessing.R” script distributed with polyDFE v2.0.
Data availability
All code and SLiM configuration files needed to reproduce the results shown in this study are available at https://github.com/TBooker/PositiveSelection_uSFS. Supplemental material available at figshare: https://doi.org/10.25387/g3.12233630.
Results
Population genomic simulations
I performed simulations that modeled genes subject to mutation-selection-drift balance with fitness effects drawn from a distribution that incorporated both deleterious and advantageous mutations. The DFE for harmful mutations was constant, but I varied the fraction (pa) and fitness effects (γa) of beneficial mutations across simulated datasets (Table 2). For each set of advantageous mutation parameters, 21Mbp of coding sequences was simulated, of which 14Mbp were nonsynonymous and 7Mbp were synonymous sites. Variants present in the simulated populations were used to construct the uSFS for a sample of 20 diploid individuals (Figure S1), a sample size which is fairly typical of current population genomic datasets (e.g., Castellano et al., 2019; Laenen et al., 2018; Williamson et al., 2014).
Table 2. Parameters of positive selection assumed in simulations and the proportion of polyDFE runs for which modeling positive selection gave a significantly better fit to the data.
| γa | pa | γa pa | Proportion of likelihood ratio tests significant | Proportion of analyses with gradient < 0.01 | ||
|---|---|---|---|---|---|---|
| With divergence | Without divergence | With divergence | Without divergence | |||
| 10 | 0.0001 | 0.001 | 0.02 | 0.07 | 0.11 | 0.71 |
| 50 | 0.005 | 0.98 | 0.86 | 0.10 | 0.77 | |
| 100 | 0.01 | 0.98 | 0.02 | 0.03 | 0.58 | |
| 500 | 0.05 | 1.00 | 0.39 | 0.00 | 0.99 | |
| 1,000 | 0.10 | 1.00 | 1.00 | 0.00 | 0.71 | |
| 10 | 0.001 | 0.01 | 0.99 | 0.96 | 0.15 | 0.71 |
| 50 | 0.05 | 1.00 | 1.00 | 0.06 | 0.98 | |
| 100 | 0.10 | 1.00 | 1.00 | 0.00 | 0.97 | |
| 500 | 0.50 | 1.00 | 1.00 | 0.00 | 0.94 | |
| 1,000 | 1.00 | 1.00 | 1.00 | 0.00 | 0.71 | |
| 10 | 0.01 | 0.10 | 1.00 | 1.00 | 0.03 | 0.80 |
| 50 | 0.50 | 1.00 | 1.00 | 0.02 | 0.99 | |
| 100 | 1.00 | 1.00 | 1.00 | 0.02 | 0.95 | |
| 500 | 5.00 | 1.00 | 1.00 | 0.00 | 0.72 | |
| 1,000 | 10.0 | 1.00 | 1.00 | 0.00 | 0.41 | |
Across simulations, the strength of selection acting on advantageous mutations ranged from γa = 10 to γa = 1,000. For a given pa parameter, increasing the strength of selection increased the observed proportion of adaptive substitutions, αObs (Figure 1A). This is expected and is due to the monotonic increasing relationship between fixation probability and the strength of positive selection first described by Haldane (1927). Additionally, parameter combinations for which γapa were equal had similar proportions of adaptive substitutions, for example compare γa = 10 and pa = 0.01 to γa = 1,000 and pa = 0.0001 (Figure 1A). This was also expected because the rate of adaptive substitutions is proportional to γapa. In some datasets, particularly when pa = 0.01 and advantageous mutations were very strongly selected (i.e., γa ≥ 500), αObs exceeded 0.75, which is higher than is typically estimated from empirical data (Galtier 2016), so these parameter combinations may not be biologically relevant.
Figure 1.
Population genetic summary statistics collated across all simulated genes. αObs is the observed proportion of substitutions fixed by positive selection. πs/π0 is genetic diversity relative to neutral expectation (π0 = 0.01). SAdv./S is the proportion of segregating nonsynonymous sites that are advantageous in the simulated datasets.
