Abstract
Simple Summary
When collecting blood samples in dogs for evaluating inflammatory conditions, this method is invasive and stressful to dogs. We compared the C-reactive protein (CRP) level in both blood and saliva samples to investigate the non-invasive test method for inflammation. There was a correlation between the blood and saliva CRP levels. This non-invasive test using saliva seems to be a useful method with which to assess inflammatory conditions in dogs.
Abstract
We performed this study to characterize the difference between the inflammatory and non-inflammatory status in diseased dogs by measuring salivary C-reactive protein (CRP) levels. In addition, we assessed whether a correlation exists between CRP levels in saliva and those in serum. CRP levels were measured in 32 client-owned dogs, which were then divided into inflammation and non-inflammation groups based on the serum CRP level. The salivary CRP level was higher in the inflammation group than in the non-inflammation group (p < 0.05). Furthermore, there was a positive correlation between the salivary and serum CRP levels (R = 0.866, p < 0.001). These data suggest that canine salivary CRP measurements can effectively and non-invasively detect an inflammatory state in dogs.
Keywords: C-reactive protein, dogs, saliva
1. Introduction
In dogs, C-reactive protein (CRP) is a major acute phase protein produced by non-specific tissue injury [1]. Its resting serum concentration is low; however, it could increase rapidly after exposure to inflammatory stimuli, then decrease after the resolution of inflammation [2]. Due to these characteristics, CRP comprises a sensitive and specific biomarker of systemic inflammation that is useful for the diagnosis and monitoring of various inflammatory diseases [3,4].
CRP levels are typically measured using serum collected by venipuncture, a painful and stress-inducing procedure that requires the involvement of professional and skilled clinicians or technicians, as well as laboratory equipment. Saliva collection is non-invasive, low-stress, and pain-free; therefore, it is an attractive alternative method for the evaluation of individual immune activity via CRP levels [5]. Saliva is an easily available biological fluid that contains many local and systemic factors that enter the oral cavity [6]. In humans, saliva samples have been successfully used for the detection of several biomarkers, such as cortisol and alpha-amylase [7,8]. In addition, saliva samples have been used in several studies of cortisol, catecholamine, and phenobarbital in veterinary medicine [9,10,11]. A previous study measured the CRP level from saliva and serum in dogs [12]. In this study, salivary CRP and serum CRP show a positive correlation, and it is considered that there is a significant difference between healthy and inflamed dogs. However, it has not been studied in diseased dogs with no inflammation.
The objectives of this study were to examine the differences in the salivary CRP levels between diseased dogs classified into inflammation or non-inflammation groups, without the use of chemical stimulants. Moreover, we assessed whether a correlation exists between CRP levels in serum and those in saliva.
2. Materials and Methods
2.1. Animals and Sample Collection
Thirty-two client-owned dogs with various diseases were presented to the Veterinary Medical Teaching Hospital at Chungnam National University during the period from May 2017 to September 2017 and were included in our study. Clients were informed of this study and agreed to participate. All dogs underwent a complete physical examination, blood count (Advia2120; Siemens Healthcare Diagnostics, Deerfield, IL, USA), CRP measurement, and serum biochemistry profile (Mindray BS-300; Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China). Dogs were classified into inflammation or non-inflammation groups according to the laboratory results: dogs with serum CRP values higher than the reference range of 0–2 mg/dL were included in the inflammation group, whereas dogs with lower serum CRP values were included in the non-inflammation group. Both serum and saliva specimens were collected from each dog at a single time point. Before collection of saliva samples, dogs were fasted for 6–12 hours. No acid stimulants were used to increase salivary secretion. Dogs that had apparent periodontal disease or bleeding gums in the physical examination were excluded from the study.
Blood samples were obtained from each dog by venipuncture and collected in tubes containing a coagulation activator. Blood was allowed to clot at room temperature and centrifuged at 3000× g at room temperature for 10 min. Serum samples were stored at −70 °C until the biochemical analysis. After taking a blood sample, saliva was obtained using a cotton pad and a 1.5-mL tube (Greiner Bio-One, Kremsmünster, Austria). The cotton pad was placed in the oral cavity in contact with the buccal mucosa, or the dogs were allowed to chew the cotton pad for 1–2 min. The pad was then placed in the tube and centrifuged for 15 min at 3000× g. Immediately after centrifugation, salivary samples were stored in Eppendorf tubes and frozen at −70 °C until the biochemical analysis. All saliva and serum samples were frozen in aliquots, and only vials needed for each assay were thawed to prevent any potential variation as a result of repetitive freeze-thaw cycles. The sample collection protocol was approved by the Institutional Animal Care and Use Committee at Chungnam National University (approval number CNU-00950).
