Abstract
The cardiac sodium channel NaV1.5, encoded by the SCN5A gene, is responsible for the fast upstroke of the action potential. Mutations in SCN5A may cause sodium channel dysfunction by decreasing peak sodium current, which slows conduction and facilitates reentry-based arrhythmias, and by enhancing late sodium current, which prolongs the action potential and sets the stage for early afterdepolarization and arrhythmias. Yet, some NaV1.5-related disorders, in particular structural abnormalities, cannot be directly or solely explained on the basis of defective NaV1.5 expression or biophysics. An emerging concept that may explain the large disease spectrum associated with SCN5A mutations centres around the multifunctionality of the NaV1.5 complex. In this alternative view, alterations in NaV1.5 affect processes that are independent of its canonical ion-conducting role. We here propose a novel classification of NaV1.5 (dys)function, categorized into (i) direct ionic effects of sodium influx through NaV1.5 on membrane potential and consequent action potential generation, (ii) indirect ionic effects of sodium influx on intracellular homeostasis and signalling, and (iii) non-ionic effects of NaV1.5, independent of sodium influx, through interactions with macromolecular complexes within the different microdomains of the cardiomyocyte. These indirect ionic and non-ionic processes may, acting alone or in concert, contribute significantly to arrhythmogenesis. Hence, further exploration of these multifunctional effects of NaV1.5 is essential for the development of novel preventive and therapeutic strategies.
Keywords: SCN5A, NaV1.5, Sodium channelopathies, Mechanisms, Therapies
This article is part of the Spotlight Issue on Inherited Conditions of Arrhythmia.
1. Introduction
The cardiac voltage-gated sodium channel (NaV1.5) encoded by the SCN5A gene, is responsible for the fast, initial upstroke of the action potential and as such is a critical determinant of cardiomyocyte excitability and conduction of the electrical impulse through the myocardium. Mutations in SCN5A are a long-established cause of a broad spectrum of inherited electrical disorders associated with sudden cardiac death, including long QT syndrome type 3 (LQT3), Brugada syndrome (BrS), progressive and non-progressive cardiac conduction disease (CCD), atrial fibrillation (AF), and sick sinus syndrome.1,2 In addition to these electrical manifestations, consequences of sodium channel dysfunction may also include structural cardiac abnormalities that range from (micro)structural degenerative changes in the myocardium,3–5 to dilated cardiomyopathy (DCM)6,7 and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/ARVD).8–10 Not uncommonly, inherited defects in SCN5A also manifest with a combination of these clinical phenotypes.11,12
Considerable knowledge has been gained into the function of NaV1.5 and its role in disease through the study of specific SCN5A variants found in patients. Clinical phenotypes associated with SCN5A mutations have so far been explained by defects in channel expression, trafficking, or its biophysical properties and these defects have been categorized into those that cause a loss or gain of channel function, or a combination of the two. Yet, some of these NaV1.5-related disorders, in particular structural abnormalities, cannot be directly or solely explained on the basis of defective NaV1.5 expression or biophysics.1,2 While factors other than the SCN5A genetic defect itself, such as the inheritance of other genetic factors, undoubtedly play a role in modulating the different phenotypes and severity of SCN5A mutations, an emerging concept that may explain the large disease spectrum associated with SCN5A mutations centres around the multifunctionality of the sodium channel complex. While classically considered in light of the essential role of the channel in mediating electrical activity in the heart, in this alternative view, mutations affecting the sodium channel are considered to lead to disease by affecting processes that are independent of its canonical ion-conducting role. While some of these processes have been studied in detail, others, particularly those mediated through protein–protein interactions within the sodium channel complex await elucidation. A more complete understanding of the various functions of NaV1.5 in cardiomyocytes will undoubtedly favour the development of novel therapeutic approaches for inherited rhythm disorders and possibly in acquired disturbances of sodium channel function as occurs in ischaemia and heart failure. We here first provide a brief description of the cardiac sodium channel and a succinct overview of inherited disorders associated with NaV1.5 dysfunction. We subsequently review in detail current knowledge of the multiple functions of NaV1.5 and propose a new classification for these various functional roles of NaV1.5. These include direct ionic effects of sodium influx on cardiomyocyte electrophysiology, indirect ionic effects of sodium influx on intracellular ion homeostasis and signalling, and non-ionic effects of NaV1.5 independent of sodium influx. In particular, we discuss their respective contribution to the detrimental effects of cardiac sodium channel dysfunction.
2. The cardiac sodium channel: the pillar of action potential initiation
2.1. Sodium channel structure and function
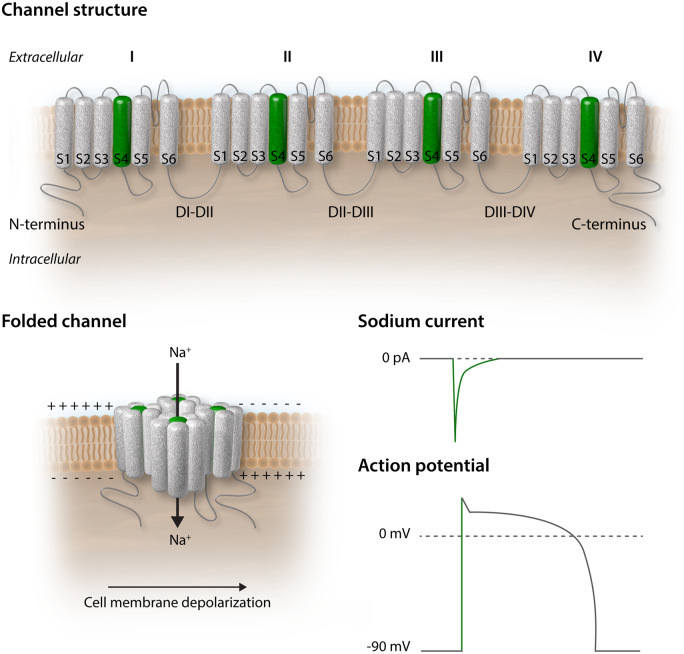
The plasma membrane being a natural barrier to the passage of most large polar molecules and ions, membrane transport proteins assure travel across the bilayer. In cardiomyocytes, the voltage-gated, pore-forming alpha subunit of the cardiac sodium channel NaV1.5 (encoded by the SCN5A gene) is a transmembrane protein allowing the passage of sodium ions from the extracellular to the intracellular space.13 It is composed of a cytoplasmic N-terminus, four transmembrane domains (DI–DIV) interconnected by cytoplasmic and extracellular loops, and a cytoplasmic C-terminal domain. The homologous DI–DIV domains each comprise six segments (S1–S6), of which S5 and S6 form the ion-conducting channel pore, and the highly charged S4 segment acts as the voltage sensor (Figure 1, upper panel).14 The resting membrane potential of a cardiomyocyte is around −90 mV, which is determined by the equilibrium potential of potassium. At this voltage, the sodium channel is closed. As NaV1.5 is voltage-sensitive, a small depolarization of the membrane causes the charged segments S4, the voltage sensors, to move outward leading to opening of the channel pore formed by segments S5 and S6 and the linker between them. The rapid activation of NaV1.5 causes sodium ions to flow into the cell along the electrochemical gradient generating a large inward current (peak sodium current). The net charge of sodium ions entering the cell further depolarizes the membrane thereby initiating phase 0 of the action potential and is essential for cardiac excitability (Figure 1, lower panels).13,15 Following activation, fast and complete NaV1.5 channel inactivation ensures proper repolarization, mediated by the DIII–DIV intracellular loop, the extracellular S5–S6 linker, and the C-terminal domain of the channel.16,17 Because of the fast activation and fast inactivation of the sodium channel, the peak sodium current is brief (<1 ms) under normal physiological conditions. A small fraction of channels, however, do not completely inactivate and a small sodium current persists throughout the duration of the action potential, called the ‘persistent’ or ‘late’ sodium current (INa,L).18 Many studies have now established that late sodium current significantly contributes to action potential duration in cardiomyocytes of different mammalian species (including human) and that it is enhanced in cardiac diseases.18–22 In a recent study, Jiang et al.23 used cryo-electron microscopy to solve the molecular structure of NaV1.5 at a resolution of 3.2–3.5 Angstroms, providing deep, unparalleled insight into the relation between the structure of the channel and its function. The three-dimensional solution of the various molecular domains allows a better understanding of the mechanisms that lead to gating alterations and from there, to the loss or gain of function that can act as substrate for an arrhythmic phenotype.
Figure 1.
The cardiac voltage-gated sodium channel NaV1.5. Upper panel: unfolded NaV1.5 protein at the cardiomyocyte plasma membrane featuring four domains (I, II, III, IV) each containing six segments (S1–S6). The charged segment S4 is represented in green. Lower left panel: folded NaV1.5 at the cardiomyocyte plasma membrane. The rapid inward sodium current depolarizes the cell membrane. Lower right panel: relation between the sodium current and the upstroke of the action potential (green segments).
