Key Points
Question
Among US veterans 75 years and older and free of atherosclerotic cardiovascular disease at baseline, is statin use associated with lower risk of mortality?
Findings
In this retrospective cohort study that used propensity score overlap weighting and included 326 981 participants, statin use, compared with no statin use, was significantly associated with a lower risk of all-cause and cardiovascular mortality (hazard ratios, 0.75 and 0.80, respectively).
Meaning
Among older US veterans without atherosclerotic cardiovascular disease at baseline, statin therapy was significantly associated with a lower risk of mortality.
Abstract
Importance
Data are limited regarding statin therapy for primary prevention of atherosclerotic cardiovascular disease (ASCVD) in adults 75 years and older.
Objective
To evaluate the role of statin use for mortality and primary prevention of ASCVD in veterans 75 years and older.
Design, Setting, and Participants
Retrospective cohort study that used Veterans Health Administration (VHA) data on adults 75 years and older, free of ASCVD, and with a clinical visit in 2002-2012. Follow-up continued through December 31, 2016. All data were linked to Medicare and Medicaid claims and pharmaceutical data. A new-user design was used, excluding those with any prior statin use. Cox proportional hazards models were fit to evaluate the association of statin use with outcomes. Analyses were conducted using propensity score overlap weighting to balance baseline characteristics.
Exposures
Any new statin prescription.
Main Outcomes and Measures
The primary outcomes were all-cause and cardiovascular mortality. Secondary outcomes included a composite of ASCVD events (myocardial infarction, ischemic stroke, and revascularization with coronary artery bypass graft surgery or percutaneous coronary intervention).
Results
Of 326 981 eligible veterans (mean [SD] age, 81.1 [4.1] years; 97% men; 91% white), 57 178 (17.5%) newly initiated statins during the study period. During a mean follow-up of 6.8 (SD, 3.9) years, a total 206 902 deaths occurred including 53 296 cardiovascular deaths, with 78.7 and 98.2 total deaths/1000 person-years among statin users and nonusers, respectively (weighted incidence rate difference [IRD]/1000 person-years, –19.5 [95% CI, –20.4 to –18.5]). There were 22.6 and 25.7 cardiovascular deaths per 1000 person-years among statin users and nonusers, respectively (weighted IRD/1000 person-years, –3.1 [95 CI, –3.6 to –2.6]). For the composite ASCVD outcome there were 123 379 events, with 66.3 and 70.4 events/1000 person-years among statin users and nonusers, respectively (weighted IRD/1000 person-years, –4.1 [95% CI, –5.1 to –3.0]). After propensity score overlap weighting was applied, the hazard ratio was 0.75 (95% CI, 0.74-0.76) for all-cause mortality, 0.80 (95% CI, 0.78-0.81) for cardiovascular mortality, and 0.92 (95% CI, 0.91-0.94) for a composite of ASCVD events when comparing statin users with nonusers.
Conclusions and Relevance
Among US veterans 75 years and older and free of ASCVD at baseline, new statin use was significantly associated with a lower risk of all-cause and cardiovascular mortality. Further research, including from randomized clinical trials, is needed to more definitively determine the role of statin therapy in older adults for primary prevention of ASCVD.
This retrospective cohort study uses Veterans Health Administration data on adults free of atherosclerotic cardiovascular disease (ASCVD) to evaluate the association between new statin use and all-cause and cardiovascular mortality, and a composite of ASCVD events (myocardial infarction, ischemic stroke, and revascularization with CABG surgery or PCI), in veterans 75 years and older.
Introduction
With improvements in medicine and technology, life expectancy has increased and adults 75 years and older are the fastest-growing segment of the population. By 2050 more than 45 million Americans will be 75 years and older, with a proportional rate of increase greatest in those 85 years and older.1 Incidence and prevalence of atherosclerotic cardiovascular disease (ASCVD) rises with age and remains the leading cause of death, reduced quality of life and increased medical costs.2 However, although older adults bear the majority of the global ASCVD burden, they are infrequently included in clinical trials that provide the evidence for prevention and treatment guidelines.3,4
Statins are a mainstay of ASCVD primary prevention, but guidelines remain equivocal on their role for persons 75 years and older, primarily because of a paucity of data.4,5,6,7 This gap is mostly attributable to lack of enrollment of individuals 75 and older in all major statin trials: fewer than 2% of 186 854 participants in 28 statin trials were 75 years and older.4 However, life tables estimate that an individual who has reached age 80 will live on average 8 to 9 additional years8 and may benefit from preventive strategies. This is one reason the updated 2018 cholesterol guidelines recommend statins as a reasonable choice for primary prevention of ASCVD for individuals 75 years and older without a life-limiting disease.6
Emerging evidence for the potential benefits of statins in elderly persons remains limited, and it will be years before trials are able to answer this question. To address this, data were used from the entire US Veterans Health Administration (VHA) services, a health care system that includes more than 24 million users nationwide over 20 years, to examine the association of statin use with incidence of all-cause mortality, cardiovascular mortality, and nonfatal ASCVD events in those 75 years and older.
