Abstract
Encapsulation in alginate beads has always been limited by the leakage due to the too wide distribution of pore sizes. Mixing alginate with other polymers have sometimes reduced the problem. Hydrocolloids from seven tropical vegetal species (barks of Triumfetta cordifolia and Bridelia thermifolia, seeds of Irvingia gabonensis and Beilschmiedia obscura, and leaves of Ceratotheca sesamoides, Adansonia digitata and Corchorus olitorius) were screened for synergistic interactions with alginate in dilute aqueous solution. Mixtures with alginate were made at different volume proportions and deviations from the initial viscosity set at 1 were evaluated. In distilled water, the gums from T. cordifolia, B. obscura, C. sesamoides and C. olitorius presented synergies with alginate. In 2 mM calcium chloride, the seven gums showed positive synergy. Interactions are favored by gum flexibility and the presence of charges, although high charges reduced the interactions. Alginate fraction of maximum viscosity enhancement depends on the ability to conformational order of the gum. The measure by laser diffraction of alginate-gum particles sizes at different fractions showed that the cooperative interactions did not always involve the largest complexes formed in gums associations. The occurrence of these interactions predicts the formation of homogeneous mixed gels at higher polymer and calcium concentrations.
Keywords: Food science, Food technology, Physical chemistry, Alginate, Calcium chloride, Synergistic interactions, Tropical vegetal gums
Food Science; Food Technology; Physical Chemistry; Alginate; Calcium Chloride; Synergistic interactions; Tropical vegetal gums.
1. Introduction
Alginate gel is widely used as encapsulation matrix for active macromolecules and probiotics. However, it possesses a too wide range of pore sizes, leading to macromolecules leakage (Rahim et al., 2008). Since pore sizes of mixed gels from synthetic or natural polymers can be better controlled (Foster and Wolf, 2011; Ullah et al., 2015), mixing alginate with other polymers has sometimes allowed to reduce beads swelling and leakage (Bonine et al., 2014; Dolatabati-Farahani et al., 2006). This suggests that mixed gels of alginate can reduce the leakage. In fact, gels made up of particulate aggregates are more compact and tight than those made up of linear polymer chains (Jones and McClements, 2010).
Homogeneous mixed gels are obtained with compatible polymers, that is polymers able to associate. Polysaccharides associations usually yield synergies which can be observed on systems properties (gel strength, viscosity…). But synergistic interactions with alginate are rather rare, although on a molecular point of view, alginate is able to form hydrogen bonds with any polysaccharide (Helgerud et al., 2010). Synergies so far identified are those with pectin and xanthan gum (Pongjanyakul and Puttipipatkhachorn, 2007; Thom et al., 1982; Toft et al., 1986; Walkenström et al., 2003). Both gums are known to possess an ordered structure (Endreß and Christensen, 2009; Goycoolea et al., 2001) and their interactions with alginate can occur in absence of cations. The association of alginate with xanthan is achieved through the formation of hydrogen bonds between hydroxyl and carboxyl groups of either gums while xanthan loses its rheological properties to adopt those of alginate (Pongjanyakul and Puttipipatkhachorn, 2007). In interactions with galactomannans, xanthan gum goes from an ordered or a disordered conformation to adopt finally an ordered conformation in the complex formed (Goycoolea et al., 2001; Higiro et al., 2006). These show the conformational adaptability (and thus the flexibility) of xanthan gum.