The effects of selection at linked sites varied across simulated datasets. The DFE for deleterious mutations was kept constant across simulations, so the extent of background selection should be fairly similar across all parameter sets and thus variation in πS/π0 reflects the effects of selective sweeps. Under neutrality πS/π0 had an expected value of 1.0 and I found that selection at linked sites reduced nucleotide diversity below that expectation in all simulations (Figure 1B). Increasing the fitness effects or frequency of advantageous mutations had a strong effect on genetic diversity at synonymous sites, as shown by πS/π0 in Figure 1B. The highlighted points in Figure 1 indicate parameter combinations for which γapa = 0.01. As expected, αObs for these three parameter sets was very similar (Figure 1A). Figure 1B shows that πS/π0 decreased across these three parameter combinations as the strength of positive selection increased. Finally, differences in pa explained most of the variation in the proportion of segregating advantageous mutations (SAdv./S) across simulated datasets, but SAdv./S. also increased with the strength of positive selection (Figure 1C). From these results, it is clear that there will be lower power to estimate positive selection on the basis of standing variation when advantageous mutations are rare (i.e., pa = 0.0001) than when they are comparatively frequent (i.e., pa = 0.01).
Analysis of the unfolded site frequency spectrum
Figure 2 shows the observed (bars) and expected (lines) distribution of derived allele frequencies for beneficial mutations segregating in simulated populations. The three panels of Figure 2 correspond to three parameter combinations for which γapa = 0.01 (γa = 1,000 and pa = 0.0001, γa = 100 and pa = 0.001 and γa = 10 and pa = 0.01). The lines in each of the panels of Figure 2 show the analytical expectation for the uSFS of advantageous mutations calculated using Equation 2 from Tataru et al., (2017). The analytical expectation closely matches the observed data for all three combinations (Figure 2). However, for a given value of pa, the analytical expectation for models with increasing fitness effects were very similar, which likely makes it difficult to distinguish them on the basis of polymorphism alone (Figure 2). For the three parameter sets shown in Figure 2, the overall contribution that advantageous alleles make to the uSFS for nonsynonymous sites is small relative to deleterious ones (Figure S1). Accurate estimation of positive selection parameters from the uSFS requires that the distribution of advantageous alleles can be distinguished from deleterious variants, so when pa is small it seems likely that uSFS analyses will be unable to easily distinguish competing models.
Figure 2.
The uSFS for advantageous mutations under different combinations of positive selection parameters. The three bar charts show observed uSFS from simulations that model positive selection parameters that yield similar α. The lines in each panel show the expected frequency spectra for different strengths of beneficial mutations and were obtained using Equation 2 from Tataru et al., (2017).
When analyzing a particular uSFS dataset in polyDFE, I either modeled the full DFE (i.e., a gamma distribution of deleterious mutations and a discrete class of advantageous mutational effects), or just a gamma DFE for harmful mutations (dDFE). I compared the two models using likelihood ratio tests, which tested the null hypothesis that the fit of the full DFE model is similar to that of a model containing only deleterious mutations. For each of the combinations of positive selection parameters shown in Table 2, I ran polyDFE on uSFS data from 100 bootstrap replicates. When modeling the full uSFS (i.e., with divergence), polyDFE identified models containing positive selection consistently for all but one (pa = 0.0001 and γa = 10) of the parameter combinations tested (Table 2). When the DFE was inferred from polymorphism data alone (i.e., without divergence), models containing positive selection were identified less often, particularly when beneficial mutations were rare (pa = 0.0001; Table 2). Table 2 also shows the proportion of analysis runs for which the gradient of the likelihood exceeded 0.1. The polyDFE manual (Tataru and Bataillon 2019) suggests that gradients >0 indicate that the program has failed to identify a unique likelihood maximum. When the full uSFS was modeled, the gradient of the likelihood was frequently >0, indicating that the model did not converge on a unique optimum. When modeling the uSFS without divergence, polyDFE reported gradients <0.01 for a large proportion of replicate analyses (Table 2).