Methods
Ethics
This study was approved, and the requirement for obtaining patient informed consent was waived, by the VHA Boston institutional review board.
Study Population
All regular VHA users from 2002-2012, 75 years and older and free of statin use, were identified through the VHA corporate data warehouse (CDW).9 Regular use of VHA was defined as at least 2 years of utilization prior to entry into the cohort. Veterans who only had visits where medications could not be prescribed, such as for prosthetics or hearing aids, were excluded. Those with invalid death dates (death before clinical encounter) or unlikely birth dates (age >109 years) were excluded (eFigure in the Supplement). Those with prevalent ASCVD, defined as a history of myocardial infarction (MI), transient ischemic attack (TIA) or stroke, peripheral vascular disease, or coronary revascularization were excluded. VHA data were linked to Centers for Medicare & Medicaid Services (CMS) data for complete capture of claims data.
To create a cohort of older adults that reflects usual clinical practice, those with cancer, dementia, or paralysis were not excluded, in contrast to other studies.10 Those who died within 150 days of entry into the cohort were excluded to avoid selection bias, as statins for primary prevention are estimated to take 2 to 5 years for benefit and are generally not recommended for those with very limited life expectancy.11,12
Exposure
A new-user design13 was used, and persons with any prior statin use in VHA pharmacy or CMS records were excluded. The date a first statin was prescribed was the index date, and subsequent statin use was captured.13 All statins approved for use in the US were identified according to their generic or trade name; drug names were adjudicated by the study team (eTable 1 in the Supplement). VHA data were linked to CMS drug information to identify medication use both inside and outside of the VHA.14 Statin nonusers were included in the cohort as soon as they had 2 years of utilization and were not receiving a statin. As such, the entire cohort at entry consisted of statin nonusers. Any patient who subsequently started a statin was assigned to the exposed group on statin prescription and contributed time to statin nonusers until the first date of statin prescription.
Outcome
The primary outcomes were all-cause and cardiovascular mortality from the National Death Index (NDI), as detailed in eTable 1 in the Supplement.15 Secondary outcomes were MI, ischemic stroke, revascularization with coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI), and composite ASCVD events (MI, ischemic stroke, and revascularization procedures).16 Both VHA and CMS claims were used to identify events (eTable 1 in the Supplement).
Follow-up time was defined as time from entry into the cohort until date of death was recorded in the NDI or an individual in the control group switched to statin use for primary outcomes. If an event was observed on the same day as a new statin prescription, this was counted as an event in the statin nonuse group. For secondary outcomes, follow-up time was defined as time from entry into cohort to time of event, or loss of follow-up, or switch to statin use.
Covariates
Information on age, sex, race, ethnicity, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and region of the country was collected at the index date. Race and ethnicity were included because these can influence survival and cardiovascular outcomes. Categories were determined according to self-reported responses to fixed categories on enrollment in the VHA. Diagnosis codes within CDW and CMS claims were used to identify comorbidities associated with both exposure (statin use) and outcome (mortality, ASCVD events).17 These included hyperlipidemia, hypertension, diabetes, liver disease, nonstatin cholesterol-lowering medications, smoking status, anemia, cancer, heart failure, atrial fibrillation, chronic kidney disease, substance abuse, and mental health disorders (eTable 1 in the Supplement). Additionally, aging-specific variables were included to identify frailty and reasons why an older individual might or might not have been prescribed a statin, such as arthritis, dementia, polypharmacy, fatigue and gait abnormality.
Smoking status at index date was defined using an algorithm developed and validated in VHA electronic health records.18 Use of blood pressure medications was defined as active prescriptions 180 days prior to within 30 days of index date. Polypharmacy was defined as 5 or more drug classes at the index date.
Statistical Analysis
Veterans with questionable demographic information (missing sex, race, or BMI) were excluded as they were presumed to not be regular VHA users. To account for confounding by indication a propensity score using the above variables was created, followed by use of overlap weighting to minimize the influence of extreme propensity scores on model output.17,19,20,21 Interaction terms included year and region to account for varying practice over the duration of follow-up.
Cox proportional hazards models with robust estimator were fit to evaluate the association of statin use with outcomes using overlap weighting. Because trial data suggest that time to benefit for statins begins at 2 to 5 years,11 as a sensitivity analysis hazard ratios (HRs) were plotted at 2-year intervals after the index date to examine the association between statin use and mortality within a short time frame. In additional sensitivity analysis, those who died in the first 150 days were included.
Generalized linear model with time to event as offset and repeated measurements were used to estimate event rates comparing statin users with nonusers. E-values were run to assess unmeasured confounding.
In secondary analyses, prespecified subgroup analyses were conducted according to age groups (5-year increments from 75 to ≥90), sex, race, and prevalent diabetes, dementia, and arthritis. Tests of interaction were run for all subgroups. The test parameter from the propensity weighting procedure was used to re-create the overlap weighting. Then the Cox proportional hazard model was fit with new weights, and the interaction term was added for testing. Because of the potential for type I error due to multiple comparisons, findings from secondary analyses and secondary end points should be interpreted as exploratory. The proportional hazards assumption was assessed using graphic plot of ln {–ln[S(t)]} curves of 2 statin groups, and reasonably parallel lines were observed indicating no violation.