Like xanthan, many gums extracted from tropical vegetal species (Triumfetta cordifolia, Bridelia thermifolia, Irvingia gabonensis, Beilschmiedia obscura, Ceratotheca sesamoides, Corchorus olitorius and Adansonia digitata) are charged. Along with neutral osidic residues, they contain galacturonyl and glucuronyl residues, representing 10 %–90 % of total sugar residues. Their size is found between 940 kDa for some fractions of C. olitorius gum and 4070 kDa for T. cordifolia gum (Ndjouenkeu et al., 1995; Nwokocha and Williams, 2014, 2016; Saidou et al., 2014; Saidou et al., 2011; Woolfe et al., 1977; Yamazaki et al., 2008, 2009). These sizes are higher than that of alginate generally found between 10 kDa and 600 kDa (Helgerud et al., 2010). Although these gums present mainly random coil conformations, some possess weak gel properties and that from C. olitorius has presented a synergy with κ-carrageenan (Ndjouenkeu et al., 1995; Nwokocha and Williams, 2014, 2016; Saidou et al., 2011, 2014; Woolfe et al., 1977; Yamazaki et al., 2008, 2009). These gums are all comestible and used in households as thickeners in sauces and dough and as local beer clarifiers.
When two polymers are mixed, they either exclude mutually or associate. In both cases, changes in the viscosity of the system can be observed. Mutual exclusion is caused by repulsive interactions between polymers; it leads to phase separation or co solubility. In case of attractive interactions, the gums associate to form complexes which can either remain in solution or precipitate. However, in dilute solution, repulsion effects are negligible (Foster and Wolf, 2011; Goycoolea et al., 1995). Therefore when mixing two dilute polymer solutions, viscosity reduction indicates associative interactions with reduction of hydrodynamic volume of polymers, while viscosity enhancement indicates associative interactions with intrapolymer and interpolymer cooperative interactions that can, in some cases, induce gelation (Goycoolea et al., 1995; Menchicchi et al., 2014, 2015). The aim of the present work is then to screen synergistic interactions, in dilute solutions, between alginate and hydrocolloid gums from the above named vegetal species taking into account the conformation and charges of the gums.
2. Material and methods
2.1. Source and preparation of vegetal material
The seven plants used as gum source, are found in the intertropical region of Africa. They are all domesticated, except Beilschmiedia obscura, which is an endemic plant. Irvingia gabonensis is exploited on large scale for its oil (Tchoundjeu and Atangana, 2007). The others are mainly used at domestic level and thus cultivated at small scale. Information on colloids containing part of the plants, their local name and their market prices are shown on Table 1.
Table 1.
Colloids containing parts, local names and market prices of the plants.
| Vegetal specy | Colloids containing part | Local name | Cost ($/kg) |
|---|---|---|---|
| Adansonia digitata | Leaves (dry) | Bocco | 2–4 |
| Beislchmiedia obscura | Nuts (dry) | Khan | 5 |
| Bridelia thermifolia | Barks (dry) | Kelly | 3–5 |
| Ceratotheca sesamoides | Leaves (dry) | Gougdo | 2–4 |
| Corchorus olitorius | Leaves (dry) | Lalo/Nkeling-nkeling | 2–4 |
| Irvingia gabonensis | Almond (dry) | Mango | 14 |
| Triumfettta cordifolia | Shoots (fresh) | Nkui | 0.3 |
Shoots of Triumfetta cordifolia, dry barks of Bridelia thermifolia, seeds of Irvingia gabonensis and Beilschmiedia obscura, dry leaves of Ceratotheca sesamoides, Adansonia digitata and Corchorus olitorius were obtained from farms and markets of the savannah region of Cameroon, Central Africa.
For T. cordifolia, the film was removed from shoots, and the barks pealed using a stainless-steel knife, cut into pieces of 5 cm of length and dried at 45 °C for 24 h in an electric drier (Saidou et al., 2013). The seeds of I. gabonensis and B. obscura were dried at 40 °C for 24 h and ground using a micro grinder Culatti to obtain particle size of 300 μm, then defatted by Soxhlet extraction with hexane. The leaves of C. sesamoides, A. digitata and C. olitorius were ground using a micro grinder Culatti to particle size of 300 μm.