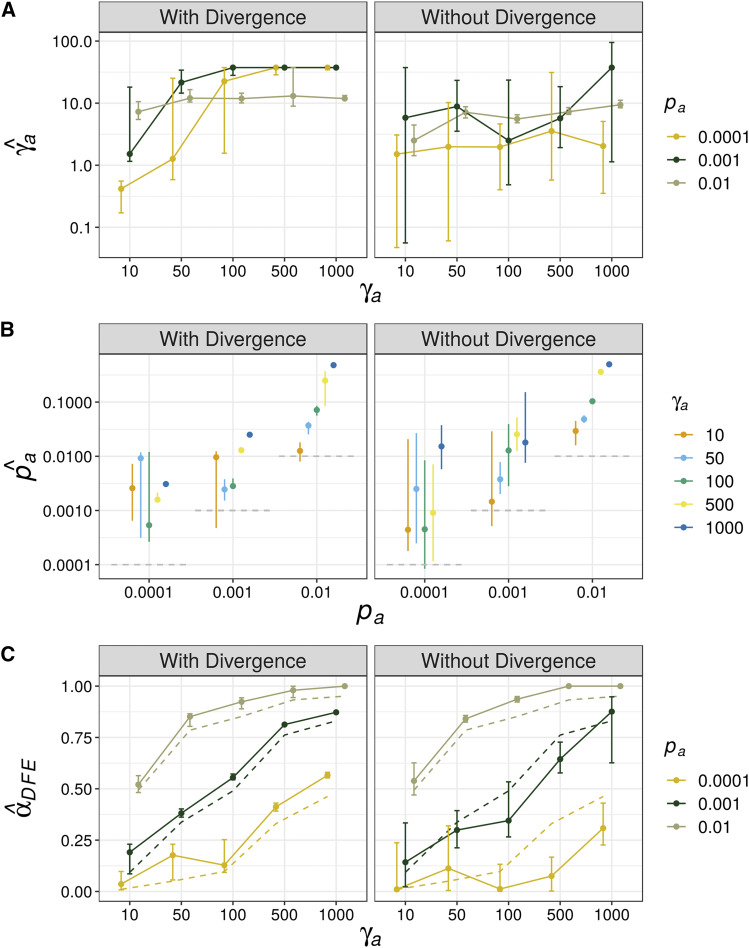
Figures 3A and 3B show the parameters of positive selection estimated by analysis of uSFS from simulated datasets. I found that when simulated beneficial mutations were mildly advantageous (γa = 10) but relatively frequent (pa = 0.01), both γa and pa were estimated accurately regardless of whether divergence was modeled or not (Figures 3A-B). This finding is consistent with both Schneider et al., (2011) and Tataru et al., (2017). When pa = 0.01 and γa > 10, the analysis of the uSFS with or without divergence yielded very similar parameter estimates, but in both cases, the strength of positive selection seemed to be positively correlated with the estimated pa (Figure 3). In all cases, when beneficial mutations had γa ≥ 50, neither γa nor pa were accurately estimated (Figure 3).
Figure 3.
Estimates of the parameters of advantageous mutations and the proportion of adaptive substitutions they imply from simulated datasets. A) γa is the inferred selective effect of a new advantageous mutation; B) pa is the proportion of new mutations that are beneficial, the horizontal dashed gray lines indicate the simulated values in each case; C) αDFE is the proportion of adaptive substitutions expected under the inferred DFE, the dashed lines indicate αObs, the proportion of adaptive substitutions observed in the simulated datasets. Error bars indicate the 95% range of 100 bootstrap replicates.
Tataru et al., (2017) pointed out that, if one had an estimate of the full DFE (i.e., with divergence), the proportion of adaptive substitutions could be obtained by taking the ratio of the fixation probability for a new beneficial mutation over the fixation probability for a random mutation integrating over the full DFE (Equation 10; Tataru et al., 2017). The proportion of adaptive substitutions obtained in this way is denoted αDFE. When modeling the full uSFS, αDFE was estimated with high accuracy, but with a slight upward bias (Figure 3C). When the DFE was inferred without divergence αDFE was underestimated when beneficial mutations were strongly selected and rare (Figure 3).
In the presence of infrequent, strongly beneficial mutations the parameters of the DFE for deleterious mutations estimated by polyDFE were very accurate (Figure S2). Estimates of the DFE for harmful mutations were less accurate when beneficial mutations occurred with pa ≥ 0.001 and γa ≥ 100. This is presumably because in such cases recurrent selective sweeps eliminate a large amount of neutral diversity and distort the distribution of standing genetic variation at nonsynonymous sites. However, as stated above, the parameter range where the DFE for harmful mutations was poorly estimated in this study may not be biologically relevant.
