Abstract
Aim:
To explore fecal short-chain fatty acids and neurotransmitter alterations in a mouse–glioma model and glioma patients.
Methods:
Liquid chromatography–mass spectrometry and 16S rRNA-sequencing from fecal samples were performed to measure metabolite levels and taxa abundance in mice/humans. Mice underwent GL261 implantation with/without temozolomide. Glioma patients were compared with healthy controls.
Results:
Glioma altered several short-chain fatty acids and neurotransmitter levels. Reduced 5-hydroxyindoleaceic acid and norepinephrine levels were seen in mice and humans. Interestingly, temozolomide treatment abrogates the effects of glioma on fecal metabolites.
Conclusion:
Our findings demonstrate the interplay between glioma and the gut–brain axis. Further work is required to identify pathways within the gut–brain axis by which glioma influences and promotes the modulation of fecal metabolites and microbiome.
Keywords: : 5-HIAA, 5-hydroxyindoleacetic acid, fecal metabolites, glioblastoma, glioma, microbiome, norepinephrine, serotonin, temozolomide
Gliomas are the most common primary malignant tumor of the CNS, with 50% of patients presenting with the most aggressive form of the disease: glioblastoma [1]. Despite research and surgical advances over the last two decades, the median survival remains poor [1–5].
The human gastrointestinal tract harbors millions of microorganisms with diverse characteristics and functions with the relative composition dependent on genetic and environmental factors [6]. The gut microbiome plays an important role in intestinal metabolism, intestinal barrier integrity, antigenic diversity and immunity [7,8]. Recent studies suggest that certain microbes may confer susceptibility to cancers and may also influence response to therapies through humoral, immunologic and metabolomic pathways; however, our understanding of the effects of the microbiome is far from complete [9].
Metabolites are small molecules that are intermediates or products of biochemical reactions, such as glycolysis or synthesis of cellular macromolecules [10]. Short-chain fatty acids (SCFAs), of which 95% consists of acetate, propionate and butyrate, have been implicated in a variety of physiological processes and (neuro) immune functions [6,11]. In animal models, decreased gastrointestinal SCFA levels have been associated with Alzheimer's disease and chronic stress [12,13]. In humans, alterations of fecal SCFA levels have been reported in various neurological conditions, including stroke [14,15]. Such data implicate SCFAs as a possible key player in microbiota–gut–brain axis communication.
Prior studies have demonstrated that bacteria respond to host neurotransmitter signaling [16]. The gut microbiome has been shown to synthesize and respond to several key neurotransmitters (e.g., serotonin [5-hydroxytryptamine], GABA, norepinephrine, epinephrine) which are involved in various host functions such as mood, behavior and cognition [6,17]. Many of these host- and microbial-derived neuroactive molecules are also important signaling molecules in host–microbiota interactions at the intestinal interface [6].
In observational studies, the microbiome and fecal metabolites have been shown to confer susceptibility to certain CNS diseases and cancer, and may potentially influence the response to therapies [6,9,18–20]. However, the role of these metabolites and the microbiome in CNS malignancies, particularly gliomas, are unknown. The goal of this preliminary study was to explore alterations in fecal SCFAs and neurotransmitters in a glioma mouse model and a preliminary human glioma cohort. We hypothesize that glioma growth may induce changes in the fecal composition of various metabolites, including neurotransmitters.
Methods
Animals
20 month old male C57BL/6 mice from the National Institute on Aging (MD, USA) were allowed to acclimate for a minimum of 4 weeks before use. All mice were housed in a temperature- and humidity-controlled vivarium, five per cage (11” L, 6" W, 6" H) with a 12 hour light/dark schedule with ad libitum access to food and water. All experimental work was approved by The University of Texas Health Science Center at Houston, Center for Laboratory Animal Medicine and Care (TX, USA).
Alterations in the expression of fecal metabolites in the setting of glioma +/- oral temozolomide (TMZ) were evaluated using 15 mice assigned to four experimental groups (first experiment). Group 1: Sham/Saline (n = 3), Group 2: Glioma/Saline (n = 4), Group 3: Glioma/TMZ 5 mg/kg (n = 4) and Group 4: Glioma/TMZ 25 mg/kg (n = 4). All animals were housed in cages according to treatment groups. Tumor implantation was performed on day 0. Starting on day 14, mice were orally gavaged with TMZ or dimethyl sulfoxide (DMSO) in saline, 5 days/week (2 days off) for 3 weeks. Fecal samples were collected and stored in sterile tubes at –80°C prior to tumor implantation (first sample), prior to TMZ/Saline treatment (second sample) and at sacrifice/post treatment (third sample).
To determine the effect of oral TMZ on fecal metabolites and microbiome composition (second experiment), nine tumor naïve mice were divided into two groups: Group 1: Oral Saline (n = 4) and Group 2: Oral TMZ 5 mg/kg (n = 5). Mice were orally gavaged with TMZ 5 mg/kg or DMSO in saline 5 days/week (2 days off) for 3 weeks. Fecal samples were collected at day 0 and prior to sacrifice.
Per Institutional Animal Care and Use Committees protocol, any animal displaying signs of significant weight loss (>10% from baseline) or immobility were euthanized. All surviving animals were sacrificed on day 42 and 21 at the experimental endpoint (first and second experiments, respectively) with an intraperitoneal injection of 0.1μl/10g body weight dose of Tribromoethanol (Avertin; Sigma-Aldrich, MO, USA) dissolved in 2-Methyl-2-Butanol. Stool samples were collected from the large intestine (cecum) at the time of sacrifice. Fresh major organs (including the brain) were stored in formalin and processed for paraffin sections and Hematoxylin & Eosin staining. Brains that were implanted with GL261 cells were macroscopically and histologically examined by a certified neuropathologist blinded to the treatment group to confirm the presence of tumor.
