Abstract
Autochtonous leptospirosis is an emerging zoonotic disease in Europe, particularly in France. We report a case of leptospirosis in a 36 year-old man, who is a recently arrived migrant from Tunisia and lives in a squat. He suffered from pulmonary and neurological involvement as well as hepatitis. Seven rats (Rattus norvegicus) were trapped in the squat where the patient lived. Leptospira spp. DNA was detected in the kidney of one rat, highlighting the most likely source of contamination. In addition to the classic recreational or professional exposure to fresh water and practice of outdoor sports as a source of leptospirosis contamination, unhealthy living conditions (homeless or squatting) and therefore frequent exposure to rats, are another risk factor for leptospirosis in Europe.
Keywords: France, Leptospirosis, Leptospira interrogans, Rattus norvegicus, Zoonosis
Introduction
Leptospirosis is an endemic zoonosis worldwide with a million cases per year, including 500,000 severe cases, and a mortality of around 60,000 cases per year [1]. This infectious disease is mainly encountered in South America, the Caribbean, South Asia and Oceania, favored by the tropical climate, stagnant waters and floods, as well as precariousness in urban areas [1]. Severe forms of human leptospirosis are mainly associated with the serovar Icterohaemorrhagiae present in Rattus norvegicus [2,3]. In France, the incidence of all forms of leptospirosis is one of the highest in Europe with an increase in the number of cases since 2014, i.e. 600 cases per year with an incidence of 1 case per 100,000 inhabitants [4]. Nevertheless, most of the cases (85 %) are imported [4]. Here, we report the clinical case of a patient suffering from autochthonous leptospirosis in Marseille, France, as well as the epidemiological survey of this patient living in a squat.
Case report
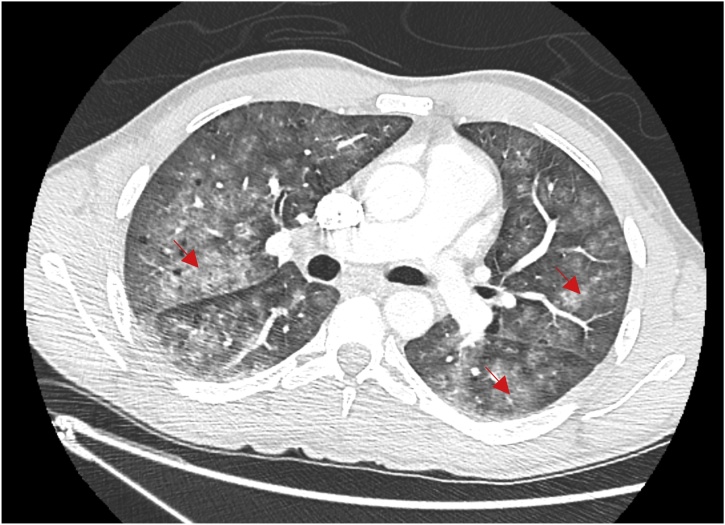
In 2018, during the winter season in December, a 36-year-old man was admitted in infectious diseases ward in Marseille (France) with fever, cough with hemoptysis, headache, stiff neck and photophobia, arthralgia, myalgia and jaundice. The symptoms were present for five days ago. This man was born in Tunisia and arrived in France three months ago. He left with his two children and wife in unsanitary conditions in a squat with presence of rat’s infestation. Upon arrival to the emergency department, the patient had fever at 38.5 °C and dyspnea (respiratory rate 28/min) with cough and hemoptysis. He also presented meningismus syndrome with headache, neck stiffness, photophobia, phonophobia and scleral icterus. His main complaint was diffuse body pain with severe myalgia, especially his involving calves. Laboratory revelated an inflammatory syndrome with white blood cells at 18 giga/L, hepatic dysfunction (AST: 70 UI/L, ALT: 105 IU/L), jaundice (Total bilirubin: 54 μmol/L, conjugated bilirubin: 44 μmol/L,) and acute kidney injury (creatinin116 μmol/L). A chest and CT scan showed bilateral interstitial infiltrates (Fig. 1). A lumbar puncture was performed and revealed liquid that looks like rock water with hypercellularity (lymphocyte count at: 100 cells/mm3, including 80 % mononuclear cells); glucose was 2.10 mmol/L and proteins were 0.67 g/L. Cerebrospinal fluid (CSF) cultures were sterile. Treatment with ceftriaxone 100 mg/kg every 12 h daily and acyclovir 15 mg/kg every 8 h was empirically initiated. After negative Herpes virus specific PCR, acyclovir was stopped and ceftriaxone was continued alone. Leptospirosis specific PCR targeting the 16S rRNA gene [6] was performed as from blood and urine and was found to be positive while detection was negative from CSF. In addition, the serology performed with a commercially available ELISA kit for both IgG and IgM (Institut Virion Serion GmbH, Warburg, Germany) showed positive IgM. The microscopic agglutination test (MAT) performed on serum was positive antibody titers for Leptospira interrogans Icterohaemorrhagiae Copenhageni (1/640), Bratislava (1/10) and Serjoe (1/160). Two and a half months later, the patient's serum was positive for Copenhageni (1/640), Icterohaemorrhagiae (1/160) and Serjoe (1/80). The patient received seven day of ceftriaxone resulting clinical improvement and discharge from hospital. He had complete clinical recovery one month post hospitalization. Interrogation of the patient showed that he lived with his family on the ground floor of a building. He had observed rat infestation outside his