2.2. Sodium channel distribution and interacting proteins
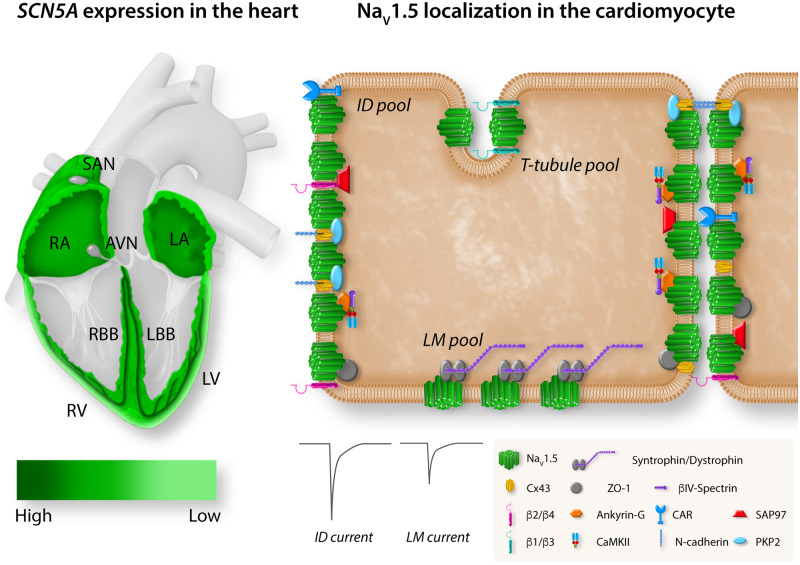
NaV1.5 expression levels vary throughout different regions of the heart, in line with regional differences in speed of conduction (Figure 2, left panel).24,25 Although very lowly expressed in the central part of the slowly conducting sinus node, NaV1.5 expression is found in the periphery of the sinoatrial node, where its presence is required for proper sinus node function.26–28 NaV1.5 is present at low levels in the inferior nodal extension (INE) and transitional zone of the atrioventricular (AV) ring, where conduction is slow.29–31 However, NaV1.5 is not expressed in the compact atrioventricular node (AVN) and its absence is crucial for proper AV function by ensuring a delay between atrial and ventricular activation.29,30,32 In contrast, NaV1.5 expression is prominent in the AV-bundle, His and Purkinje fibres where conduction is fastest (up to 2 m/s).24,26 The sodium channel is furthermore broadly expressed in atrial and ventricular working myocardium.26,33 In both ventricles, a transmural gradient is observed with higher expression of SCN5A and NaV1.5 in the subendocardium than in the subepicardium.26SCN5A and NaV1.5 are moreover expressed at lower levels in the right ventricular outflow tract as compared to the ventricles; nevertheless, in healthy myocardium, this does not lead to conduction slowing within this region.34 Differential patterning of NaV1.5 also exists within subcellular microdomains of the cardiomyocyte (Figure 2, right panel). The combination of nanometre precision microscopy and patch-clamp techniques has shown that NaV1.5 channels gather at the membrane in clusters,35 with biophysical properties of NaV1.5 clusters differing between cardiomyocyte microdomains. Indeed, sodium channels located at the intercalated disc (ID) generate larger currents than those located at the lateral membrane (LM).36,37 In addition, gating properties also differ according to location with a positive shift in steady-state activation, a negative shift in steady-state inactivation, and a slower recovery from inactivation observed for channels located at the LM as compared to those at the ID.36 In the latter, three additional distinct pools of NaV1.5 channels have been identified (sarcolemmal crests, grooves, and T-tubules), with crests carrying the largest currents and T-tubules the smallest.35,38,39 NaV1.5 channels associate with partner proteins to form region-specific macromolecular complexes.40 The large variety in interacting proteins already suggests that different macromolecular complexes may be relevant for specific functions at different locations and may be differentially regulated. NaV1.5 can be found, for example, in proximity to N-cadherin, connexin 43, plakophilin-2 (PKP2), ankyrin-G, βIV-spectrin, calcium/calmodulin-dependent protein kinase II (CaMKII), coxsackie and adeno virus receptor, zonula occludens 1, and synapse-associated protein 97 at the ID, whereas it associates mainly with the dystrophin–syntrophin complex at the LM.40 Moreover, it has been suggested that of the accessory β-subunits (which modulate NaV1.5 channel density and kinetics41) β2 and β4 are located preferentially at the ID while β1 and β3 are localized mainly in the T-tubules.42 The functional relevance of these distinct sodium channel complexes is exemplified by the fact that mutations in NaV1.5 interacting proteins are associated with various arrhythmia syndromes.43 These observations furthermore shed light on potential microdomain-specific function of NaV1.5, as will be discussed in more detail below.
Figure 2.
Regional and subcellular distribution of SCN5A/NaV1.5 in the heart and cardiomyocyte. Left panel: schematic representation of a heart showing the expression level of SCN5A in the different compartments. Expression of SCN5A is highest in the AV bundle, His bundle, and RBB and LBB (dark green). SCN5A is broadly expressed in RA and LA and RV and LV with an epi/endo gradient in the ventricles. SCN5A is absent from the central SAN and AVN. Right panel: schematic representation of the localization of NaV1.5 with specific regional partner proteins in the microdomains of the cardiomyocyte: ID, LM, and T-tubules. The sodium current generated at the ID is larger than the sodium current generated at the LM. LA, left atria; LBB, left bundle branch; LV, left ventricle; RA, right atria; RBB, right bundle branch; RV, right ventricle; SAN, sinoatrial node.
3. Inherited disorders associated with NaV1.5 dysfunction
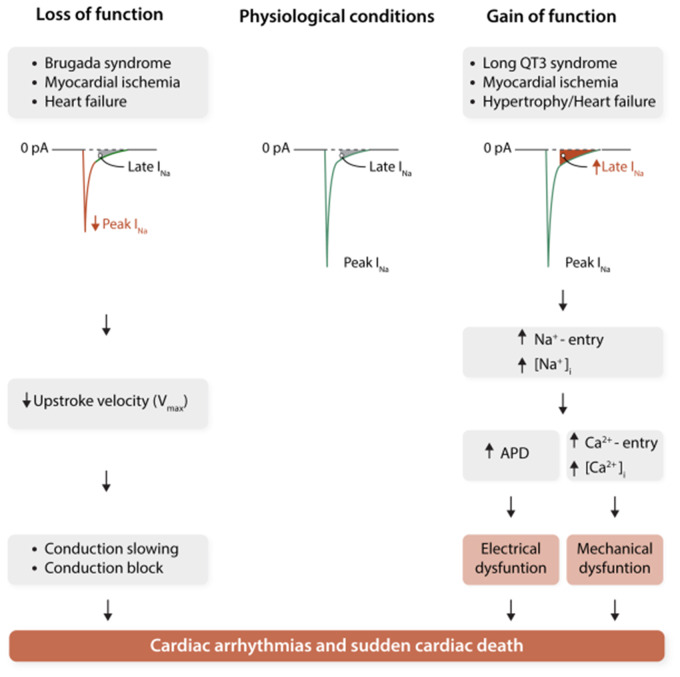
Mutations in the SCN5A gene, leading to NaV1.5 dysfunction, have been linked to various types of cardiac electrical diseases. As discussed in more detail below, these syndromes each display distinct clinical and electrophysiological characteristics, despite the fact that they are caused by mutations in the same ion channel. From a biophysical point of view, these alterations may be divided into those leading to a loss or to a gain of NaV1.5 function (Figure 3). Loss of function mutations in SCN5A lead to decreased peak sodium current and conduction slowing. On the other hand, gain of function SCN5A mutations affecting (recovery from) inactivation are associated with increased late sodium current, leading to persistent sodium influx during the course of the action potential, thereby prolonging repolarization. In some cases, a single SCN5A mutation may cause at the same time gain and loss of function biophysical defects leading to an overlap phenotype (see below), and occasionally mutations are also associated with cardiac structural abnormalities.4,5,45 This diversity in phenotypic expression furthermore underscores the importance of the diversity and multifunctionality of SCN5A/NaV1.5. We first describe briefly the main phenotypical characteristics of the various inherited syndromes associated with NaV1.5 dysfunction, before exploring in more detail the diverse actions of sodium channels in the next section.
Figure 3.
Schematic representation of the arrhythmogenic consequences of reduced peak sodium current (peak INa; left) and increased late sodium current (late INa; right). APD, action potential duration; [Ca2+]i, intracellular calcium concentration; [Na+]i, intracellular sodium concentration. Redrawn from Remme and Wilde44 with permission.
3.1. Long QT syndrome type 3
LQT3 is characterized by prolonged QT intervals on the electrocardiogram (ECG) and increased risk for sudden death due to ventricular tachyarrhythmias, in particular Torsades de Pointes. LQT3 patients often display bradycardia, and ventricular arrhythmias occur predominantly at slow heart rates, i.e. during rest.46,47 Unfortunately, cardiac arrest (rather than syncope) is often the first clinical event.46,48 The underlying biophysical alterations include action potential prolongation due to enhanced INa,L, secondary to mutation-induced altered or incomplete NaV1.5 inactivation.49,50 Management of LQT3 patients includes beta-blocker therapy, pacemaker implantation in selected cases, and implantable cardioverter-defibrillator (ICD) implantation in patients at high risk of arrhythmias. Additional strategies aimed at inhibiting INa,L are currently under investigation.51
3.2. Brugada syndrome
BrS is characterized by ST-segment elevation in the right-precordial leads on the ECG, which may be variably present but unmasked or increased by Class 1A or 1C anti-arrhythmic sodium channel blocking drugs (ajmaline, flecainide). BrS has an increased prevalence in males and is associated with an increased risk for ventricular arrhythmias and sudden death occurring mostly during rest or sleep.52 In approximately 20% of BrS patients, SCN5A mutations are identified, which are typically ‘loss-of-function’ mutations leading to reduced sodium channel availability.53,54 Mechanisms involved in BrS include conduction abnormalities, increased (transmural) heterogeneity in action potential duration and right ventricular structural abnormalities.55 Management options include ICD implantation and measures aimed at preventing known arrhythmia-provoking factors such as fever; quinidine may be added as an adjunct therapy to decrease the incidence of ventricular arrhythmias.56
3.3. Progressive cardiac conduction defect and sick sinus syndrome
Loss-of-function mutations in SCN5A leading to reduced sodium channel availability have been associated with inherited sick sinus syndrome and progressive cardiac conduction defect (PCCD).57–59 PCCD, also called Lenègre or Lev disease, is characterized by progressive conduction slowing through the His–Purkinje system, with right and/or left bundle branch block and QRS-widening, leading to complete AV block, syncope, and sudden death.
3.4. Atrial fibrillation
In addition to acquired, age-related conditions, AF may also occur as a hereditary disease in young patients with structurally normal hearts. Both loss of function and gain of function mutations in SCN5A have been associated with familial AF, which may induce AF through decreased atrial conduction velocity and increased atrial action potential duration and excitability, respectively.60,61
3.5. Dilated cardiomyopathy
In rare cases (1.7%62) SCN5A mutations are also associated with DCM,3 often presenting in combination with atrial arrhythmias and/or fibrillation.7 Recently a common polymorphism in SCN5A (rs1805124) has been associated with the development of DCM.63SCN5A mutations leading to DCM have been associated with both loss and gain of sodium channel function.64–66 The mechanisms underlying DCM secondary to SCN5A mutations are likely complex, involving altered (late) sodium current or proton leak current (pre-existent) myocardial structural abnormalities, and the presence of long-standing (atrial) arrhythmias.66,67
3.6. Sodium channel overlap syndrome
A single SCN5A mutation may also result in multiple disease phenotypes, referred to as ‘sodium channel overlap syndrome’. For example, the SCN5A-1795insD mutation is associated with sinus node dysfunction, bradycardia, conduction disease, BrS, and LQT3 in a large Dutch family with a high risk for nocturnal sudden death.11,68 The simultaneous presence of LQT3 (i.e. gain of function) and BrS or conduction disease (i.e. loss of function) due to one single SCN5A mutation may seem unlikely given the apparent opposing biophysical alterations underlying both clinical entities. However, studies by us and others have shown that SCN5A-1795insD as well as the overlap mutations SCN5A-E1784K and SCN5A-delK1500 reduce peak current density while simultaneously increasing INa,L magnitude, providing the biophysical basis for the clinical overlap syndrome phenotype.69–71
3.7. Arrhythmogenic cardiomyopathy/ARVC
Inherited arrhythmogenic cardiomyopathy (ACM), previously designated ARVC is associated with cardiomyopathic changes, heart failure, ventricular arrhythmias, and sudden death. Mutations in desmosomal proteins are identified in most affected individuals, including plakoglobin, PKP2, and desmoglein-2.72 A number of these desmosomal proteins have been shown to interact with NaV1.5 and as a consequence, reduced INa is a common feature in ACM/ARVC disease models.9,73,74 More recently, rare variants in SCN5A have been identified in ACM/ARVC patients.8,75 For one of these mutations, studies in human-induced pluripotent stem cells-derived cardiomyocytes demonstrated a reduction in INa and a potential detrimental effect on cell adhesion, potentially (partly) explaining the observed ACM phenotype.8
4. Multifunctionality of NaV1.5
The diversity in electrical and structural phenotypes secondary to SCN5A mutations described in the previous section may be considered unexpected and disputes the idea that the sole role of the cardiac sodium channel lies in the initiation and propagation of the cardiac action potential. Indeed, aided by advanced imaging and electrophysiological techniques, studies in the last decade have revealed an increasing diversity and complexity of sodium channels in the heart and the cardiomyocyte. In this section, we explore in more detail the diverse ionic and non-ionic mechanisms by which sodium channel (dys)function leads to electrical and structural alterations.