GL261 mouse model of glioma
Mice were implanted with GL261 cells maintained in Kaur's laboratory (Courtesy of Dr. Maria Castro, University of Michigan, MI, USA). GL261 is an invasive but nonmetastatic murine glioma model with high tumor take rate that harbors both p53 and K-ras mutations [21]. GL261 cells were cultured in Dulbecco's Modified Eagle's Minimal with 10% fetal bovine serum (Sigma-Aldrich), penicillin and streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Mice were anesthetized and stabilized in a stereotactic frame, a burr hole was drilled 2 mm lateral and 1 mm anterior to the bregma in the right hemisphere, to a depth of 3.5 mm [22]. GL261 (1 × 105 cells) in 2 μl of Hank's buffered salt solution or sham-treated with the same amount of solution were implanted over 5 minutes using autoinjectors.
A total of 100 mg of TMZ (Sigma-Aldrich) was dissolved in 1.5 μl of DMSO (Sigma-Aldrich) and sonicated three times. The solution was further dissolved in 38.5 μl of sterile saline for a final working concentration of TMZ of 2.5 mg/μl.
Patient characteristics & fecal sample collection
This pilot prospective study was approved by the institutional review board of the University of Texas Health Science Center at Houston and Memorial Hermann Hospital, Houston (TX, USA). The study was conducted from January 2018 to July 2019. Patients for which a fecal sample was obtained prior to surgical resection for newly diagnosed gliomas at our institution were included in the study. Patients with recent antibiotic exposure (30 days) for other conditions, under 18 year of age, history of other cancers or gastrointestinal diseases (e.g., inflammatory bowel disease) were excluded from the study. Patients’ age, sex, race, Karnofsky performance score (KPS), body mass index (BMI), diagnosis (2016 WHO classification of brain tumors) [23], tumor volume, adjuvant therapy, progression and survival were obtained from the electronic medical record and stored in a prospective REDCap database (Table 1). Patients underwent biopsy or maximum safe tumor resection at the discretion of the treating neurosurgeon, followed by radiation therapy with concomitant TMZ (75 mg/m2) [4]. IDH1 p.R132H immunohistochemistry was performed with a mutant protein-specific antibody (1:40; H09 monoclonal; Dianova, Hamburg, Germany) in a Dako Omnis (Agilent Technologies, CA, USA) autostainer. Fecal samples were collected before resection (Pre-Sx) and prior to a prophylactic intraoperative single dose of cephalosporin. Six household family members or nonrelated individuals were utilized as controls. Fecal samples for metabolomic analysis and 16S rRNA sequencing were collected using microbial collection and stabilization kits (OMNIgene Gut; DNA Genotek, Kanata, Canada).
Table 1. . Demographic and clinical characteristics of patients.
| Pt's ID | Sex | Age | Race | Pre-op KPS | Dx | WHO grade | IDH | Tumor location |
|---|---|---|---|---|---|---|---|---|
| G1 | M | 76 | A | 60 | GBM | 4 | WT | Parietal |
| G2 | F | 18 | H | 80 | DA | 2 | WT | Temporal-Insular |
| G3 | F | 70 | W | 80 | GBM | 4 | WT | Frontal |
| G4 | M | 29 | H | 80 | GBM | 2 | Mut | Fronto-Parietal |
| G5 | F | 53 | W | 70 | GBM | 4 | WT | Frontal |
| G6 | M | 64 | H | 40 | GBM | 4 | WT | Temporal |
| G7† | M | 31 | W | 60 | GBM | 4 | WT | Frontal |
| G8† | M | 69 | W | 70 | GBM | 4 | WT | Parieto-Occipital |
| G9† | M | 21 | W | 90 | GBM | 4 | WT | Temporal |
| G10† | F | 60 | A | 90 | AA | 3 | WT | Parietal |
These patients only had a pre-treatment (second) and post treatment (third) sample, thus they were only use for metabolite analysis following treatment.
A: Asian; AA: Anaplastic astrocytoma; DA: Diffuse astrocytoma; Dx: Diagnosis; F: Female; G: Glioma; GBM: Glioblastoma; H: Hispanic; KPS: Karfnosky performance status; M: Male; Mut: Mutant; W: White nonhispanic; WHO: World Health Organization; WT: Wild-type.
Neurotransmitters & SCFAs extraction & analysis
Targeted Metabolic profiling of neurotransmitters and SCFA was performed at the Alkek Center for Molecular Discovery at Baylor College of Medicine, Houston (TX, USA), as previously described [24,25]. We identified 26 and 23 metabolites in mice and humans, respectively. Briefly, fecal neurotransmitters were obtained after the addition of 4:3:2 methanol: chloroform: water. The organic and aqueous layers were collected, dried and resuspended with 1:1 water: methanol and were subjected to liquid chromatography–mass spectrometry. The neurotransmitters were separated using High-performance liquid chromatography (HPLC) column Zorbax eclipse XDB C-18, 1.8 microns, 4.6 × 100 mm (Agilent Technologies). Neurotransmitters were measured in electrospray ionization positive mode using a 6490 triple quadrupole mass spectrometer coupled to an HPLC system (Agilent Technologies) with multiple reaction monitoring. The acquired data were analyzed using Agilent mass hunter quantitative software. (Agilent Technologies).