All analyses were conducted in SAS Enterprise Guide 7.1 (SAS Inc). P < .05 (2-sided) was considered statistically significant.
Results
Participant Characteristics
Of 7 242 193 veterans seen at the VHA between 2002-2016 who were 75 years and older, 648 677 had no prior statin prescription. Of these, 138 055 were excluded because of missing demographic data, 179 428 because of prior ASCVD events, and 4213 because of death in the first 150 days from baseline. In total 326 981 veterans were eligible for participation (eFigure in the Supplement). There were 57 178 (17.5%) new users of a statin, and 53 727 had additional prescriptions during follow-up. There were 269 803 who never had a statin prescription during follow-up. Mean age was 81.1 (SD, 4.1) years (range, 75-107 years), 91.0% were white, and 97.3% were men. Simvastatin was most commonly prescribed (84.8%), followed by lovastatin (11.0%), pravastatin (2.5%), and fluvastatin (1.2%). Atorvastatin and rosuvastatin were used by 0.5%. Table 1 shows patient demographics at baseline before and after propensity score weighting. Those who initiated a statin were more likely to have diagnostic codes for hyperlipidemia, hypertension, and diabetes. They were also more likely to have arthritis; polypharmacy; use diuretics, angiotensin-converting enzyme inhibitors, β-blockers, and take nonstatin cholesterol-lowering medications; and less likely to have dementia.
Table 1. Demographics of 326 981 Veterans 75 Years and Older, Free of Atherosclerotic Cardiovascular Disease, Before and After Propensity Score Overlap Weighting.
| Variable | Crude | After propensity score weighting | ||||
|---|---|---|---|---|---|---|
| No statin (n = 326 981)a | Statin (n = 57 178) | Standardized difference | No statin (n = 326 981) | Statin (n = 57 178) | Standardized difference | |
| Age, mean (SD), y | 80.7 (4.0) | 81.2 (3.6) | 14.4 | 81.1 (1.5) | 81.1 (3.0) | 0.0 |
| Body mass index, mean, (SD)b | 26.7 (4.4) | 27.5 (4.3) | 18.6 | 27.4 (1.6) | 27.4 (3.6) | 0.0 |
| Sex, % | ||||||
| Men | 97.3 | 97.3 | 0.3 | 97.3 | 97.3 | 0.0 |
| Women | 2.7 | 2.7 | 0.3 | 2.7 | 2.7 | 0.0 |
| Race, %c | ||||||
| White | 90.7 | 90.2 | 1.8 | 90.3 | 90.3 | 0.0 |
| Black/African American | 7.5 | 7.9 | 1.5 | 7.9 | 7.9 | 0.0 |
| Other | 1.7 | 1.9 | 1.0 | 1.9 | 1.9 | 0.0 |
| Hispanic/Latinx ethnicity, % | 3.6 | 4.4 | 4.3 | 3.6 | 4.4 | 4.4 |
| Smoking status, % | ||||||
| Current | 7.3 | 7.4 | 0.6 | 7.5 | 7.5 | 0.0 |
| Former | 71.9 | 63.5 | 18.2 | 65.7 | 65.7 | 0.0 |
| Never | 20.8 | 29.1 | 19.3 | 26.9 | 26.9 | 0.0 |
| Comorbidities, % | ||||||
| Hypertension | 66.2 | 80.4 | 32.6 | 77.8 | 77.8 | 0.0 |
| Hyperlipidemia | 15.8 | 53.8 | 87.1 | 43.1 | 43.1 | 0.0 |
| Arthritis | 37.7 | 46.0 | 16.8 | 43.9 | 43.9 | 0.0 |
| Cancer | 36.2 | 42.8 | 13.5 | 41.1 | 41.1 | 0.0 |
| Anemia | 16.8 | 19.3 | 6.6 | 18.5 | 18.5 | 0.0 |
| Depression | 12.6 | 15.7 | 9.0 | 14.9 | 14.9 | 0.0 |
| Diabetes | 13.1 | 27.0 | 35.1 | 23.4 | 23.4 | 0.0 |
| Atrial fibrillation | 8.8 | 9.5 | 2.4 | 9.5 | 9.5 | 0.0 |
| Dementia | 8.5 | 7.5 | 3.6 | 7.6 | 7.6 | 0.0 |
| Fatigue | 5.6 | 6.7 | 4.8 | 6.4 | 6.4 | 0.0 |
| Congestive heart failure | 4.9 | 5.7 | 3.3 | 5.6 | 5.6 | 0.0 |
| Gait abnormality | 4.0 | 5.1 | 5.5 | 4.7 | 4.7 | 0.0 |
| Posttraumatic stress disorder | 2.1 | 3.2 | 6.7 | 2.9 | 2.9 | 0.0 |
| Sleep apnea | 2.0 | 3.0 | 5.9 | 2.7 | 2.7 | 0.0 |
| Chronic kidney disease | 1.1 | 2.3 | 8.8 | 1.9 | 1.9 | 0.0 |
| Schizophrenia | 0.8 | 0.6 | 1.5 | 0.7 | 0.7 | 0.0 |
| Liver disease | 0.4 | 0.2 | 3.4 | 0.2 | 0.2 | 0.0 |
| Substance abuse | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 |
| Medication use, % | ||||||
| Polypharmacy (>5 drug classes) | 38.6 | 63.7 | 51.9 | 58.2 | 58.2 | 0.0 |
| Diuretics | 27.2 | 35.7 | 18.3 | 34.0 | 34.0 | 0.0 |
| ACE inhibitor | 26.2 | 39.6 | 28.8 | 36.6 | 36.6 | 0.0 |
| α-Blocker | 23.4 | 25.1 | 4.1 | 24.8 | 24.8 | 0.0 |
| Calcium channel blocker | 20.0 | 26.9 | 16.3 | 25.4 | 25.4 | 0.0 |
| β-Blocker | 15.7 | 23.1 | 18.9 | 21.5 | 21.5 | 0.0 |
| Angiotensin receptor blocker | 4.0 | 6.4 | 10.9 | 5.8 | 5.8 | 0.0 |
| Nonstatin lipid-lowering drug | 2.5 | 5.1 | 13.8 | 4.8 | 4.8 | 0.0 |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index.
