Key Points
Question
Does a dose of a daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 reduce cumulative systemic antibiotic administration days for all-cause, acute infections in care home residents?
Findings
In this randomized clinical trial that included 310 participants, this daily probiotic combination, compared with placebo, did not significantly reduce antibiotic administration over 1 year (mean cumulative antibiotic administration days, 12.9 vs 12.0).
Meaning
The findings do not support the use of probiotics for reducing antibiotic administration in older adults living in care homes.
Abstract
Importance
Probiotics are frequently used by residents in care homes (residential homes or nursing homes that provide residents with 24-hour support for personal care or nursing care), although the evidence on whether probiotics prevent infections and reduce antibiotic use in these settings is limited.
Objective
To determine whether a daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 compared with placebo reduces antibiotic administration in care home residents.
Design, Setting, and Participants
Placebo-controlled randomized clinical trial of 310 care home residents, aged 65 years and older, recruited from 23 care homes in the United Kingdom between December 2016 and May 2018, with last follow-up on October 31, 2018.
Interventions
Study participants were randomized to receive a daily capsule containing a probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 (total cell count per capsule, 1.3 × 1010 to 1.6 × 1010) (n = 155), or daily matched placebo (n = 155), for up to 1 year.
Main Outcomes and Measures
The primary outcome was cumulative antibiotic administration days for all-cause infections measured from randomization for up to 1 year.
Results
Among 310 randomized care home residents (mean age, 85.3 years; 66.8% women), 195 (62.9%) remained alive and completed the trial. Participant diary data (daily data including study product use, antibiotic administration, and signs of infection) were available for 98.7% randomized to the probiotic group and 97.4% randomized to placebo. Care home residents randomized to the probiotic group had a mean of 12.9 cumulative systemic antibiotic administration days (95% CI, 0 to 18.05), and residents randomized to placebo had a mean of 12.0 days (95% CI, 0 to 16.95) (absolute difference, 0.9 days [95% CI, –3.25 to 5.05]; adjusted incidence rate ratio, 1.13 [95% CI, 0.79 to 1.63]; P = .50). A total of 120 care home residents experienced 283 adverse events (150 adverse events in the probiotic group and 133 in the placebo group). Hospitalizations accounted for 94 of the events in probiotic group and 78 events in the placebo group, and deaths accounted for 33 of the events in the probiotic group and 32 of the events in the placebo group.
Conclusions and Relevance
Among care home residents in the United Kingdom, a daily dose of a probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 did not significantly reduce antibiotic administration for all-cause infections. These findings do not support the use of probiotics in this setting.
Trial Registration
ISRCTN Identifier:16392920
This randomized clinical trial compares the effects of daily combination probiotics (Lactobacillus and Bifidobacterium spp) vs placebo on cumulative antibiotic use for all-cause infections over 1 year among care home residents in Wales.
Introduction
The global human probiotics market size was more than $34 billion (US dollars) in 2015, and may be worth $64 billion by 2023.1 The US hospital and nursing home market for probiotics was estimated at $92.4 million in 2016, and is projected to expand at an estimated compound annual growth rate of 9.3% from 2017 to 2025.2 Probiotics are often promoted for health indications3 and may be an inexpensive and safe intervention to reduce antibiotic use and resistance through preventing infections.4,5
A systematic review of probiotics to reduce antibiotic use for common infections in infants and children included 17 randomized clinical trials (RCTs) that evaluated 13 probiotic formulations of Lactobacillus and Bifidobacterium strains singly or combined and found that probiotic use was associated with reduced risk of antibiotic prescription relative to placebo.6 A further systematic review of 20 RCTs in otherwise healthy children and adults found that use of Lactobacillus and Bifidobacterium probiotic strains was associated with reduced duration of respiratory illness in children.7 However, the quality of this supporting evidence was variable, and the authors called for additional well-designed studies to substantiate the findings and explore effects in other populations.6,7
With the aging population, care homes are an increasingly important care sector; care home residents are more prone to infections and consume more antibiotics than the general population,8 increasing the risk of antimicrobial resistance and poor outcomes.9 The Probiotics to Reduce Infections in Care Home Residents (PRINCESS) trial was designed to test the hypothesis that daily administration of a combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 probiotics to care home residents would reduce cumulative systemic antibiotic administration days for all-cause acute infections.
Methods
Trial Design
This study was designed as a multicenter, parallel, individually randomized, placebo-controlled, double-blind clinical trial and was conducted between December 2016 and May 2018 in UK care homes. The trial was approved by the research ethics committee (REC) for Wales (Wales REC 3; recognized by the UK Ethics Committee Authority [15/WA/0306]), which approved all recruitment sites. National Health Service (NHS) health boards and clinical commissioning groups gave research and development approval to sites. Written informed consent was obtained from those participants with capacity to do so, and for those who lacked capacity to provide consent, a consultee (either a legal representative or guardian) could complete a consultee declaration for participation on their behalf. The protocol has been published elsewhere,10 and the final protocol, amendments, and statistical analysis plan are available in Supplement 1.
Participants
Care home residents in this trial included those living in residential, nursing, and dual registered homes. Care home residents were eligible if they were aged 65 years or older. Exclusions were being immunocompromised (ongoing immune-suppressants; long-term, high-dose, oral, intramuscular, or intravenous steroids) or taking ongoing regular probiotics. Full eligibility criteria are provided in eAppendix 1 in Supplement 2.
Treatment Allocation
Participants were randomized using an online process in a 1:1 ratio using minimization to balance groups by care home and resident sex, with a random component set at 80%.
Procedures
Nurses registered with the UK Nursing and Midwifery Council and blind to group allocation made weekly visits to each care home and recorded weekly diary data for each participant in an online database. Participant data included the amount of study product (probiotic or placebo) taken each day, signs of infection, use of antibiotics including route, diarrhea, hospitalization, and serious or trial-related adverse events. Data were obtained from participants’ daily medical administration records, care home clinical records, observation of the participant, and discussion with participants or their friends and family, care home staff, and hospital discharge summaries. The EuroQol Group 5-Dimension Self-Report (EQ-5D) for health utility and the Icepop Capability Measure for Older People (ICECAP-O) well-being questionnaires were collected at baseline, at 3-month follow-up, and at the 12-month follow-up point (or as close to 12 months as the study would allow [some participant follow-up was truncated]). Participants were asked to provide stool and saliva samples at baseline, month 3, and at 12-month follow-up (or as close to 12 months as the study would allow), but this was not a requirement for participation.