4.1 Direct ionic (dys)function of NaV1.5
4.1.1. Direct ionic consequences of inherited NaV1.5 (dys)function
Clearly, the ion-conducting role of NaV1.5 is essential for cardiac electrical function, with alterations in sodium ion influx, i.e. through loss of function or gain of function of NaV1.5, directly impacting on either depolarization or repolarization characteristics of the cardiomyocyte. In inherited arrhythmia syndromes, loss of function mutations in SCN5A can lead to a decreased number of functional channels on the membrane due to misfolding of the channel and/or altered trafficking.53,54,76 Sodium current magnitude may also be decreased secondary to reduced conductivity or a shift in the voltage dependence of (in)activation. Similar alterations in sodium channel trafficking and/or kinetics may also occur as a consequence of alterations in proteins interacting with NaV1.5. In all situations, reduced sodium channel availability leads to decreased cardiac excitability and pro-arrhythmic conduction slowing. Gain of function SCN5A mutations may lead to enhanced late sodium current through a number of potential mechanisms: (i) disruption of fast inactivation, thereby allowing for sodium channels to re-open,49 (ii) incomplete or slowed inactivation, resulting in channel openings of longer duration, and (iii) a shift in voltage dependence of inactivation with a consequent increased voltage range of incomplete current inactivation (resulting in an increase in window current). In addition, faster recovery from inactivation (causing increased sodium channel availability), or increased peak INa density may occur.50,77,78 Finally, enhanced INa,L has also been observed secondary to mutations in proteins interacting with NaV1.5. Independent of the underlying mechanism, the consequence is an enhanced and prolonged sodium influx during the action potential resulting in delayed repolarization, action potential prolongation, and early afterdepolarizations which may subsequently trigger Torsades de Pointes arrhythmias and sudden death. These direct ionic mechanisms underlying Nav1.5 dysfunction appear straightforward, but it must be remembered that the biophysical effects of SCN5A mutations are usually investigated in heterologous expression systems which may not adequately reflect the cardiomyocyte environment.79 Indeed, the actual situation in the heart is likely more complex due to transcriptional and post-translational regulation, and NaV1.5 complex diversity within subcellular microdomains.
4.1.2. Modulation of the direct ionic effect of NaV1.5
Splicing of SCN5A is age- and species-dependent and leads to transcript variants with different functional characteristics, including peak and late sodium current magnitude.80,81 Alternative splicing of SCN5A may be of significant functional relevance for disease expressivity in sodium channelopathy. For instance, the SCN5A-L409P mutation leads to more severe biophysical defects in the presence of the neonatal isoform, including an increased INa,L, potentially explaining the unusual severity and early onset of long QT syndrome in the affected foetus.82 In addition, splice variants have been shown to differentially modulate the reduced sodium channel membrane expression consequent to the BrS mutation SCN5A-G1406R.83 Post-translationally, NaV1.5 is predominantly regulated by phosphorylation, ubiquitylation, and glycosylation (reviewed in84,85). While glycosylation may affect sodium channel gating,84,86 ubiquitylation is mainly responsible for regulating the number of plasma membrane proteins at the cell surface by subjecting NaV1.5 to proteosomal or lysosomal degradation.87 NaV1.5 trafficking and the density of channels at the membrane has furthermore been shown to be regulated by a large variety of mechanisms including protein kinases A and C, CaMKII,85 reactive oxygen species (ROS),88 intracellular calcium levels,89 temperature,90 extracellular protons and pH,91 and stretch.92 Hence, various modulatory mechanisms may alter the ion-conducting properties of NaV1.5, thereby directly impacting on cardiac excitability and/or repolarization, and consequent arrhythmogenesis.
4.1.3. Direct ionic (dys)function of NaV1.5 in microdomains
As described above, NaV1.5 channels are not randomly distributed along the cardiomyocyte membrane but are regionally organized in clusters and macromolecular complexes. The differences in NaV1.5 expression, sodium current density and kinetics observed between these subcellular microdomains (i.e. ID vs. LM), may be explained at least in part by the regional variation in modulatory NaV1.5 interacting proteins. Hence, mutations in SCN5A or in genes coding for NaV1.5 partner proteins may not only affect ionic NaV1.5 function, but in fact may do so in a microdomain-specific manner. Sodium channels located at the LM are associated with the syntrophin–dystrophin complex, and dystrophin-deficient mdx mice display reduced NaV1.5 expression levels predominantly at the LM.93 NaV1.5 channels lacking the three last amino acids at the C-terminal domain (ΔSIV) no longer interact with alpha-1-syntrophin and Scn5a-ΔSIV mice display reduced sodium current specifically at the LM, where syntrophin is exclusively located.94 This LM-specific decrease in sodium current resulted in reduced transversal conduction velocity and PR- and QRS-prolongation in Scn5a-ΔSIV mice. The A257G-SNTA1 mutation in alpha-1-syntrophin on the other hand causes a gain of function of NaV1.5 as seen in LQTS.95 Similarly, a mutation in caveolin-3, another LM partner of NaV1.5, causes a gain-of-function of NaV1.5 and LQTS in patients.96 The impact of these mutations on the subcellular ionic function of NaV1.5 has not been assessed; however, since both alpha-1-syntrophin and caveolin-3 are both LM partner proteins of NaV1.5, one may speculate that the observed gain of function is mediated specifically by LM-based NaV1.5 channels. In contrast, alterations in NaV1.5 partner proteins at the ID have mostly resulted in loss of sodium channel function in this microdomain and consequently conduction slowing and arrhythmias. For instance, the Gja1-D378stop mutation in Cx43 was associated with lethal arrhythmic events in mice and reduced sodium current at the ID due to trafficking defects.97,98 In addition, heterozygous deletion of the coxsackie and adeno virus receptor resulted in loss-of-function of the sodium channel specifically at the ID and enhanced arrhythmia susceptibility during myocardial infarction in both humans and mice.37 Moreover, mutations in the desmosomal proteins plakophilin 2, desmoglein 2, and plakoglobin lead to ACM/ARVC, decreased NaV1.5 current at the ID region and consequent conduction slowing and pro-arrhythmia.9,10,73 Patients harbouring mutations in sodium channel β subunit genes display a diversity of arrhythmic syndromes similar to SCN5A channelopathies, including BrS and/or CCD (β1 and β3), LQTS (β4), AF (β1, β2, and β3), and idiopathic ventricular fibrillation (β3).99–103 While preferential localization of certain β subunits at the ID (β2 and β4) vs. T-tubules (β1 and β3) has been suggested,42 any microdomain-specific alterations in NaV1.5 function secondary to these mutations remain to be elucidated.99–102 Similarly, mutations in GPDL1 and MOG1 encoding the probable glycerophosphoryl diester phosphodiesterase 1 and the nucleocytoplasmic transport protein multicopy suppressor of Gsp1, respectively, have been linked to BrS and reduced sodium current, but their distinct subcellular consequences remain as yet unknown.104–106 Overall, these studies have provided some additional insight into the ionic function of NaV1.5 within distinct microdomains. It may be tempting to speculate that NaV1.5-dysfunction primarily affects conduction at the ID and ionic homeostasis mostly at the LM, or loss-of-function SCN5A mutations predominantly impact the ID and gain of function mutations mostly the LM. However, further investigations are necessary to fully elucidate the potential microdomain-specific effects and consequences of mutations in SCN5A and NaV1.5 partner proteins.
4.2 Indirect ionic effects of NaV1.5 (dys)function
Indirect ionic effects of cardiac sodium channels are related to mechanisms or pathways that are activated by the alterations in intracellular sodium concentration consequent to the influx of sodium ions through NaV1.5 into the cardiomyocyte. In addition to implications for (pro-arrhythmic) alterations in intracellular calcium concentrations, other consequences include metabolic dysregulation and activation of calcium-dependent signalling pathways. Here, essential insight may also be gained from findings in cell types other than cardiomyocytes.
