Abstract
Prior studies have demonstrated that infants and toddlers who later go on to develop autism spectrum disorder (ASD) show atypical functional connectivity as well as altered neural processing of language and other auditory stimuli, but the timeline underlying the emergence of these altered developmental trajectories is still unclear. Here we used resting-state fMRI (rsfMRI) during natural sleep to examine the longitudinal development of functional connectivity in language-related networks from 1.5 to 9 months of age. We found that functional connectivity of networks that underlie the integration of sensory and motor representations, which is crucial for language development, is disrupted in infants at high familial risk (HR) for developing ASD as early as 1.5 months of age. By 9 months of age, HR infants showed hyperconnectivity between auditory and somatosensory regions whereas low risk (LR) infants displayed greater intrahemispheric connectivity between auditory cortex and higher-order temporal regions as well as the hippocampus. Furthermore, while LR infants showed robust changes in functional connectivity during the first year of life with increasing long-range connectivity accompanied by decreasing short-range connectivity over time, HR infants displayed limited developmental changes. Our findings demonstrate that early disruptions in the development of language-related network connectivity may provide an early marker for the later emergence of ASD symptomatology.
Keywords: Autism, Connectivity, Development, Infant, Network, rsfMRI
1. Introduction
Altered functional connectivity is characteristic of developmental disorders involving language impairment such as autism spectrum disorder (ASD). Children with ASD show atypical connectivity in several resting-state networks compared to neurotypical controls (Hernandez et al., 2015; Jack, 2018; Uddin et al., 2013). In particular, youth with ASD show aberrant interhemispheric functional connectivity between primary and secondary auditory cortices as well as atypical temporo-thalamic connectivity associated with social behavioral symptoms (Linke et al., 2017). Furthermore, toddlers with ASD display disrupted synchronization of language areas (Dinstein et al., 2011), suggesting that early disruptions in functional connectivity may be predictive of later language impairments associated with the disorder. Prior research has examined the emergence of functional network atypicalities in various large-scale networks from 12 to 24 months of age in infants who later developed ASD (Keehn et al., 2013; McKinnon et al., 2019), but little is known about the development of functional connectivity within early auditory-language networks in at-risk infants. In addition, the earliest age at which various alterations in connectivity (Emerson et al., 2017; Pruett et al., 2015) and structural morphometry (Hazlett et al., 2017) have been observed in this population is 6 months of age; thus, little is known about the emergence of functional connectivity prior to this age. Here we used resting-state functional magnetic resonance imaging (rsfMRI) to characterize the development of functional connectivity between auditory and language processing regions in infants at high and low familial risk for ASD during the first year of life from 1.5 to 9 months of age.
Early infancy is a period of dynamic changes in the functional architecture of the typically developing infant brain. A growing body of work has begun to identify the development of resting-state networks in neurotypical infants whereby primary sensorimotor and auditory networks are the first to resemble adult-like patterns whereas higher-order networks such as the dorsal attention network and default mode network develop more gradually over the course of the first year of life (Gao et al., 2015, 2016; Smyser et al., 2011; Uddin et al., 2010). Auditory networks can already be detected shortly after birth (Fransson et al., 2011, 2009; Perani et al., 2011), but the connectivity of language regions continues to develop with an increase in leftward asymmetry and interhemispheric connectivity as a function of increasing age (Emerson et al., 2016). Thalamocortical networks that support the integration of sensory information, a crucial aspect of early language development, are also evident in neonates and develop dynamically during the first year (Alcauter et al., 2014). During this period, long-range connectivity, including cortical-subcortical and intrahemispheric connectivity, increases with age (Smyser et al., 2010; Thomason et al., 2015; Wen et al., 2019). Findings in typically-developing infants collectively indicate that different resting-state networks show unique timings and developmental trajectories, but little is known about the development of early intrinsic functional connectivity of language-related networks in infants at familial risk for ASD.
Functional connectivity of auditory networks is altered in ASD; indeed, toddlers with ASD already show reduced interhemispheric connectivity in language-processing regions compared to both toddlers with language delay as well as typically-developing (TD) toddlers (Dinstein et al., 2011). This pattern continues throughout development; findings in older youth with ASD show altered connectivity between regions associated with language processing (Gao et al., 2019a, 2019b) as well as reduced interhemispheric connectivity in auditory cortices (Anderson et al., 2011), which is associated with greater severity of sensory processing deficits and lower verbal IQ (Linke et al., 2017). While much of the extant literature examining auditory networks focuses on connectivity between primary and secondary auditory cortices, the thalamus is also important for integrating cortical information and has been shown to support both receptive and expressive aspects of language processing (Klostermann et al., 2013). Individuals with ASD show increased thalamocortical connectivity (Cerliani et al., 2015; Nair et al., 2013); hyperconnectivity between thalamus and primary/secondary auditory cortices has been associated with reduced cognitive and behavioral symptomatology in youth with ASD (Linke et al., 2017). However, little is known about the early development of thalamocortical networks in infants at risk for ASD.
Infant siblings of children with ASD are at elevated risk for the disorder with a recurrence rate of around 20 % (Ozonoff et al., 2011). These infants often experience atypical developmental trajectories and exhibit difficulties with language even if they do not go on to develop ASD; indeed, this population has higher rates of language delay in both the receptive and expressive domains (Garrido et al., 2017; Hudry et al., 2014). Although behavioral symptoms only begin to emerge towards the end of the second year and infants are typically not reliably diagnosed until age 3 (Jones et al., 2014), prior findings from infant sibling studies suggest that early differences in functional connectivity of the brain associated with ASD can be detected before the first birthday (Wolff et al., 2017a, 2017b). Thus, prospective longitudinal studies of infants at risk for ASD provide a unique window into the emergence of atypical patterns of connectivity in early development. Given that these infants are at elevated risk for ASD, this sample is particularly suited for investigating how developmental trajectories of functional connectivity may contribute to the etiology of ASD risk and later outcome.