Interventions
Participants were randomized to receive a daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 (total cell count per capsule, 1.3 × 1010 to 1.6 × 1010) or a matched placebo (capsule containing maltodextrin, microcrystalline cellulose, magnesium stearate, and silicon dioxide) once daily (eAppendix 2 in Supplement 2). The study product was not administered while care home residents were away from care homes, such as when hospitalized or after withdrawal from the study.
Outcomes
The primary outcome was cumulative systemic antibiotic administration days for all-cause infections, defined as the total number of days of systemic antibiotic administration, as recorded in care home medical records and hospital discharge summaries, with the denominator calculated as the total number of days participants were observed in the study.
Secondary outcomes were the total number of days of antibiotic administration for each infection category recorded in care home medical records (urinary tract infection, gastrointestinal infection, respiratory tract infections [divided into upper and lower respiratory tract infections post hoc after the trial management group decided it would be more informative to evaluate these outcomes separately], skin and soft tissue infection, unexplained fever, and other); number, site, and duration of infection (mean and cumulative values reported); duration of diarrhea when oral antibiotics were taken and not taken; antibiotic-associated diarrhea; incidence of Clostridioides difficile infection; antibiotic sensitivity of stool gram-negative Enterobacteriaceae and vancomycin-resistant enterococci (VRE) and counts of Lactobacillus rhamnosus and Bifidobacterium animalis subsp lactis; oral Candida spp; self- or proxy-reported (or both) health-related quality of life measured by EQ-5D-5L (index value range, −0.594 [worst] to 1 [best]); health status range, 0 [worst] to 100 [best]) and ICECAP-O (range, 0 [worst] to 1 [best])11; number and duration of hospital stays; and deaths. eAppendix 3 in Supplement 2 provides further details on the derivation of some outcomes.
Statistical Analyses
An estimated 330 participants from 20 care homes in the UK would provide 90% power at the 5% level to demonstrate a 10% relative reduction in cumulative systemic antibiotic administration days, assuming a mean number of cumulative systemic antibiotic administration days of 17.4 and a 10% reduction in the probiotic group to 15.6 days per resident-year.8 We considered a 10% reduction feasible and clinically important12 because physician-targeted interventions to reduce antibiotic use for respiratory tract infections have been associated with a mean reduction in antibiotic prescriptions of 11.6%13; longer duration of antibiotic exposure has been associated with increased risk of subsequent infections with drug-resistant organisms14; approximately 20% of all antibiotics prescribed in primary care in England are considered inappropriate15; and a UK government initiative to halve inappropriate prescribing would amount to a 10% relative reduction.16
This sample size accounted for 30% of participants contributing no outcome data (ie, randomized but contributing to neither the numerator or denominator). The target sample size was adjusted after a planned interim assessment of outcome data availability after 3 months (33 participants) to be at least 258. Assuming a mean number of days for which primary outcome data could be available (ie, accounting for follow-up time) of approximately 250 days, this would provide at least 82% power to detect a 10% relative reduction in cumulative systemic antibiotic administration days.
Primary and secondary comparative analyses were prespecified and included all randomized participants who provided outcome data, analyzed in the group to which they were randomized without imputation to account for loss of observation time. The mean cumulative systemic antibiotic administration days per resident-year was compared between groups by fitting a 2-level negative binomial regression model, accounting for participants nested within care homes, the length of time observed, and the sex of care home residents. Similarly, the majority of secondary outcome analyses (cumulative systemic antibiotic administration days by infection type, rates of infections, rates of diarrhea) involved the between-group comparison of rate variables using 2-level Poisson or negative binomial regression (depending on the presence of overdispersion). The decision to analyze lower and upper respiratory tract infections separately was made post hoc by the trial management group because reporting lower respiratory tract infections separately was considered important, as these infections typically cause greater morbidity in the study population than upper respiratory tract infections. The consistency of conclusions drawn from the primary analysis was investigated by conducting the following prespecified sensitivity analyses: (1) including prophylactic antibiotic use in the definition of cumulative systemic antibiotic administration days; (2) ignoring periods of hospitalization from both the numerator and denominator; (3) handling data truncated due to death from infection by imputing participants as having been administered antibiotics for the remainder of the time they should have been observed in the trial (a composite strategy)17; and (4) accounting for study product consumption (see eAppendix 4 in Supplement 2). Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary endpoints should be interpreted as exploratory. For all analyses, 2-sided 95% CIs and P values were calculated. P values of less than .05 were considered statistically significant. Statistical analyses were conducted using IBM SPSS version 25 and STATA version 15. Further details of statistical analyses are provided in eAppendix 4 of Supplement 2.