front door. Five weeks post hospitalization, an epidemiological veterinary investigation was conducted at the patient's home. Trapping was performed for four consecutive nights along the vicinity of a river and seven rats (Rattus norvegicus) were captured. After general anesthesia (ketamine) and euthanasia, the rats were autopsied. Serological analyses of these rats were performed using a MAT with a dilution threshold of 1:10 of 23 leptospire serovars. All the rats were serologically negative. The detection of L. interrogans DNA was performed according to the amplification protocol of the 16S locus of ribosomal DNA to which duplex PCR, amplification of the secY locus was added [5,6]. PCR was positive on the kidneys of one rat. Sequencing was not possible. All liver, lungs and urine samples were negative.
Fig. 1.
Chest Ct-Scan of the patient showing interstitial bilateral pneumonia.
Discussion
We report a case of autochtonous human leptospirosis in Marseille (France) assessed by a veterinary survey exploring the source of contamination. We detected Leptospiral DNA in the kidney of one rat surrounding the housing of the patient, suggesting that it could be a possible source of contamination. We used a validated PCR technique with validated positive and negative controls, allowing prompt diagnosis in the patient, and DNA detection in the rat. The rat with positive kidney PCR had negative serology (at 1:10), which can be explained by the fact that animal reservoirs generally have immune tolerance to host strains [5]. It is also possible that the infection in the trapped rat had occurred recently before it seroconverted. The genus Leptospira includes 24 species, including 10 pathogens and more than 300 serovars. Some mammals are likely to harbor leptospires. Nevertheless, rodents are asymptomatic carriers, while dogs and livestock are susceptible animals [1,7]. Classical risks factors for Leptospirosis in Europe are recreational or professional exposure to fresh water and practice of outdoor sports, especially in the rivers after heavy rain [8,9]. However, urban or peri-urban leptospirosis has been reemerging in Europe in recent years, with reports from Italy, Greece and south of France (Marseille) [3,7,9,10]. France reports one of the highest endemicity levels in Europe, but this is mainly due to cases from the French overseas territories, with an incidence in these regions up to 10–100 times higher than in mainland France [4]. In a clinically compatible context, the association of fever, hepatitis with jaundice, acute kidney injury, conjontcivitis and neurological symptoms sould evoke the diagnosis. In most leptospirosis human cases diagnosed in metropolitan France, the origin of leptospires is unknown. They are often summer cases related to the aquatic activities [4,11]. No case of infection in the city among precarious people (immigrants without papers living in squats) has been described in France and only few case in the USA, Japan and Portugal, in a homeless person [[12], [13], [14], [15]]. The proliferation of rats has already been implicated as a possible reservoir and source of transmission of leptospirosis in Marseille France, associated with the accumulation of garbage and rainfall [7]. Our case is original and the associated veterinary investigations, rarely highlighted in the literature, illustrate the persistence of this reservoir in Marseille. This case also highlights that recent immigration and unstable housing (homeless or squatting) with subsequent rat exposure is another risk factor for leptospirosis that should be taken into account by the clinicians. Our case is in addition to those already described recently. Leptospirosis of the homeless in cities is a topical disease [16].
Funding source
This work has benefited from the French State support, managed by ‘Agence Nationale pour la Recherche’ including the ‘Programme d’Investissements d’avenir’ under the reference Méditerranée Infection 10-IAHU-03. This work was supported by the Région Provence-Alpes-Côte d’Azur and European funding FEDER PRIMI.
Contribution
Study design: JCL, BD; Management of the patient: PS, CE, GD, JCL; Data collection: PS, AK, HM, YL, GD, PP; Data analysis: PS, CE, GD, PP, BD, JCL; Writing: PS, PP, BD, JCL
Ethical approval
Not required.
Declaration of Competing Interest
None.
Acknowledgments
We thank Audrey Chapron and Sergei Castañeda for their kind cooperation.
Contributor Information
Pablo Sanchez Fernandez, Email: sanchezpfernandez@gmail.com.