Here, we used resting-state fMRI (rsfMRI) to characterize the development of functional connectivity networks underlying auditory and language processing during the first year of life in infants at high and low familial risk for developing ASD to assess whether atypicalities could already be observed this early as a function of risk status. We first examined seed-based connectivity of primary and secondary auditory cortices at 1.5 and 9 months of age. We expected to find robust networks for primary auditory cortices in both groups at 1.5 months of age with additional maturation of networks for secondary auditory cortices by 9 months of age. Next, we modeled longitudinal changes over time in ROI-based network connectivity to examine differences in the functional connectivity between auditory and language-processing regions as well as the thalamus. Based on prior findings in typically-developing adolescents, we expected to see growth in longer range cortio-cortical connections in the low risk group over time. Given ample evidence of disrupted connectivity in toddlers (Dinstein et al., 2011; Emerson et al., 2017; Keehn et al., 2013; McKinnon et al., 2019; Pruett et al., 2015) as well as older youth with ASD (Anderson et al., 2011; Cerliani et al., 2015; Linke et al., 2017; Nair et al., 2013), we expected to see atypical developmental trajectories in the high risk group. To our knowledge, this is the first study to examine the longitudinal development of connectivity within language-related networks in infants at high familial risk for developing ASD.
2. Materials and methods
2.1. Participants
Participants in this study were enrolled as part of a longitudinal project examining early brain-based markers of ASD during the first year. Infants were assigned to risk cohorts based on family history: High risk (HR) infants had at least one older sibling with a confirmed ASD diagnosis, whereas low risk (LR) infants had no family history of ASD (i.e., no first- or second-degree relatives with ASD) or any other neurodevelopmental disorders. All infant participants were enrolled prior to 1.5 months of age. Informed consent was obtained from parents/legal guardians of infant participants under protocols approved by the UCLA Institutional Review Board (IRB). Exclusionary criteria for both groups included: 1) genetic syndromes or neurological conditions (e.g., fragile X syndrome, tuberous sclerosis), 2) chronic medical conditions or significant perinatal insult impacting development, 3) severe visual, hearing, or motor impairment, and 4) contraindication for MRI.
At the 1.5 month time point, 74 infants (41 HR, 33 LR) underwent MRI; 4 HR and 1 LR did not successfully complete the rsfMRI scan (i.e., woke up before or during this scan). Data from four HR infants did not pass quality control during data preprocessing (as described below) and were also excluded, yielding a final sample of 65 infants (33 HR, 32 LR) for this time point. At the 9 month time point, 78 infants (48 HR, 30 LR) underwent MRI; 7 HR and 5 LR did not successfully complete the rsfMRI scan. Data from 3 HR and 3 LR did not pass quality control during data preprocessing and were also excluded, yielding a final sample of 60 infants (38 HR, 22 LR) for this time point. Risk-based cohorts were matched on age, sex, and race (Table 1). A total of 38 infants (18 HR, 20 LR) provided data at both time points; longitudinal ROI-based functional connectivity analyses were computed using mixed effect models to capitalize on the entire sample of infants. In contrast with HR infants, whose inclusionary criteria required them to be younger siblings of children with ASD, some infants in the LR group were first born children (1.5 months: N = 17; 9 months: N = 11). In order to ensure that birth order did not influence the results, we extracted and compared connectivity estimates between first-born LR infants and LR infants who had older siblings for all comparisons where there was a significant effect in the LR group (seed-based analyses) and significant interactions between risk and time point (ROI-based functional connectivity analyses). There were no significant differences based on birth order (all ps > 0.09).
Table 1.
Subject demographics.
| HR |
LR |
HR v LR P-Values |
1.5 v 9 Month P-Values |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.5 Months N=33 | 9 Months N=38 | 1.5 Months N=32 | 9 Months N=22 |
1.5 Months | 9 Months | HR | LR | HR v LR | |
| Male/Female Subjects | 19/14 | 23/15 | 19/13 | 11/11 | 0.88 | 0.43 | 0.96 | 0.93 | 0.92 |
| Age (months) | 1.50 + 0.29 | 9.23 + 0.38 | 1.55 + 0.24 | 9.16 + 0.37 | 0.41 | 0.47 | – | – | 0.24 |
| Mean Relative Motion (mm) | 0.10 + 0.07 | 0.08 + 0.05 | 0.08 + 0.05 | 0.06 + 0.03 | 0.35 | 0.13 | 0.12 | 0.12 | 0.85 |
| # of Motion/Noise Components | 26.67 + 9.66 | 22.13 + 7.78 | 27.22 + 11.19 | 22.86 + 8.25 | 0.83 | 0.73 | 0.049 | 0.10 | 0.96 |
| Race (white/nonwhite) | 22/9 | 25/9 | 21/9 | 13/8 | 0.93 | 0.36 | 0.97 | 0.95 | 0.94 |
2.2. MRI data acquisition
We followed recommended guidelines for pediatric neuroimaging in early infancy (Raschle et al., 2012). During natural sleep, 8-minute rsfMRI scans were collected in infants at 1.5 and 9 months of age. Parents were instructed to put their infant to sleep using their regular bedtime routine; once asleep, swaddled infants were transferred to the scanner bed. Soft and malleable silicone earplugs as well as MiniMuffs Neonatal Noise Attenuators (Natus Medical Inc., San Carlos, CA) were used as hearing protection; headphones used to convey auditory stimuli during a different scan further dampened scanner noise. Infants were placed on a custom-made bed, which fit inside the head coil, and secured to the scanner bed with a Velcro strap. To minimize movement, a weighted blanket was used and foam pads were positioned around each infant’s head. A study staff member remained in the scan room for the duration of the scan to monitor infants for overt movement, waking, or signs of distress.
All MRI data were collected on a Siemens 3 T Tim Trio (12-channel head coil; 1.5 months: 24 HR, 29 LR; 9 months: 19 HR, 15 LR) or Prisma scanner (32-channel head coil; 1.5 months: 9 HR, 3 LR; 9 months: 19 HR, 7 LR) during natural sleep. Scanner was included as a covariate of no-interest in all subsequent analyses. Resting state data were collected during an 8-minute rsfMRI scan (Siemens Trio: TR = 2000 ms, TE = 28 ms, matrix size 64 × 64, FOV = 192 mm, 34 slices, 3 mm in-plane resolution, with 4mm-thick axial slices; Siemens Prisma: identical parameters except with 33 slices). A matched bandwidth T2-weighted high-resolution echo planar scan was acquired co-planar to the functional scan to allow for spatial registration (Siemens Trio: TR = 5000 ms, TE = 34 ms, matrix size 128 × 128, FOV = 192 mm, 34 slices, 1.5 mm in-plane resolution, with 4mm-thick axial slices; Siemens Prisma: identical parameters except with TE = 45 ms, 33 slices).