2.2. Measurement of CRP Concentration in Both Saliva and Plasma
CRP levels in both the serum and saliva samples were measured using a commercial canine CRP ELISA kit (Abcam, Cambridge, MA, USA). Dilutions of the serum and saliva samples used in analyses were 1:1000, 1:10, and undiluted. All thawed salivary samples were centrifuged at 1500× g for 15 min at 4 °C to remove cellular debris and minimize the turbidity of the saliva, which could negatively impact the accuracy of analysis [13]. Supernatants were transferred into fresh Eppendorf tubes and appropriately labeled. A total of 64 samples, consisting of 32 serum and 32 saliva samples, were assayed in duplicate. For ELISA analysis, all samples were thawed and mixed thoroughly; ELISA was then performed in accordance with the manufacturer’s instructions. Absorbance was detected at a wavelength of 450 nm on a microplate reader (Biotek Instruments Inc., Winooski, VT, USA).
2.3. Statistical Analysis
Statistical analyses were performed using IBM SPSS software (version 24, IBM Corp., Ehningen, Germany). CRP levels were expressed as both mean and standard deviation by Kolmogorov–Smirnov test. Pearson’s product-moment correlation and simple regression analysis was used to compare CRP levels in saliva and serum. Differences in salivary CRP were analyzed using unpaired t-tests and F-test was used to compare variances. Differences were considered statistically significant when p < 0.001.
3. Results
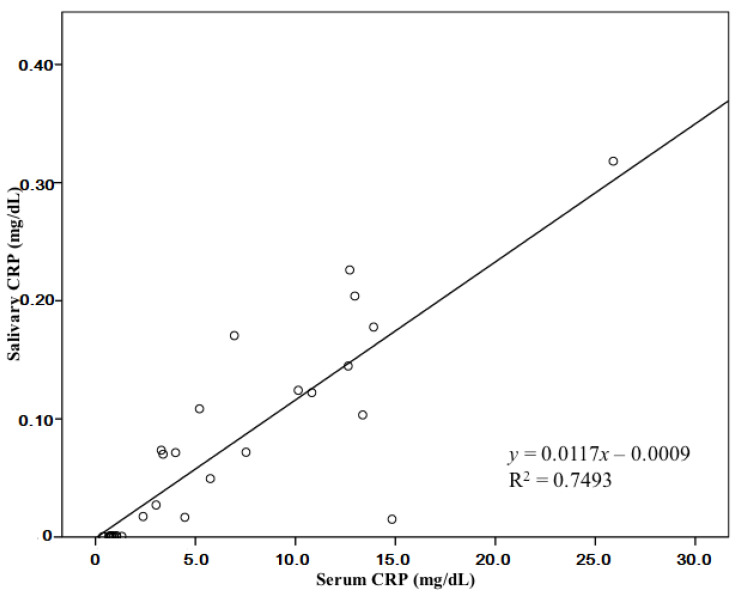
The 32 client-owned diseased dogs consisted of 15 different breeds, including Maltese (n = 9), Shih Tzu (n = 4), Schnauzer (n = 3), Yorkshire Terrier (n = 2), Welsh Corgi (n = 2), Pomeranian (n = 2), Beagle (n = 2), Sapsaree (n = 1), Poodle (n = 1), German Shepherd (n = 1), French Bulldog (n = 1), Cocker Spaniel (n = 1), Chihuahua (n = 1), Cane Corso (n = 1), and Australian Shepherd (n = 1). Based on a general health screening and laboratory evaluation, the dogs were diagnosed with various diseases. Animals were divided into two groups (inflammation and non-inflammation) according to CRP seric levels, regardless of the conditions present. Cardiologic disease was present in a large proportion of the non-inflammation group. Well-managed or congenital cardiological disease and acute pancreatitis (recovered) were included in the non-inflammation group. Post-operative inflammation within four days and acute pancreatitis at the time of diagnosis were the most common conditions in the inflammation group. The clinical characteristics of all dogs are shown in Table 1 and Table 2. Salivary CRP levels were significantly higher in the inflammation group than in the non-inflammation group. Furthermore, there was a positive correlation between the serum and salivary CRP levels in all dogs (R = 0.866, p < 0.001; Figure 1).
Table 1.