Angeli Kodjo, Email: angeli.kodjo@vetagro-sup.fr.
Hacène Medkour, Email: hacenevet1990@yahoo.fr.
Younes Laidoudi, Email: younes.laidoudi@yahoo.com.
Grègory Dubourg, Email: gregory.dubourg@univ-amu.fr.
Carole Eldin, Email: carole.eldin@univ-amu.fr.
Philippe Parola, Email: philippe.parola@univ-amu.fr.
Bernard Davoust, Email: bernard.davoust@gmail.com.
Jean-Christophe Lagier, Email: jean-christophe.lagier@univ-amu.fr.
References
- 1.Levett P.N. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupouey J., Faucher B., Edouard S., Richet H., Kodjo A., Drancourt M. Human leptospirosis: an emerging risk in Europe? Comp Immunol Microbiol Infect Dis. 2014;37(2):77–83. doi: 10.1016/j.cimid.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Dupouey J., Faucher B., Edouard S., Richet H., de Broucker C.-A., Marié J.-L. Epidemiological investigation of a human leptospirosis case reported in a suburban area near Marseille. New Microbes New Infect. 2014;2(3):82–83. doi: 10.1002/nmi2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourhy P., Septfons A., Picardeau M. Diagnostic, surveillance et épidémiologie de la leptospirose en France. Bull Epidémiol Hebd. 2017;(8-9):131–137. http://invs.santepubliquefrance.fr/beh/2017/8-9/2017_8-9_1.html [Google Scholar]
- 5.Morel A.S., Dubourg G., Prudent E., Edouard S., Gouriet F., Casalta J.P. Complementarity between targeted real-time specific PCR and conventional broad-range 16S rDNA PCR in the syndrome-driven diagnosis of infectious diseases. Eur J Clin Microbiol Infect Dis. 2015;34(3):561–570. doi: 10.1007/s10096-014-2263-z. [DOI] [PubMed] [Google Scholar]
- 6.Mérien F., Amouriaux P., Perolat P., Baranton G., Saint Girons I. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J Clin Microbiol. 1992;30(9):2219–2224. doi: 10.1128/jcm.30.9.2219-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socolovschi C., Angelakis E., Renvoisé A., Fournier P.-E., Marié J.L., Davoust B. Strikes, flooding, rats, and leptospirosis in Marseille, France. Int J Infect Dis. 2011;15(10):e710–715. doi: 10.1016/j.ijid.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita N., Ng C.F.S., Kim Y., Suzuki M., Saito N., Ariyoshi K. The non-linear and lagged short-term relationship between rainfall and leptospirosis and the intermediate role of floods in the Philippines. PLoS Negl Trop Dis. 2018;12(4) doi: 10.1371/journal.pntd.0006331. e0006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitale M., Agnello S., Chetta M., Amato B., Vitale G., Bella C.D. Human leptospirosis cases in Palermo Italy. The role of rodents and climate. J Infect Public Health. 2018;11(2):209–214. doi: 10.1016/j.jiph.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Panagopoulos P., Ganitis A., Papanas N., Iosifidou G., Maltsan T., Kioutsouk S. Leptospirosis: a report on a series of five autochthonous cases in a Greek region. J Chemother. 2016;28(5):428–431. doi: 10.1179/1973947815Y.0000000073. [DOI] [PubMed] [Google Scholar]
- 11.Brockmann S., Piechotowski I., Bock-Hensley O., Winter C., Oehme R., Zimmermann S. Outbreak of leptospirosis among triathlon participants in Germany, 2006. BMC Infect Dis. 2010;10:91. doi: 10.1186/1471-2334-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones S., Kim T. Fulminant leptospirosis in a patient with human immunodeficiency virus infection: case report and review of the literature. Clin Infect Dis. 2001;33(5):31–33. doi: 10.1086/322645. [DOI] [PubMed] [Google Scholar]
- 13.Bini Viotti J., Chan J.C., Rivera C., Tuda C. Sporadic leptospirosis case in Florida presenting as Weil`s disease. IDCases. 2019;19:e00686. doi: 10.1016/j.idcr.2019.e00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang Ym, Hagiwara A., Uemura T. Leptospirosis infection in a homeless patient in December in Tokyo: a case report. J Med Case Rep. 2015;9:198. doi: 10.1186/s13256-015-0687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira Marques T., Nascimento P.O., Almeida A., Tosatto V. Weil’s disease in a young homeless man living in Lisbon. BMJ Case Rep. 2020;13(6):e233543. doi: 10.1136/bcr-2019-233543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagan J.E., Moraga P., Costa F., Capian N., Ribeiro G.S., Wunder E.A., Jr Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl Trop Dis. 2016;10(1) doi: 10.1371/journal.pntd.0002927. e0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]