2.3. fMRI data preprocessing
Functional imaging data were preprocessed and analyzed using FMRIB’s Software Library (FSL; Smith et al., 2004). Preprocessing included skull stripping, motion correction, spatial smoothing with a Gaussian kernel of 6 mm full width at half maximum (FWHM), and affine registration with 6 degrees of freedom to the subject’s corresponding high-resolution anatomical scan, followed by affine registration with 12 degrees of freedom to an infant brain template (Shi et al., 2011). Scans collected at the 1.5-month time point were registered to a neonate brain template whereas scans collected at the 9-month time point were registered to the 1-year infant brain template (Shi et al., 2011); each registration was visually inspected as part of quality control procedures. The automatic independent component classifier ICA-AROMA (Pruim et al., 2015a, 2015b) was used to regress out components labeled as motion or noise. This approach was used instead of deleting individual motion-contaminated volumes ("scrubbing" as per Power et al., 2012, 2014) to effectively control for motion while retaining data from the full scan. Indeed, ICA-AROMA has been demonstrated to not only perform equivalently to scrubbing (Pruim et al., 2015a, 2015b), but also to improve resting-state network reproducibility while reducing the loss in temporal degrees of freedom (Carone et al., 2017; Pruim et al., 2015a, 2015b). The two groups were matched on the total number of components removed as well as the amount of mean relative motion (i.e., inter-volume motion; Table 1). To further reduce noise and other confounds, data were bandpass filtered (0.01 Hz < t < 0.1 Hz). In addition, mean white matter time series, mean cerebrospinal fluid time series, and mean global time series (Power et al., 2014) were included as nuisance regressors at the single-subject level. Global signal regression (GSR) was applied as it is effective at removing motion and respiratory related global artifacts and has been shown to improve the correspondence between functional connectivity and neuroanatomical structures (Fox et al., 2009; Murphy and Fox, 2017). Furthermore, GSR is particularly effective at denoising data when used in conjunction with ICA-AROMA (Parkes et al., 2018). However, to ensure that our functional connectivity results were not driven by GSR (Murphy and Fox, 2017), all group-level analyses (described below) were conducted again using a preprocessing pipeline which did not include global signal as a nuisance regressor. A very similar pattern of results emerged from these analyses; importantly, all interactions between risk group and time point (see Section 3.4 below) remained significant except for the interaction involving changes in connectivity between the right pSTG and the right thalamus which only approached significance (p = .08).
2.4. fMRI data analysis
2.4.1. Seed-based analysis
To examine whole-brain functional connectivity of the auditory network, average residual time series were extracted from anatomical regions-of-interest (ROIs) for left and right Heschl’s Gyrus (HG) and posterior superior temporal gyrus (pSTG), as derived from an infant brain atlas (Shi et al., 2011). For each ROI, the time series extracted from processed residuals in standard space was tested for correlations with that of every other voxel in the brain, and the resulting correlation map for each ROI/network was then converted into a z-statistic map using Fisher’s r-to-z transformation.
All whole-brain group-level contrasts were conducted in FSL using FMRIB’s Local Analysis of Mixed Effects (FLAME 1 + 2) since equal variances were not assumed between the HR and LR groups. Scanner was included as a covariate of no-interest in all group-level analyses. Initial within-group analyses were pre-threshold masked with an age-appropriate anatomical gray matter mask (Shi et al., 2011) and were corrected for multiple comparisons at a threshold of Z > 3.1, P < 0.05. Between-group analyses were pre-threshold masked using a joint connectivity mask across both risk groups (HR + LR), and were corrected for multiple comparisons at a threshold of Z > 2.3, P < 0.05.
2.4.2. Language-related ROI-Based connectivity analyses
ROI-based functional connectivity was further examined by correlating mean time series extracted from each ROI. These regions included primary auditory cortex (left/right HG), canonical language regions including left/right pSTG as well as Broca’s Area and its right hemisphere homologue (left/right IFG pars triangularis and pars opercularis), and left/right thalamus. Correlations were computed for all ROI pairings, resulting in a 10 × 10 ROI matrix. Linear models in R (i.e., linear regression models; R Core Team, 2018) were used to investigate 1) the effects of risk group within each time point (i.e., between-group differences in ROI-based functional connectivity at each time point), as well as 2) the effects of time point within each risk group (i.e., differences in ROI-based functional connectivity between 1.5 and 9 months of age within each group). Lastly, linear mixed effect models in R (lme function from the nlme package; Pinheiro et al., 2020) were used to investigate the interaction between risk group and time point to examine how the two risk groups differed in the longitudinal change in language-related ROI-based connectivity from 1.5 to 9 months of age. All reported results survived false discovery rate (FDR) correction at p < .05; for completeness, results significant at p < .05, uncorrected, are also shown in the figures.
3. Results
3.1. Seed-based connectivity
HG and pSTG were used as seeds in the initial analysis to determine how primary and secondary auditory cortex established connectivity with the whole brain. At both time points and for both groups, connectivity maps generated from left and right HG as well as pSTG showed robust connectivity with a widespread network of bilateral temporal, limbic, and sensorimotor regions (see Supplementary Information).
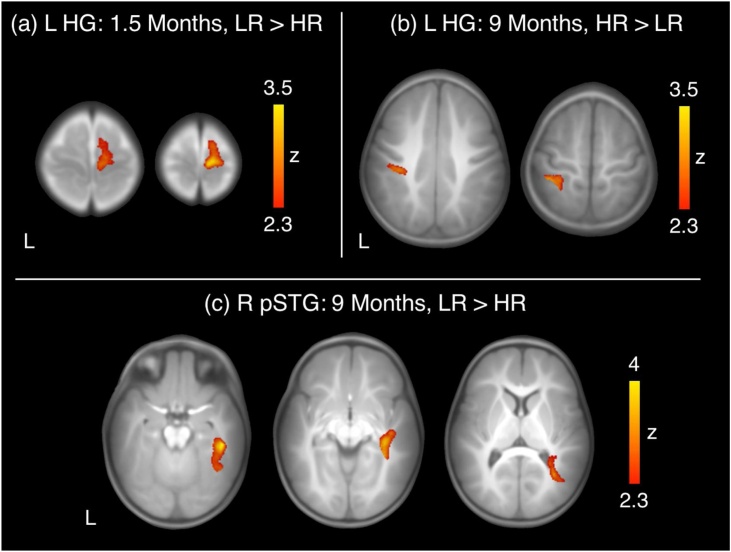
We next examined group differences in connectivity with primary and secondary auditory cortex at each time point. At 1.5 months, compared to HR infants, the LR group showed greater interhemispheric connectivity between left HG and right sensorimotor regions (supplementary motor area, precentral gyrus, postcentral gyrus; Fig. 1a, Table 2). At 9 months of age, HR infants showed greater intrahemispheric connectivity between left HG and left postcentral gyrus and parietal regions (inferior parietal lobule, superior parietal gyrus, supramarginal gyrus) compared to the LR group (Fig. 1b, Table 2). At this time point, LR infants showed greater intrahemispheric connectivity between right pSTG and the right hippocampus as well as other higher-order cortical regions, including right inferior temporal gyrus, fusiform gyrus, middle temporal gyrus, and parahippocampal gyrus compared to the HR group (Fig. 1c, Table 2).