The SCFA were analyzed by derivatization procedures. After the addition of 500 μl of acetonitrile, homogenization and supernatant collection, a 2:1 supernatant:200 mM 12C6-3NPH and 120 mM EDC were added and incubated. The mixture was cooled and reconstituted with a 10% aqueous acetonitrile. 10 μl of the solution injected into liquid chromatography/tandem mass spectrometry using acquity UPLC HSS T3 1.8 um (2.1 × 100 mM) (Waters Corporation, MA, USA). The SCFA were measured in electrospray ionization negative mode using a 6490 triple quadrupole mass spectrometer coupled to an HPLC system (Agilent Technologies) with multiple reaction monitoring. The acquired data were analyzed using Agilent mass hunter quantitative software (Agilent Technologies).
Microbial DNA extraction & 16S rRNA gene sequencing
16S rRNA gene compositional analysis provides a summary of the composition and structure of the bacterial component of the microbiome. Genomic bacterial DNA extraction methods were optimized to maximize the yield of bacterial DNA while keeping background amplification to a minimum. 16S rRNA gene sequencing methods were adapted from the methods developed for the Earth Microbiome Project and NIH-Human Microbiome Project and performed at the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine, Houston (TX, USA), as previously described [26,27]. A custom script that constructs an operational taxonomic unit table from the output files was used for downstream analyses using a visualization toolkit developed at the Alkek Center named ATIMA (Agile Toolkit for Incisive Microbial Analyses).
Statistical analysis
The ATIMA software was used for the analysis of 16S rRNA sequencing. ATIMA is an R software suite combining publicly available packages (i.e., APE and VEGAN) and purpose written code to import sample data and identify trends in taxa abundance and other variables. For neurotransmitters and SCFA metabolites analysis, the data were log2-transformed and normalized with internal standard per-sample, per-method basis. For every metabolite in the normalized dataset, two-sample t-tests were conducted to compare expression levels between different groups. Differential metabolites were identified by adjusting the p-values for multiple testing at an false discovery rate (FDR) threshold of <0.25. For individual metabolites comparison, scattered plots were performed in Prism v 8.4.1 (GraphPad, CA, USA).
Results
Glioma alters murine fecal metabolites
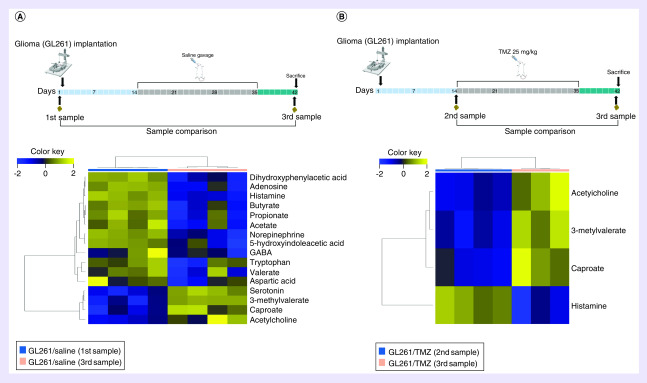
Comparison of samples collected prior to tumor implantation (first sample-baseline) versus samples collected at the time of sacrifice (third sample) in GL261/Saline mice revealed that 12 metabolites were decreased and four metabolites were increased after tumor development, Figure 1A. Metabolites with reduced levels following glioma growth included dihydroxy phenyl acetic acid (DOPAC), adenosine, histamine, butyrate, propionate, acetate, norepinephrine, 5-hydroxyindoleacetic acid (5-HIAA), GABA, tryptophan, valerate and aspartic acid. In contrast, serotonin 3-methyl valerate, caproate and acetylcholine were increased after tumor growth.
Figure 1. . Alterations in fecal metabolites in mice following glioma growth with and without temozolomide.
(A) Alterations in fecal metabolites following glioma growth. Heat map of unsupervised hierarchical clustering showing fecal metabolite levels between the first sample (n = 4) and third sample (n = 4) in the GL261/Saline group. There were four increased and 12 decreased fecal metabolites following glioma growth (FDR < 0.25). (B) Changes in fecal metabolites following glioma growth in the setting of TMZ treatment. Heat map of unsupervised hierarchical clustering showing fecal metabolite levels before (second sample, n = 4) and after (third sample, n = 3, fecal quantity not sufficient for analysis in one mouse) in the GL261/TMZ (25 mg/kg) group. There were only three increased and one decreased fecal metabolite. Metabolite changes are fewer in the setting of TMZ. (FDR < 0.25).
FDR: False discovery rate; TMZ: Temozolomide.
Additional comparison of all baseline samples (to increase sample size) from this experimental group compared with the third sample of the GL261/Saline mice demonstrated consistent findings (Supplementary Figure 1).
Glioma-Induced fecal metabolite changes in the setting of TMZ in mice
To investigate alterations in fecal metabolites in the setting of TMZ, mice were orally gavaged with TMZ for 3 weeks after tumor implantation. Fecal metabolite analysis was performed between the second and third sample (before and after TMZ) in the GL261/TMZ group demonstrating an increase in 3 metabolites (acetylcholine, 3-methyl valerate, caproate) and decrease in histamine at sacrificed (third sample), Figure 1B. Moreover, the fecal metabolite analysis performed between the first and third sample (before tumor implantation and after TMZ) did not reveal statistically significant differences in mice treated with TMZ, contrasting the changes observed in the GL261/Saline group (Supplementary Figure 2). No significant differences in weight loss were found between GL261/Saline and GL261/TMZ. A comparison between mice undergoing oral gavage with either saline or TMZ (second experiment) did not reveal significant differences of fecal metabolites at the administered dose (5 mg/kg).