All participants entered as statin nonusers. Characteristics are shown for the entire cohort at entry and at the time of first statin prescription for those who initiated a statin.
Calculated as weight in kilograms divided by height in meters squared.
Race categories were determined according to self-reported responses to fixed categories on enrollment in the Veterans Health Administration. Other race was defined as all responses that are nonwhite, black, or African American, including Native American, Asian, Pacific Islander.
Primary Outcomes
During a mean follow-up of 6.8 (SD, 3.9) years, a total of 206 902 deaths occurred including 53 296 cardiovascular deaths. Before adjustment, there were 78.7 and 98.2 total deaths per 1000 person-years among statin users and nonusers, respectively (weighted incidence rate difference [IRD]/1000 person-years, –19.5 [95% CI, –20.4 to –18.5]). There were 22.6 and 25.7 cardiovascular deaths per 1000 person-years among statin users and nonusers, respectively (weighted IRD/1000 person-years, –3.1 [95% CI, –3.6 to –2.6]). After propensity score overlap weighting was applied, statin use was significantly associated with a lower risk of all-cause mortality (HR, 0.75 [95% CI, 0.74 to 76]) and cardiovascular death (HR, 0.80 [95% CI, 0.78 to 0.81]) (Table 2).
Table 2. Association Between Statin Use, All-Cause Mortality, and Major Cardiovascular Events in 326 981 US Veterans 75 Years and Older Free of Atherosclerotic Cardiovascular Disease at Baseline, After Propensity Score Overlap Weighting.
| Outcome | Crude rate/1000 person-years | Weighted incidence rate difference/1000 person-years (95% CI)a | HR (95% CI) | P value | |
|---|---|---|---|---|---|
| Statin user (N = 57 178) | Statin nonuser (N = 269 803) | ||||
| Primary outcomes | |||||
| All-cause mortality (n = 206 902) | 78.7 | 98.2 | −19.45 (−20.38 to −18.52) | 0.75 (0.74 to 0.76) | <.001 |
| All CV death (n = 53 296) | 22.6 | 25.7 | −3.09 (−3.63 to −2.55) | 0.80 (0.78 to 0.81) | <.001 |
| Secondary outcomes | |||||
| ASCVD composite (n = 123 379)b | 66.3 | 70.4 | −4.05 (−5.09 to −3.02) | 0.92 (0.91 to 0.94) | <.001 |
| Myocardial infarction (n = 24 951) | 13.2 | 12.6 | 0.56 (0.13 to 0.98) | 0.99 (0.97 to 1.03) | .94 |
| Ischemic stroke (n = 35 630) | 18.4 | 18.2 | 0.25 (−0.26 to 0.76) | 0.98 (0.96 to 1.01) | .20 |
| CABG surgery/PCI (n = 74 362) | 35.2 | 39.2 | −3.38 (−4.12 to −2.64) | 0.89 (0.88 to 0.91) | <.001 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass graft; CV, cardiovascular; HR, hazard ratio; PCI, percutaneous coronary intervention.
Weighted incidence rate difference comparing statin users to nonusers after overlap weighting was applied.
ASCVD composite: time to first MI or ischemic stroke or CABG/PCI. There were fewer composite ASCVD events compared with total individual events, as participants were censored at first event of interest.
In sensitivity analysis, HRs plotted at 2-year intervals following the index date indicated that statin use was significantly associated with lower mortality within the first three 2-year intervals. For all-cause mortality, HRs at years 2, 4, and 6 were 0.68 (95% CI, 0.66-0.69), 0.79 (95% CI, 0.77-0.81), and 0.87 (95% CI, 0.84-0.91), with a similar pattern for cardiovascular death (eTable 2 in the Supplement). Results were unchanged when veterans who died in the first 150 days (n = 4213) were included (HR, 0.75 [95% CI, 0.74 to 0.76] for all-cause mortality and 0.80 [95% CI, 0.78 to 0.82] for cardiovascular mortality). E-values are reported in eTable 3 in the Supplement.