Fig. 1.
Between-group differences in seed-based whole-brain functional connectivity. At 1.5 months, LR infants showed greater interhemispheric connectivity between left HG and right somatosensory regions compared to HR infants (a). At 9 months, HR infants displayed greater intrahemispheric connectivity between left HG and left sensory regions (b) whereas LR infants exhibited greater connectivity between right pSTG and right hippocampus as well as higher-order cortical regions including right fusiform gyrus, inferior temporal gyrus, middle temporal gyrus, and parahippocampal gyrus (c). (HG: Heschl’s gyrus; HR: high risk; LR: low risk; pSTG: posterior superior temporal gyrus).
Table 2.
Between-Group Differences in Seed-Based Whole-Brain Connectivity.
| Cluster Location | L/R | Max Z | Peak (mm) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| L HG Seed: 1.5 Months, LR > HR | |||||
| Supplementary Motor Area | R | 2.87 | 5 | −10 | 37 |
| Precentral Gyrus | R | 3.47 | 9 | −20 | 47 |
| Paracentral Lobule | R | 3.27 | 6 | −22 | 46 |
| Postcentral Gyrus | R | 3.05 | 7 | −23 | 47 |
| Supplementary Motor Area | L | 2.65 | 1 | −7 | 38 |
| L HG Seed: 9 Months, HR > LR | |||||
| Postcentral Gyrus | L | 3.06 | −36 | −35 | 50 |
| Inferior Parietal Lobule | L | 3.25 | −27 | −38 | 43 |
| Superior Parietal Gyrus | L | 3.01 | −34 | −36 | 50 |
| Supramarginal Gyrus | L | 2.35 | −40 | −18 | 25 |
| R pSTG Seed: 9 Months, LR > HR | |||||
| Inferior Temporal Gyrus | R | 3.98 | 38 | −25 | −16 |
| Fusiform Gyrus | R | 4.06 | 31 | −34 | −10 |
| Hippocampus | R | 3.54 | 30 | −25 | −6 |
| Middle Temporal Gyrus | R | 3.08 | 30 | −50 | 7 |
| Superior Temporal Gyrus | R | 3.04 | 35 | −16 | −6 |
| Parahippocampal Gyrus | R | 3.44 | 28 | −34 | −4 |
| Calcarine Cortex | R | 2.52 | 26 | −49 | 5 |
HG: Heschl’s gyrus; HR: high risk; LR: low risk; pSTG: posterior superior temporal gyrus.
3.2. ROI-based connectivity: effects of group within time point
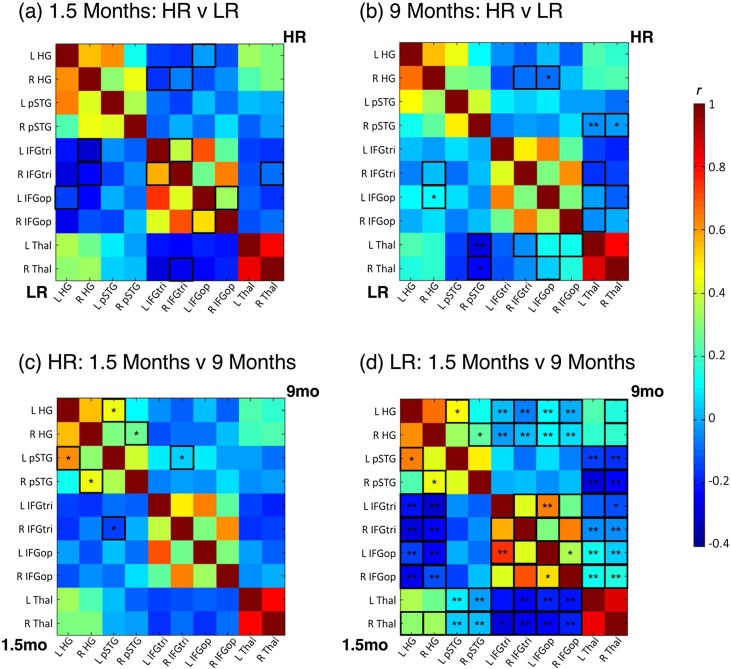
To examine alterations in functional connectivity within a network of language-related regions as well as thalamus, we first investigated between-group differences at each time point. At 1.5 months, no group differences survived FDR-correction for multiple comparisons (Fig. 2a). At 9 months, compared to the LR group, HR infants showed decreased connectivity between right auditory cortex and frontal regions (Fig. 2b). The HR group also showed increased connectivity between bilateral thalamus and right pSTG compared to LR infants (Fig. 2b).
Fig. 2.
ROI-based functional connectivity: Effects of group within time point and effects of time point within group. At 1.5 months, there were no significant between-group differences (a), but by 9 months HR infants showed greater thalamic connectivity (bilateral thalamus to right pSTG) and less frontotemporal connectivity (right HG to left IFGop) than LR infants. The HR group displayed few significant differences in connectivity over time (c) including increasing interhemispheric frontotemporal connectivity (left pSTG to right IFGtri) and decreasing intrahemispheric connectivity between primary and secondary auditory cortex (left HG to left pSTG; right HG to right pSTG). In contrast, the LR group exhibited widespread changes from 1.5 to 9 months (d) with increasing long-range connectivity (temporal to frontal, thalamus to frontal regions) and decreasing short-range connectivity (temporal to temporal, frontal to frontal, thalamus to temporal regions). Black-outlined boxes indicate significance at p < .05, uncorrected; * indicates significance at p < .05, FDR-corrected; ** indicates significance at p < .01, FDR-corrected. (HG: Heschl’s gyrus; HR: high risk; IFGtri: inferior frontal gyrus pars triangularis; IFGop: inferior frontal gyrus pars opercularis; LR: low risk; pSTG: posterior superior temporal gyrus; thal = thalamus).