Effects of glioma & TMZ in the murine fecal microbiome
A comparison between mice at baseline (first sample, n = 14), GL261 implanted mice before chemotherapy (second sample, n = 8), GL261/Saline (third sample, n = 4) and GL261/TMZ (third sample, n = 4) demonstrated no significant differences in α-diversity operative taxonomic units (p = 0.38) or Shannon Diversity Index (p = 0.12). Principal coordinate analysis (PCoA) demonstrated significant β-diversity changes between these groups (Weighted Bray-Curtis PCoA p = 0.0001).
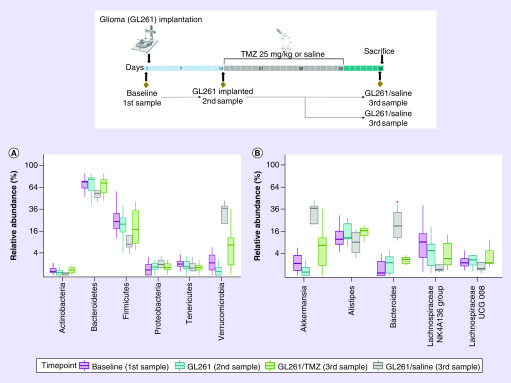
Taxa relative abundance differences at the phylum level revealed a non-significant reduction in both Bacteroidetes and Firmicutes with an increased abundance of Verrucomicrobia after tumor growth, Figure 2A. At the genus level, contrary to its phylum the relative abundance of Bacteroides significantly increased after tumor development (p = 0.04), Figure 2B. Also, the Akkermansia genus from the Verrucomicrobia phylum was increased after tumor growth (1.2% Baseline vs 37% GL261/Saline at sacrifice); however, this was not statistically significant (Figure 2B). Interestingly, in the setting of TMZ (GL261/TMZ third sample), the glioma induced microbiome changes were diminished, reaching similar levels as the baseline (first sample) or following glioma implantation (second sample), Figure 2A & B.
Figure 2. . Alterations in fecal microbiome following glioma growth with and without temozolomide.
(A) Taxa at the Phylum level. Relative abundance levels of Bacteroidetes and Firmicutes are decreased and Verrucomicrobia increased following tumor growth; however, these changes are diminished in the GL261/TMZ group. (B) Taxa at the Genus level. Relative abundance levels of Akkermansia and Bacteroides levels are increased after tumor growth; however, these changes are diminished in the GL261/TMZ group. Baseline of all mice in the first experiment (first Sample, n = 14), GL261 bearing mice before treatment (second Sample, n = 8), GL261/Saline at sacrifice (third Sample, n = 4) and GL261/TMZ 25 mg/kg at sacrifice (third Sample, n = 4). A red asterisk marks the significant changes.
TMZ: Temozolomide.
Glioma induced changes in fecal metabolites & microbiome in humans
Patients included in the study were diagnosed according to the WHO 2016 classification by a neuropathologist as glioblastoma, IDH-wild type (GBM IDH-WT) (n = 4), glioblastoma, IDH-mutant (GBM IDH-Mut) (n = 1) and diffuse astrocytoma IDH-WT (n = 1). The median follow-up for the cohort in this preliminary study was 14.2 months. The demographic and clinical characteristics of patients are depicted in Table 1.
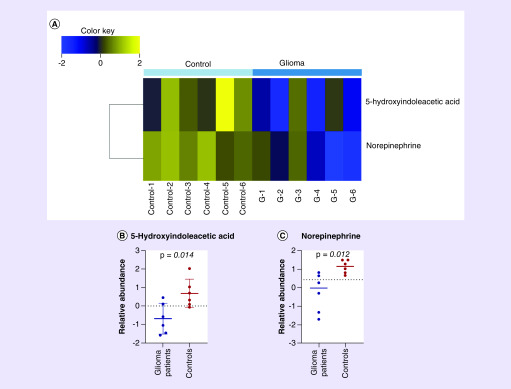
Fecal samples for metabolite analysis from six glioma patients were collected prior to surgical resection (first sample) and compared with six control samples. There was no significant difference in the median age of both groups (glioma, 58.5- vs Control, 62 years). Demographic characteristics of the control group are displayed in Supplementary Table 1. Fecal metabolite analysis in glioma cases demonstrated decreased levels of 5-HIAA and norepinephrine compared with controls, Figure 3A–C. Bacterial α-diversity, β-diversity and relative taxa abundance from the six glioma patients compared with controls did not reveal significant differences.
Figure 3. . Alterations in fecal metabolites of glioma patients and controls.
(A) Heat map of unsupervised hierarchical clustering showing fecal metabolites levels demonstrating decrease 5-hydroxyindoleaceic acid and norepinephrine in stool samples from glioma patients before surgical resection compared with controls. (B) Scattered plot of fecal 5-hydroxyindoleaceic acid showing decrease level in glioma patients before surgical resection compared with controls. (C) Scattered plot of fecal norepinephrine showing decrease level in glioma patients before surgical resection compared with controls. Glioma patients n = 6. Controls n = 6. (False discovery rate < 0.25).
Fecal samples for metabolite analysis from ten glioma patients before and after chemoradiotherapy (second and third samples) were compared. No significant differences were found between this comparison.