Results
Participants
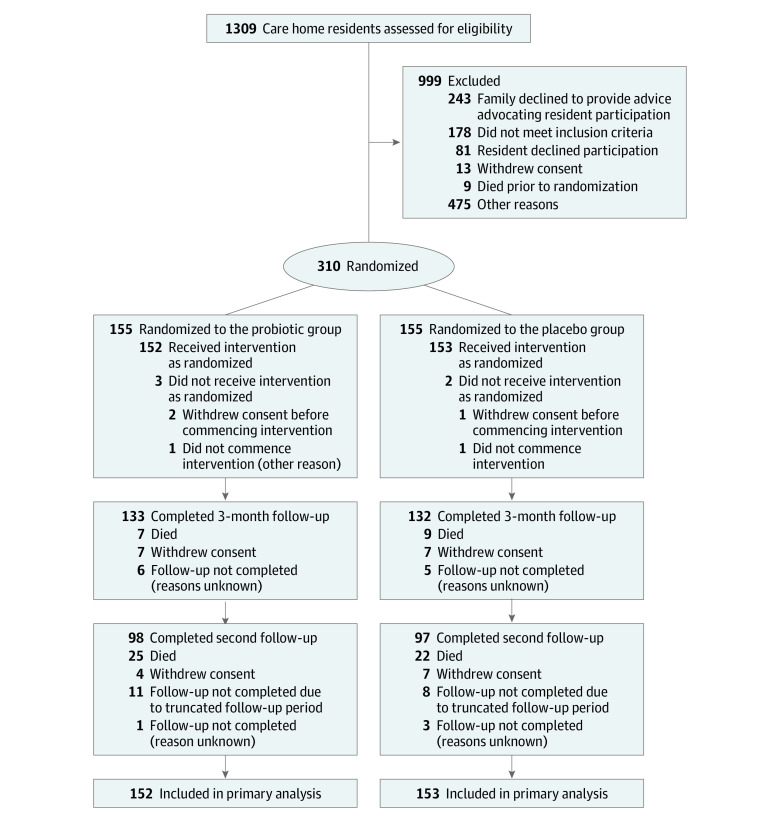
Of 310 care home residents, 155 in each group were randomized from 23 care homes in the UK between December 2016 and May 2018. Due to slower than anticipated recruitment, follow-up was truncated for 106 care home residents, with these care home residents followed up for between 147 and 362 days in total. Among the 199 participants who remained alive, had not withdrawn from the study, and could have undergone a second follow up at 12 months postrandomization (or earlier for those whose follow-up was truncated), responses were available for 195 (98.0%) care home residents, 98 in the probiotic group and 97 in the placebo group (Figure). The mean (SD) age was 85.3 (7.39) years; 66.8% (207/310) were women; 65.8% (204/310) lacked capacity to consent. Care home residents in trial groups were well matched for these and most other characteristics at baseline, including stool sample culture for probiotic organisms. However, more care home residents in the probiotic group had C difficile cultured from their stool (6/83 [7.2%]) compared with the placebo group (0/75) (Table 1). Care home residents allocated to the probiotic group contributed 39 798 person days (mean [SD] number of days per probiotic participant, 252.4 [110.51]) and care home residents allocated to placebo contributed 37 974 person days (mean days, 242.9 [115.24]). The primary cause of unobserved data was truncation due to death, with postrandomization deaths occurring in 33 care home residents randomized to the probiotic group and 32 randomized to receive placebo (total number of unobserved days due to death, 7578 for the probiotic group and 6978 for the placebo group). Other reasons for unobserved data were resident absence from the care home (56 days in the probiotic group and 114 days in the placebo group), waiting for a capacity assessment (7 days in the probiotic group and zero in the placebo group), and data not collected for an unknown reason (1898 days in the probiotic group and 972 days in the placebo group). There were 305 (98.4%) care home residents who contributed to the primary analysis and secondary analyses relating to infections and diarrhea, with 5 care home residents excluded from these analyses due to death or withdrawal following randomization and prior to contributing data.
Figure. Enrollment, Randomization, and Follow-up of Care Home Residents.
Table 1. Participant Characteristics at Baseline of Care Home Residents.
| Characteristic | Probiotic (n = 155)a | Placebo (n = 155)a |
|---|---|---|
| Age, mean (SD), y | 85.1 (7.6) | 85.6 (7.21) |
| Men | 52 (33.5) | 51 (32.9) |
| Women | 103 (66.5) | 104 (67.1) |
| Consent provided by proxyb | 98 (63.2) | 106 (68.4) |
| Consent self-providedb | 57 (36.8) | 49 (31.6) |
| Years of residency in care home, median (IQR)c | 1 (0-2) | 1 (0-3) |
| Height, mean (SD), cmd | 162 (7.8) | 165 (8.8) |
| Weight, median (IQR), kg | 60 (52.1-70.6) | 63 (55.6-72.9) |
| Ulna lengthe, mean (SD), cm | 25 (2.5) | 26 (2.5) |
| Mid–upper arm circumference, mean (SD), cmf | 27 (4.5) | 27 (4.1) |
| Clinical frailty scaleg | ||
| Very fit to managing well | 13 (8.4) | 18 (11.6) |
| Vulnerable to moderately frail | 64 (41.3) | 51 (32.9) |
| Severely frail to terminally ill | 78 (50.3) | 86 (55.5) |
| Prescribed antimicrobials in the last 4 weeks | 45 (29.0) | 37 (23.9) |
| Used a proton pump inhibitor in the last 4 weeks | 61 (39.4) | 52 (33.5) |
| Used a laxative in the last 4 weeks | 75 (48.4) | 85 (54.8) |
| Used vitamin D in the last 4 weeks | 50 (32.3) | 44 (28.4) |
| Lactobacillus rhamnosus growth on plate, No./total No. (%)h | 28/83 (33.7) | 19/75 (25.3) |
| Bifidobacterium animalis subsp lactis growth on plate, No./total No. (%)h | 3/83 (3.6) | 4/75 (5.3) |
| Growth of Clostridioides difficile, No./total No. (%)h | 6/83 (7.2) | 0/75 |
Values are reported as No. (%) unless otherwise specified. Some characteristic categories, as indicated by footnote, did not count all group participants.
If participants lacked capacity to consent, a consultee (legal representative or guardian) advised about their participation in accordance with the governing legislation.
Categorical count included 153 in the probiotic group and 154 in the placebo group.
Categorical count included 70 in the probiotic group and 74 in the placebo group.
Indicates length between the point of the elbow and the midpoint of the prominent bone of the wrist. Categorical count included 152 in the probiotic group and 150 in the placebo group.
Indicates the distance between the bony protrusion on the shoulder and the point of the elbow, marking the midpoint and measuring around the arm at this point. Categorical count included 151 in the probiotic group and 150 in the placebo group.
Scale assesses the level of fitness or frailty in an older adult (score ranges and interpretations: 1-3, very fit to managing well; 4-6, vulnerable to moderately frail; and 7-8, severely frail to terminally ill).
Assessed from participant stool samples.
Intervention Fidelity
Among study participants, 302 (97.4%) initiated at least 1 dose of study product (152 [98.1%] in the placebo group and 150 [96.8%] in the probiotic group). Of the remaining 8 care home residents, 5 withdrew following randomization and 3 died soon after randomization. For the 302 care home residents who initiated at least 1 study product dose, a median of 93.3% (interquartile range [IQR], 93.56% to 99.45%) full or partial doses were taken, and 89.4% (68 356/73 302) were either swallowed as capsules or sprinkled on food (that was not hot) prior to ingestion, 4.4% (3258/73 302) in liquid form, and 2.3% (1688/73 302) by method unknown.