2.2. Extraction and purification of gums
Gum extraction was done as described by Saidou et al. (2014), Ndjouenkeu et al. (1995) and Ndjouenkeu et al. (1996). The barks/water ratio used was 4g/150 mL and 4 g/60 mL for T. cordifolia and B. thermifolia respectively. The barks were infused in water at 60 °C for 1 h under agitation with a blade, the mixture sieved, and the extract centrifuged at 3600 rpm for 30 min. The supernatant constituted the crude extract. The powder from the seeds of I. gabonensis, B. obscura and leaves of C. olitorius, A. digitata and C. sesamoides were infused in distilled water (10 g/250 mL) at 60 °C for 1 h under agitation with a magnetic stirrer. The solution was then centrifuged at 3600 rpm for 30 min and the supernatant collected as crude extract.
The crude extracts were purified as described by Saidou et al. (2014), freeze dried and the powders stored at room temperature in sealed polyethylene tubes.
Sodium alginate (ALGOGEK 3001) was purchased from Degussa France. The molecular weight was calculated from intrinsic viscosity using Mark-Houwink-Sakaruda relationship with the constants determined by Martinsen and co-workers in 1991 (Pamies et al., 2010). The value was (106.5 ± 1.6) kDa.
2.3. Viscosity measurements
Viscosity measurements were undergone on water and salt (calcium chloride) solutions of gum using an Ostwald capillary viscometer in a water bath at 30 °C.
Gum powders (1 g) were suspended in distilled water (100 mL) and stirred overnight at room temperature using a magnetic stirrer. The obtained stock solutions were then diluted in distilled water to concentrations between 0.01 % and 0.5 % depending on the gum. For salty gum solutions, calcium chloride from a stock solution of CaCl2 20 mM, was added to the aqueous gum solution in order to obtain a final concentration of 2 mM. This concentration of 2 mM corresponds to half saturation of alginate solution of specific viscosity 1.
The constant k of the viscometer was first determined through the measurement of the flow time of distilled water and calculated from Eq. (1) using the known kinematic viscosity of water (0.896 cSt at 30 °C). Then, for each gum solution the flow time (s) in the viscometer was measured and used for the determination of kinematic viscosity given by Eq. (1).
| V = k.t | (1) |
with V kinematic viscosity (cSt), k constant, t (s) flow time of the solution.
The dynamic viscosity was then calculated using Eq. (2), the fluid density (ρ) being measured using a pycnometer. The specific viscosity sp) was calculated from the viscosities of the solution and the solvent through Eq. (3)
| η = V.p | (2) |
with dynamic viscosity, ρ density of the solution,
| (3) |
with dynamic viscosity of the solution, s dynamic viscosity of the solvent, sp specific viscosity of the solution.
To appreciate gums conformations in the two solvents, the power law coefficient b of Eq. (4) was determined by plotting log (sp) as a function of log (C).
| (4) |
C is the gum concentration in the solution, a and b are constants.
The intrinsic viscosity () of the gums was determined by extrapolation to zero concentration of Huggins (Eq. (5)), Kraemer (Eq. (6)) and Single-point (Eq. (7)) plots.
| (5) |
| (6) |
| (7) |
With the intrinsic viscosity; the relative viscosity; k′, k″ and d constants.
The degree of contraction of each gum was calculated as the ratio of the intrinsic viscosity in distilled water and in calcium chloride 2 mM (Eq. (8)).
| (8) |
2.4. Determination of gum charge
The method used is derived from acid base titration of surface charges of solid particles presented by Ntalikwa (2007). 0.1 g of gum was dissolved in 50 mL distilled water and titration undergone with HCl 1 N coupled to pH measurement until a final pH of 1.4. 50 mL of distilled water was used as blank. The charge was expressed by Eq. (9)
| (9) |
with q being the gum charge in millimoles per gram; C, the normality of hydrochloric acid; ve the volume of acid used in the gum solution; v0 the volume of acid used in the blank, and me the mass of gum in the solution.