Measured C-reactive protein (CRP) levels in the serum and saliva samples and the clinical conditions of the inflammation group (n = 19) and non-inflammation group (n = 13).
| Non-Inflammation Group | |||
| No. |
Serum CRP Levels
(mg/dl) |
Salivary CRP Levels
(mg/dl) |
Clinical Condition |
| 1 | 0.6938 | 0.00060 | Peripheral vestibular syndrome |
| 2 | 1.3186 | 0.00054 | Acute pancreatitis (Being recovered) |
| 3 | 0.6841 | 0.00048 | Urolithiasis (No cystitis) |
| 4 | 0.3895 | 0.00038 | Mast cell tumorectomy (2 years ago) |
| 5 | 0.3772 | 0.00037 | Mitral valve insufficiency (Being treated) |
| 6 | 0.9053 | 0.00073 | Mitral valve insufficiency (Being treated) |
| 7 | 0.7934 | 0.00069 | Hyperthyroidism |
| 8 | 1.0628 | 0.00085 | Tricuspid valve insufficiency (Being treated) |
| 9 | 0.9215 | 0.00075 | Lymphoma (Being treated) |
| 10 | 1.0376 | 0.00094 | Patent ductus arteriosus |
| 11 | 0.7037 | 0.00070 | Patent ductus arteriosus |
| 12 | 0.8106 | 0.00076 | Atopic dermatitis (Being treated with oclacitinib) |
| 13 | 0.7889 | 0.00010 | Narcolepsy |
| Inflammation Group | |||
| No. |
Serum CRP Levels
(mg/dl) |
Salivary CRP levels
(mg/dl) |
Clinical Condition |
| 1 | 10.14104 | 0.12419 | Orthopedics |
| 2 | 14.83298 | 0.01509 | Thyroid tumor |
| 3 | 12.71612 | 0.22608 | Gastrointestinal stromal tumor, peritonitis |
| 4 | 6.94354 | 0.17053 | Thyroid tumor |
| 5 | 13.36547 | 0.10334 | Acute pancreatitis, anaplasmosis |
| 6 | 10.82076 | 0.12226 | Cystotomy, nephrotomy |
| 7 | 12.63759 | 0.14473 | Orthopedics |
| 8 | 13.91174 | 0.17774 | Orthopedics |
| 9 | 12.97129 | 0.20399 | Tumorectomy |
| 10 | 3.03367 | 0.02713 | Tumorectomy |
| 11 | 5.74407 | 0.04943 | Trauma |
| 12 | 5.20193 | 0.10855 | Acute pancreatitis |
| 13 | 3.29112 | 0.07348 | Cardiogenic pulmonary edema, mitral valve insufficiency |
| 14 | 4.46760 | 0.01669 | Pyometra, mammary gland tumor, acute pancreatitis |
| 15 | 2.38347 | 0.01738 | Urolithiasis, cystitis |
| 16 | 4.00526 | 0.07147 | Acute colitis |
| 17 | 25.89826 | 0.31818 | Pneumonia, Chronic kidney disease |
| 18 | 7.53124 | 0.07178 | Orthopedics |
Table 2.
Comparison of the signalment and median CRP levels of the inflammation group and non-inflammation group.
| Inflammation Group (n = 19) |
Non-Inflammation Group (n = 13) |
|
|---|---|---|
| Signalment | ||
| Age 1 | 9 (2–18) | 11 (1–16) |
| Sex 2 | 3M, 7CM, 6F, 3SF | 1M, 10CM, 1F, 1SF |
| CRP levels (mg/dL) | ||
| Serum 3 | 9.11994 ± 0.11117 * | 0.80669 ± 0.00061 |
| Saliva 3 | 5.75988 ± 0.07887 | 0.24827 ± 0.00022 |
1 Age is represented in years as mean (range). 2 Sex is represented as the number of dogs in each subcategory of sex. 3 Serum and saliva are represented as mean ± standard deviation. * Significant statistical difference between the inflammation group and non-inflammation group indicates p < 0.0001. M, male; CM, castrated male; F, female; SF, spayed female; CRP, C-reactive protein.
Figure 1.
Correlation between C-reactive protein (CRP) levels in serum and saliva.
4. Discussion
The results of this study indicate that salivary CRP is an accurate method for identifying inflammation in diseased dogs. Saliva can be collected without the use of invasive methods, and is advantageous for pediatric patients or those with a specific clinical pathologic state (e.g., anemia or hemostatic disease), physical sensitivity (e.g., to needles), and geographic handicaps (e.g., those who reside far from the hospital) [6,12].
In this study, salivary CRP was able to serve as an indicator of inflammatory status and showed a moderate-to-strong association (R = 0.866 and R2 = 0.749) with the serum CRP level. The quantification of salivary CRP and its correlation with the serum CRP has been assessed in human [5], porcine [14], and canine [12] studies. Although this correlation in dogs (R2 = 0.69) was higher than that in humans (R2 = 0.49) or pigs (R2 = 0.52), a moderate association was detected in all groups.