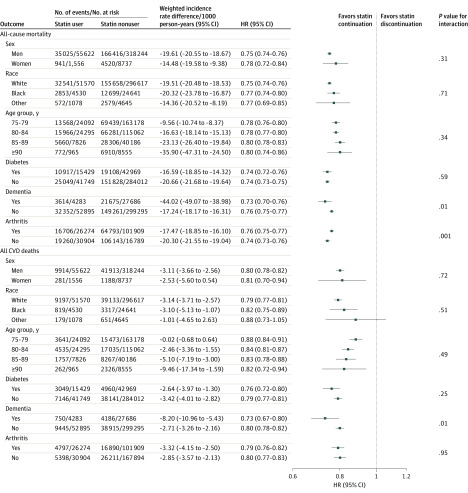
In subgroup analysis, the association between statin use and lower mortality remained statistically significant across all age groups, with significant lower risk of both all-cause and cardiovascular mortality even in those 90 years and older (HR, 0.80 [95% CI, 0.74 to 0.87]; P = .34 for interaction for all-cause mortality and HR, 0.81 [95% CI, 0.70 to 0.94]; P = .49 for interaction for cardiovascular mortality) (Figure 1).
Figure 1. Association Between Statin Use and All-Cause and Cardiovascular Mortality in 326 981 US Veterans 75 Years and Older Free of Atherosclerotic Cardiovascular Disease at Baseline, Stratified by Age, Sex, Race, Diabetes, Dementia, and Arthritis.
Race categories were determined according to self-reported responses to fixed categories on enrollment in the Veterans Health Administration. Other race was defined as all responses that are nonwhite, black, or African American, including Native American, Asian, Pacific Islander. CVD indicates cardiovascular disease; HR, hazard ratio.
In total, 8737 women were included, and results did not change when stratified by sex (P = .31 for interaction for all-cause mortality and P = .72 for interaction for cardiovascular mortality). There were no statistically significant differences for the primary outcome when stratified by race or diabetes (P > .05 for all for interactions). There was a statistically significant interaction by dementia and arthritis status for all-cause mortality and according to dementia status for cardiovascular mortality (P < .05 for all) (Figure 1).
Secondary Outcomes
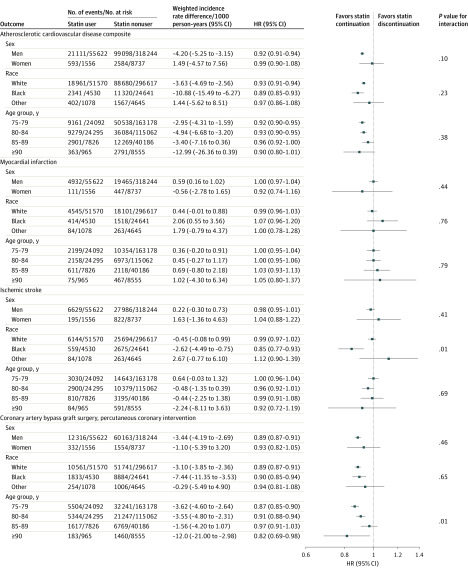
The ASCVD composite end point occurred in 123 379 persons, with a total of 24 951 MIs, 35 630 ischemic strokes, and 74 362 revascularization procedures. There were 66.3 and 70.4 composite cardiovascular events per 1000 person-years for statin users vs nonusers (weighted IRD, –4.05 [95% CI, –3.0 to –5.1]). After adjustment, the HR for the composite ASCVD events was 0.92 (95% CI, 0.91-0.94). Corresponding HRs were 0.99 (95% CI, 0.97 to 1.03) for MI, 0.98 (95% CI, 0.96 to 1.01) for ischemic stroke, and 0.89 (95% CI, 0.88 to 0.91) for revascularization (Table 2).
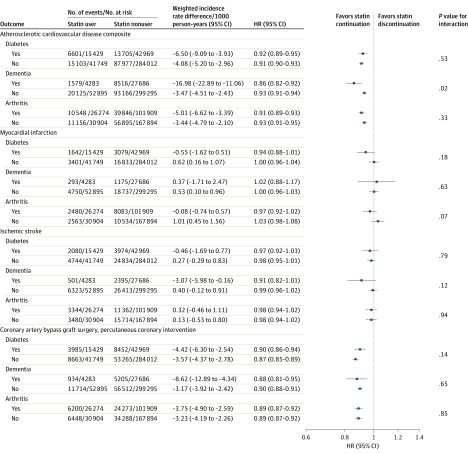
Results for all secondary outcomes stratified by age, sex, race, diabetes, dementia, and arthritis are shown in Figure 2 and Figure 3. Among 24 prespecified stratified analyses there were statistically significant interactions for 3 subgroups: for the composite ASCVD outcome among those with prior dementia vs those without prior dementia (HR, 0.86 [95% CI, 0.82 to 0.92] vs 0.93 [95% CI, 0.91 to 0.94]; P = .02 for interaction), for ischemic stroke according to race (white: HR, 0.99 [95% CI, 0.97 to 1.02]; black: HR, 0.85 [95% CI, 0.77 to 0.93]; other race: HR, 1.00 [95% CI, 0.78 to 1.28]; P = .01 for interaction), and according to age category for revascularization (P = .01 for interaction).