At 3 months postrandomization, significantly more stool samples were found to contain Lactobacillus rhamnosus among care home residents randomized to the probiotic group vs from those randomized to the placebo group (83.9% [47/56] vs 36.5% [19/52]; absolute risk difference [ARD], –47.4% [95% CI, –64.8% to –29.0%]; adjusted odds ratio [AOR], 9.19 [95% CI, 3.51 to 24.07]; P < .001), and mean (SD) concentrations were 7.04 × 105 (3.05 × 106) for those randomized to the probiotic group and 4.67 × 104 (2.77 × 105) in the placebo group. This finding persisted at the second follow-up time point, with stool samples containing Lactobacillus rhamnosus in 73.0% (27/37) of participants in the probiotic group vs 31.0% (9/29) of participants in the placebo group (ARD, –41.9% [95% CI, –66.1% to –17.7%]; AOR, 6.41 [95% CI, 2.14 to 19.20]; P = .001), mean (SD) concentrations, 1.52 × 105 (5.27 × 105) in the probiotic group vs 1.40 × 104 (4.31 × 104) in the placebo group (eTable 5 in Supplement 2).
Care home residents randomized to the probiotic group provided stool samples containing Bifidobacterium animalis subsp lactis significantly more frequently than those randomized to placebo at 3 months (51.8% [29/56] vs 3.8% [2/52]; ARD, –47.9% [95% CI, –65.0% to –30.9%]; AOR, 26.90 [95% CI, 5.94 to 121.66]; P < .001), mean (SD) concentrations, 1.72 × 106 (5.11 × 106) in the probiotic group and 2.88 × 104 (1.71 × 105) in the placebo group. This finding persisted at the second follow-up time point (56.8% [21/37] in the probiotic group vs 6.9% [2/29]; ARD, –49.9% [95% CI, –73.0% to –26.7%]; AOR, 21.96 [95% CI, 2.97 to 162.43]; P = .002), with mean (SD) concentrations of 2.15 × 105 (4.45 × 105) in the probiotic group vs 3.62 × 102 (1.86 × 103) in the placebo group (eTable 5 in Supplement 2).
There were 202 (66.2%) care home residents who were prescribed at least 1 nonprophylactic antibiotic (63.4% [97/155] in the probiotic group vs 69.1% [105/155] in the placebo group). There were 287 courses of nonprophylactic antibiotics prescribed in the probiotic group vs 336 courses in the placebo group.
Primary Outcome
Care home residents randomized to the probiotic group had a mean (SD) of 12.9 (18.4) cumulative systemic antibiotic administration days (95% CI, 0 to 18.05 days), and care home residents randomized to the placebo group had a mean of 12.0 (18.6) cumulative systemic antibiotic administration days (95% CI, 0 to 16.95 days). The distribution was positively skewed, with 37% of residents having 0 days due to not being administered antibiotics (eFigure 1 in Supplement 2). The absolute difference in cumulative systemic antibiotic administration days was 0.9 days (95% CI, –3.25 to 5.05 days) and the adjusted incidence rate ratio (IRR) was 1.13 (95% CI, 0.79 to 1.63; P = .50). Death due to infection was reported for 12 care home residents in the probiotic group and 6 in the placebo group, with 6 care home residents in the probiotic group and 1 in placebo group taking an antibiotic until death. Further details of sensitivity analyses for the primary outcome measure are provided in eTable 1, eTable 2, and eTable 3 in Supplement 2.
Secondary Outcomes
Care home residents randomized to receive a daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 were administered significantly more antibiotics for lower respiratory tract infections than those randomized to the placebo group (mean 6.2 days in the probiotic group vs 4.0 days in the placebo group; absolute difference, 2.2 days [95% CI, –0.41 to 4.81 days]; adjusted IRR, 1.42 [95% CI, 1.05 to 1.93]; P = .02). There were no statistically significant between-group differences in antibiotic use for urinary tract infections (mean 7.1 days in the probiotic group vs mean 6.7 days in the placebo group; absolute difference, 0.4 days [95% CI, –2.81 to 3.61 days]; adjusted IRR, 1.17 [95% CI, 0.75 to 1.84]; P = .48), upper respiratory tract infections (mean 3.3 days in the probiotic group vs mean 3.4 days in the placebo group; absolute difference, 0.1 days [95% CI, –2.09 to 2.29 days]; adjusted IRR, 1.13 [95% CI, 0.71 to 1.78]; P = .61), skin infections (mean 3.4 days in the probiotic group vs mean 3.7 days in the placebo group; absolute difference, 0.3 days [95% CI, –2.20 to 2.80 days]; adjusted IRR, 0.92 [95% CI, 0.54 to 1.57]; P = .76), and duration of infection (median 6 days in the probiotic group vs median 5 days in the placebo group; adjusted mean difference, 0.08 [95% CI, –0.001 to 0.16]; P = .05; Table 2; eFigure 2 in Supplement 2). Unexplained fever was not reported for any participants during the trial.
Table 2. Between-Group Differences for Infection-Related Outcome Measuresa.
| Analysis | Probiotic (n = 155) | Placebo (n = 155) | Absolute difference (95% CI) | Adjusted incidence rate ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Primary outcome, No. (%) with data | 152 (98.1) | 153 (98.7) | |||
| Cumulative antibiotic administration, mean (SD), days | 12.9 (18.4) | 12.0 (18.6) | 0.9 (–3.25 to 5.05) | 1.13 (0.79 to 1.63) | .50 |
| Secondary outcome, No. (%) with dataa | 152 (98.1) | 153 (98.7) | |||
| Cumulative systemic antibiotic administration, mean (SD), days | |||||
| For urinary tract infectionb | 7.1 (15.0) | 6.7 (13.6) | 0.4 (–2.81 to 3.61) | 1.17 (0.75 to 1.84) | .48 |
| For upper respiratory tract infectionsb | 3.3 (9.4) | 3.4 (10.1) | 0.1 (–2.09 to 2.29) | 1.13 (0.71 to 1.78) | .61 |
| For lower respiratory tract infectionsb | 6.2 (14.6) | 4.0 (7.6) | 2.2 (–0.4 to 4.8) | 1.4 (1.1 to 1.9) | .02 |
| For skin infectionsb | 3.4 (8.7) | 3.7 (13.1) | 0.3 (–2.20 to 2.80) | 0.92 (0.54 to 1.57) | .76 |
| Incidence of infection, mean (SD), No. per person | |||||
| Of any infection | 2.5 (2.5) | 2.4 (2.7) | 0.1 (–1.3 to 1.5) | 1.0 (0.8 to 1.2) | .92 |
| Of urinary tract infections | 0.8 (1.4) | 0.8 (1.4) | 0 (–0.3 to 0.3) | 1.1 (0.6 to 2.1) | .68 |
| Of gastrointestinal infections | 0.03 (0.2) | 0.04 (0.2) | 0 (0 to 0.1) | 0.8 (0.2 to 2.6) | .68 |
| Of upper respiratory tract infections | 0.4 (0.8) | 0.5 (0.9) | 0.1 (–0.1 to 0.3) | 0.8 (0.5 to 1.2) | .31 |
| Of lower respiratory tract infections | 0.6 (1.0) | 0.5 (0.9) | 0.1 (–0.1 to 0.3) | 1.2 (0.8 to 1.7) | .41 |
| Of skin infections | 0.6 (1.2) | 0.5 (1.1) | 0.1 (–0.2 to 0.4) | 1.2 (0.7 to 2.0) | .49 |
| ≥1 Infection, No. (%) | 111 (73.0) | 102 (66.7) | 0.1 (0 to 0.2) | 1.4 (0.8 to 2.4)c | .20 |
| Duration of infection for those with ≥1 infection, mean (SD)d | 6.8 (4.7) | 6.0 (4.9) | 0.9 (–0.4 to 2.2) | 0.1 (0 to 0.2)e | .05 |
| Cumulative number of infection days per person-year, mean (SD)f | 22 (30.8) | 21 (40.7) | 1 (–7.1 to 9.1) | 1.1 (0.8 to 1.5) | .67 |
Abbreviation: IQR, interquartile range.