4.2.1. NaV1.5 dysfunction and dysregulation of intracellular sodium–calcium homeostasis
The most established indirect ionic action of NaV1.5 is its secondary effect on intracellular sodium–calcium homeostasis. The increase in intracellular Na+ concentration ([Na+]i) following opening of NaV1.5 channels induces membrane depolarization which facilitates opening of L-type calcium channels and subsequent calcium-induced calcium release from the sarcoplasmic reticulum (SR). As a consequence, the cytosolic Ca2+ concentration ([Ca2+]i) increases which is essential for excitation–contraction coupling. Following the upstroke of the action potential Na+ ions will be pumped out of the cell by the Na+/Ca2+-exchanger (NCX) acting in reverse mode exchanging 3 Na+ for 1 Ca2+. Rise in cytosolic Ca2+ concentration ([Ca2+]i) due to Ca2+ release from the SR will force the NCX in the forward mode pumping 1 Ca2+ out and 3 Na+ in the cell. The [Na+]i will return to its normal level in diastole due to activity of the sodium potassium pump, Na+/K+-ATPase, which exchanges 3 Na+ ions for 2 K+. Any condition leading to increased diastolic [Na+]i will in turn, enhance reverse mode activity of the NCX, consequently resulting in increased diastolic [Ca2+]i.107 Indeed, enhanced INa,L in the setting of gain of function SCN5A mutations or heart failure has been shown to increase both [Na+]i and [Ca2+]i in ventricular cardiomyocytes.108,109 Deletion of the NaV1.5 accessory subunit β1 also led to increase in tetrodotoxin (TTX)-sensitive sodium current and disrupted calcium homeostasis.110 Transcriptional regulation of calcium-handling genes was observed in Scn5a-N1325S mice,111 suggesting a link between sodium channel activity and calcium-dependent transcriptional pathways. Moreover, elevated diastolic [Ca2+]i affects gene expression by activation of signalling pathways (discussed in the next paragraph) but also increases SR Ca2+ concentration ([Ca2+]SR). Both elevated [Ca2+]i and [Ca2+]SR increase the open probability of the ryanodine receptors causing calcium aftertransients leading to delayed afterdepolarizations, which may trigger an action potential and initiate arrhythmias. Elevated diastolic [Ca2+]i may also impair cardiomyocyte relaxation, leading to diastolic dysfunction, and additionally decrease L-type Ca2+ current. Interestingly, high Ca2+ concentrations have been shown to prolong AV-conduction in isolated rabbit hearts.112 Hence, it may be speculated that elevated diastolic [Ca2+]i reduces L-type Ca2+ current in transitional cells surrounding the sinus node and AVN (where SCN5A and NaV1.5 are present at low levels), thereby affecting action potential upstroke and contributing to the phenotype of the bradycardia and prolonged PR interval often observed in patients with SCN5A mutations.11,71 Thus, these indirect, ionic effects of NaV1.5 dysfunction acting through Na+ and Ca2+ homeostasis have important consequences for AV-conduction, pro-arrhythmia, and possibly mechanical dysfunction (see Section 5). In addition, DCM-related mutations (such as SCN5A-R219H) have been shown to cause a proton leak current, suggesting that intracellular acidification may contribute to the cardiomyopathy (and arrhythmias) observed in mutation carriers.67
4.2.2. Effects of NaV1.5 (dys)function on calcium-dependent signalling pathways
While increased [Ca2+]i is detrimental to the contractile function of myocytes and the development of arrhythmias, enhanced calcium signalling can also have unfavourable consequences in the long run. General or local increases in [Ca2+]i in the cardiomyocyte are predominantly sensed by calmodulin, a ubiquitous calcium sensor. Ca2+-calmodulin can activate, for example, CaMKII and protein phosphatase calcineurin (CaN). Both the CaMKII and the CaN signalling pathways control gene transcription leading to the development of cardiac hypertrophy and fibrosis, via histone deacetylases and the nuclear factor of activated T cells, respectively, and this process is referred to as ‘excitation–transcription coupling’.113,114 Both CaMKII and CaN pathways are activated in heart failure due to elevated [Ca2+]i. The cause of elevated diastolic [Ca2+]i in heart failure is multifactorial and may involve INa, ICaL, NCX, SR Ca-ATPase, ryanodine receptors, and other factors.115 In this setting, the indirect ionic effect of the sodium channel may be mediated by INa,L which is increased in heart failure.22,116 Calcium-activated CaMKII may in turn feedback on the sodium channel and regulate its (in)direct functions as has been shown in the setting of heart failure.117,118 CaMKII phosphorylates NaV1.5 at several residues in the DI-DII loop,119 and CaMKII-mediated phosphorylation of NaV1.5 at Ser571 increases INa,L.120 On the other hand, phosphorylation of the Thr594 and Ser516 residues lead to a loss of function phenotype as seen in BrS.121 The C-terminus of NaV1.5 harbours an EF-hand Ca2+ binding site. The EF-hand interacts with the IQ motif [binding site for calmodulin (CaM)] and the DIII–DIV linker to control inactivation of the channel.122,123 Therefore, increases in [Ca2+]i may not only activate calcium-dependent (pro-hypertrophic) signalling pathways, but also directly feedback on NaV1.5 either directly or via CaM/CaMKII and contribute to arrhythmogenesis. Finally, dysregulated intracellular Na+ and Ca2+ homeostasis may also affect mitochondrial (dys)function,124 potentially leading to pro-arrhythmic ROS production.125
4.2.3. Evidence from cells other than cardiomyocytes
Sodium and calcium signalling pathways have much broader targets than the ones described above and interest in sodium channels in non-excitable cells has yielded precious information on other indirect ionic functions of the sodium channel. NaV1.5 channels are also expressed in non-excitable cells where they participate in diverse cell functions.126 In CD4+ CD8+ double positive (DP) thymocytes, stage-specific expression of SCN5A controls positive selection (maturation of thymocytes into T cells).127 TTX treatment or shRNA knockdown prevented positive selection of DP thymocytes, by decreasing the secondary sustained Ca2+ flux. Indirect ionic functions of sodium channels can also be achieved when not expressed at the plasma membrane. NaV1.5 is located intracellularly at the membrane of late endosomes in human monocyte-derived macrophages.128 Phagocytosis was inhibited by 10 µM TTX in these cells which blocked the sodium current generated by NaV1.5 controlling acidification of late endosomes during this process.128 Sodium channels are furthermore known to play a crucial role in tumour activity and metastasis, with NaV1.5 being most relevant in breast cancer, colon cancer, and ovary cancer.126 Increased expression of NaV1.5 in these tumours is associated with more aggressive properties, including invasion of the lymph nodes and recurrence of metastasis.129,130 Indirect ionic mechanisms of NaV1.5 are thought to be involved in this process.129 Increased INa, through its effect on the sodium–hydrogen exchanger, induces acidification of the extracellular environment, which increases cathepsin activity, invadopodial formation, and extracellular matrix degeneration, and thus facilitates tumour invasion.129,131 Another mechanism by which altered NaV1.5 function may affect tumour behaviour is through its effects on calcium-dependent SNARE-mediated vesicle fusion. Here, increased [Ca2+]i levels (secondary to enhanced INa,L) may stimulate podosome activity and increase motility of the tumour cell, thus contributing to cellular invasion.129,132 Indeed, in MDA-MB-231 breast cancer cells, NaV1.5 was shown to generate an INa,L that could be inhibited by 30 µM TTX.130 Reduction of either the peak or late sodium current decreased invasiveness of cancer cells, while increasing INa,L promoted it. Other studies have confirmed the beneficial effects of (late) sodium current inhibition on tumour growth, invasion, proliferation, and metastasis.133,134 These observations clearly demonstrate that voltage-gated sodium channels are capable of controlling diverse cell functions in non-excitable cells such as activation of calcium signalling pathways and local acidification. Mechanistic insight drawn from these studies may facilitate further exploration of indirect ionic effects of NaV1.5 in cardiomyocytes.
4.3 Non-ionic effects of NaV1.5 (dys)function
4.3.1. Non-ionic function of NaV1.5 during cardiac development
Non-ionic functions of cardiac sodium channels are related to effects mediated by the NaV1.5 protein itself or the protein complexes it is associated with, independent of the influx of sodium ions through the channel pore. Evidence for such non-ionic function of NaV1.5 in the heart was first demonstrated during cardiac development. NaV1.5 is expressed from embryonic day 9.5 (ED9.5) onward in the working myocardium of the murine embryonic ventricle.135 Mice with homozygous deficiency of NaV1.5 die in utero around ED9.5 showing ventricular malformation including absence of trabeculae.71,135,136 Whether ionic and/or non-ionic functions of NaV1.5 are involved here is as yet unclear. However, one could speculate that a role for direct ionic actions of NaV1.5 is less likely since cardiomyocyte membrane potentials during early development are relatively depolarized, leaving NaV1.5-based channels mostly inactivated and cardiac electrical activity driven predominantly by calcium channels.137 A key study in zebrafish embryos showed that knockdown of Scn5a led to decreased expression of the myocardial precursor genes Nkx2-5, Gata4, and Hand2, resulting in impaired cardiac development and cardiomyocyte proliferation. Crucially, abnormalities in cardiac development only occurred when the NaV1.5 protein was absent but not when INa was pharmacologically inhibited, indicating that the direct ionic function of NaV1.5 was not involved.138 Although these data clearly point to a role for non-ionic function of NaV1.5 in ventricular malformation and embryonic death, the underlying mechanism requires further investigation, in addition to the potential relevance of the embryonic Scn5a isoform in this process.
4.3.2. Actions of NaV1.5 through its macromolecular complex
As described above, NaV1.5 forms part of a macromolecular complex where it interacts with a variety of other proteins. While it is clear that these interacting proteins modulate NaV1.5 function, it is as yet unknown if the opposite is also true, i.e. whether NaV1.5 (dys)function also impacts on other components of the macromolecular complex. Through this complex, NaV1.5 may directly or indirectly interact with numerous proteins involved in cytoskeletal anchoring, signal transduction, and cell adhesion, including dystrophin, laminin, integrins, and components of the extracellular matrix. For example, NaV1.5 binds to fibroblast growth factor homologous factor (FHF); since the latter has been shown to interact with Cx43139 (located at the ID) and with the L-type calcium channel140 (located at the T-tubules in the LM), one could speculate that mutation-induced alterations in NaV1.5-FHF binding could lead to various (non-)ionic consequences, which may be microdomain-dependent.141 The recently demonstrated interaction and co-regulation of NaV1.5 and potassium channels provides additional pathways by which NaV1.5 can indirectly affect cardiomyocyte (electrical) function.142,143 It is not yet clear whether NaV1.5 dysfunction disrupts stability of the macromolecular complexes, thereby potentially affecting cell structure and integrity. If so, the consequences could be numerous and diverse, including alterations in mechanical function and development of cardiac structural abnormalities. Importantly, given the fact that the composition of the macromolecular complex differs between LM and ID, such non-ionic effects of NaV1.5 are likely subdomain-specific. During heart failure, for example, NaV1.5 is differentially regulated in the different cardiomyocyte microdomains.144 Hence, NaV1.5 dysfunction could theoretically lead to disruption of the dystrophin–syntrophin complex and caveolin-3 at the LM, and of desmosomal proteins and connexins at the ID. Indeed, evidence is emerging which points towards a secondary effect of NaV1.5 dysfunction on ID remodelling.
4.3.3. Non-ionic function of NaV1.5 at the ID
Strong evidence for a non-ionic action of NaV1.5 comes from its function at the ID, where it has a potential role in cell adhesion. A large body of work identifies NaV1.5 as part of a larger complex at the ID, including N-cadherin, PKP2, β1, and connexin 43 clustered together and regulating each other to keep the integrity of cell–cell communication.8–10,145,146 Loss of NaV1.5 has been shown to compromise the size and density of N-cadherin clusters at the ID membrane thereby altering adhesion of neighbouring cardiomyocytes, which, in turn, reduces peak INa.35 N-cadherin clusters are anchoring points for the microtubule network.9,147 Hence, by indirectly altering N-cadherin integrity at the ID, sodium channel deficiency may disturb trafficking of molecules along microtubules to the ID. Evidence for a role of NaV1.5 in cell adhesion is further demonstrated by studies in ACM patients and mouse models of ACM. First, it has been shown that PKP2-heterozygous mice show decreased sodium current and a widening of the inter-cellular space at the ID.10 This was seconded by the observation that many human variants in PKP2 lead to reduced sodium current.9 Finally, in a recent study the SCN5A-R1898H variant was identified in an ARVC patient. In vitro analysis of the consequences of the mutation showed reduced abundance of NaV1.5 (and reduced sodium current) and N-cadherin at the ID thereby compromising cell adhesion.8 NaV1.5 has been identified in an additional signalling ID complex including ankyrin-G, βIV-spectrin, and CaMKII.148 Further studies in neurons have shown that ankyrin-G is essential for clustering sodium channels at the membrane where they exert their function.149 Thus, non-ionic effects of NaV1.5 dysfunction not only modulate electrophysiological properties of the myocardium, but may also affect cardiac structure and integrity, thereby further predisposing to arrhythmias. From these observations, one may argue that clinical management of sodium channelopathies may benefit from targeting not only NaV1.5, but also other components of its macromolecular complex.