3.3. ROI-based connectivity: effects of time point within group
We next examined longitudinal changes in ROI-based functional connectivity within each risk group to explore the development of functional connectivity over time. From 1.5 to 9 months of age, HR infants showed small, circumscribed changes in functional connectivity, limited to increasing interhemispheric frontotemporal connectivity and decreasing within-hemispheric connectivity between primary and secondary auditory cortex (Fig. 2c). In contrast, LR infants showed widespread changes in connectivity during the first year of life with increasing long-range connectivity (i.e., frontotemporal: bilateral HG to Broca’s area and its right hemisphere homologue; fronto-thalamic: bilateral thalamus to Broca’s area and its right hemisphere homologue) and decreasing short-range connectivity (i.e., temporal to temporal: intrahemispheric primary to secondary auditory cortex; frontal to frontal: left IFG pars triangularis to left IFG pars opercularis, left IFG pars opercularis to right IFG pars opercularis; temporo-thalamic: bilateral thalamus to bilateral pSTG) from 1.5 to 9 months of age (Fig. 2d).
3.4. ROI-based connectivity: interactions between group and time point
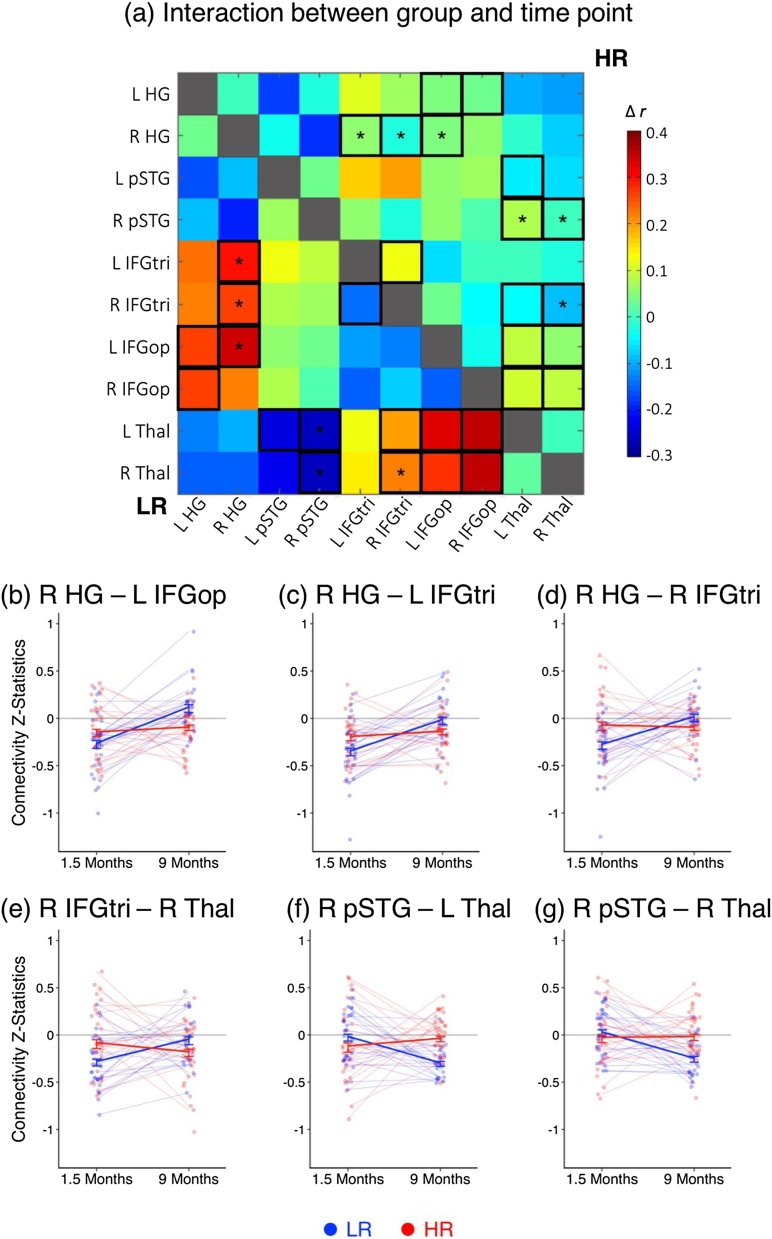
Lastly, we sought to examine group differences in longitudinal trajectories of language-related ROI-based functional connectivity by investigating the interaction between risk group and time point. Significant differences in change over time between the two risk groups clustered in two main areas: connectivity with right HG and connectivity with the thalamus (Fig. 3a). Across time, LR infants demonstrated increasing connectivity between right primary auditory cortex and frontal regions including Broca’s area (left IFG pars opercularis and pars triangularis) as well as right IFG pars triangularis, whereas HR infants did not show differences in connectivity between these regions from 1.5 to 9 months of age (Fig. 3b-d). A similar pattern was observed in the intrahemispheric connectivity between right IFG pars triangularis and right thalamus whereby LR infants displayed increasing fronto-thalamic connectivity whereas HR infants did not show significant differences in connectivity over time between these regions (Fig. 3e). However, the opposite pattern was found in temporo-thalamic connectivity between right pSTG and bilateral thalamus; LR infants showed decreasing intrahemispheric connectivity between right temporal cortex and thalamus from 1.5 to 9 months whereas HR infants did not display significant differences in temporo-thalamic connectivity over time such that by 9 months of age, the LR group showed reduced connectivity compared to the HR group (Fig. 3f-g).
Fig. 3.
Group differences in longitudinal development of language-related ROI-based functional connectivity. Significant interactions between group and time point were observed in frontotemporal connectivity and thalamocortical connectivity (a). Across time, LR infants showed increasing connectivity between right auditory cortex and frontal regions whereas HR infants displayed little change from 1.5 to 9 months (b-d). A similar pattern was observed in frontothalamic connectivity where LR infants exhibited increasing connectivity between right frontal cortex and thalamus whereas HR infants showed decreasing connectivity (e). The LR group showed decreasing connectivity between right temporal cortex and bilateral thalamus (f-g). In matrix (a),: black-outlined boxes indicate significance at p < .05, uncorrected; * indicates significance at p < .05, FDR-corrected. In graphs (b-g): red indicates HR group, blue indicates LR group. (HG: Heschl’s gyrus; HR: high risk; IFGtri: inferior frontal gyrus pars triangularis, IFGop: inferior frontal gyrus pars opercularis; LR: low risk; pSTG: posterior superior temporal gyrus; thal: thalamus). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study, we examined the development of functional networks associated with language processing in very young infants at high and low familial risk for ASD. As early as 1.5 months of age, functional connectivity underlying the integration of auditory and motor representations, which is crucial for language development, was already atypical in HR infants. By 9 months of age, HR infants showed hyperconnectivity with somatosensory regions whereas LR infants displayed greater connectivity with higher-order cortical regions. Over the first year of life, widespread changes with increasing long-range connectivity and decreasing short-range connectivity were observed in the LR group, whereas HR infants showed very limited developmental changes. More specifically, the LR group exhibited increasing connectivity between frontotemporal language regions and decreasing temporo-thalamic connectivity from 1.5 to 9 months of age, whereas HR infants displayed more static developmental profiles.