Model Identifiability
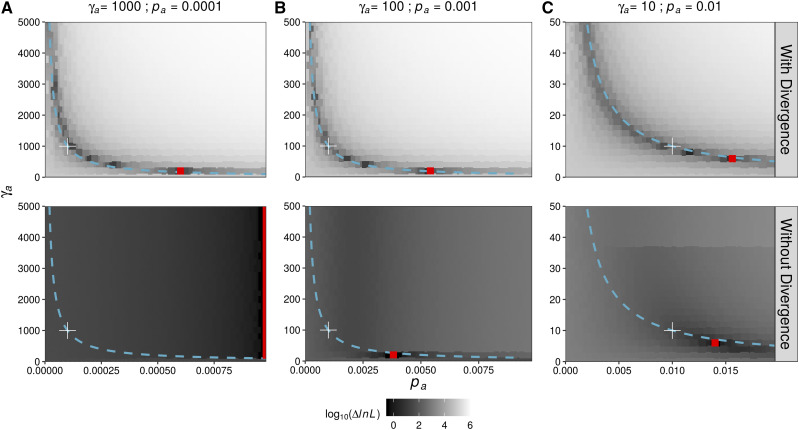
It is very difficult to tease apart the parameters of positive selection from the uSFS by maximum likelihood. Figure 4 shows the likelihood surface for the three sets of positive selection parameters that satisfy the condition γapa = 0.1. The proportion of adaptive substitutions is largely determined by the product γapa (Kimura and Ohta 1971) and, as expected, the three parameter combinations shown in Figure 4 all exhibit a similar αObs (Figure 1A). However, the extent by which neutral genetic diversity is reduced and the number of segregating advantageous mutations differ substantially across the three parameter combinations (Figure 1). The top row of panels in Figure 4 shows that when modeling the full uSFS, the likelihood surface closely tracks the relation γapa = 0.1. Focusing on the top panel in Figure 4A, the maximum likelihood estimates (MLEs) of the positive selection parameters (the red dot) are far from the true parameter values (indicated by the plus sign), but the MLEs obtained satisfy γapa = 0.1. The ridge in the likelihood surface observed when modeling the full uSFS was described by both Schneider et al., (2011) and Tataru et al., (2017). It comes about because between-species divergence carries information about α, and α is proportional to γapa.
Figure 4.
The likelihood surface for the γa and pa parameters for three simulated datasets. Hue indicates differences in log likelihood between a particular parameter combination and the best-fitting model. Best fitting models are indicated by red points and the true parameters are given above the plots and indicated by the white plus signs on the likelihood surface. The relation γapa = 0.1 is shown as a turquoise line and is constant across the three datasets shown.
Inferring the parameters of the DFE from polymorphism alone avoids the assumption of an invariant DFE, but when doing so it may be difficult to distinguish competing models. Indeed, across the three parameter combinations shown, values close to the truth were only obtained from simulated data when γa = 10 and pa = 0.01 (bottom panel Figure 4C). In the case of γa = 1000 and pa = 0.0001, the likelihood surface about the true parameters was very flat (Figure 4A). Increasing the pa parameter increased likelihood for all strengths of selection, so that the MLEs shown in Figure 4A are simply the values with the highest pa in the range tested (the vertical red line in Figure 4A). When γa = 100 and pa = 0.001, the likelihood surface about the estimates was steep, but the selection parameters identified by maximum likelihood were incorrect (Figure 4B).
Discussion
In this study, I analyzed simulated datasets modeling a range of positive selection parameter combinations. I found that estimates of positive selection parameters obtained by analysis of the uSFS were only accurate when beneficial mutations had γa < 50, under stronger selection the individual parameters of positive selection were not accurately estimated (Figure 3). This is not particularly surprising and is consistent with verbal arguments made in published studies (Booker & Keightley 2018; Campos et al., 2017). However, it is troubling that when beneficial mutations are strongly selected and rare, the uSFS may often indicate a significant signal of positive selection, but erroneous parameter estimates are obtained. If one were to analyze an empirical dataset and estimate parameters of positive selection of the order γa ∼10 and pa ∼0.01, it would be difficult to know whether those were reflective of the true underlying parameters or an artifact of strong selection.