We observed a difference of the salivary and serum CRP levels which do not meet the regression equation. The low ratio of the saliva CRP level to the serum CRP level could reduce the precision of salivary CRP measures, particularly at low serum CRP concentrations in a non-inflammation state [5]. Although the mechanisms by which CRP is transported from serum to saliva remain unclear [5], CRP could potentially enter in the serum in its high-molecular weight form, and its two glycosylated subunits could increase its lipid-insolubility [15,16], preventing CRP from entering saliva. For large molecules such as proteins and charged steroids, the primary route of entry into the oral cavity is through plasma exudates of systemic origin from gingival crevicular fluid (GCF) [17], as well as from minor abrasions in the mouth, instead of diffusion or ultrafiltration [9,18]. Whole saliva used in this study comprised oral fluids from the major and minor salivary gland, as well as fluids of non-salivary origin, including GCF, serum transudate from the mucosa and sites of inflammation, epithelial and immune cells, food debris, and many microbes [18,19]. Poor oral health in diseased dogs might have caused minor bleeding or GCF overflow from micro-injuries, which could have increased salivary CRP in the absence of elevated serum CRP. Furthermore, steroid hormones are known to be metabolized by oral bacteria and epithelial cells in the salivary gland during transcellular movement [20]. Although this mechanism is not known in salivary CRP, this modification and subsequent metabolism could considerably modify or reduce detection in saliva.
Our investigation has different experimental settings and collection methods compared to previously published studies. The composition of whole saliva can be rapidly altered by flow rate, the degree of stimulation in various glands, and the time of day [21]. Our protocol controlled for animal factors by fasting, acid stimulant exclusion, and collection time, as well as by the exclusion of dogs with periodontitis and other oral diseases. In addition, we collected samples before treatment, which may have increased the association with inflammation. A previous study in humans reported that mechanical stimulation, but not acid stimulation, is the most viable option for acquisition of saliva without changes in CRP levels [21]. Based on these results, we used mechanical stimulation for saliva collection in our study. Further studies in veterinary medicine are needed to confirm the optimal collection protocol.
There were some limitations in the present study. Firstly, we included a relatively small number of dogs. Most diseased dogs who were presented to the clinic showed signs of dehydration and dry mucosa in the oral cavity; therefore, we were unable to obtain a sufficient volume of saliva for analysis. Secondly, our study used sample pretreatment (centrifugation) to remove cellular debris. Processing methods can adversely affect the concentration of CRP in humans [21]; therefore, it is necessary to verify whether the same adverse effect is present in veterinary medicine samples.
5. Conclusions
Taken together, the results of this study suggest that salivary CRP measurements in dogs can provide a straightforward, adaptable, and non-invasive method for the detection of inflammation. This method could be used as a tool to monitor health and assess inflammation in diseased animals. To use saliva for the assessment of inflammation in clinical practice, further studies should focus on creating reference ranges in different species, as well as the stability of saliva samples in various storage conditions.
Acknowledgments
This research was supported by a program of “Development of Health Monitoring Devices based on Mobile System” in the Ministry of Science, ICT and Future Planning (NRF-2014M3A9D7070779) and by the Cooperative Research Program of Center for Companion Animal Research (Project No. PJ014045022018): Rural Development Administration, Republic of Korea. This manuscript was written based on the master’s thesis of author Yoo-Ra Cho.
Author Contributions
Conceptualization, Y.-R.C., G.-H.S., Y.J.K. and K.-W.S.; Data curation, Y.-R.C., G.-H.S. and K.-W.S.; Formal analysis, Y.-R.C., Y.-I.O., G.-H.S. and Y.J.K.; Funding acquisition, Y.J.K. and K.-W.S.; Investigation, Y.-R.C., Y.-I.O. and K.-W.S.; Methodology, G.-H.S. and Y.J.K.; Software, Y.-R.C., Y.-I.O. and Y.J.K.; Supervision, Y.-I.O., G.-H.S., Y.J.K. and K.-W.S.; Validation, Y.-I.O. and K.-W.S.; Visualization, Y.-R.C. and Y.-I.O.; Writing—original draft, Y.-R.C.; Writing—review & editing, Y.-I.O., Y.J.K. and K.-W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and ICT, South Korea: NRF-2014M3A9D7070779 and Rural Development Administration: Project No. PJ014045022018.
Conflicts of Interest
The authors should declare no conflict of interests.