Across the first year of life, HR infants exhibited altered connectivity between temporal and somatosensory regions that support auditory-motor integration, which is particularly critical in early language learning. Indeed, prior task-based fMRI studies have demonstrated that typically developing neonates already activate a network of temporal and frontal regions similar to that observed in adults in response to speech stimuli (Perani et al., 2011; Sato et al., 2012), indicating that these regions are important for speech processing very early in life. By 4–7 months of age, LR infants show early specialization for voice processing in frontal and temporal regions whereas HR infants do not (Blasi et al., 2015). Prior task-related fMRI findings in toddlers with ASD have also shown that activation to speech stimuli is stratified by language outcome; specifically, as compared to ASD toddlers who did not exhibit language delays, ASD toddlers with poor language outcome showed attenuated neural responses in both frontal and temporal regions (Lombardo et al., 2015). In addition, anatomical connections between frontal and temporal language regions subserve language production and syntactic processing (Friederici, 2009, 2011). These white matter tracts, which are critical for language development, are present in early infancy (Liu et al., 2018; Perani et al., 2011) and are altered in HR infants as early as 1.5 months of age (Liu et al., 2018). Our findings highlight that functional connectivity of these regions is also disrupted in HR infants.
At 1.5 months, HR infants displayed less interhemispheric connectivity between left HG and right sensorimotor regions compared to LR infants, but this pattern changed by 9 months such that HR infants showed greater intrahemispheric connectivity between left HG and left sensory cortex compared to the LR group. Prior research has demonstrated that long-range connectivity, including interhemispheric connectivity, increases during typical development (Smyser et al., 2010; Thomason et al., 2015). By contrast, the results we observed here, whereby the HR infants show greater intrahemispheric connectivity and a lack of interhemispheric connectivity between primary auditory cortex and sensorimotor regions at 9 months of age, are consistent with prior studies reporting reduced interhemispheric connectivity in toddlers (Dinstein et al., 2011) as well as adults with ASD (Anderson et al., 2011). Taken together, altered connectivity between frontal and temporal regions important for sensorimotor functions supporting language processing may provide an underlying mechanism for altered neural activity to speech observed in older youth with ASD.
At 9 months of age, compared to the HR group, LR infants displayed greater intrahemispheric connectivity between secondary auditory cortex and cortical regions implicated in higher-order language processing, as well as the hippocampus. In adults, the right pSTG is known to process prosody (Fruhholz et al., 2012), a critical cue for early language learning in infancy (Romberg and Saffran, 2010). Using near-infrared optical topography to examine the neural basis of prosodic processing in 3-month-old infants, Homae et al. (2006) demonstrated that right temporoparietal areas are robustly activated in response to normal speech sounds containing pitch contours. In the present study, we found that compared to HR infants, 9-month-old LR infants displayed greater connectivity between this prosodic processing region and hippocampus as well as higher-order cortical regions. This is in line with prior neuroimaging findings in older youth indicating that TD adolescents show greater connectivity between right posterior superior temporal sulcus and other cortical regions compared to the ASD group (Abrams et al., 2013; Gervais et al., 2004). Whereas the LR infants started to exhibit robust longer-range connectivity by 9 months, HR infants displayed a more immature connectivity profile underlying higher-order language processing, which could cascade into later language deficits.
Our ROI-based functional connectivity analyses focused on a specific network including canonical language-related regions as well as the thalamus. Compared to the HR group, LR infants displayed significant developments in connectivity over the course of the first year of life. Of note, LR infants showed a significant increase in interhemispheric frontotemporal connectivity and a significant decrease in intrahemispheric temporo-temporal connectivity between primary and secondary auditory cortex. In a recent study comparing HR infants who later developed ASD (HR+) with HR infants who did not develop the disorder (HR–) as well as LR infants, Lewis et al. (2017) used graph theory methods to show that across the first year of life, HR + infants displayed reduced efficiency of many frontotemporal language processing regions, including HG, STG, Broca’s area, and its right hemisphere homologue. In our study, the relative lack of developmental change in the HR group may reflect limited growth in functional connectivity that results from reduced efficiency of these brain regions overall. By contrast, LR infants exhibited dynamic changes from 1.5 to 9 months of age with increasing long-range connectivity and decreasing short-range connectivity. This is consistent with prior findings in neurotypical neonates showing increases in long-range connectivity, especially cortical-subcortical and intrahemispheric connectivity, with advancing fetal age (Smyser et al., 2010; Thomason et al., 2015). In line with prior work demonstrating that TD youth display reduced thalamocortical connectivity with primary/secondary auditory cortices compared to adolescents with ASD (Linke et al., 2017), decreasing thalamocortical connectivity was observed in our LR infants across time such that by 9 months of age, the LR group displayed reduced connectivity between bilateral thalamus and right pSTG compared to HR infants. Taken together, these findings suggest that LR infants followed an expected pattern of development whereby long-range connectivity matures over the first year of life whereas HR infants exhibited a relatively static, more immature developmental profile in functional connectivity of language-related ROI-based networks.
In the present study, among the HR infants for whom data was available at 36 months of age, only eight HR infants qualified for an ASD diagnosis (with five and seven of them contributing data at the 1.5-month and 9-month time points, respectively). Accordingly, given the small sample, we were unable to meaningfully examine differences in connectivity between infants who did receive a diagnosis and those who did not. Future studies should examine how early differences in developmental trajectories of functional connectivity may relate to later diagnostic outcome and ASD symptomatology. Indeed, infants at high familial risk for developing ASD often go on to exhibit a range of heterogeneous outcomes including other neurodevelopmental, learning, or language disorders (Zwaigenbaum et al., 2007). As such, our findings may be more indicative of altered functional connectivity of language-related networks that may reflect a more general susceptibility to language impairment common to other developmental disorders. Indeed, recent imaging genetics studies have linked common variants on several genes associated with language impairment, including CNTNAP2, to delayed language development as well as atypical functional and structural connectivity (Alarcón et al., 2008; Scott-Van Zeeland et al., 2010; von Hohenberg et al., 2013). Furthermore, ASD is a complex and heterogeneous disorder involving both elevated environmental (Elsabbagh et al., 2012) and polygenic (Grove et al., 2019) risk factors that may already be impacting early development in the first year of life (Gao et al., 2019a, 2019b). Accordingly, future large-scale investigations should examine the impact of genetic and environmental factors on the developmental trajectory of functional connectivity in the infant brain.