Cumulative systemic antibiotic administration days for gastrointestinal infection was not reported due to a small number of participants having gastrointestinal infection (2 participants in the probiotic group and 0 in the placebo group).
Cumulative infection-site–specific antibiotic administration days were rate variables expressed per person-year. The mean rates were calculated by dividing the number of days that an antibiotic was administered for a specific infection (as indicated in the care home medical records) by the period of exposure days.
Indicates adjusted odds ratio (95% CI).
Duration of infection was calculated by dividing the number of infection days by the total number of infections. Values in this category are based on a count of 111 in the probiotic group and 102 in the placebo group. See eFigure 2 in Supplement 2 for the distribution.
Indicates adjusted mean difference (95% CI).
Cumulative number of infection days was a rate variable expressed as infection days per person-year, with the number of suspected infection days as the numerator over the period of exposure days. During weekly visits, research nurses would record whether care home residents displayed signs of infection (and if so, record which infection[s]) following discussions with care home staff. This was asked and recorded separately from whether a care home resident received an antibiotic on a given day.
Care home residents allocated to the probiotic group had statistically significant lower self-reported generic well-being/capability scores at 3 months (mean score, 0.72 in the probiotic group vs mean score, 0.69 in the placebo group; absolute difference, 0.03 [95% CI, –0.05 to 0.11]; adjusted mean difference, –0.06 [95% CI, –0.11 to –0.001]; P = .05). There were no statistically significant differences for other self-reported and proxy well-being and quality of life outcomes (Table 3).
Table 3. Between-Group Differences for Secondary Outcome Measures.
| Secondary analysis | Probiotic (n = 155) | Placebo (n = 155) | Absolute difference (95% CI) | Adjusted difference (95% CI)a | P value |
|---|---|---|---|---|---|
| 3-mo EQ-5D index valueb,c | |||||
| Self-report, mean (SD) [No.] | 0.6 (0.3) [49] | 0.6 (0.2) [43] | 0 (–0.1 to 0.2) | Mean, –0.1 (–0.1 to 0) | .13 |
| Proxy, mean (SD) [No.] | 0.5 (0.3) [130] | 0.5 (0.3) [129] | 0 (–0.1 to 0.1) | Mean, 0 (–0.1 to 0) | .66 |
| 3-mo EQ-5D health statusb,c | |||||
| Self-report, mean (SD) [No.] | 65 (18.3) [44] | 65 (20.6) [42] | 0.1 (–8.1 to 8.3) | Mean, –0.3 (–8.0 to 7.5) | .95 |
| Proxy, mean (SD) [No.] | 71 (19.1) [128] | 70 (20.6) [130] | 0.4 (–4.4 to 5.2) | Mean, 0.4 (–4.1 to 4.8) | .87 |
| Second follow-up EQ-5D index valueb,c | |||||
| Self-report, mean (SD) [No.] | 0.6 (0.4) [38] | 0.6 (0.3) [31] | 0 (–0.2 to 0.2) | Mean, 0 (–0.1 to 0.1) | .92 |
| Proxy, mean (SD) [No.] | 0.5 (0.3) [97] | 0.5 (0.3) [95] | 0 (0 to 0.1) | Mean, 0 (–0.1 to 0.1) | .79 |
| Second follow-up EQ-5D health statusb,c | |||||
| Self-report, mean (SD) [No.] | 65 (21.4) [34] | 66 (21.5) [29] | 0.5 (–10.1 to 11.1) | Mean, 24.4 (–1267.9 to 1316.6)d | .97 |
| Proxy, mean (SD) [No.] | 65 (21.8) [98] | 64 (21.0) [96] | 0.6 (–5.4 to 6.6) | Mean, 0.6 (–4.9 to 6.2) | .82 |
| 3-mo ICECAP-O valuee | |||||
| Self-report, mean (SD) [No.] | 0.7 (0.2) [47] | 0.7 (0.2) [40] | 0 (–0.1 to 0.1) | Mean, –0.1 (–0.1 to –0) | .05 |
| Proxy, mean (SD) [No.] | 0.7 (0.2) [117] | 0.7 (0.2) [118] | 0 (0 to 0.1) | Mean, 0 (0 to 0) | .85 |
| Second follow-up ICECAP-O valuee | |||||
| Self-report, mean (SD) [No.] | 0.7 (0.3) [35] | 0.7 (0.2) [27] | 0.1 (–0.1 to 0.2) | Mean, –0.1 (–0.2 to 0) | .15 |
| Proxy, mean (SD) [No.] | 0.7 (0.2) [84] | 0.7 (0.2) [90] | 0 (–0.1 to 0.1) | Mean, 0 (–0.1 to 0) | .69 |
| No. ever hospitalized/total No. (%) | 42/152 (27.6) | 36/153 (23.5) | 0 (–0.1 to 0.1) | OR, 1.25 (0.74 to 2.11) | .41 |
| Death, No. (%) | 33 (21.3) | 32 (20.6) | 0 (–0.1 to 0.1) | OR, 1.03 (0.59 to 1.80) | .90 |
| No. of hospital stays, mean (SD)f | 0.4 (0.7) | 0.3 (0.6) | 0.08 (–0.06 to 0.22) | IRR, 1.17 (0.72 to 1.90) | .53 |
| Cumulative No. of hospital days, mean (SD)f | 4.5 (12.5) | 5.4 (19.4) | 0.9 (–2.77 to 4.57) | IRR, 1.00 (0.43 to 2.29) | >.99 |
| Incidence of antibiotic-associated diarrhea, mean (SD)f | 0.8 (2.0) | 0.6 (1.8) | 0.2 (–0.16 to 0.50) | IRR, 1.39 (0.79 to 2.46) | .25 |