4.3.4. Non-ionic function of NaV1.5 in non-excitable cells
As indicated above, increased expression of NaV1.5 in tumours is associated with more aggressive properties, including tissue invasion and metastasis.126,131 In addition to pro-metastatic pathways induced by alterations in intracellular calcium homeostasis, non-ionic mechanisms may also be involved but remain as yet unexplored. Through its interaction with both cytoskeletal and ID proteins, alterations in NaV1.5 may impact on (intracellular) communication, cell organization, and morphology. A similar role in tumour invasion has already been demonstrated for the accessory β subunits, which additionally function as cell adhesion molecules. In particular, β1 expression in tumour cells has been shown to modulate their capacity for adhesion, migration, and invasion, while effects on angiogenesis have also been reported.150 A functional role for NaV1.5 in gastrointestinal function has also been demonstrated, and SCN5A mutations have been linked to gut motility disorders such as irritable bowel syndrome.151 Although alterations in excitability likely play a crucial role in mediating smooth muscle cell motility, it would be interesting to see whether non-ionic mechanisms are also involved.
5. (Non-)ionic consequences of NaV1.5 dysfunction: clinical and therapeutic considerations
The majority of disease characteristics associated with NaV1.5 dysfunction are clearly the consequence of the electrophysiological defects of the channel leading to conduction slowing, repolarization abnormalities, and consequent arrhythmias. However, it is now increasingly recognized that SCN5A mutations may also lead to the development of cardiac fibrosis, dilatation, and hypertrophy, which cannot be readily explained by the sole electrical malfunction of NaV1.5 and that these diverse disease entities may co-exist with electrical abnormalities.6,7 BrS patients carrying loss of function SCN5A mutations may suffer myocardium remodelling including right ventricular hypertrophy, fibrosis, epicardial fatty infiltration, and myocyte cytoplasm degeneration.4,5 As mentioned in Section 2, DCM has been observed in patients with SCN5A mutations,3 often in combination with AF.7 Strikingly, there appears to be an age-dependent development of disease severity suggesting that structural changes develop secondary to sodium channel dysfunction and might manifest later in life. Slow and gradual development of structural abnormalities within the myocardium will influence the genesis and severity of arrhythmias. This could explain in part why the onset of disease is relatively late (mid 30s–40s) in patients carrying SCN5A mutations linked to BrS.152 These observations are seconded by results obtained from mouse studies. In mice heterozygous for Scn5a (Scn5a+/−), cardiac fibrosis developed with age, and the occurrence and severity of ventricular arrhythmias correlated with the extent of structural abnormalities developed in the myocardium with ageing.153,154 In the Scn5a1798insD/+ mouse model, arrhythmic events and sudden cardiac death were more frequent with age and development of cardiac hypertrophy.155 In addition, mice overexpressing the LQTS mutation Scn5a-N1325S developed extensive myocardial fibrosis at relatively advanced age (6–10 months) as well as loss of cardiomyocytes due to apoptosis.156 Although it is not completely clear how a mutation in SCN5A leads to structural changes in the myocardium, we have here proposed a number of mechanisms, involving both (in)direct ionic and non-ionic roles of NaV1.5. In the case of loss of function, as seen in ARVC/ACM, the physical disassociation of NaV1.5 from structural proteins may create structural abnormalities, indicating a role for non-ionic actions of NaV1.5. In the case of gain of function mutations in SCN5A or acquired diseases leading to enhanced INa,L, increases in intracellular calcium may, in addition to providing an arrhythmic substrate, activate calcium-dependent signalling pathways and lead to the development of fibrosis.108,109
In addition to the potential mechanisms described above, electrical activity-dependent stimulation of pro-fibrotic factors of the transforming growth factor β pathway has been described in the setting of sodium channel dysfunction.157 Moreover, de novo expression of NaV1.5 in myofibroblasts could contribute to direct ionic function by coupling myofibroblasts to cardiomyocytes, but also to an indirect ionic effect by activating fibrotic signalling pathways.158 Irrespective of the underlying mechanisms, i.e. gain or loss of function, and ionic vs. non-ionic, development of structural cardiac abnormalities may increase the likelihood of arrhythmias by itself, and by acting in concert with the electrophysiological alterations induced by the mutation. Overall, these subtle structural abnormalities are generally not detectable on non-invasive examinations, which render the correlation with indirect ionic and non-ionic effects of NaV1.5 dysfunction challenging. However, an echocardiography study in SCN5A-1795insD mutation carriers revealed ventricular diastolic dysfunction in a number of patients, confirming the potential clinical relevance and opportunities for continued monitoring of ventricular function and identification of structural changes during follow-up.45 Crucially, this may also allow the detection of potential switches in arrhythmogenic substrate from a direct ionic to indirect or non-ionic effects with age. Here, it is important to realize that co-development of structural changes commonly observed in older individuals, such as fibrosis and cardiac hypertrophy secondary to hypertension may further increase arrhythmia risk in SCN5A mutation carriers.155 Similarly, the presence of co-morbidities associated with calcium abnormalities, enhanced late sodium current, metabolic dysregulation, and increased mitochondrial ROS production (e.g. heart failure, diabetes mellitus, obesity)159,160 may further enhance calcium-dependent pro-arrhythmic events, particularly in the setting of gain of function SCN5A mutation. Clearly, these considerations may have significant consequences for disease outcome and patient management, making it essential to further unravel the ionic and non-ionic indirect consequences of NaV1.5 dysfunction.
As yet, therapeutic options for patients with SCN5A mutations are limited. In some cases, loss of sodium channel function may be targeted by certain sodium channel blockers (for instance mexiletine) which can restore membrane expression of trafficking defective sodium channels.161 This, however, does not constitute an attractive therapeutic approach for clinical practice due to the potential deleterious effects of INa reduction. Other approaches to safely enhance NaV1.5 trafficking and/or function are currently under investigation. Meanwhile, options to prevent the detrimental consequences of loss of NaV1.5 function may be further explored, including for instance anti-fibrotic therapy. In the case of gain of function SCN5A mutations, current therapy is predominantly aimed at preventing arrhythmia triggers (e.g. beta-blockade, pacemaker implantation), although pharmacological INa,L inhibition is increasingly recognized as an attractive therapeutic target.51,162 In addition to normalizing repolarization, INa,L inhibition may also be beneficial by restoring pro-arrhythmic intracellular calcium dysregulation, decreasing diastolic dysfunction, and preventing activation of calcium-dependent pro-hypertrophic signalling pathways.109,155,163 Therapies aimed at improving cardiomyocyte metabolism and mitochondrial function (e.g. ROS scavengers) may also prove of benefit in these patients. Thus, preventing the development of cardiac structural defects and metabolic derangements may prove a valuable way of reducing risk for arrhythmias and sudden cardiac death in patients with sodium channel dysfunction.
6. Conclusions
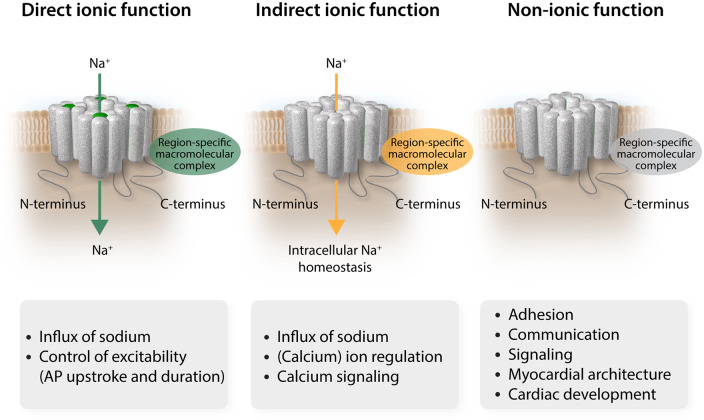
In the past two decades, clinical and genetic research combined with basic studies employing patch-clamp and nanometre precision techniques have enabled colossal advances in our understanding of sodium channel (dys)function. These investigations have provided insight into the relation between the structure of the channel, the structure of the channel interacting with its protein partners, and the effects of mutations on its function. More recently, studies have focused on the diversity and complexity of sodium channel localization, composition, and regulation, in addition to the multifunctionality of the sodium channel complex. Increasing evidence now points to sodium channel functions other than the classically well-described electrical effects secondary to influx of sodium ions into the cardiomyocyte, which may explain the observed phenotypic diversity observed in SCN5A mutation carriers (Figure 4). Based on the evidence we presented, we propose that sodium channel (dys)function and its consequences may now be categorized into (i) direct ionic effects of sodium influx through NaV1.5 on membrane potential and consequent action potential generation, (ii) indirect ionic effects of sodium influx on intracellular homeostasis and signalling, and (iii) non-ionic effects of NaV1.5, independent of sodium influx, through interactions with macromolecular complexes within the different microdomains of the cardiomyocyte. These indirect ionic and non-ionic processes may, acting alone or in concert, contribute significantly to arrhythmogenesis in inherited sodium channel (dys)function. Hence, further exploration of these multifunctional effects of NaV1.5 will be essential for the development of novel therapeutic strategies aimed at preventing arrhythmias and sudden cardiac death not only in inherited syndromes but also in acquired diseases such as myocardial ischaemia and heart failure. Given the rapid development of highly innovative techniques, potential novel targets are expected to be identified in the (near) future.
Figure 4.
Novel classification of NaV1.5 (dys)function. The direct ionic function controls excitability by modification of membrane potential and initiation of AP. The indirect ionic function regulates calcium levels and downstream calcium signalling. The non-ionic function relies on the integrity of region-specific macromolecular complexes to control cell adhesion, cell–cell communication, signalling (other than calcium), myocardium architecture (extracellular matrix), and developmental aspects. AP, action potentials.
Data availability
No new data were generated or analysed in support of this research.
Conflict of interest: none declared.
Funding
This work was funded by an Innovational Research Incentives Scheme Vidi grant from the Netherlands Organisation for Health Research and Development (ZonMw; 91714371 to C.A.R.); the Dutch Heart Foundation (NHS2010/B201 to C.A.R.); the Netherlands CardioVascular Research Initiative CVON (Dutch Heart Foundation, Dutch Federation of University Medical Centres, ZonMw, and the Royal Netherlands Academy of Sciences; projects CVON2018-30 PREDICT2 and CVON2015-12 e-DETECT to C.A.R.); and Fondation Leducq Transatlantic Network of Excellence grants (17CVD02 to C.A.R. and M.D.; 16CVD02 RHYTHM, M.R.R.).