Figure 2. Association Between Statin Use and and Major Cardiovascular Events in 326 981 US Veterans 75 Years and Older Free of Atherosclerotic Cardiovascular Disease at Baseline, Stratified by Age, Sex, and Race.
Race categories were determined according to self-reported responses to fixed categories on enrollment in the Veterans Health Administration. Other race was defined as all responses that are nonwhite, black, or African American, including Native American, Asian, Pacific Islander. HR indicates hazard ratio.
Figure 3. Association Between Statin Use and Major Cardiovascular Events in 326 981 US Veterans 75 Years and Older Free of Atherosclerotic Cardiovascular Disease at Baseline, Stratified by Diabetes, Dementia, and Arthritis.
HR indicates hazard ratio.
Discussion
In a cohort of veterans 75 years and older, new statin use was significantly associated with a lower risk of all-cause and cardiovascular mortality. Results remained consistent even at advanced ages and in those with comorbidities, with similar lower risk for mortality observed in statin users 90 years and older or with dementia. In secondary analyses, new statin use was also significantly associated with a lower risk of a composite of ASCVD events.
Although the number of individuals 75 years and older in statin trials is limited, some data suggest that statins are effective for prevention of mortality when taken for primary prevention of ASCVD.22,23,24 The Heart Protection Study (HPS) was the first statin primary prevention trial to include individuals 75 and older. In subgroup analysis of 1263 participants aged 75 to 80 years, the risk of mortality was significantly reduced in those randomized to simvastatin vs placebo (12.9% vs 14.7%, P = .0003).25 The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial randomized 5804 participants aged 70 to 82 years to pravastatin or placebo.26 Overall, a 15% reduction in a composite end point of major ASCVD events was reported for those taking pravastatin (P = .014), with a 24% reduction in cardiovascular mortality (P = .043). However, in subgroup analysis of those without ASCVD at baseline (56% of participants), pravastatin did not lower the risk of major ASCVD events (HR, 0.94 [95% CI, 0.77 to 1.15]).
In contrast, a post hoc exploratory analysis of 726 participants 75 years and older in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial—Lipid-Lowering Trial (ALLHAT-LLT) showed a possible increased risk of mortality in those randomized to statins.27 However, there were high rates of crossover, limited sample size for those 75 years and older, and low numbers of absolute events. One of the benefits of using routine clinical practice data is access to large amounts of patient-level information that captures relevant characteristics. This is particularly important for older adults specifically excluded from trials because of the comorbidities that put them at higher risk of ASCVD and mortality. This may explain the higher event rate and larger magnitude of the inverse association of statins with mortality seen in this study compared with clinical trials. Individuals enrolled in clinical trials tend to be healthier than those in the community, particularly older adults in cardiovascular trials, which may limit event rates.3 Furthermore, the greater associations for all-cause mortality in this study compared with cardiovascular events that are typically prevented with statins could reflect the pleotropic benefits of statins.
Observational data on statin use for primary prevention have been mixed.28,29 A retrospective cohort study of 46 864 primary care patients in Spain demonstrated that statin use was associated with a significant lower risk of first ASCVD events among new users aged 74 to 85 years with diabetes (HR, 0.76 [95% CI, 0.65 to 0.89]), with similar findings among those aged 85 years and older.10 Two Korean cohort studies that included patients 75 years and older revealed a significant inverse association of statin use with all-cause mortality and primary ASCVD events.30,31 A French cohort of 7284 matched pairs 75 years and older also reported a significant lower risk of primary ASCVD events among statin users with modifiable cardiovascular risk factors.32 Another population-based study in France of 120 173 patients 75 years and older demonstrated a significant association between higher risk of cardiovascular events and discontinuation of statin therapy over a 2-year period.33
As with available trial data, prior studies have been limited by small sample sizes of very old adults, limited outcomes, and healthier populations, which may not reflect the general aging population who may benefit from a statin. The association with lower mortality among statin users seen in the Spanish study by Ramos et al10 among those with diabetes is mirrored in the current study. It is possible that the exclusion of chronically ill older adults with cancer and dementia in prior studies that did not find an inverse association were because of a bias toward the null in a healthier population at lower risk of mortality and ASCVD compared with the presented results.
The findings presented herein support the VHA 2014 cholesterol guidelines,34 which did not have a cutoff for age for primary prevention, and the updated American Heart Association/American College of Cardiology 2018 recommendation to consider prescribing statins to those 75 years and older.6 While many older adults are likely to benefit from statins for primary prevention, some evidence suggests that individuals with very limited life expectancy (eg, <6 months) may rather benefit from stopping statins.12 For this reason, veterans who died within 150 days of baseline were not included in the main analysis; however, in sensitivity analysis including these veterans, results did not change.