2.5. Screening of synergistic interactions in dilute solution
Synergistic interactions with alginate were screened according to the method described by Goycoolea et al. (1995) with slight modifications. The solvents used were distilled water and calcium chloride (2 mM). Gums solutions with specific viscosity 1 were used. In each case, alginate solution and the studied gum solution were mixed at different volume proportions and the viscosity of the mixture determined as described above. Solutions were prepared in triplicates.
The interactions between gums were estimated by measuring the surface of viscosity deviation as a function of alginate fraction in the mixture. The theoretical viscosities to obtain in case of no interaction were calculated from Eq. (10).
| (10) |
With ηtheo being the theoretical specific viscosity of the solution, ηalg the specific viscosity of alginate solution, ηg the specific viscosity of the gum solution, vg the volume of gum solution in the mixture and valg the volume of alginate solution in the mixture.
The weight fraction of alginate in the mixture was calculated from Eq. (11).
| (11) |
With falg the weight fraction of alginate in the mixture, Calg the concentration of alginate solution, Cg the concentration of the gum solution.
The difference between experimental value and theoretical value of viscosity was expressed as deviation percentage from the theoretical value (Eq. (12)).
| (12) |
with ηexp specific viscosity experimental value.
The interaction surfaces were determined by integration with the software Scilab-6.0.0- alpha-2, using three methods: (i) the polynomial interpolation of the line using Statgraphics Centurion XVI, and integration of the polynomial, (ii) the spline integration, and (iii) the trapezoidal integration of experimental data. The average surface of the three methods was considered.
2.6. Determination of particle size of interacting gums
The volumetric average diameter of interacting gums was determined by light scattering (laser diffraction) using the Mastersizer Hydro 2000SM of Malvern Technologies. The solvent used was a solution of calcium Chloride 2 mM. 20 mL of the solutions used for viscosity measurements were put in the dispersion unit where the agitation was maintained at 2000 rpm. The laser beam with a power of 5 mW was applied at 2 wavelengths; 632.8 nm and 466 nm. The particle size was computed with the software Mastersizer 2000 v5.17 using the raw data from the optical unit. The measurements were done in duplicates and the results expressed as the volumetric particle size distribution from which the average diameter is derived.
Gums characteristics and interactions surfaces were statistically compared by the Least Significant Difference of Fisher using the software Statgraphics Centurion XVI.
3. Results and discussion
3.1. Gums characteristics
Ionic and viscosity characteristics of the gums are shown on Table 2. All the gums are negatively charged and responsive to the presence of salt. The conformations and intrinsic viscosities change in presence of salt. In distilled water, the power law coefficients are generally lower and the intrinsic viscosities higher than in calcium chloride solution. Because of the mutual repulsion of charges in absence of ions, the gums are forced to expand and adopt a somehow rigid conformation, this imposes a higher gyration radius and thus a higher intrinsic viscosity. Calcium ions in solution reduce the repulsion and allow the gums to adopt their real functional conformation. However, the power law coefficients of the gums from A. digitata, B. obscura and C. olitorius instead fall from 1.01, 1.39 and 1.19 in distilled water to 0.93, 1.01 and 1.07 respectively in calcium chloride solution while their intrinsic viscosities decrease (except for B. obscura), showing that they are more ordered in presence of ions. According to Higiro et al. (2006) and Lapasin and Pricl, as cited by Lai and Chiang (2002), a power law coefficient lower than 1 is associated to a rod-like conformation while a coefficient between 1.1 and 1.4 indicates a random coil conformation. Therefore, in distilled water, alginate and the gums from I. gabonensis and A. digitata have a rod-like conformation; the gum from T. cordifolia is found at the limit of random coils while the others are random coils. In calcium chloride, the gums from A. digitata, B. obscura and C. olitorius have a rod-like conformation while the others are random coils.
Table 2.