References
- 1.Eckersall P.D., Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 2010;185:23–27. doi: 10.1016/j.tvjl.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Conner J.G., Eckersall P.D., Ferguson J., Douglas T.A. Acute phase response in the dog following surgical trauma. Res. Vet. Sci. 1988;45:107–110. doi: 10.1016/S0034-5288(18)30902-0. [DOI] [PubMed] [Google Scholar]
- 3.Griebsch C., Arndt G., Raila J., Schweigert F.J., Kohn B. C-reactive protein concentration in dogs with primary immune-mediated hemolytic anemia. Vet. Clin. Pathol. 2009;38:421–425. doi: 10.1111/j.1939-165X.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- 4.Viitanen S.J., Laurila H.P., Lilja-Maula L.I., Melamies M.A., Rantala M., Rajamaki M.M. Serum C-Reactive Protein as a Diagnostic Biomarker in Dogs with Bacterial Respiratory Diseases. J. Vet. Int. Med. 2013;28:84–91. doi: 10.1111/jvim.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouellet-Morin I., Danese A., Williams B., Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav. Immun. 2011;25:640–646. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Hofman L.F. Human Saliva as a Diagnostic Specimen. J. Nutr. 2001;131:1621S–1625S. doi: 10.1093/jn/131.5.1621S. [DOI] [PubMed] [Google Scholar]
- 7.Hellhammer D.H., Wüst S., Kudielka B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Engert V., Vogel S., Efanov S.I., Duchesne A., Corbo V., Ali N., Pruessner J.C. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology. 2011;36:1294–1302. doi: 10.1016/j.psyneuen.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Beerda B., Schilder M.B., Janssen N.S., Mol J.A. The Use of Saliva Cortisol, Urinary Cortisol, and Catecholamine Measurements for a Noninvasive Assessment of Stress Responses in Dogs. Horm. Behav. 1996;30:272–279. doi: 10.1006/hbeh.1996.0033. [DOI] [PubMed] [Google Scholar]
- 10.Dunnett M., Littleford A., Lees P. Phenobarbitone concentrations in the hair, saliva and plasma of eight epileptic dogs. Vet. Rec. 2002;150:718–724. doi: 10.1136/vr.150.23.718. [DOI] [PubMed] [Google Scholar]
- 11.German A.J., Hall E.J., Day M.J. Measurement of IgG, IgM and IgA concentrations in canine serum, saliva, tears and bile. Vet. Immunol. Immunopathol. 1998;64:107–121. doi: 10.1016/S0165-2427(98)00132-9. [DOI] [PubMed] [Google Scholar]
- 12.Parra M.D., Tecles F., Martinez-Subiela S., Ceron J.J. C-reactive protein measurement in canine saliva. J. Vet. Diagn. Investig. 2005;17:139–144. doi: 10.1177/104063870501700207. [DOI] [PubMed] [Google Scholar]
- 13.Schipper R.G., Silletti E., Vingerhoeds M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007;52:1114–1135. doi: 10.1016/j.archoralbio.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez A.M., Martinez-Subiela S., Eckersall P.D., Ceron J.J. C-reactive protein quantification in porcine saliva: A minimally invasive test for pig health monitoring. Vet. J. 2009;181:261–265. doi: 10.1016/j.tvjl.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Caspi D., Baltz M.L., Snel F., Gruys E., Niv D., Batt R.M., Munn E.A., Buttress N., Pepys M.B. Isolation and characterization of C-reactive protein from the dog. Immunology. 1984;53:307–313. [PMC free article] [PubMed] [Google Scholar]
- 16.Eckersall P.D., Conner J.G. Bovine and canine acute phase proteins. Vet. Res. Commun. 1988;12:169–178. doi: 10.1007/BF00362798. [DOI] [PubMed] [Google Scholar]
- 17.Megson E., Fitzsimmons T., Dharmapatni K., Bartold P.M. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J. Clin. Periodontol. 2010;37:797–804. doi: 10.1111/j.1600-051X.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman E., Lamster I.B. The diagnostic applications of saliva--a review. Crit. Rev. Oral Biol. Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 19.Miller C.S., Foley J.D., Bailey A.L., Campell C.L., Humphries R.L., Christodoulides N., Floriano P.N., Simmons G., Bhagwandin B., Jacobson J.W., et al. Current developments in salivary diagnostics. Biomark. Med. 2010;4:171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quissell D.O. Steroid hormone analysis in human saliva. Ann. N. Y. Acad. Sci. 1993;694:143–145. doi: 10.1111/j.1749-6632.1993.tb18348.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed R., Campbell J.L., Cooper-White J., Dimeski G., Punyadeera C. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. Clin. Transl. Med. 2012;1:19. doi: 10.1186/2001-1326-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]