In the present study, we focused on examining connectivity between regions canonically associated with language processing and sensorimotor integration. Structural factors such as cortical surface area (Hazlett et al., 2017), subcortical volume (Swanson et al., 2017), and white matter development (Liu et al., 2018; Wolff et al., 2012, 2017a, 2017b) are also altered in HR infants and may influence functional connectivity of these networks. Future research should thus aim to integrate structural metrics with measures of functional connectivity in the developing brain in at-risk populations. In addition, functional connectivity has previously been shown to be related to later behavioral phenotypes such as expressive language outcome in TD infants (Emerson et al., 2016) and restricted/repetitive behaviors in HR infants (McKinnon et al., 2019). Though this was beyond the scope of the present study, as we focused on examining longitudinal changes within these language-related networks, future research should examine brain-behavior relationships to enhance the power to detect brain-based biomarkers for the disorder. This could also help improve the predictive value of classification models that use early rsfMRI data to predict later diagnosis (Emerson et al., 2017; Pruett et al., 2015).
4.1. Conclusions
Extending prior work showing altered functional connectivity in infant siblings at high risk for developing ASD, this is the first study to characterize longitudinal development of functional connectivity in language-related networks in this population. Collectively, our findings suggest that early differences in language-related network connectivity can be detected in the first year of life which may provide an early marker of risk for ASD or other suboptimal developmental outcomes.
Funding
This work was supported by the National Institute of Child Health and Human Development (P50 HD055784 to S.Y.B. and F31 HD088102 to J.L.) and the National Institute on Drug Abuse (T90 DA022768 to J.L.). We are grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
J.L., N.J.O., K.K.C., J.J., and G.P. contributed to data collection, and J.L. conducted image processing and data analysis. This manuscript has been reviewed by all authors: J.L. wrote the manuscript with M.D.; S.Y.B. and S.S.J. provided additional feedback. The authors thank the families who generously gave their time to participate in this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100814.
Contributor Information
Janelle Liu, Email: janelle.j.liu@ucla.edu.
Nana J. Okada, Email: nokada@mednet.ucla.edu.
Kaitlin K. Cummings, Email: kkcummings@mednet.ucla.edu.
Jiwon Jung, Email: JiwonJung@mednet.ucla.edu.
Genevieve Patterson, Email: GPatterson@mednet.ucla.edu.
Susan Y. Bookheimer, Email: sbook@ucla.edu.
Shafali S. Jeste, Email: sjeste@mednet.ucla.edu.
Mirella Dapretto, Email: mirella@ucla.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abrams D.A., Lynch C.J., Cheng K.M., Phillips J., Supekar K., Ryali S., Uddin L.Q., Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc. Natl. Acad. Sci. U. S. A. 2013;110(29):12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M., Abrahams B.S., Stone J.L., Duvall J.A., Perederiy J.V., Bomar J.M., Sebat J., Wigler M., Martin C.L., Ledbetter D.H. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am. J. Hum. Genet. 2008;82(1):150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S., Lin W., Smith J.K., Short S.J., Goldman B.D., Reznick J.S., Gilmore J.H., Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 2014;34(27):9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S., Druzgal T.J., Froehlich A., DuBray M.B., Lange N., Alexander A.L., Abildskov T., Nielsen J.A., Cariello A.N., Cooperrider J.R. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A., Lloyd-Fox S., Sethna V., Brammer M.J., Mercure E., Murray L., Williams S.C., Simmons A., Murphy D.G., Johnson M.H. Atypical processing of voice sounds in infants at risk for autism spectrum disorder. Cortex. 2015;71:122–133. doi: 10.1016/j.cortex.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone D., Licenik R., Suri S., Griffanti L., Filippini N., Kennedy J. Impact of automated ICA-based denoising of fMRI data in acute stroke patients. Neuroimage Clin. 2017;16:23–31. doi: 10.1016/j.nicl.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L., Mennes M., Thomas R.M., Di Martino A., Thioux M., Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism Spectrum disorder. JAMA Psychiatry. 2015;72(8):767–777. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I., Pierce K., Eyler L., Solso S., Malach R., Behrmann M., Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Divan G., Koh Y.J., Kim Y.S., Kauchali S., Marcin C., Montiel-Nava C., Patel V., Paula C.S., Wang C. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.W., Gao W., Lin W. Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J. Neurosci. 2016;36(42):10883–10892. doi: 10.1523/JNEUROSCI.3980-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.W., Adams C., Nishino T., Hazlett H., Wolff J., Zwaigenbaum L., Constantino J., Shen M.D., Swanson M.R., Elison J.T. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med. 2017:9. doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Engström M., Hallberg B., Mosskin M., Åden U., Lagercrantz H., Blennow M. Spontaneous brain activity in the newborn brain during natural sleep—an fMRI study in infants born at full term. Pediatr. Res. 2009;66(3):301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends Cogn. Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Fruhholz S., Ceravolo L., Grandjean D. Specific brain networks during explicit and implicit decoding of emotional prosody. Cereb. Cortex. 2012;22(5):1107–1117. doi: 10.1093/cercor/bhr184. [DOI] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex. 2015;25(9):2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Lin W., Grewen K., Gilmore J.H. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 2016 doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Grewen K., Knickmeyer R.C., Qiu A., Salzwedel A., Lin W., Gilmore J.H. A review on neuroimaging studies of genetic and environmental influences on early brain development. Neuroimage. 2019;185:802–812. doi: 10.1016/j.neuroimage.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Linke A., Jao Keehn R.J., Punyamurthula S., Jahedi A., Gates K., Fishman I., Muller R.A. The language network in autism: atypical functional connectivity with default mode and visual regions. Autism Res. 2019 doi: 10.1002/aur.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido D., Petrova D., Watson L.R., Garcia-Retamero R., Carballo G. Language and motor skills in siblings of children with autism spectrum disorder: a meta-analytic review. Autism Res. 2017;10(11):1737–1750. doi: 10.1002/aur.1829. [DOI] [PubMed] [Google Scholar]