On the basis of this study, it seems that researchers should treat parameters of positive selection obtained by analysis of the uSFS with caution. Schneider et al., (2011) and Tataru et al., (2017) showed that uSFS analysis methods perform well when beneficial mutations are mildly beneficial and somewhat frequent. However, I found that fitting the uSFS when advantageous mutations are strongly selected and rare, models incorporating positive selection were often statistically supported (Table 1), but parameter estimates obtained were spurious (Figure 3). When fitting uSFS models to empirical data, researchers should keep this limitation of the method in mind, particularly when analyzing small samples, and use these results to help build intuition about specific analyses. The expected uSFS for advantageous mutations is very similar for DFE models that share the same pa parameter, and in such cases differing models can only be distinguished by the density of high frequency derived variants (Figure 2). Polarization error when estimating the uSFS can generate an excess in the number of high frequency derived variants (Keightley & Jackson 2018), so may generate a spurious signal of strong positive selection. Analysis methods have been proposed which attempt to estimate the rate of polarization error when modeling the uSFS (Barton & Zeng 2018; Tataru et al., 2017), but further study is required to determine whether such methods reduce the signal of positive selection in uSFS-based analyses. Accounting for positive selection when analyzing the uSFS yielded robust estimates of the DFE for harmful mutations across the simulated datasets (Figure S2). Although I only examined a single DFE for harmful mutations in this study, Tataru et al., (2017) showed that polyDFE accurately recovered the parameters of a range of DFE models if positive selection is accounted for.
Estimates of α based on analysis of the uSFS may be biased when beneficial mutations are strongly selected and infrequent. Calculating α using the rearranged MK-test makes the problematic assumption that the DFE has remained invariant in the time since the focal species began to diverge from the outgroup (Tataru et al., 2017). However, Tataru et al., (2017) pointed out that one can avoid that assumption if αDFE is calculated from a DFE estimated without divergence data. In this study, estimates of αDFE obtained when the full uSFS was analyzed were very precise, but with a slight upward bias (Figure 3). When simulated beneficial mutations were strongly selected and rare, the parameters inferred using polymorphism data alone (i.e., without divergence) yielded spurious estimates of αDFE (Figure 3). When analyzing datasets from real populations, αDFE may not capture the contribution that strongly beneficial mutations make to molecular evolution. This may make it difficult to contrast αDFE between species with large differences in Ne, because the number of segregating advantageous mutations and thus ability to accurately estimate selection parameters will depend on the population size.
The nature of the distribution of fitness effects for natural populations is largely unknown. In this study, I analyzed the uSFS data under the exact DFE model that had been simulated (i.e., a gamma distribution of deleterious mutational effects plus a discrete class of beneficial effects). When analyzing empirical data, researchers have to make assumptions about the probability distribution that best describes the DFE of focal populations. A gamma distribution is often assumed for deleterious mutations as it is flexible and is described by only two parameters (Eyre-Walker and Keightley 2007). However, when estimating the DFE from empirical data, one may bias their analyses by strictly adhering to one particular family of probability distributions (Kousathanas and Keightley 2013). When analyzing empirical data model averaging provides a way to estimate key features of the DFE while remaining agnostic to the exact shape of the distribution (Tataru and Bataillon 2020). I performed model averaging, combining parameters estimates obtained under both the null model with no beneficial mutations with the model including beneficial mutation parameters, applying weights that were proportional to the differences in fit between the null and alternative models. Figure S4 shows parameter estimates obtained by model averaging, it is structured similarly to Figure 3 from the main text. For most of the parameter combinations I tested in this study, there was significant difference in likelihood between models with and without positive selection (Table 1). Because of that, models with positive selection were given greater weightings in the model averages and there was little impact of the averaging on parameter estimates (Figure S4). Note, however, that the discrete class of beneficial mutations I simulated is highly abstract and was used to explore the limits of the uSFS analysis methods. In reality, the DFE for beneficial mutations is likely continuous and model averaging is potentially useful for fitting DFE models to unknown distributions.