| Cumulative days of antibiotic-associated diarrhea, mean (SD)f | 6.8 (22.3) | 4.4 (16.1) | 2.4 (–2.00 to 6.71) | IRR, 1.83 (0.95 to 3.54) | .07 |
| Incidence of all-cause diarrhea, mean (SD)f | 1.8 (3.9) | 1.6 (3.5) | 0.2 (–0.6 to 1.1) | IRR, 1.1 (0.7 to 1.6) | .80 |
| Cumulative days of all-cause diarrhea, mean (SD)f | 4.4 (10.2) | 4.4 (10.8) | 0 (–2.3 to 2.4) | IRR, 1.2 (0.78 to 2.0) | .39 |
| ≥1 All-cause diarrhea episode, No./total No. (%) | 64/152 (42.1) | 61/153 (39.9) | 0 (–0.1 to 0.1) | OR, 1.0 (0.6 to 1.8) | .89 |
| Mean duration of diarrhea episodes for those with ≥1 episode, mean (SD), No. of persons | 1.4 (0.6) [64] | 1.4 (0.6) [61] | 0.1 (–0.1 to 0.3) | Mean, 0.1 (–0.1 to 0.2) | .27 |
Abbreviations: EQ-5D-5L, EuroQol Group 5-Dimension Self-Report; ICECAP-O, Icepop Capability Measure for Older People; IRR, incidence rate ratio; OR, odds ratio.
Column includes adjusted mean difference, adjusted OR, and adjusted IRR values and 95% CIs (indicated as mean, OR, and IRR).
Column includes adjusted mean difference, adjusted OR, and adjusted IRR values and 95% CIs (indicated as mean, OR, and IRR).
Responses by proxy were completed by relatives, consultees, (or the legal representative or guardian) on behalf of participants without capacity to self-report.
EQ-5D index values range from −0.594 to 1 (higher score indicates better health utility), and EQ-5D health status values range from 0 to 100 (higher score indicates better overall health).
Indicates transformed outcome (power of 2).
ICECAP-O score ranges from 0 to 1 (higher score indicates higher capability).
Analysis for this category included 152 participants in the probiotic group and 153 in the placebo group.
There were no statistically significant between-group differences for being hospitalized at least once during the postrandomization study period (42/152 [27.6%] in probiotic group vs 36/153 [23.5%] in placebo group; absolute percentage risk difference, –4.1% [95% CI, –13.9% to 5.7%]; adjusted OR, 1.25 [95% CI, 0.74 to 2.11]; P = .41), number of hospital stays (mean [SD], 0.4 [0.7] in the probiotic group vs 0.3 [0.6] in the placebo group; absolute difference, 0.08 [95% CI, –0.06 to 0.22]; adjusted IRR, 1.17 [95% CI, 0.72 to 1.90]; P = .53), cumulative number of hospital days (mean [SD], 4.5 [12.5] days in the probiotic group vs 5.4 [19.4] days in the placebo group; absolute difference, 0.9 days [95% CI, –2.77 to 4.57 days]; adjusted IRR, 1.00 [95% CI, 0.43 to 2.29]; P >.99), or death (33/155 [21.3%] in the probiotic group vs 32/155 [20.6%] in the placebo group; absolute percentage risk difference, –0.6% [95% CI, –9.7% to 8.4%]; AOR, 1.03 [95% CI, 0.59 to 1.80]; P = .90) (Table 3). Similarly, there were no statistically significant between-group differences for incidence of antibiotic-associated diarrhea (mean 0.8 vs 0.6; absolute difference, 0.2 [95% CI, –0.16 to 0.50]; adjusted IRR, 1.39 [95% CI, 0.79 to 2.46]; P = .25), and cumulative days of antibiotic-associated diarrhea (mean 6.8 days vs 4.4 days; absolute difference, 2.4 days [95% CI, –2.00 to 6.71 days]; adjusted IRR, 1.83 [95% CI, 0.95 to 3.54]; P = .07) (Table 3).
There were no statistically significant between-group differences with regards to Enterobacterales resistant to at least 1 of the tested antibiotics in stool samples at 3 months (37/55 [67.3%] in the probiotic group vs 39/52 [75.0%] in the placebo group; ARD, 7.7% [95% CI, –9.5% to 25.9%]; AOR, 0.61 [95% CI, 0.24 to 1.56]; P = .30), at second follow-up (23/33 [69.7%] in the probiotic group vs 19/27 [70.0%] in the placebo group; ARD, 0.7% [95% CI, –22.6 to 24.0%]; AOR, 0.76 [95% CI, 0.20 to 2.89]; P = .68), in the presence of oral candida at 3 months (88/113 [77.9%] in the probiotic group vs 80/105 [76.2%] in the placebo group; ARD, –0.2% [95% CI, –11.3 to 10.9%]; AOR, 1.23 [95% CI, 0.54 to 2.83]; P = .62), at second follow-up (70/85 [82.4%] in the probiotic group vs 57/76 [75.0%] in the placebo group; ARD, –7.4% [95% CI, –20.0% to 5.3%]; AOR, 1.27 [95% CI, 0.50 to 3.21]; P = .62). Analysis of the outcome measures related to candidiasis are provided in eTable 4 in Supplement 2. Three stool samples were positive for vancomycin-resistant Enterococci at baseline, 3 months, and at the final follow-up time point. Further details of analysis of microbiology outcome measures are provided in eTable 5 in Supplement 2.