Summary of gums characteristics in distilled water and in 2 mM calcium chloride.
| Gums | Parameters |
|||||
|---|---|---|---|---|---|---|
| Charge (mmol/g) | (dl/g) | (dl/g) | Degree of contraction | |||
| Alginate | 0.88 ± 0.02a | 1.16 ± 0.09cd | -4.68 ± 0.24b | 8.45 ± 0.44b | 7.56 ± 0.35d | 1.12 ± 0.11a |
| T. cordifolia | 1.09 ± 0.02d | 1.42 ± 0.06f | -3.60 ± 0.86bc | 18.29 ± 0.64e | 5.63 ± 0.54b | 3.25 ± 0.43d |
| B. thermifolia | 1.16 ± 0.04e | 1.26 ± 0.04e | -6.67 ± 0.97a | 33.76 ± 1.32g | 13.66 ± 0.36e | 2.47 ± 0.17c |
| I. gabonensis | 0.94 ± 0.03b | 1.18 ± 0.12de | -3.28 ± 0.88bc | 7.46 ± 0.31b | 4.50 ± 0.08a | 1.66 ± 0.10b |
| B. obscura | 1.39 ± 0.04g | 1.01 ± 0.05ab | -1.96 ± 1.22c | 3.55 ± 0.22a | 5.08 ± 0.11ab | 0.70 ± 0.06a |
| C. sesamoides | 1.20 ± 0.02f | 1.40 ± 0.04f | -8.31 ± 1.67a | 21.26 ± 0.87f | 6.99 ± 0.60cd | 3.04 ± 0.39d |
| C. olitorius | 1.19 ± 0.02ef | 1.07 ± 0.12bc | -2.73 ± 0.62c | 15.47 ± 0.88d | 7.32 ± 0.91cd | 2.11 ± 0.39c |
| A. digitata | 1.01 ± 0.03c | 0.93 ± 0.02a | -7.81 ± 1.06a | 11.00 ± 0.18c | 6.70 ± 0.11c | 1.64 ± 0.06b |
a, b, c … Values on the same column with the same letter as superscript are not significantly different (p ≤ 0.05).
: power law coefficient of the gum specific viscosity as a function of the concentration in water; : power law coefficient of the gum specific viscosity as a function of the concentration in 2mM CaCl2 solution; Charge: expressed as the quantity of protons needed to neutralize one gram of gum; : intrinsic viscosity of the gum in water; : intrinsic viscosity of the gum in 2mM CaCl2; Degree of contraction: the ratio of intrinsic viscosity in water on the intrinsic viscosity in calcium chloride solution.
These intrinsic viscosities are similar to those found previously for the gums from T. cordifolia, B. thermifolia and I. gabonensis (Ndjouenkeu et al., 1996; Nwokocha and Williams, 2014; Saidou et al., 2011, 2014). B. obscura gave a lower value than Ndjouenkeu and coworkers (1995); and C. olitorius and A. digitata gave higher values (Nwokocha and Williams, 2016; Saidou et al., 2011). These differences can be due to the difference of the nature and the concentration of ions, for the solvents used in this study were distilled water and 2 mM calcium chloride solutions instead of 0.1 M sodium chloride in literature.
The degrees of contraction (ratio of intrinsic viscosities in water and in salt solution) have values similar to those obtained with natural polymers by Menchicchi et al. (2015). The evolution tendency of power law coefficient values (Table 2) in calcium chloride and the degrees of contraction are comparable, showing that there may exist a correlation between both parameters. The rigidity (low degree of contraction) of the gum from B. obscura can be explained by its low charge and its ability to form weak gels (Ndjouenkeu et al., 1995). Uncharged gums are not influenced by the presence of ions, and negatively charged gums contract in presence of monovalent cations. In presence of an increasing concentration of divalent cations, gums charges are screened in a first time, making possible gum contraction and a subsequent viscosity reduction. When all the charges are screened, the excess cations form intermolecular junctions which instead increase the viscosity (Saidou et al., 2014). Intrinsic viscosity reduction was possible in this study because of the low calcium concentration used. The low charge of B. obscura gum allowed this concentration to expand the molecule. From Table 3, it is seen that in calcium chloride, the concentration needed to get a viscosity of 1 is higher than in water for all gums. This is consistent with the intrinsic viscosity reduction in presence of ions. The gum from B. obscura gives a viscosity of 1 at the same concentration (0.12%) in both solvents. Alginate, which forms dimers at this calcium concentration, shows the lower concentration increase. The degree of contraction of alginate and B. obscura gum are statistically the same (Table 2). This allows us to conclude that both gums aggregate at the used calcium chloride concentration. Therefore in presence of calcium chloride, B. obscura gum has a limited contraction because of its low charge, but since it aggregates, the intrinsic viscosity increases.