Discussion
In the present study, we investigated for the first-time fecal SCFAs and neurotransmitter changes in a GL261 murine glioma model, as well as in a preliminary prospective cohort of adult glioma patients. Additionally, we also evaluated changes in these metabolites in the setting of oral TMZ. Our murine experiments found 16 differentially expressed metabolites following glioma growth.
The immune and inflammatory response that occurs following blood–brain barrier (BBB) disruption induced by tumor growth is likely to affect the sympathetic and parasympathetic modulation of the enteric nervous system, as observed in other diseases such as stroke [28]. These systems have a critical role in modulating gut function, including regulation of mucus and fluid production, acid secretion, mucosal immune response, secretion of antimicrobial peptides, release of neurotransmitters into the gut and modifying the access of luminal bacteria and antigens to immune cell in the gut [6,28].
SCFAs are almost exclusively derived from bacterial metabolism in the gut [28]. Changes in both microbiome and SCFAs’ have established the role of the microbiome-gut–brain axis in various neurological conditions [6,15,28]. SCFAs influence the immune system and have been implicated in the regulation of neutrophil chemotaxis, induction of regulatory T cell development, IL-10 secretion, inhibition of NF-κ B and the suppression of cytokine production from myeloid cells. [6,28–31].
In this study, we found that propionate, butyrate and acetate, the three most abundant SCFAs, all decrease following glioma growth (Figure 1A). While prior studies have established a relationship between the gut microbiome, SCFA and cancer development [32,33], such a relationship has not yet been defined in CNS tumors. Changes in SCFA after glioma development appear to be due to the effect of CNS damage and BBB disruption that lead to changes in the microbial community via gut–bran axis interactions with a subsequent decrease in the production of bacterial SCFAs. Moreover, our results demonstrate that the extent of SCFAs changes in our murine glioma model were not observed in the setting of TMZ (Figure 1B). Such findings could be explained as a result of tumor control from chemotherapy rather than the intrinsic effect of the chemotherapy on the gut, as no metabolite changes were observed in either mice or humans following TMZ. However, these findings warrant further study to better understand the role of SCFA changes as signal molecules in glioma, the interplay with the immune system and the effects on tumor microenvironment, which has potential therapeutic implications.
In addition to the changes in SCFAs, our study demonstrated that fecal 5-HIAA and norepinephrine levels significantly decreased following glioma growth in mice and humans compared with controls. Interestingly, retrospective studies demonstrated a reduced incidence of glioma in patients on long-term therapy with tricyclic antidepressants, which work through reuptake inhibition of serotonin and norepinephrine allowing for increased local levels of these neurotransmitters [34]. Prior studies have demonstrated that gut luminal serotonin is regulated by the commensal microbiome in mice and that fecal levels changes correspond to brain tissue serotonin levels [35].
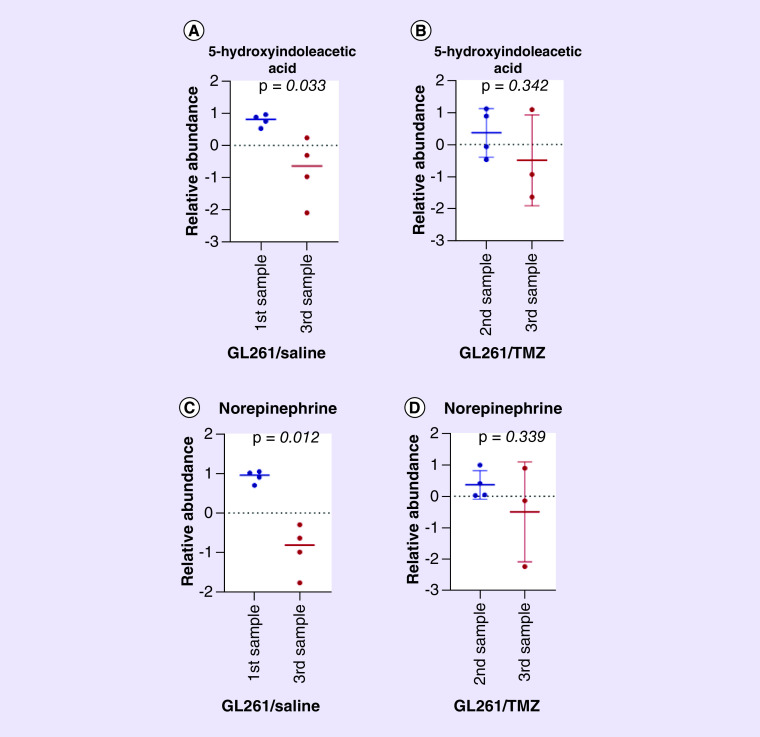
Recent studies demonstrate that genes related to dopamine and serotonin signaling influence survival in patients with GBM. These survival differences based on monoamine gene expression suggest that these signaling mechanisms may influence GBM growth and progression [36]. Serotonin has been shown to influence canonical growth pathways and affect cancer progression and oncogenesis in several types of tumors. Additionally, serotonin has a potential ability to influence blood flow and angiogenesis, which are critical factors affecting tumor growth in GBM [36]. In our study, fecal serotonin increased after tumor development and 5-HIAA was decreased in the mice glioma model. GL261 models have been reported to recapitulate histologic and biological characteristics of GBM [37]. Moreover, our results appear to be translational to humans as 5-HIAA levels were also decreased in glioma patients. These changes were not observed in the setting of TMZ treatment (Figure 3A & B and 4A & B).