At 3 months postrandomization, 7 of the 107 stool samples tested (6.5%) were positive for C difficile, with a greater number detected in samples belonging to care home residents randomized to the probiotic group than the placebo group (6/55 [10.9%] vs 1/52 [1.9%]; ARD, –9.0% [95% CI, –18.4% to 0.4%]; AOR, 6.51 [95% CI, 0.75 to 56.57]; P = .09). At the second follow-up, 2 of 64 samples tested (3.1%) yielded C difficile. Both of these samples were from care home residents randomized to the probiotic group.
Subgroup Effects
There were no statistically significant different intervention effects for any of the prespecified subgroups. Further details are provided in eTable 6 in Supplement 2.
Adverse Events
A total of 120 care home residents experienced 283 adverse events (150 adverse events in the probiotic group and 133 in the placebo group). Hospitalizations accounted for 94 events in the probiotic group and 78 events in placebo group, and deaths accounted for 33 of the events in the probiotic group and 32 of the events in the placebo group (Table 3).
Three trial-related adverse events were identified and all were in the placebo group; study product was stopped because of choking risk for 1 participant, because of participant-reported worsening diarrhea for another, and because of participant-reported bloating for the third.
Discussion
This double-blind, placebo-controlled clinical trial found that the administration of a daily dose of the probiotic combination, Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 to care home residents did not result in significantly fewer cumulative systemic antibiotic administration days for all-cause acute infections.
Prior studies of probiotics have produced contradictory findings and have been criticized for poor design, selective reporting, poorly described and verified outcomes, inadequate reporting of harms, and poor ascertainment of outcomes.3 In this trial, a registered nurse, blind to randomization status, visited study participants each week to complete participant diary data from multiple sources, with data for only 1.3% of eligible study days missing, and probiotic organisms were identified more often and in greater counts in the stool of care home residents in the probiotic group.
A recent meta-analysis on the effectiveness of probiotics in preventing infections in older adults included 15 studies covering 5916 participants with a mean age of 75.21 years.18 Three of the included studies recruited institutionalized older adults: Mañé and colleagues19 randomized 50 participants to receive a low or high daily dose of Lactobacillus plamtarum or placebo for up to 12 weeks and found that the high dose significantly increased the percentages of markers of immunogenicity and significantly lowered incidence of infections. Van Puyenbroeck and colleagues20 randomized 737 nursing home residents to receive a fermented milk containing Lactobacillus casei Shirota or placebo for 176 days and found no significant effect on the number of days with respiratory symptoms or anti-influenza antibody titers after influenza vaccination. Nagata and colleagues21 randomized 72 residents and staff members of facilities for older adults to receive Lactobacillus casei Shirota in fermented milk or placebo each day for 6 months and found a lower incidence of fever and improved bowel movements in those taking the probiotic. The authors of the review concluded that the overall quality of evidence was poor, that the evidence did not support the use of probiotics for reducing infections in older adults, that safety outcomes were similar between probiotics and placebo, and that more research was needed.18
A subsequent, double-blind, placebo-controlled pilot trial of Lactobacillus rhamnosus GG or placebo daily for 6 months to prevent respiratory infections in 209 nursing home residents identified laboratory-confirmed respiratory viral infections in 14 (15.0%) and 21 (22.9%) in the placebo and probiotic groups respectively, and called for a larger trial.5 A large trial of hospitalized patients found no benefit from short term lactobacilli and bifidobacteria with regard to antibiotic associated diarrhea,22 which conflicted with findings from several systematic reviews.23
This trial found no beneficial effect of probiotic use compared with placebo on antibiotic use overall or for the main categories of infections that commonly affect the population studied, duration of infections, health utility and well-being, hospitalizations, death, antibiotic-associated diarrhea, or carriage of antibiotic-resistant stool organisms. However, participants who were randomized to the probiotic group were administered significantly more antibiotics for lower respiratory tract infections, had small but statistically significant lower self-reported generic well-being/capability scores at 3 months, and a prespecified sensitivity analysis found a significant increase in cumulative systemic antibiotic days. These findings should be interpreted with caution, given multiple testing. However, this study does not rule out harm from probiotics. Certain probiotics may delay the return of the host gut microbiome to its normal state after antibiotic treatment,24 and a retrospective single-center study found probiotic exposure was associated with C difficile infection in hospitalized patients.25
Limitations
This study has several limitations. First, although all care home residents remaining in the trial were followed up for at least 6 months, some had their follow-up truncated before the originally planned 12 months due to longer than expected study set-up.
Second, a higher than expected proportion of stool cultures were positive for the study probiotics at baseline, and probiotic organisms were isolated from some of the stool samples obtained from the placebo group at follow-up, albeit at low counts. More sensitive microbiological techniques may partially explain isolation of these organisms at low counts. Exposure to the probiotic organisms in the placebo group would dilute any between-group differences in outcomes.
Third, infection related outcomes were not based on standard definitions, as presentation of infections in this population is often nonspecific, and care home residents were not tested for etiology using microbiological sampling. This may limit generalizability of some secondary outcomes.
Fourth, given a lower than expected event rate, this study was underpowered to detect statistical significance for the minimal clinically important difference in the primary outcome. Fifth, these findings are not necessarily generalizable to other probiotics or probiotic combinations or applicable to other populations since the effects of probiotic supplementation may be strain specific and vary according to setting, immune status, and age.