Table 3.
Concentration (in g/dl) of gums stock solutions used to screen interactions (specific viscosity fixed of 1 at 30 °C in different solvents).
| Gums | Solvents |
|
|---|---|---|
| Distilled water | CaCl2 2 mM | |
| Alginate | 0.10 | 0.14 |
| T. cordifolia | 0.02 | 0.05 |
| B. thermifolia | 0.02 | 0.05 |
| I. gabonensis | 0.10 | 0.19 |
| B. obscura | 0.12 | 0.12 |
| C. sesamoides | 0.035 | 0.07 |
| C. olitorius | 0.30 | 0.50 |
| A. digitata | 0.035 | 0.15 |
From these gums' characteristics, two main groups can be defined, the random coil gums and those able to adopt an ordered conformation.
-
-
The random coil gums present a random coil conformation both in distilled water and in calcium chloride solution (b1.1). Their degree of contraction is higher than 2.2. The gums from T. cordifolia, B. thermifolia and C. sesamoides constitute this group.
-
-
The gums able to adopt an ordered conformation possess a rod-like shape conformation in at least one solvent. Their degree of contraction is lower than 2.2. The gums from B. obscura, I. gabonensis, A. digitata and C. olitorius constitute this group. B. obscura gum is the only one with degree of contraction lower than unity and could be considered as a sub-group.
3.2. Interactions with alginate
The concentration dependency of specific viscosity of gums solutions in water and calcium chloride allowed to locate the specific viscosity of 1. These concentrations, recorded in Table 3, were used to screen synergistic interactions. Apart from B. obscura, all the gums need a higher concentration in calcium chloride to maintain the specific viscosity of 1. They are more soluble in presence of ions. This confirms the presence on their chains of chemical groups able to interact with ions, the carboxyl groups of uronyl residues.
In calcium chloride solution, all the gums interact with alginate, while in distilled water, three of them, the gums from B. thermifolia, I. gabonensis and A. digitata show positive and negative viscosity deviations, meaning an absence of interaction. The interaction parameters (surface and alginate ratio of maximum viscosity enhancement or reduction) obtained in distilled water and in calcium chloride solution are recorded in Table 4.
Table 4.
Summary of alginate-gum interaction parameters in distilled water and 2 mM calcium chloride solution.
| Gums | Distilled water |
CaCl2 2 mM |
||||
|---|---|---|---|---|---|---|
| Alginate fraction1 | Alginate solution percentage2 | Interaction surface | Alginate fraction1 | Alginate solution percentage2 | Interaction surface | |
| I. gabonensis | / | / | 0.44 ± 0.01b | 50 % | 51.0 ± 0.2a | |
| C. olitorius | 0.33 ± 0.14a | 60 % | 5.3 ± 0.3b | 0.38 ± 0.04a | 65 % | 51.2 ± 0.9a |
| A. digitata | / | / | 0.35 ± 0.01a | 40 % | 39.9 ± 0.7b | |
| T. cordifolia | 0.73 ± 0.07b | 40 % | -7.7 ± 0.3a | 0.70 ± 0.04c | 45 % | 37.7 ± 0.4c |
| C. sesamoides | 0.60 ± 0.08b | 30 % | 8.8 ± 0.4c | 0.78 ± 0.01d | 65 % | 36.0 ± 0.7d |
| B. thermifolia | / | / | 0.76 ± 0.06d | 60 % | 36.1 ± 1.0d | |
| B. obscura | 0.36 ± 0.10a | 30 % | 18.4 ± 0.2d | 0.45 ± 0.01b | 40 % | 33.1 ± 0.3e |
a, b, c… Values on the same column with the same letter as superscript are not significantly different (p ≤ 0.05).