For most older adults, consideration of the time to benefit is critical before beginning a new therapy.11 Trial data suggest that the benefit for statins is realized within 2 to 5 years, and in the presented results an association between statin use and lower mortality was evident within 2 years.
Cardiovascular risk factors were not required for entry into this cohort, as nearly all current cardiovascular risk calculators assign an elevated risk of future ASCVD events in older adults based on age alone. The burden of cardiovascular risk factors and ASCVD is high in older adults, and the risk of stroke in particular rises with older age.35 Results in this study did not differ even in those at very advanced ages, suggesting that age alone should not be the determinant to stop or start a statin. Furthermore, there were no statistical differences according to race, sex, or diabetes status. However, in subgroups, there was a statistically significant lower risk of ischemic stroke in black vs nonblack individuals, but because these were subgroup analysis, this finding is considered exploratory.
Two clinical trials may add evidence to answer this question. The ongoing Statins Therapy for Reducing Events in the Elderly (STAREE) trial (NCT02099123) enrolled adults 70 years and older in Australia and results are expected in 2020, although numbers of individuals older than 75 years will still be limited. The recently funded Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults (PREVENTABLE) trial (NCT04262206) will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older. While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.
This study has several strengths, including a large data set with both electronic health records and claims data to identify events. The use of the entire VHA data set identified large numbers of new-users of statins older than 90 years and helped increase the ability to identify both clinically and statistically important associations with lower mortality risk and ASCVD events. The size of the data set also allowed exploring outcomes in populations often underrepresented in clinical trials, such as those with dementia and those of nonwhite race. Additional strength includes the use of rigorous statistical methods to reduce the risk of confounding by indication including new-user design and propensity score weighting. Veterans overall bear a greater burden of both cardiovascular disease risk factors and cardiovascular disease than the general population, which ensured adequate event rates in this cohort.36
Limitations
This study has several limitations. First, proportionally few women comprise VHA users, and results in VHA data may not generalize to the general population. However, a study comparing VHA patients with Medicare patients found greater overlap than previously hypothesized.37
Second, despite the use of new user design and propensity score methods to minimize bias, there may be residual and unmeasured confounding in this retrospective cohort study.
Third, this study did not specifically address frailty, although variables included in the model such as fatigue, slow walking, and polypharmacy may be proxies for frailty. Furthermore, individuals with dementia or cancer were not excluded, as has been done in other studies.
Fourth, adverse effects of statins such as myalgias, increased risk of diabetes, postulated decline in cognition,38 drug-drug interactions, and polypharmacy were not assessed in this study. Previous research has shown a slight elevated risk of type 2 diabetes in the VHA with high-potency statin use.39
Fifth, surveillance data were not available to evaluate the effect of statin discontinuation on the results. Statins are expected to have a legacy effect of at least 2 years, so that even individuals who stop taking a statin for a few months before the next refill can reasonably be considered statin users; furthermore, inclusion of person-time during which statins were discontinued in the user group might have prevented observing larger point estimates.
Sixth, in this study, simvastatin was most commonly used, which does not reflect current practice. Since the publication of the 2013 American College of Cardiology/American Heart Association guidelines, high-dose, high-intensity statins such as atorvastatin and rosuvastatin are recommended for ASCVD prevention in individuals at high risk. However, because guidelines have recommended increasing intensity of statins over time, the presented results may underestimate the statin-ASCVD association in contemporary practice.
Seventh, as this investigation relies on claims data, it is possible that relevant covariates were not coded or were undercoded.
Conclusions
Among US veterans 75 years and older and free of ASCVD at baseline, statin use was significantly associated with a lower risk of all-cause and cardiovascular mortality. Further research, including from randomized clinical trials, is needed to more definitively determine the role of statin therapy in older adults for primary prevention of ASCVD.