References
- 1. Remme CA, Bezzina CR.. Review: sodium channel (dys)function and cardiac arrhythmias. Cardiovasc Ther 2010;28:287–294. [DOI] [PubMed] [Google Scholar]
- 2. Ruan Y, Liu N, Priori SG.. Sodium channel mutations and arrhythmias. Nat Rev Cardiol 2009;6:337–348. [DOI] [PubMed] [Google Scholar]
- 3. Bezzina CR, Rook MB, Groenewegen WA, Herfst LJ, van der Wal AC, Lam J, Jongsma HJ, Wilde AAM, Mannens M.. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ Res 2003;92:159–168. [DOI] [PubMed] [Google Scholar]
- 4. Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJG, Verkerk AO, De Groot JR, Bhuiyan Z, Bezzina CR, Veldkamp MW, Linnenbank AC, Van Der Wal AC, Tan HL, Brugada P, Wilde AAM, De Bakker JMT.. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 2005;112:2769–2777. [DOI] [PubMed] [Google Scholar]
- 5. Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, Sanna T, Bellocci F, Russo MA.. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation 2005;112:3680–3687. [DOI] [PubMed] [Google Scholar]
- 6. Olson TM, Keating MT.. Mapping a cardiomyopathy locus to chromosome 3p22-p25. J Clin Invest 1996;97:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL.. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005;293:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Te Riele ASJM, Agullo-Pascual E, James CA, Leo-Macias A, Cerrone M, Zhang M, Lin X, Lin B, Rothenberg E, Sobreira NL, Amat-Alarcon N, Marsman RF, Murray B, Tichnell C, van der Heijden JF, Dooijes D, van Veen TAB, Tandri H, Fowler SJ, Hauer RNW, Tomaselli G, van den Berg MP, Taylor MRG, Brun F, Sinagra G, Wilde AAM, Mestroni L, Bezzina CR, Calkins H, Peter van Tintelen J, Bu L, Delmar M, Judge DP.. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc Res 2017;113:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, Rothenberg E, Chen HSV, Napolitano C, Priori SG, Delmar M.. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014;129:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, Van Der Nagel R, Hund T, Birchmeier W, Mohler P, Van Veen TA, Van Rijen HV, Delmar M.. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 2012;95:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bezzina C, Veldkamp MW, van den Berg MP, Postma AV, Rook MB, Viersma J-W, van Langen IM, Tan-Sindhunata G, Bink-Boelkens MTE, van der Hout AH, Mannens MMAM, Wilde AAM.. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res 1999;85:1206–1213. [DOI] [PubMed] [Google Scholar]
- 12. Veltmann C, Barajas-Martinez H, Wolpert C, Borggrefe M, Schimpf R, Pfeiffer R, Caceres G, Burashnikov E, Antzelevitch C, Hu D.. Further insights in the most common SCN5A mutation causing overlapping phenotype of long QT syndrome, Brugada syndrome, and conduction defect. J Am Heart Assoc 2016;5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev 1992;72:S15–S48. [DOI] [PubMed] [Google Scholar]
- 14. Sato C, Sato M, Iwasaki A, Doi T, Engel A.. The sodium channel has four domains surrounding a central pore. J Struct Biol 1998;121:314–325. [DOI] [PubMed] [Google Scholar]
- 15. Whalley DW, Wendt DJ, Starmer CF, Rudy Y, Grant AO.. Voltage-independent effects of extracellular K+ on the Na+ current and phase 0 of the action potential in isolated cardiac myocytes. Circ Res 1994;75:491–502. [DOI] [PubMed] [Google Scholar]
- 16. Kass RS. Sodium channel inactivation in heart: a novel role of the carboxy-terminal domain. J Cardiovasc Electrophysiol 2006;17:S21–S25. [DOI] [PubMed] [Google Scholar]
- 17. Casini S, Tan HL, Bhuiyan ZA, Bezzina CR, Barnett P, Cerbai E, Mugelli A, Wilde AAM, Veldkamp MW.. Characterization of a novel SCN5A mutation associated with Brugada syndrome reveals involvement of DIIIS4-S5 linker in slow inactivation. Cardiovasc Res 2007;76:418–429. [DOI] [PubMed] [Google Scholar]
- 18. Maltsev VA, Sabbah HN, Higgins RSD, Silverman N, Lesch M, Undrovinas AI.. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 1998;98:2545–2552. [DOI] [PubMed] [Google Scholar]
- 19. Kiyosue T, Arita M.. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res 1989;64:389–397. [DOI] [PubMed] [Google Scholar]
- 20. Undrovinas AI, Fleidervish IA, Makielski JC.. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res 1992;71:1231–1241. [DOI] [PubMed] [Google Scholar]
- 21. Song Y, Belardinelli L.. Basal late sodium current is a significant contributor to the duration of action potential of guinea pig ventricular myocytes. Physiol Rep 2017;5:e13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI.. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 2007;9:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang D, Shi H, Tonggu L, Gamal El-Din TM, Lenaeus MJ, Zhao Y, Yoshioka C, Zheng N, Catterall WA.. Structure of the cardiac sodium channel. Cell 2020;180:122–134.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kléber AG, Janse MJ, Fast VG.. Normal and abnormal conduction in the heart. Handbook of Physiology. Section 2: The Cardiovascular System Oxford University Press; 2001. p. 455–530. [Google Scholar]
- 25. Boukens BJ, Christoffels VM.. Electrophysiological patterning of the heart. Pediatr Cardiol 2012;33:900–906. [DOI] [PubMed] [Google Scholar]
- 26. Remme CA, Verkerk AO, Hoogaars WMH, Aanhaanen WTJ, Scicluna BP, Annink C, den Hoff MJB, Wilde AAM, Veen TAB, Veldkamp MW, Bakker JMT, Christoffels VM, Bezzina CR.. The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium. Basic Res Cardiol 2009;104:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lei M, Goddard C, Liu J, Leoni AL, Royer A, Fung SS, Xiao G, Ma A, Zhang H, Charpentier F, Vandenberg JI, Colledge WH, Grace AA, Huang CL.. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 2005;567:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lei M, Jones SA, Liu J, Lancaster MK, Fung SS-M, Dobrzynski H, Camelliti P, Maier SKG, Noble D, Boyett MR.. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol 2004;559:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrecca K, Amellal F, Laird DW, Cohen SA, Shrier A.. Sodium channel distribution within the rabbit atrioventricular node as analysed by confocal microscopy. J Physiol 1997;501:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoo S, Dobrzynski H, Fedorov VV, Xu SZ, Yamanushi TT, Jones SA, Yamamoto M, Nikolski VP, Efimov IR, Boyett MR.. Localization of Na+ channel isoforms at the atrioventricular junction and atrioventricular node in the rat. Circulation 2006;114:1360–1371. [DOI] [PubMed] [Google Scholar]
- 31. Greener ID, Tellez JO, Dobrzynski H, Yamamoto M, Graham GM, Billeter R, Boyett MR.. Ion channel transcript expression at the rabbit atrioventricular conduction axis. Circ Arrhythm Electrophysiol 2009;2:305–315. [DOI] [PubMed] [Google Scholar]
- 32. Liu GX, Remme CA, Boukens BJ, Belardinelli L, Rajamani S.. Overexpression of SCN5A in mouse heart mimics human syndrome of enhanced atrioventricular nodal conduction. Hear Rhythm 2015;12:1036–1045. [DOI] [PubMed] [Google Scholar]
- 33. Kaufmann SG, Westenbroek RE, Maass AH, Lange V, Renner A, Wischmeyer E, Bonz A, Muck J, Ertl G, Catterall WA, Scheuer T, Maier S.. Distribution and function of sodium channel subtypes in human atrial myocardium. J Mol Cell Cardiol 2013;61:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boukens BJ, Sylva M, Vries C de G-D, Remme CA, Bezzina CR, Christoffels VM, Coronel R.. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res 2013;113:137–141. [DOI] [PubMed] [Google Scholar]
- 35. Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng-Xia-Liang Korchev YE, Gorelik J, Fenyö D, Rothenberg E, Delmar M.. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun 2016;7:10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin X, Liu N, Lu J, Zhang J, Anumonwo JMB, Isom LL, Fishman GI, Delmar M.. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Hear Rhythm 2011;8:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsman RFJ, Bezzina CR, Freiberg F, Verkerk AO, Adriaens ME, Podliesna S, Chen C, Purfürst B, Spallek B, Koopmann TT, Baczko I, Dos Remedios CG, George AL, Bishopric NH, Lodder EM, de Bakker JMT, Fischer R, Coronel R, Wilde AAM, Gotthardt M, Remme CA.. Coxsackie and adenovirus receptor is a modifier of cardiac conduction and arrhythmia vulnerability in the setting of myocardial ischemia. J Am Coll Cardiol 2014;63:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhargava A, Lin X, Novak P, Mehta K, Korchev Y, Delmar M, Gorelik J.. Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte. Circ Res 2013;112:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rougier J-S, Essers MC, Gillet L, Guichard S, Sonntag S, Shmerling D, Abriel H.. A distinct pool of Nav1.5 channels at the lateral membrane of murine ventricular cardiomyocytes. Front Physiol 2019;10:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shy D, Gillet L, Abriel H.. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta 2013;1833:886–894. [DOI] [PubMed] [Google Scholar]
- 41. Brackenbury WJ, Isom LL.. Na channel β subunits: overachievers of the ion channel family. Front Pharmacol 2011;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maier SKG, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA.. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 2004;109:1421–1427. [DOI] [PubMed] [Google Scholar]
- 43. Kyle JW, Makielski JC.. Diseases caused by mutations in Nav1.5 interacting proteins. Card Electrophysiol Clin 2014;6:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Remme CA, Wilde A.. Late sodium current inhibition in acquired and inherited ventricular (dys)function and arrhythmias. Cardiovasc Drugs Ther 2013;27:91–101. [DOI] [PubMed] [Google Scholar]
- 45. Hummel YM, Wilde AAM, Voors AA, Bugatti S, Hillege HL, van den Berg MP.. Ventricular dysfunction in a family with long QT syndrome type 3. Europace 2013;15:1516–1521. [DOI] [PubMed] [Google Scholar]
- 46. Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde A, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R.. Genotype-phenotype correlation in the long-QT syndrome. Circulation 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 47. Schwartz PJ. The congenital long QT syndromes from genotype to phenotype: clinical implications. J Intern Med 2006;259:39–47. [DOI] [PubMed] [Google Scholar]
- 48. Zareba W, Sattari MN, Rosero S, Couderc JP, Moss AJ.. Altered atrial, atrioventricular, and ventricular conduction in patients with the long QT syndrome caused by the DeltaKPQ SCN5A sodium channel gene mutation. Am J Cardiol 2001;88:1311–1314. [DOI] [PubMed] [Google Scholar]
- 49. Bennett PB, Yazawa K, Makita N, George AL.. Molecular mechanism for an inherited cardiac arrhythmia. Nature 1995;376:683–685. [DOI] [PubMed] [Google Scholar]
- 50. Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P, Napolitano C, Priori SG, Kass RS.. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem 2001;276:30623–30630. [DOI] [PubMed] [Google Scholar]
- 51. Wilde AAM, Remme CA.. Therapeutic approaches for long QT syndrome type 3: an update. Europace 2018;20:222–224. [DOI] [PubMed] [Google Scholar]
- 52. Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol 2006;29:1130–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tan H, Bezzina C, Smits J, Verkerk A, Wilde A.. Genetic control of sodium channel function. Cardiovasc Res 2003;57:961–973. [DOI] [PubMed] [Google Scholar]
- 54. Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AAM, Brugada R, Schott J-J, Ackerman MJ.. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Hear Rhythm 2010;7:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoogendijk MG, Potse M, Linnenbank AC, Verkerk AO, den Ruijter HM, van Amersfoorth SCM, Klaver EC, Beekman L, Bezzina CR, Postema PG, Tan HL, Reimer AG, van der Wal AC, Ten Harkel ADJ, Dalinghaus M, Vinet A, Wilde AAM, de Bakker JMT, Coronel R.. Mechanism of right precordial ST-segment elevation in structural heart disease: excitation failure by current-to-load mismatch. Hear Rhythm 2010;7:238–248. [DOI] [PubMed] [Google Scholar]