Figure 4. . Alterations in fecal 5-hydroxyindoleaceic acid and norepinephrine in mice following glioma growth with and without temozolomide.
(A) Scatter plot showing decrease fecal 5-hydroxyindoleaceic acid levels following tumor growth in GL261/Saline mice. First sample n = 4 and third Sample n = 4. (B) Scatter plot showing no differences between the second sample (prechemotherapy) and third sample (postchemotherapy/sacrifice) in fecal 5-hydroxyindoleaceic acid levels in tumor-bearing mice treated with TMZ (GL261/TMZ) group. second Sample n = 4 and third Sample n = 3. (C) Scatterplot showing decrease fecal norepinephrine levels following tumor growth in GL261/Saline mice. first Sample n = 4 and third Sample n = 4. (D) Scatter plot showing no differences between the second Sample (prechemotherapy) and third Sample (postchemotherapy/sacrifice) in fecal norepinephrine levels in tumor-bearing mice treated with TMZ (GL261/TMZ) group. Second sample n = 4 and third sample n = 3. The fecal quantity was not sufficient for analysis in the third sample of one mouse in the GL261/TMZ group).
TMZ: Temozolomide.
5-HIAA, a major metabolite of serotonin, is formed by the mitochondrial enzyme monoamine oxidase [38]. Approximately 90% of the serotonin is synthesized in the gastrointestinal tract and regulates gastrointestinal, cardiac, respiratory and endocrine functions. Also, it crosses the BBB where it is involved in brain function [39]. A recent study evaluating the SERT protein, which plays a critical role in regulating the extracellular availability of serotonin in the gut and brain, and the microbiome found that SERT knockout mice displayed higher abundances of Bacilli species and lower abundances of Bifidobacterium species and Akkermansia muciniphilia [40]. Importantly, Bacilli is part of the Firmicutes phylum and Akkermansia muciniphila is the most important species of Akkermansia genus and Verrucomicrobia phylum. Our preliminary findings demonstrated decreased levels of Firmicutes and increased abundance of Akkermansia and Verrucomicrobia following tumor growth and significant increase in Bacteroides (Figure 2A & B). These microbiome alterations could drive the corresponding changes in fecal 5-HIAA levels (Figure 1A). It is well established that a variety of malignancies and CNS insults result in alterations to the gut microbiome, which in turn are able to modify peripheral immune cell and CNS function. Therefore, glioma is very likely to alter the gut microbiome, which will, in turn, modulate the colonic neurotransmitter production and de novo synthesis [41–43]. Studies utilizing germ-free mice found reduced serum and colonic levels of serotonin, suggesting that local environmental changes promote systemic changes. Furthermore, spore-forming bacteria, their metabolites and SCFAs can stimulate the production of serotonin from enterochromaffin cells [41,42]. However, further investigation is needed to identify the specificity of these changes.
Even though, no statistically significant differences between microbial taxa in humans were observed, there were significant differences in fecal 5-HIAA and norepinephrine between glioma patients and controls, which most likely correspond to subtle differences in the fecal microbiome that were enough to alter the colonic metabolite production and de novo synthesis, both known to be the effect of the gut microbiome [41–43]. However, larger studies to identify such microbial taxa are needed.
Catecholamines including norepinephrine are traditionally considered to be a part of the fight-or-flight stress response [44]. In addition, recent studies have demonstrated that catecholamines also modulate host–microbial interface. Interestingly, in germ-free mice, serum norepinephrine is increased when compared with controls [45]. As described above, increased norepinephrine through the activity of tricyclic antidepressants is implicated in limiting glioma growth thus, our finding of decreased norepinephrine may help in driving glioma growth and progression.
It has been demonstrated that the gut is a major source of early norepinephrine release during sepsis. This release initiates and helps to propagate the systemic inflammatory cascade driving increased TNF-α production and downstream inflammatory cytokines [46]. In our study, fecal norepinephrine was significantly decreased in tumor-bearing mice and humans with glioma, compared with controls (Figure 3A & C & 4C & D) suggesting that the tumor may be blunting the ’type 1’ inflammatory effects. The changes observed in fecal norepinephrine levels might be related to microbiome changes as observed in other metabolites. Fecal microbiome and metabolites changes may occur with CNS injury (BBB disruption) such as tumor growth, which may disrupt functions of the autonomic nervous system as well as the hypothalamic–pituitary–adrenal axis [47,48]. This plays a role in regulating gut motility, permeability, bicarbonate and acid secretion resulting in a dysbiosis. Alterations in microbial constitution have been shown to alter not only the local immune environment but also in the CNS [28,49,50], accelerating pathology or improving outcomes, such as the case of γδ T cells in ischemic stroke [51]. Furthermore, it is understood that microorganisms communicate with each other via the secretion of SCFAs, catecholamines, serotonin and other neurotransmitters. These signaling molecules are able to interact with receptors of vagal afferents [52,53], as well as diffuse into circulation where they may act in an endocrine fashion [47,54]. Additionally, differences have been observed in serum norepinephrine levels between germ-free and controls in previous studies [45].
While prior studies have demonstrated changes in gut microbiome following chemotherapy, particularly alkylating agents [55], our study did not reveal significant differences of fecal metabolites at the administered doses (5 mg/kg). However, further studies, including the utilization of higher doses in mice and larger human cohorts are required to evaluate microbiome and metabolite changes from oral TMZ.