- Gervais H., Belin P., Boddaert N., Leboyer M., Coez A., Sfaello I., Barthelemy C., Brunelle F., Samson Y., Zilbovicius M. Abnormal cortical voice processing in autism. Nat. Neurosci. 2004;7(8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Grove J., Ripke S., Als T.D., Mattheisen M., Walters R.K., Won H., Pallesen J., Agerbo E., Andreassen O.A., Anney R. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019;51(3):431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., Elison J.T., Swanson M.R., Zhu H., Botteron K.N. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L.M., Rudie J.D., Green S.A., Bookheimer S., Dapretto M. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 2015;40(1):171–189. doi: 10.1038/npp.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Asakawa K., Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci. Res. 2006;54(4):276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hudry K., Chandler S., Bedford R., Pasco G., Gliga T., Elsabbagh M., Johnson M.H., Charman T. Early language profiles in infants at high-risk for autism spectrum disorders. J. Autism Dev. Disord. 2014;44(1):154–167. doi: 10.1007/s10803-013-1861-4. [DOI] [PubMed] [Google Scholar]
- Jack A. Neuroimaging in neurodevelopmental disorders: focus on resting-state fMRI analysis of intrinsic functional brain connectivity. Curr. Opin. Neurol. 2018;31(2):140–148. doi: 10.1097/WCO.0000000000000536. [DOI] [PubMed] [Google Scholar]
- Jones E.J., Gliga T., Bedford R., Charman T., Johnson M.H. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B., Wagner J.B., Tager-Flusberg H., Nelson C.A. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front. Hum. Neurosci. 2013;7:444. doi: 10.3389/fnhum.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann F., Krugel L.K., Ehlen F. Functional roles of the thalamus for language capacities. Front. Syst. Neurosci. 2013;7:32. doi: 10.3389/fnsys.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.D., Evans A.C., Pruett J.R., Jr., Botteron K.N., McKinstry R.C., Zwaigenbaum L., Estes A.M., Collins D.L., Kostopoulos P., Gerig G. The emergence of network inefficiencies in infants with autism Spectrum disorder. Biol. Psychiatry. 2017;82(3):176–185. doi: 10.1016/j.biopsych.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A.C., Jao Keehn R.J., Pueschel E.B., Fishman I., Muller R.A. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Tsang T., Jackson L., Ponting C., Jeste S.S., Bookheimer S.Y., Dapretto M. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev. Sci. 2018 doi: 10.1111/desc.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Pierce K., Eyler L.T., Carter Barnes C., Ahrens-Barbeau C., Solso S., Campbell K., Courchesne E. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015;86(2):567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon C.J., Eggebrecht A.T., Todorov A., Wolff J.J., Elison J.T., Adams C.M., Snyder A.Z., Estes A.M., Zwaigenbaum L., Botteron K.N. Restricted and repetitive behavior and brain functional connectivity in infants at risk for developing autism Spectrum disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4(1):50–61. doi: 10.1016/j.bpsc.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Treiber J.M., Shukla D.K., Shih P., Muller R.A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136(Pt 6):1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Young G.S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L., Bryson S., Carver L., Constantino J., Dobkins K. Recurrence risk for autism Spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yucel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C., Paoloniato A., Lohmann G., Friederici A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. 2011;108(38):16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D. 2020. Team RC. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-147. [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett J.R., Jr., Kandala S., Hoertel S., Snyder A.Z., Elison J.T., Nishino T., Feczko E., Dosenbach N.U., Nardos B., Power J.D. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Dev. Cogn. Neurosci. 2015;12:123–133. doi: 10.1016/j.dcn.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg A.R., Saffran J.R. Statistical learning and language acquisition. Wiley Interdiscip. Rev. Cogn. Sci. 2010;1(6):906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Hirabayashi Y., Tsubokura H., Kanai M., Ashida T., Konishi I., Uchida-Ota M., Konishi Y., Maki A. Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum. Brain Mapp. 2012;33(9):2092–2103. doi: 10.1002/hbm.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland A.A., Abrahams B.S., Alvarez-Retuerto A.I., Sonnenblick L.I., Rudie J.D., Ghahremani D., Mumford J.A., Poldrack R.A., Dapretto M., Geschwind D.H. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci. Transl. Med. 2010;2(56) doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Yap P.T., Wu G., Jia H., Gilmore J.H., Lin W., Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Snyder A.Z., Neil J.J. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56(3):1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M.R., Wolff J.J., Elison J.T., Gu H., Hazlett H.C., Botteron K., Styner M., Paterson S., Gerig G., Constantino J. Splenium development and early spoken language in human infants. Dev. Sci. 2017;20(2) doi: 10.1111/desc.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Grove L.E., Lozon T.A., Jr., Vila A.M., Ye Y., Nye M.J., Manning J.H., Pappas A., Hernandez-Andrade E., Yeo L. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev. Cogn. Neurosci. 2015;11:96–104. doi: 10.1016/j.dcn.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front. Syst. Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Khouzam A., Phillips J., Feinstein C., Ryali S., Menon V. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hohenberg C., Wigand M.C., Kubicki M., Leicht G., Giegling I., Karch S., Hartmann A.M., Konte B., Friedl M., Ballinger T. CNTNAP2 polymorphisms and structural brain connectivity: a diffusion-tensor imaging study. J. Psychiatr. Res. 2013;47(10):1349–1356. doi: 10.1016/j.jpsychires.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Zhang H., Li G., Liu M., Yin W., Lin W., Zhang J., Shen D. First-year development of modules and hubs in infant brain functional networks. Neuroimage. 2019;185:222–235. doi: 10.1016/j.neuroimage.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Gu H., Gerig G., Elison J.T., Styner M., Gouttard S., Botteron K.N., Dager S.R., Dawson G., Estes A.M. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Jacob S., Elison J.T. The journey to autism: insights from neuroimaging studies of infants and toddlers. Dev. Psychopathol. 2017:1–17. doi: 10.1017/S0954579417000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Swanson M.R., Elison J.T., Gerig G., Pruett J.R., Jr., Styner M.A., Vachet C., Botteron K.N., Dager S.R., Estes A.M. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism. 2017;8:8. doi: 10.1186/s13229-017-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L., Thurm A., Stone W., Baranek G., Bryson S., Iverson J., Kau A., Klin A., Lord C., Landa R. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J. Autism Dev. Disord. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.