The simulations I performed in this study generated the ideal dataset for estimating parameters of selection from the uSFS. I simulated 21Mbp of coding sites in which genotypes and whether sites were selected or not was unambiguously known. When analyzing real data this is not the case and researchers often have to filter a large proportion of sites out of their analyses or choose to analyze a subset of genes that have orthology with outgroups or other biological properties of interest. Even with perfect knowledge, strongly beneficial mutations only represented a small proportion of the standing genetic variation at nonsynonymous sites (Figure 1, S1). In addition, the populations I simulated were randomly mating and had constant sizes over time. The results I present in this study suggest that even with perfect knowledge of a population that adheres to the assumptions of a Wright-Fisher model, it is inherently difficult to infer the parameters of strongly beneficial mutations from the uSFS, particularly so when beneficial mutations occur infrequently.
Estimating parameters of positive selection from the uSFS vs. estimates from patterns of diversity
As discussed above, studies based on analysis of the uSFS and those based on selective sweep models have yielded vastly different estimates of the parameters of positive selection. Patterns of neutral genetic diversity in both humans and wild mice cannot be explained by the effects of background selection alone, and in both species it has been suggested that strongly beneficial mutations are required to explain the observed patterns (Nam et al. 2017; Booker and Keightley 2018). In the case of wild house mice, positive selection parameters obtained by analysis of the uSFS do not explain dips in nucleotide diversity around functional elements (Booker and Keightley 2018). Recently, Castellano et al. (2019) analyzed the uSFS for nonsynonymous sites in great ape species but did not find significant evidence for positive selection. In their dataset, Castellano et al. (2019) had at least 8 haploid genome sequences for each of great ape species they analyzed, and they argued that they were underpowered to detect positive selection on the basis of the uSFS. In this study, I analyzed datasets of 20 diploid individuals and found that it was very difficult to accurately capture positive selection parameters. Increasing the number of sampled individuals even further may increase the power to estimate the strength of positive selection, but this study suggests that the increase in power will depend on the underlying DFE. When pa is small, the expected number of advantageous mutations present in the uSFS for 200 diploids is less than 10 for most frequency classes when 14Mbp of nonsynonymous sites have been used to construct the uSFS (Figure S3). Indeed, Figure S3 shows that even with very large sample sizes, the expected uSFS for beneficial mutations are very similar and may only be distinguished on the basis of a small number of high frequency derived alleles. Thus, it may be that the uSFS is inherently limited in the information it carries on the DFE for beneficial mutations so other sources of information may have to be used to accurately recover parameters.
In this study, I modeled beneficial mutations using a discrete class of selection coefficients when, in reality, there is likely a continuous distribution of fitness effects. Indeed, studies in both humans and D. melanogaster have found evidence for a bimodal distribution containing both strongly and weakly beneficial mutations contributing to adaptive evolution using methods which incorporate linkage information but do not explicitly estimate selection parameters (Elyashiv et al. 2016; Uricchio et al. 2019). There are currently no methods that estimate the DFE using an analytical expression for the uSFS expected under the combined effects of BGS and sweeps. Rather, nuisance parameters or demographic models are used to correct for the contribution that selection at linked sites may make to the shape of the SFS (Eyre-Walker et al. 2006; Galtier 2016; Tataru et al. 2017). However, as this study shows, the parameters of positive selection are not reliably estimated when analyzing the uSFS alone. A way forward may be in using computational approaches to make use of all of the available data, while not necessitating an expression for the uSFS expected under the combined effects of BGS, sweeps, population size change and direct selection. An advance in this direction has recently been made by Uricchio et al., (2019) who developed an ABC method for estimating α which makes use of the distortions to the uSFS generated by BGS and sweeps. By applying their method to data from humans, Uricchio et al., (2019) found that α = 0.13 for nonsynonymous sites, 72% of which was generated by mildly beneficial mutations and 28% by strongly beneficial mutations. However, the computational approach developed by Uricchio et al., (2019) could readily be extended to model an arbitrarily complex DFE for beneficial mutations. Their methods could be implemented in a machine-learning context, with training data generated by forward-simulations that capture confounding factors such as population structure and population size change as well as the effects of selection at linked sites.