Recent studies demonstrate how certain microbial strains affect survival in melanoma and epithelial tumors treated with immunotherapy [19,20]. It is conceivable that similar findings may occur in CNS malignancies and potentially affect survival, via immunomodulatory responses through metabolites, neurotransmitters and cytokines [29]. However, further study of the relationship between CNS malignancies, metabolites, microbiome and response to treatment are warranted. Future investigations should be directed to identify how specific fecal microbiome and metabolites interplay with the immune system and its effect on tumor growth, response to therapies and outcome; specifically, isolated depletion and reconstitution with individual microbial taxa.
We acknowledge several limitations in this preliminary study, including the relatively small number of humans and mice in the experimental groups, lack of circulating immune markers, blood and brain metabolites and absence of associations with outcomes and response to TMZ. Additionally, our study group was very heterogeneous in terms of sex, age, glioma subtype (IDH-Mut vs IDH-WT) and other factors that are known to affect the microbiome [56,57], which may account for the lack of significant differences found in our human cohort. However, the results in this study demonstrate for the first-time glioma induced changes in both SCFAs and neurotransmitters and demonstrate the well-established microbiome–gut–brain axis. Finally, we did not observe fecal metabolite changes at the TMZ administered doses, but fecal metabolite changes with higher TMZ doses cannot be excluded and deserves further study. Future studies with larger sample sizes are needed to identify differences in fecal microbiome and metabolites accounting for glioma subtypes, which have different behavior, growth patterns and prognosis.
Conclusion
Our preliminary findings demonstrate the interplay between glioma and the gut–brain axis in mice and humans. Several fecal metabolites are differentially expressed in mice following tumor growth. Norepinephrine and 5-HIAA are significantly decreased in humans with glioma, as well as in tumor-implanted mice. Interestingly, the modulation of fecal metabolites is likely driven by glioma feedback as treatment with TMZ, which limits glioma growth and abrogates the effects of glioma on murine fecal metabolites. Further work is required to identify the pathways within the microbiome-gut–brain axis that influence and promote the changes of fecal metabolites. Elucidating these pathways may provide additional therapeutic strategies to limit glioma growth and improve survival.
Summary points.
Glioma growth induces fecal metabolite alterations in a mouse GL261 model.
Short-chain fatty acids and neurotransmitters, including butyrate, propionate, acetate, norepinephrine and 5-hydroxyindoleaceic acid, were decreased following tumor growth.
Serotonin, 3-methyl valerate, caproate and acetylcholine increase following tumor growth.
Temozolomide diminished fecal metabolite changes in tumor-bearing mice.
5-Hydroxyindoleaceic acid and norepinephrine were decreased in tumor-bearing mice and glioma patients.
There is a decrease in Bacteroidetes and Firmicutes phyla levels and an increase in Verrucomicrobia phylum after tumor growth in mice.
There is an increased relative abundance of Akkermansia and Bacteroides genera.
Modulation of the microbiome could expand therapeutics options for glioma patients and deserves further study.
Supplementary Material
Acknowledgments
We would like to acknowledge our patients and family members for participating in the study. We also greatly appreciate the help of Memorial Hermann Hospital nurses.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/cns-2020-0007
Author contributions
Study design: Y Esquenazi and LY Ballester. Performed experiments: A Patrizz, N Putluri and Y Esquenazi. Human samples and data collection: A Dono, A Patrizz and Y Esquenazi. Analysis of data: A Dono, N Putluri and Y Esquenazi. Data interpretation: A Dono, RM McCormack, LY Ballester and Y Esquenazi. Manuscript draft writing: A Dono, RM McCormack, N Putluri and Y Esquenazi. Manuscript editing: A Dono, RM McCormack, BP Ganesh, B Kaur, LD McCullough, LY Ballester and Y Esquenazi. Manuscript approval: all authors.
Financial & competing interests disclosure
This work was supported by the Vivian L. Smith Foundation. The metabolomics core was supported by the CPRIT Core Facility Support Award RP170005 ‘Proteomic and Metabolomic Core Facility’, NCI Cancer Center Support Grant P30CA125123, intramural funds from the Dan L. Duncan Cancer Center (DLDCC).
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors obtained appropriate were approved for this prospective human study by the institutional review board of The University of Texas Health Science Center at Houston and Memorial Hermann Hospital, Houston, TX, following the 1964 Helsinki Declaration and its later amendments. Informed consent has been obtained from the participants involved. All experimental work was approved by The University of Texas Health Science Center at Houston Center for Laboratory Animal Medicine and Care.
Data sharing statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Ferlay J, Colombet M, Soerjomataram I. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144(8), 1941–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 2.McLendon R, Friedman A, Bigner D. et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216), 1061–1068 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H, Parsons DW, Jin G. et al. Mutations in Gliomas. N. Engl. J. Med. 360(8), 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352(10), 987–996 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Esquenazi Y, Friedman E, Liu Z, Zhu J-J, Hsu S, Tandon N. The survival advantage of “supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery 81(2), 275–288 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Cryan JF, O'Riordan KJ, Cowan CSM. et al. The microbiota–gut–brain axis. Physiol. Rev. 99(4), 1877–2013 (2019). [DOI] [PubMed] [Google Scholar]; •• Recent review article explaining the microbiome–gut–brain axis in great detail.