Conclusions
Among care home residents in the UK, a daily oral probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp lactis BB-12 did not significantly reduce antibiotic administration for all-cause infections. The findings do not support the use of probiotics in this setting.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Eligibility Criteria
eAppendix 2. Further Details of Trial Intervention
eAppendix 3. Details on Derivation of Outcome Measures
eAppendix 4. Further Details of Statistical Analyses
eTable 1. Analysis of Primary Outcome Measure With Modified Definitions
eTable 2. Analysis of Primary Outcome Measure Accounting for Study Product Adherence
eTable 3. Analysis of Primary Outcome Measure Accounting for Missing Data
eTable 4. Between-Arm Differences for Amount of Oral Candidiasis Outcome Measures*
eTable 5. Between-Arm Differences for Microbiology Outcome Measures*
eTable 6. Subgroup Effects for Cumulative Antibiotic Administration Days (Primary Outcome)
eFigure 1. Cumulative Antibiotic Administration Days by Group (N=305)
eFigure 2. Mean Duration of All-Cause Infections (Days) by Group (N=305)*
Data Sharing Statement
References
- 1.Global Market Insights Inc Probiotics Market Size to Exceed USD 64 Billion by 2023. Published 2016. Accessed October 06, 2019. https: //www.prnewswire.com/news-releases/probiotics-market-size-to-exceed-usd-64-billion-by-2023-global-market-insights-inc-578769201.html
- 2.Grand View Research US Hospital and Nursing Home Probiotics Market Size, Share & Trends Analysis Report By Channel (Hospitals, Nursing Homes), by Function (Gut Health, Immunity, Wellness), and Segment Forecasts, 2018-2025. Published 2018. Accessed 06 October 2019. https://www.grandviewresearch.com/industry-analysis/us-hospital-nursing-home-probiotics-market
- 3.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716-729. doi: 10.1038/s41591-019-0439-x [DOI] [PubMed] [Google Scholar]
- 4.Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B. Probiotic approach to prevent antibiotic resistance. Ann Med. 2016;48(4):246-255. doi: 10.3109/07853890.2016.1161232 [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Hylwka T, Smieja M, Surrette M, Bowdish DME, Loeb M. Probiotics to prevent respiratory infections in nursing homes: a pilot randomized controlled trial. J Am Geriatr Soc. 2018;66(7):1346-1352. doi: 10.1111/jgs.15396 [DOI] [PubMed] [Google Scholar]
- 6.King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? a systematic review and meta-analysis. Eur J Public Health. 2019;29(3):494-499. doi: 10.1093/eurpub/cky185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41-54. doi: 10.1017/S0007114514000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie D, Hood K, Bayer A, et al. Antibiotic prescribing and associated diarrhoea: a prospective cohort study of care home residents. Age Ageing. 2015;44(5):853-860. doi: 10.1093/ageing/afv072 [DOI] [PubMed] [Google Scholar]
- 9.Vihta KD, Stoesser N, Llewelyn MJ, et al. Trends over time in Escherichia coli bloodstream infections, urinary tract infections, and antibiotic susceptibilities in Oxfordshire, UK, 1998-2016: a study of electronic health records. Lancet Infect Dis. 2018;18(10):1138-1149. doi: 10.1016/S1473-3099(18)30353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen-Jones E, Lowe R, Lown M, et al. Protocol for a double-blind placebo-controlled trial to evaluate the efficacy of probiotics in reducing antibiotics for infection in care home residents: the Probiotics to Reduce Infections iN CarE home reSidentS (PRINCESS) trial. BMJ Open. 2019;9(6):e027513. doi: 10.1136/bmjopen-2018-027513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couzner L, Crotty M, Norman R, Ratcliffe J. A comparison of the EQ-5D-3L and ICECAP-O in an older post-acute patient population relative to the general population. Appl Health Econ Health Policy. 2013;11(4):415-425. doi: 10.1007/s40258-013-0039-8 [DOI] [PubMed] [Google Scholar]
- 12.Cook JA, Julious SA, Sones W, et al. DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. BMJ. 2018;363:k3750. doi: 10.1136/bmj.k3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Velden AW, Pijpers EJ, Kuyvenhoven MM, Tonkin-Crine SK, Little P, Verheij TJ. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract. 2012;62(605):e801-e807. doi: 10.3399/bjgp12X659268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J Antimicrob Chemother. 2007;60(1):92-99. doi: 10.1093/jac/dkm141 [DOI] [PubMed] [Google Scholar]
- 15.Smieszek T, Pouwels KB, Dolk FCK, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(suppl_2):ii36-ii43. doi: 10.1093/jac/dkx500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SC. Reducing inappropriate prescribing of antibiotics in English primary care: evidence and outlook. J Antimicrob Chemother. 2018;73(4):833-834. doi: 10.1093/jac/dkx535 [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency Committee for Medicinal Products for Human Use ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. Published February 17, 2020. Accessed October 6, 2019. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical-principles_en.pdf
- 18.Wachholz PA, Nunes VDS, Polachini do Valle A, Jacinto AF, Villas-Boas PJF. Effectiveness of probiotics on the occurrence of infections in older people: systematic review and meta-analysis. Age Ageing. 2018;47(4):527-536. doi: 10.1093/ageing/afy006 [DOI] [PubMed] [Google Scholar]
- 19.Mañé J, Pedrosa E, Lorén V, et al. A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects: a dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutr Hosp. 2011;26(1):228-235. [PubMed] [Google Scholar]
- 20.Van Puyenbroeck K, Hens N, Coenen S, et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95(5):1165-1171. doi: 10.3945/ajcn.111.026831 [DOI] [PubMed] [Google Scholar]
- 21.Nagata S, Asahara T, Wang C, et al. The effectiveness of lactobacillus beverages in controlling infections among the residents of an aged care facility: a randomized placebo-controlled double-blind trial. Ann Nutr Metab. 2016;68(1):51-59. doi: 10.1159/000442305 [DOI] [PubMed] [Google Scholar]
- 22.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257. doi: 10.1016/S0140-6736(13)61218-0 [DOI] [PubMed] [Google Scholar]
- 23.Butler CC, Duncan D, Hood K. Does taking probiotics routinely with antibiotics prevent antibiotic associated diarrhoea? BMJ. 2012;344:e682. doi: 10.1136/bmj.e682 [DOI] [PubMed] [Google Scholar]
- 24.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e1416. doi: 10.1016/j.cell.2018.08.047 [DOI] [PubMed] [Google Scholar]
- 25.Carvour ML, Wilder SL, Ryan KL, et al. Predictors of Clostridium difficile infection and predictive impact of probiotic use in a diverse hospital-wide cohort. Am J Infect Control. 2019;47(1):2-8. doi: 10.1016/j.ajic.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Eligibility Criteria
eAppendix 2. Further Details of Trial Intervention
eAppendix 3. Details on Derivation of Outcome Measures
eAppendix 4. Further Details of Statistical Analyses
eTable 1. Analysis of Primary Outcome Measure With Modified Definitions
eTable 2. Analysis of Primary Outcome Measure Accounting for Study Product Adherence
eTable 3. Analysis of Primary Outcome Measure Accounting for Missing Data
eTable 4. Between-Arm Differences for Amount of Oral Candidiasis Outcome Measures*
eTable 5. Between-Arm Differences for Microbiology Outcome Measures*
eTable 6. Subgroup Effects for Cumulative Antibiotic Administration Days (Primary Outcome)
eFigure 1. Cumulative Antibiotic Administration Days by Group (N=305)
eFigure 2. Mean Duration of All-Cause Infections (Days) by Group (N=305)*
Data Sharing Statement