- 56. Belhassen B, Viskin S.. Pharmacologic approach to therapy of Brugada syndrome: quinidine as an alternative to ICD therapy In Antzelevitch C, Brugada P, Brugada J, Brugada R (eds). The Brugada Syndrome: From Bench to Bedside. Oxford: Blackwell Futura; 2004. pp. 202–211. [Google Scholar]
- 57. Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, Wilde AA, Escande D, Mannens MM, Le Marec H.. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet 1999;23:20–21. [DOI] [PubMed] [Google Scholar]
- 58. Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL.. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 2003;112:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smits JPP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, Bhuiyan ZA, Mannens M, Balser JR, Tan HL, Bezzina CR, Wilde A.. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J Mol Cell Cardiol 2005;38:969–981. [DOI] [PubMed] [Google Scholar]
- 60. Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Y, Ohno S, Nishio Y, Tsuji K, Itoh H, Kimura T, Kita T, Horie M.. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol 2008;52:1326–1334. [DOI] [PubMed] [Google Scholar]
- 61. Li Q, Huang H, Liu G, Lam K, Rutberg J, Green MS, Birnie DH, Lemery R, Chahine M, Gollob MH.. Gain-of-function mutation of Nav1.5 in atrial fibrillation enhances cellular excitability and lowers the threshold for action potential firing. Biochem Biophys Res Commun 2009;380:132–137. [DOI] [PubMed] [Google Scholar]
- 62. McNair WP, Sinagra G, Taylor MRG, Lenarda A, Di Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L; Familial Cardiomyopathy Registry Research Group. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 2011;57:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mazzaccara C, Limongelli G, Petretta M, Vastarella R, Pacileo G, Bonaduce D, Salvatore F, Frisso G.. A common polymorphism in the SCN5A gene is associated with dilated cardiomyopathy. J Cardiovasc Med 2018;19:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ge J, Sun A, Paajanen V, Wang S, Su C, Yang Z, Li Y, Wang S, Jia J, Wang K, Zou Y, Gao L, Wang K, Fan Z.. Molecular and clinical characterization of a novel SCN5A mutation associated with atrioventricular block and dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2008;1:83–92. [DOI] [PubMed] [Google Scholar]
- 65. Nguyen TP, Wang DW, Rhodes TH, George AL.. Divergent biophysical defects caused by mutant sodium channels in dilated cardiomyopathy with arrhythmia. Circ Res 2008;102:364–371. [DOI] [PubMed] [Google Scholar]
- 66. Bezzina CR, Remme CA.. Dilated cardiomyopathy due to sodium channel dysfunction: what is the connection? Circ Arrhythm Electrophysiol 2008;1:80–82. [DOI] [PubMed] [Google Scholar]
- 67. Gosselin-Badaroudine P, Keller DI, Huang H, Pouliot V, Chatelier A, Osswald S, Brink M, Chahine M.. A proton leak current through the cardiac sodium channel is linked to mixed arrhythmia and the dilated cardiomyopathy phenotype. PLoS One 2012;7:e38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berg MP, Wilde AAM, Viersma JW, Brouwer JAN, Haaksma J, Hout AH, Stolte-Dijkstra I, Bezzina CR, Langen IM, Beaufort-Krol GCM, Cornel JH, Crijns HJGM.. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol 2001;12:630–636. [DOI] [PubMed] [Google Scholar]
- 69. Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, Priori S.. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest 2002;110:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Makita N, Behr E, Shimizu W, Horie M, Sunami A, Crotti L, Schulze-Bahr E, Fukuhara S, Mochizuki N, Makiyama T, Itoh H, Christiansen M, McKeown P, Miyamoto K, Kamakura S, Tsutsui H, Schwartz PJ, George AL, Roden DM.. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest 2008;118:2219–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Remme CA, Verkerk AO, Nuyens D, van Ginneken ACG, van Brunschot S, Belterman CNW, Wilders R, van Roon MA, Tan HL, Wilde AAM, Carmeliet P, de Bakker JMT, Veldkamp MW, Bezzina CR.. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation 2006;114:2584–2594. [DOI] [PubMed] [Google Scholar]
- 72. Calkins H, Corrado D, Marcus F.. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017;136:2068–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rizzo S, Lodder EM, Verkerk AO, Wolswinkel R, Beekman L, Pilichou K, Basso C, Remme CA, Thiene G, Bezzina CR.. Intercalated disc abnormalities, reduced Na+ current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012;95:409–418. [DOI] [PubMed] [Google Scholar]
- 74. Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patiño GA, Taffet SM, Isom LL, Delmar M.. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res 2009;105:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu J, Hu J, Dai X, Cao Q, Xiong Q, Liu X, Liu X, Shen Y, Chen Q, Hua W, Hong K.. SCN5A mutation in Chinese patients with arrhythmogenic right ventricular dysplasia. Herz 2014;39:271–275. [DOI] [PubMed] [Google Scholar]
- 76. Viswanathan PC, Balser JR.. Inherited sodium channelopathies a continuum of channel dysfunction. Trends Cardiovasc Med 2004;14:28–35. [DOI] [PubMed] [Google Scholar]
- 77. Wedekind H, Smits JPP, Schulze-Bahr E, Arnold R, Veldkamp MW, Bajanowski T, Borggrefe M, Brinkmann B, Warnecke I, Funke H, Bhuiyan ZA, Wilde AAM, Breithardt G, Haverkamp W.. De novo mutation in the SCN5A gene associated with early onset of sudden infant death. Circulation 2001;104:1158–1164. [DOI] [PubMed] [Google Scholar]
- 78. Clancy CE, Tateyama M, Liu H, Wehrens XHT, Kass RS.. Non-equilibrium gating in cardiac Na+ channels. Circulation 2003;107:2233–2237. [DOI] [PubMed] [Google Scholar]
- 79. Casini S, Albesa M, Wang Z, Portero V, Ross-Kaschitza D, Rougier J-S, Marchal GA, Chung WK, Bezzina CR, Abriel H, Remme CA.. Functional consequences of the SCN5A-p.Y1977N mutation within the PY ubiquitylation motif: discrepancy between HEK293 cells and transgenic mice. Int J Mol Sci 2019;20:5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blechschmidt S, Haufe V, Benndorf K, Zimmer T.. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. Prog Biophys Mol Biol 2008;98:309–318. [DOI] [PubMed] [Google Scholar]
- 81. Shang LL, Pfahnl AE, Sanyal S, Jiao Z, Allen J, Banach K, Fahrenbach J, Weiss D, Taylor WR, Zafari AM, Dudley SC.. Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res 2007;101:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Murphy LL, Moon-Grady AJ, Cuneo BF, Wakai RT, Yu S, Kunic JD, Benson DW, George AL.. Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia. Hear Rhythm 2012;9:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan B-H, Valdivia CR, Song C, Makielski JC.. Partial expression defect for the SCN5A missense mutation G1406R depends on splice variant background Q1077 and rescue by mexiletine. Am J Physiol Heart Circ Physiol 2006;291:H1822–H1828. [DOI] [PubMed] [Google Scholar]
- 84. Rook MB, Evers MM, Vos MA, Bierhuizen M.. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res 2012;93:12–23. [DOI] [PubMed] [Google Scholar]
- 85. Marionneau C, Abriel H.. Regulation of the cardiac Na+ channel NaV1.5 by post-translational modifications. J Mol Cell Cardiol 2015;82:36–47. [DOI] [PubMed] [Google Scholar]
- 86. Zhang Y, Hartmann HA, Satin J.. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol 1999;171:195–207. [DOI] [PubMed] [Google Scholar]
- 87. Abriel H, Staub O.. Ubiquitylation of ion channels. Physiology (Bethesda) 2005;20:398–407. [DOI] [PubMed] [Google Scholar]
- 88. Liu M, Liu H, Dudley SC.. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res 2010;107:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Casini S, Verkerk AO, van Borren M, van Ginneken ACG, Veldkamp MW, de Bakker JMT, Tan HL.. Intracellular calcium modulation of voltage-gated sodium channels in ventricular myocytes. Cardiovasc Res 2009;81:72–81. [DOI] [PubMed] [Google Scholar]
- 90. Amin AS, Verkerk AO, Bhuiyan ZA, Wilde AAM, Tan HL.. Novel Brugada syndrome-causing mutation in ion-conducting pore of cardiac Na+ channel does not affect ion selectivity properties. Acta Physiol Scand 2005;185:291–301. [DOI] [PubMed] [Google Scholar]
- 91. Jones DK, Peters CH, Tolhurst SA, Claydon TW, Ruben PC.. Extracellular proton modulation of the cardiac voltage-gated sodium channel, Nav1.5. Biophys J 2011;101:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G.. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol 2010;588:4969–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H.. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 2011;108:294–304. [DOI] [PubMed] [Google Scholar]
- 94. Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, Van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H.. PDZ domain-binding motif regulates cardiomyocyte compartment-specific Nav1.5 channel expression and function. Circulation 2014;130:147–160. [DOI] [PubMed] [Google Scholar]
- 95. Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, Abbasi S, Purevjav E, Samani K, Ackerman MJ, Qi M, Moss AJ, Shimizu W, Towbin JA, Cheng J, Vatta M.. alpha-1-Syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol 2008;1:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA.. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006;114:2104–2112. [DOI] [PubMed] [Google Scholar]
- 97. Lübkemeier I, Requardt RP, Lin X, Sasse P, Andrié R, Schrickel JW, Chkourko H, Bukauskas FF, Kim J-S, Frank M, Malan D, Zhang J, Wirth A, Dobrowolski R, Mohler PJ, Offermanns S, Fleischmann BK, Delmar M, Willecke K.. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol 2013;108:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang F-X, Li Z, Pfenniger A, Lübkemeier I, Keegan S, Fenyö D, Willecke K, Rothenberg E, Delmar M.. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res 2014;104:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Watanabe H, Koopmann TT, Scouarnec S, Le YT, Ingram CR, Schott J-J, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld ACP, Pfeufer A, Kääb S, Wichmann H-E, Hasdemir C, Aizawa Y, Wilde AAM, Roden DM, Bezzina CR.. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest 2008;118:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, Kannankeril PJ, Roden DM.. Mutations in sodium channel β1- and β2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C.. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet 2009;2:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusié-Luna MT, Makielski JC, Ackerman MJ.. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation 2007;116:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Valdivia CR, Medeiros-Domingo A, Ye B, Shen W-K, Algiers TJ, Ackerman MJ, Makielski JC.. Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res 2010;86:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: physiology and pathophysiology. J Mol Cell Cardiol 2010;48:2–11. [DOI] [PubMed] [Google Scholar]
- 105. London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC.. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 2007;116:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kattygnarath D, Maugenre S, Neyroud N, Balse E, Ichai C, Denjoy I, Dilanian G, Martins RP, Fressart V, Berthet M, Schott JJ, Leenhardt A, Probst V, Le Marec H, Hainque B, Coulombe A, Hatem SN, Guicheney P.. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet 2011;4:261–268. [DOI] [PubMed] [Google Scholar]
- 107. Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet J.. [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res 2003;57:986–995. [DOI] [PubMed] [Google Scholar]