- 7.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 336(6086), 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson JK, Holmes E, Kinross J. et al. Host-gut microbiota metabolic interactions. Science 336(6086), 1262–1267 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat. Med. 25(3), 377–388 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Zorofchian S, Iqbal F, Rao M, Aung PP, Esquenazi Y, Ballester LY. Circulating tumour DNA, microRNA and metabolites in cerebrospinal fluid as biomarkers for central nervous system malignancies. J. Clin. Pathol. 72(4), 271–280 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology 150(1), 7–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Wang Y, Xiayu X. et al. Altered gut microbiota in a mouse model of Alzheimer's disease. J. Alzheimer's Dis. 60(4), 1241–1257 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Maltz RM, Keirsey J, Kim SC. et al. Prolonged restraint stressor exposure in outbred CD-1 mice impacts microbiota, colonic inflammation, and short chain fatty acids. PLoS One 13(5), e0196961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger MM, Spiegel J, Dillmann KU. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57(8), 2096–2102 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Lyte M, Ernst S. Catecholamine induced growth of Gram negative bacteria. Life Sci. 50(3), 203–212 (1992). [DOI] [PubMed] [Google Scholar]
- 17.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 65(1), 63–68 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Blacher E, Bashiardes S, Shapiro H. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572(7770), 474–480 (2019). [DOI] [PubMed] [Google Scholar]; •• Recent article that identify specific micrbiome taxa and metabolite that improve survival in a mouse model of amyotrophic lateral sclerosis.
- 19.Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359(6371), 91–97 (2018). [DOI] [PubMed] [Google Scholar]; •• Recent article that identify specific microbiome taxa that influences the efficacy of immunotherapy in epithelial cancer.
- 20.Gopalakrishnan V, Spencer CN, Nezi L. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371), 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Recent article that identify specific micorbiome taxa that influences the efficacy of immunotherapy in melanoma.
- 21.Szatmári T, Lumniczky K, Désaknai S. et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 97(6), 546–553 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo JY, Swanner J, Otani Y. et al. Oncolytic HSV therapy increases trametinib access to brain tumors and sensitizes them in vivo. Neuro Oncol. 21(9), 1131–1140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis DN, Perry A, Reifenberger G. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131(6), 803–820 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Jin F, Thaiparambil J, Donepudi SR. et al. Tobacco-specific carcinogens induce hypermethylation, DNA adducts, and DNA damage in bladder cancer. Cancer Prev. Res. 10(10), 588–597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Von Rundstedt FC, Rajapakshe K, Ma J. et al. Integrative pathway analysis of metabolic signature in bladder cancer: a linkage to the Cancer Genome Atlas project and prediction of survival. J. Urol. 195(6), 1911–1919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttenhower C, Gevers D, Knight R. et al. Structure, function and diversity of the healthy human microbiome. Nature 486(7402), 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar RC. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10(10), 996–998 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Spychala MS, Venna VR, Jandzinski M. et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 84(1), 23–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrian-Shai R, Reichardt JKV, Harris CC, Toren A. The gut–brain axis, paving the way to brain cancer. Trends Cancer 5(4), 200–207 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab. 20(5), 719–730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14(6), 277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat. Med. 25(3), 377–388 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Yachida S, Mizutani S, Shiroma H. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25(6), 968–976 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: A study using the GPRD. Br. J. Cancer 104(1), 193–197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hata T, Asano Y, Yoshihara K. et al. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One 12(7), e0180745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caragher SP, Hall RR, Ahsan R, Ahmed AU. Monoamines in glioblastoma: Complex biology with therapeutic potential. Neuro Oncol. 20(8), 1014–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kijima N, Kanemura Y. Mouse models of glioblastoma. : Glioblastoma. De Vleeschouwer S. (). Exon Publications, Brisbane, Australia, 131–139 (2017). [PubMed] [Google Scholar]
- 38.Jayamohananan H, Manoj Kumar MK, Aneesh TP. 5-HIAA as a potential biological marker for neurological and psychiatric disorders. Adv. Pharm. Bull. 9(3), 374–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu. Rev. Med. 60(1), 355–366 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singhal M, Turturice BA, Manzella CR. et al. Serotonin transporter deficiency is associated with dysbiosis and changes in metabolic function of the mouse intestinal microbiome. Sci. Rep. 9(1), 2138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reigstad CS, Salmonson CE, Rainey JF. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29(4), 1395–1403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yano JM, Yu K, Donaldson GP. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2), 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyte M. Microbial Endocrinology in the microbiome-gut–brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9(11), 9–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starkman MN, Cameron OG, Nesse RM, Zelnik T. Peripheral catecholamine levels and the symptoms of anxiety: studies in patients with and without pheochromocytoma. Psychosom. Med. 52(2), 129–142. [DOI] [PubMed] [Google Scholar]
- 45.Kingsley TR, Nekvasil NP, Snyder DL. The influence of dietary restriction, germ-free status, and aging on adrenal catecholamines in Lobund-Wistar rats. J. Gerontol. 46(4), B135–B141 (1991). [DOI] [PubMed] [Google Scholar]
- 46.Mittal R, Debs LH, Patel AP. et al. Neurotransmitters: the critical modulators regulating gut–brain axis. J. Cell. Physiol. 232(9), 2359–2372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology 146(6), 1500–1512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer EA. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 12(8), 453–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh V, Roth S, Llovera G. et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36(28), 7428–7440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20(2), 145–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benakis C, Brea D, Caballero S. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22(5), 516–523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain. Behav. Immun. 19(4), 334–344 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Bercik P, Park AJ, Sinclair D. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 23(12), 1132–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konrad D, Wueest S. The gut-adipose-liver axis in the metabolic syndrome. Physiology 29(5), 304–313 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Viaud S, Saccheri F, Mignot G. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342(6161), 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez JM, Murphy K, Stanton C. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 9(4), 279–290 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.