Acknowledgments
I wish to extend gratitude to Peter Keightley, Michael Whitlock and Sam Yeaman for valuable advice and mentorship. Thanks to Thomas Bataillon, Sam Yeaman and Peter Keightley for comments on previous versions of the manuscript. Thanks to Paula Tataru and Thomas Bataillon for help with polyDFE. Thanks to two anonymous reviewers for constructive feedback.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.12233630.
Communicating editor: Y. Kim
Literature Cited
- Bailey S. F., and Bataillon T., 2016. Can the experimental evolution programme help us elucidate the genetic basis of adaptation in nature? Mol. Ecol. 25: 203–218. 10.1111/mec.13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank C., Hietpas R. T., Wong A., Bolon D. N., and Jensen J. D., 2014. A Bayesian MCMC approach to assess the complete distribution of fitness effects of new mutations: uncovering the potential for adaptive walks in challenging environments. Genetics 196: 841–852. 10.1534/genetics.113.156190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N. H., 2000. Genetic hitchhiking. Philos Trans R Soc L. B Biol Sci 355: 1553–1562. 10.1098/rstb.2000.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton H. J., and Zeng K., 2018. New methods for inferring the distribution of fitness effects for INDELs and SNPs. Mol. Biol. Evol. 35: 1536–1546. 10.1093/molbev/msy054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böndel K. B., Kraemer S. A., Samuels T., McClean D., Lachapelle J. et al. , 2019. Inferring the distribution of fitness effects of spontaneous mutations in Chlamydomonas reinhardtii. PLoS Biol. 17: e3000192 10.1371/journal.pbio.3000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker T. R., and Keightley P. D., 2018. Understanding the factors that shape patterns of nucleotide diversity in the house mouse genome. Mol. Biol. Evol. 35: 2971–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A. R., Williamson S. H., Indap A. R., Degenhardt J. D., Hernandez R. D. et al. , 2008. Assessing the evolutionary impact of amino acid mutations in the human genome. PLoS Genet. 4: e1000083 10.1371/journal.pgen.1000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. L., and Charlesworth B., 2019. The effects on neutral variability of recurrent selective sweeps and background selection. Genetics 212: 287–303. 10.1534/genetics.119.301951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. L., Zhao L., and Charlesworth B., 2017. Estimating the parameters of background selection and selective sweeps in Drosophila in the presence of gene conversion. Proc. Natl. Acad. Sci. USA 114: E4762–E4771. 10.1073/pnas.1619434114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano D., Macià M. C., Tataru P., Bataillon T., and Munch K., 2019. Comparison of the full distribution of fitness effects of new amino acid mutations across great apes. Genetics 213: 953–966. 10.1534/genetics.119.302494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., 1994. The effect of background selection against deleterious mutations on weakly selected, linked variants. Genet. Res. 63: 213–227. 10.1017/S0016672300032365 [DOI] [PubMed] [Google Scholar]
- Elyashiv E., Sattath S., Hu T. T., Strutsovsky A., McVicker G. et al. , 2016. A genomic map of the effects of linked selection in Drosophila. PLoS Genet. 12: e1006130 10.1371/journal.pgen.1006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre-Walker A., and Keightley P. D., 2009. Estimating the rate of adaptive molecular evolution in the presence of slightly deleterious mutations and population size change. Mol. Biol. Evol. 26: 2097–2108. 10.1093/molbev/msp119 [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A., and Keightley P. D., 2007. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 8: 610–618. 10.1038/nrg2146 [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A., Woolfit M., and Phelps T., 2006. The distribution of fitness effects of new deleterious amino acid mutations in humans. Genetics 173: 891–900. 10.1534/genetics.106.057570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N., 2016. Adaptive protein evolution in animals and the effective population size hypothesis. PLoS Genet. 12: e1005774 10.1371/journal.pgen.1005774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane J. B. S., 1927. A Mathematical theory of natural and artificial selection, Part V: Selection and mutation. Math. Proc. Camb. Philos. Soc. 23: 838–844. 10.1017/S0305004100015644 [DOI] [Google Scholar]