eFigure. CONSORT Diagram for Cohort Inclusion
eTable 1. Variable Definitions
eTable 2. Hazard Ratios for Mortality and CV Outcomes for 2-8 Years After Index Date
eTable 3. E-values for Main Outcomes
References
- 1.Ortman JM, Velkoff VA, Hoga H An Aging Nation: The Older Population in the United States: Population Estimates and Projections. Published May 2014. Accessed March 30, 2020. https://www.census.gov/prod/2014pubs/p25-1140.pdf
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander KP, Newby LK, Cannon CP, et al. ; American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology . Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549-2569. doi: 10.1161/CIRCULATIONAHA.107.182615 [DOI] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ Collaboration Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407-415. doi: 10.1016/S0140-6736(18)31942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley CE, Roefaro J, Forman DE, Orkaby AR. Statins for primary prevention in those aged 70 years and older: a critical review of recent cholesterol guidelines. Drugs Aging. 2019;36(8):687-699. doi: 10.1007/s40266-019-00673-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortality Rates and Life Expectancy of Veterans from 1980 to 2014, and by Education and Income. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Published April 2017. Accessed May 26, 2020. https://www.va.gov/vetdata/docs/SpecialReports/Mortality_study_USVETS_2015_1980_2014.pdf
- 9.Price LE, Shea K, Gephart S. The Veterans Affairs’s corporate data warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39(4):311-318. doi: 10.1097/NAQ.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos R, Comas-Cufí M, Martí-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359. doi: 10.1136/bmj.k3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234. doi: 10.1111/jgs.15216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med. 2015;175(5):691-700. doi: 10.1001/jamainternmed.2015.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Veterans Affairs System of Records Notice 97VA10P1: Consolidated Data Information System-VA. 76 FR 25409. Published May 4, 2011; amended March 3, 2015. Accessed June 2, 2020. https://www.govinfo.gov/content/pkg/PAI-2019-VA/xml/PAI-2019-VA.xml#97VA10P1
- 15.Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository—National Death Index (NDI). Defense Suicide Prevention Office. Accessed June 2, 2020. https://www.dspo.mil/About-Suicide/Suicide-Data-Repository/
- 16.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016;25(4):467-471. doi: 10.1002/pds.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song RJ, Ho Y-L, Nguyen X-MT, et al. Abstract 18809: development of an electronic health record-based algorithm for smoking status using the Million Veteran Program (MVP) cohort survey response. Circulation. 2016;134:A18809. [Google Scholar]
- 19.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345-357. doi: 10.1016/j.jacc.2016.10.060 [DOI] [PubMed] [Google Scholar]
- 20.Seeger JD, Kurth T, Walker AM. Use of propensity score technique to account for exposure-related covariates: an example and lesson. Med Care. 2007;45(10)(suppl 2):S143-S148. doi: 10.1097/MLR.0b013e318074ce79 [DOI] [PubMed] [Google Scholar]
- 21.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188(1):250-257. [DOI] [PubMed] [Google Scholar]
- 22.Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62(22):2090-2099. doi: 10.1016/j.jacc.2013.07.069 [DOI] [PubMed] [Google Scholar]
- 23.Teng M, Lin L, Zhao YJ, et al. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta-analysis. Drugs Aging. 2015;32(8):649-661. doi: 10.1007/s40266-015-0290-9 [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135(20):1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271 [DOI] [PubMed] [Google Scholar]
- 25.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi: 10.1016/S0140-6736(02)09327-3 [DOI] [PubMed] [Google Scholar]
- 26.Shepherd J, Blauw GJ, Murphy MB, et al. ; PROSPER Study Group . Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi: 10.1016/S0140-6736(02)11600-X [DOI] [PubMed] [Google Scholar]
- 27.Han BH, Sutin D, Williamson JD, et al. ; ALLHAT Collaborative Research Group . Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med. 2017;177(7):955-965. doi: 10.1001/jamainternmed.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orkaby AR, Gaziano JM, Djousse L, Driver JA. Statins for primary prevention of cardiovascular events and mortality in older men. J Am Geriatr Soc. 2017;65(11):2362-2368. doi: 10.1111/jgs.14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huesch MD. Association of baseline statin use among older adults without clinical cardiovascular disease in the SPRINT trial. JAMA Intern Med. 2018;178(4):560-561. doi: 10.1001/jamainternmed.2017.7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Lee CJ, Shim CY, et al. Statin and clinical outcomes of primary prevention in individuals aged >75 years: the SCOPE-75 study. Atherosclerosis. 2019;284:31-36. doi: 10.1016/j.atherosclerosis.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 31.Jun JE, Cho IJ, Han K, et al. Statins for primary prevention in adults aged 75 years and older: a nationwide population-based case-control study. Atherosclerosis. 2019;283:28-34. doi: 10.1016/j.atherosclerosis.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 32.Bezin J, Moore N, Mansiaux Y, Steg PG, Pariente A. Real-life benefits of statins for cardiovascular prevention in elderly subjects: a population-based cohort study. Am J Med. 2019;132(6):740-748.e7. doi: 10.1016/j.amjmed.2018.12.032 [DOI] [PubMed] [Google Scholar]
- 33.Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population-based cohort study in France. Eur Heart J. 2019;40(43):3516-3525. doi: 10.1093/eurheartj/ehz458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction Version 3.0-2014. Published December 2014. Accessed July 18, 2017. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG2014.pdf
- 35.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25(4):563-577. doi: 10.1016/j.cger.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong ES, Wang V, Liu C-F, Hebert PL, Maciejewski ML. Do Veterans Health Administration enrollees generalize to other populations? Med Care Res Rev. 2016;73(4):493-507. doi: 10.1177/1077558715617382 [DOI] [PubMed] [Google Scholar]
- 38.Bitzur R. Remembering statins: do statins have adverse cognitive effects? Diabetes Care. 2016;39(suppl 2):S253-S259. doi: 10.2337/dcS15-3022 [DOI] [PubMed] [Google Scholar]
- 39.Djoussé L, Song RJ, Cho K, Gaziano JM, Gagnon DR. Association of statin therapy with incidence of type 2 diabetes among US Veterans. J Clin Cardiol Cardiovasc Ther. 2019;1(1):10.31546/JCCCVT.1002. doi: 10.31546/JCCCVT.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. CONSORT Diagram for Cohort Inclusion
eTable 1. Variable Definitions
eTable 2. Hazard Ratios for Mortality and CV Outcomes for 2-8 Years After Index Date
eTable 3. E-values for Main Outcomes