- 108. Lindegger N, Hagen BM, Marks AR, Lederer WJ, Kass RS.. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol 2009;47:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rivaud MR, Baartscheer A, Verkerk AO, Beekman L, Rajamani S, Belardinelli L, Bezzina CR, Remme CA.. Enhanced late sodium current underlies pro-arrhythmic intracellular sodium and calcium dysregulation in murine sodium channelopathy. Int J Cardiol 2018;263:54–62. [DOI] [PubMed] [Google Scholar]
- 110. Lin X, O'Malley H, Chen C, Auerbach D, Foster M, Shekhar A, Zhang M, Coetzee W, Jalife J, Fishman GI, Isom L, Delmar M.. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol 2015;593:1389–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L.. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol 2011;301:C577–C586. [DOI] [PubMed] [Google Scholar]
- 112. Watanabe Y. Effects of calcium and sodium concentrations on atrioventricular conduction: experimental study in rabbit hearts with clinical implications on heart block and slow calcium channel blocking agent usage. Am Heart J 1981;102:883–891. [DOI] [PubMed] [Google Scholar]
- 113. Santana LF. NFAT-dependent excitation-transcription coupling in heart. Circ Res 2008;103:681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bers D. CaMKII regulation of cardiac excitation-transcription coupling. Hear Rhythm 2011;8:1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wagner S, Maier LS, Bers DM.. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res 2015;116:1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Toischer K, Hartmann N, Wagner S, Fischer TH, Herting J, Danner BC, Sag CM, Hund TJ, Mohler PJ, Belardinelli L, Hasenfuss G, Maier LS, Sossalla S.. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J Mol Cell Cardiol 2013;61:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A.. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol 2008;294:H1597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS.. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 2006;116:3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ.. A βIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest 2010;120:3508–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ, Hund TJ.. Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation 2015;132:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon AC.. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem 2012;287:19856–19869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chagot B, Potet F, Balser JR, Chazin WJ.. Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel Nav1.5. J Biol Chem 2009;284:6436–6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chagot B, Chazin WJ.. Solution NMR structure of Apo-calmodulin in complex with the IQ motif of human cardiac sodium channel NaV1.5. J Mol Biol 2011;406:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kawasaki K, Suzuki Y, Yamamura H, Imaizumi Y.. Rapid Na+ accumulation by a sustained action potential impairs mitochondria function and induces apoptosis in HEK293 cells expressing non-inactivating Na+ channels. Biochem Biophys Res Commun 2019;513:269–274. [DOI] [PubMed] [Google Scholar]
- 125. Yang K-C, Bonini MG, Dudley SC.. Mitochondria and arrhythmias. Free Radic Biol Med 2014;71:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Black JA, Waxman SG.. Noncanonical roles of voltage-gated sodium channels. Neuron 2013;80:280–291. [DOI] [PubMed] [Google Scholar]
- 127. Lo W-L, Donermeyer DL, Allen PM.. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol 2012;13:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Carrithers MD, Dib-Hajj S, Carrithers LM, Tokmoulina G, Pypaert M, Jonas EA, Waxman SG.. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol 2007;178:7822–7832. [DOI] [PubMed] [Google Scholar]
- 129. Roger S, Gillet L, Guennec J-Y Le, Besson P.. Voltage-gated sodium channels and cancer: is excitability their primary role? Front Pharmacol 2015;6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gillet L, Roger S, Besson P, Lecaille F, Gore J, Bougnoux P, Lalmanach G, Le Guennec JY.. Voltage-gated sodium channel activity promotes cysteine cathepsin-dependent invasiveness and colony growth of human cancer cells. J Biol Chem 2009;284:8680–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Besson P, Driffort V, Bon É, Gradek F, Chevalier S, Roger S.. How do voltage-gated sodium channels enhance migration and invasiveness in cancer cells? Biochim Biophys Acta 2015;1848:2493–2501. [DOI] [PubMed] [Google Scholar]
- 132. Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels 2012;6:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nelson M, Yang M, Dowle AA, Thomas JR, Brackenbury WJ.. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol Cancer 2015;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu J, Liu D, Liu J, Zhao C, Yao S, Hong L.. Blocking the Nav1.5 channel using eicosapentaenoic acid reduces migration and proliferation of ovarian cancer cells. Int J Oncol 2018;53:855–865. [DOI] [PubMed] [Google Scholar]
- 135. Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA.. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA 2002;99:6210–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos M. A, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P.. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med 2001;7:1021–1027. [DOI] [PubMed] [Google Scholar]
- 137. Sakai T, Fujii S, Hirota A, Kamino K.. Optical evidence for calcium-action potentials in early embryonic precontractile chick heart using a potential-sensitive dye. J Membrain Biol 1983;72:205–212. [DOI] [PubMed] [Google Scholar]
- 138. Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, Yang T, Zhong TP, Roden DM.. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res 2010;106:1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sakurai T, Tsuchida M, Lampe PD, Murakami M.. Cardiomyocyte FGF signaling is required for Cx43 phosphorylation and cardiac gap junction maintenance. Exp Cell Res 2013;319:2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hennessey JA, Wei EQ, Pitt GS.. Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ Res 2013;113:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Musa H, Kline CF, Sturm AC, Murphy N, Adelman S, Wang C, Yan H, Johnson BL, Csepe TA, Kilic A, Higgins RSD, Janssen PML, Fedorov VV, Weiss R, Salazar C, Hund TJ, Pitt GS, Mohler PJ.. SCN5A variant that blocks fibroblast growth factor homologous factor regulation causes human arrhythmia. Proc Natl Acad Sci USA 2015;112:12528–12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Portero V, Wilders R, Casini S, Charpentier F, Verkerk AO, Remme CA.. KV4.3 expression modulates NaV1.5 sodium current. Front Physiol 2018;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Milstein ML, Musa H, Balbuena DP, Anumonwo JMB, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J.. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci U S A 2012;109:E2134–E2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rivaud MR, Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Rothenberg E, Bezzina CR, Delmar M, Remme CA.. Sodium channel remodeling in subcellular microdomains of murine failing cardiomyocytes. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M.. Interactions between ankyrin-G, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ Res 2011;109:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, Hoagland D, Wan X, King DR, Sanchez-Alonso J, Chen C, Jourdan J, Isom LL, Deschenes I, Smyth JW, Gorelik J, Poelzing S, Gourdie RG.. The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shaw RM, Fay AJ, Puthenveedu MA, von ZM, Jan YN, Jan LY.. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007;128:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ.. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res 2014;115:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bréchet A, Fache M-P, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B.. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol 2008;183:1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bouza AA, Isom LL.. Voltage-gated sodium channel β subunits and their related diseases. Handb Exp Pharmacol 2018;246:423–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, Lindberg G, Karling P, Ohlsson B, Gazouli M, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Camilleri M, Locke GR, Talley NJ, D’Amato M, Ackerman MJ, Farrugia G.. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 2014;146:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Probst V, Kyndt F, Potet F, Trochu JN, Mialet G, Demolombe S, Schott JJ, Baró I, Escande D, Marec H. Le. Haploinsufficiency in combination with aging causes SCN5A-linked hereditary Lenègre disease. J Am Coll Cardiol 2003;41:643–652. [DOI] [PubMed] [Google Scholar]
- 153. Royer A, van Veen TAB, Le Bouter S, Marionneau C, Griol-Charhbili V, Léoni A-L, Steenman M, van Rijen HVM, Demolombe S, Goddard CA, Richer C, Escoubet B, Jarry-Guichard T, Colledge WH, Gros D, de Bakker JMT, Grace AA, Escande D, Charpentier F.. Mouse model of SCN5A-linked hereditary Lenègre’s: disease age-related conduction slowing and myocardial fibrosis. Circulation 2005;111:1738–1746. [DOI] [PubMed] [Google Scholar]
- 154. Leoni AL, Gavillet B, Rougier JS, Marionneau C, Probst V, Scouarnec S, Le Schott JJ, Demolombe S, Bruneval P, Huang CLH, Colledge WH, Grace AA, Marec H, Le Wilde AA, Mohler PJ, Escande D, Abriel H, Charpentier F.. Variable Nav1.5 protein expression from the wild-type allele correlates with the penetrance of cardiac conduction disease in the Scn5a+/- mouse model. PLoS One 2010;5:e9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rivaud MR, Jansen JA, Postema PG, Nannenberg EA, Mizusawa Y, Nagel R, van der Wolswinkel R, Made I, van der Marchal GA, Rajamani S, Belardinelli L, Tintelen JP, van Tanck MWT, Wal AC, van der Bakker JMT, de Rijen HV, van Creemers EE, Wilde AAM, Berg MP, van den Veen TAB, van Bezzina CR, Remme CA.. A common co-morbidity modulates disease expression and treatment efficacy in inherited cardiac sodium channelopathy. Eur Heart J 2018;39:2898–2907. [DOI] [PubMed] [Google Scholar]
- 156. Zhang T, Yong SL, Drinko JK, Popović ZB, Shryock JC, Belardinelli L, Wang QK.. LQTS mutation N1325S in cardiac sodium channel gene SCN5A causes cardiomyocyte apoptosis, cardiac fibrosis and contractile dysfunction in mice. Int J Cardiol 2011;147:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Leask A. TGFβ, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 2007;74:207–212. [DOI] [PubMed] [Google Scholar]
- 158. Chatelier A, Mercier A, Tremblier B, Thériault O, Moubarak M, Benamer N, Corbi P, Bois P, Chahine M, Faivre JF.. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol 2012;590:4307–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sossalla S, Maier LS.. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacol Ther 2012;133:311–323. [DOI] [PubMed] [Google Scholar]
- 160. Dietl A, Maack C.. Targeting mitochondrial calcium handling and reactive oxygen species in heart failure. Curr Heart Fail Rep 2017;14:338–349. [DOI] [PubMed] [Google Scholar]
- 161. Ruan Y, Denegri M, Liu N, Bachetti T, Seregni M, Morotti S, Severi S, Napolitano C, Priori SG.. Trafficking defects and gating abnormalities of a novel SCN5A mutation question gene-specific therapy in long QT syndrome type 3. Circ Res 2010;106:1374–1383. [DOI] [PubMed] [Google Scholar]
- 162. Portero V, Casini S, Hoekstra M, Verkerk AO, Mengarelli I, Belardinelli L, Rajamani S, Wilde AAM, Bezzina CR, Veldkamp MW, Remme CA.. Anti-arrhythmic potential of the late sodium current inhibitor GS-458967 in murine Scn5a-1798insD+/− and human SCN5A-1795insD+/− iPSC-derived cardiomyocytes. Cardiovasc Res 2017;113:829–838. [DOI] [PubMed] [Google Scholar]
- 163. Maier LS, Sossalla S.. The late Na current as a therapeutic target: where are we? J Mol Cell Cardiol 2013;61:44–50. [DOI] [PubMed] [Google Scholar]