- Haller B. C., and Messer P. W., 2019. SLiM 3: Forward genetic simulations beyond the Wright-Fisher model. Mol. Biol. Evol. 36: 632–637. 10.1093/molbev/msy228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. G., 2010. Understanding and using quantitative genetic variation. Philos. Trans. R. Soc. B Biol. Sci. 365: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley P. D., Campos J. L., Booker T. R., and Charlesworth B., 2016. Inferring the frequency spectrum of derived variants to quantify adaptive molecular evolution in protein-coding genes of Drosophila melanogaster. Genetics 203: 975–984. 10.1534/genetics.116.188102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley P. D., and Eyre-Walker A., 2007. Joint inference of the distribution of fitness effects of deleterious mutations and population demography based on nucleotide polymorphism frequencies. Genetics 177: 2251–2261. 10.1534/genetics.107.080663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley P. D., and Jackson B. C., 2018. Inferring the probability of the derived vs. the ancestral allelic state at a polymorphic site. Genetics 209: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., and Ohta T., 1971. Theoretical aspects of population genetics, Princeton Univ. Press, Princeton, NJ. [PubMed] [Google Scholar]
- Kousathanas A., and Keightley P. D., 2013. A comparison of models to infer the distribution of fitness effects of new mutations. Genetics 193: 1197–1208. 10.1534/genetics.112.148023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laenen B., Tedder A., Nowak M. D., Toräng P., Wunder J. et al. , 2018. Demography and mating system shape the genome-wide impact of purifying selection in Arabis alpina. Proc. Natl. Acad. Sci. USA 115: 816–821. 10.1073/pnas.1707492115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe L., and Charlesworth B., 2006. Inferring the distribution of mutational effects on fitness in Drosophila. Biol. Lett. 2: 426–430. 10.1098/rsbl.2006.0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. M., and Kreitman M., 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351: 652–654. 10.1038/351652a0 [DOI] [PubMed] [Google Scholar]
- Nam K., Munch K., Mailund T., Nater A., Greminger M. P. et al. , 2017. Evidence that the rate of strong selective sweeps increases with population size in the great apes. Proc. Natl. Acad. Sci. USA 114: 1613–1618. 10.1073/pnas.1605660114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., 2003. The distribution of fitness effects among beneficial mutations. Genetics 163: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., and Unckless R. L., 2014. The population genetics of evolutionary rescue. PLoS Genet. 10: e1004551 10.1371/journal.pgen.1004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S. P., 2009. The evolutionary enigma of sex. Am. Nat. 174: S1–S14. 10.1086/599084 [DOI] [PubMed] [Google Scholar]
- Schneider A., Charlesworth B., Eyre-Walker A., and Keightley P. D., 2011. A method for inferring the rate of occurrence and fitness effects of advantageous mutations. Genetics 189: 1427–1437. 10.1534/genetics.111.131730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataru P., and Bataillon T., 2020. polyDFE: Inferring the distribution of fitness effects and properties of beneficial mutations from polymorphism data, pp. 125–146 in Methods in Molecular Biology, Humana Press Inc, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Tataru P., and Bataillon T., 2019. polyDFEv2.0: testing for invariance of the distribution of fitness effects within and across species. Bioinformatics 35: 2868–2869. 10.1093/bioinformatics/bty1060 [DOI] [PubMed] [Google Scholar]
- Tataru P., Mollion M., Glemin S., and Bataillon T., 2017. Inference of distribution of fitness effects and proportion of adaptive substitutions from polymorphism data. Genetics 207: 1103–1119. 10.1534/genetics.117.300323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uricchio L. H., Petrov D. A., and Enard D., 2019. Exploiting selection at linked sites to infer the rate and strength of adaptation. Nat. Ecol. Evol. 3: 977–984. 10.1038/s41559-019-0890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. J., Josephs E. B., Platts A. E., Hazzouri K. M., Haudry A. et al. , 2014. Evidence for widespread positive and negative selection in coding and conserved noncoding regions of Capsella grandiflora. PLoS Genet. 10: e1004622 10.1371/journal.pgen.1004622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S., 1937. The distribution of gene frequencies in populations. Proc. Natl. Acad. Sci. USA 23: 307–320. 10.1073/pnas.23.6.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All code and SLiM configuration files needed to reproduce the results shown in this study are available at https://github.com/TBooker/PositiveSelection_uSFS. Supplemental material available at figshare: https://doi.org/10.25387/g3.12233630.