Alginate fraction of maximum viscosity enhancement (or reduction).
Alginate solution percentage in the mixture showing the highest viscosity enhancement.
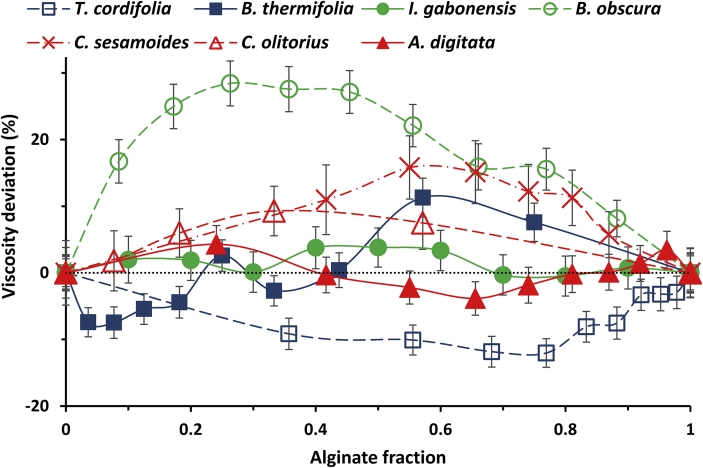
In distilled water, 4 gums show clearly visible interactions. The gums from B. obscura, C. sesamoides and C. olitorius show positive synergy with alginate while the gum from T. cordifolia shows negative synergy (Figure 1, Table 3). Apart from C. sesamoides gum which is not much studied in literature, all these interacting gums, like alginate, are able to form gels at higher concentrations (Ndjouenkeu et al., 1995; Saidou et al., 2014; Woolfe et al., 1977; Yamazaki et al., 2008, 2009). These findings can be compared with the behavior of xanthan and pectin, both possess gelling properties and form synergistic gels with alginate in absence of cations (Brejnholt, 2010; Endreß and Christensen, 2009; Sworn, 2010). This suggests that gelling gums are likely to associate with alginate. The negative synergy with T. cordifolia gum demonstrates an association leading to aggregates formation. The positives synergies instead show a dominance of cooperative interactions yielding to a coupled network formation. Therefore, the observed positive synergies indicate potential mixed gels at higher concentrations. Since the gums involved are all negatively charged, these gels are expected to be physical and of low mechanical stability. Given that alginate gel is cross-linked and thus has good mechanical properties, it is therefore important to evaluate the potential of mixed gels formation in conditions where alginate starts to gel, in presence of calcium chloride.
Figure 1.
Percentage deviation of the specific viscosity of alginate-gum mixtures in distilled water with respect to the base line of no interaction (mean ± SD, n = 3).
The viscosities registered with the three other gums (from B. thermifolia, I. gabonensis and A. digitata), do not allow to conclude about the existence of a synergy in distilled water (Figure 1). With B. thermifolia, it is observed that below an alginate fraction of 0.44, the deviation is either null or negative and beyond this value, it is positive. A. digitata gives a positive deviation at extreme fraction values and a negative deviation between alginate fractions of 0.41 and 0.81. The deviation observed with I. gabonensis is negligible although it tends to be positive. These observations might show associations of the gums with alginate in which there is a competition between aggregates formation (leading to negative deviations) and coupled network formation (leading to positive deviations). A study of interactions in presence of calcium chloride will help to conclude about the occurrence of associative interactions with these gums.
3.2.1. Influence of calcium chloride and charge
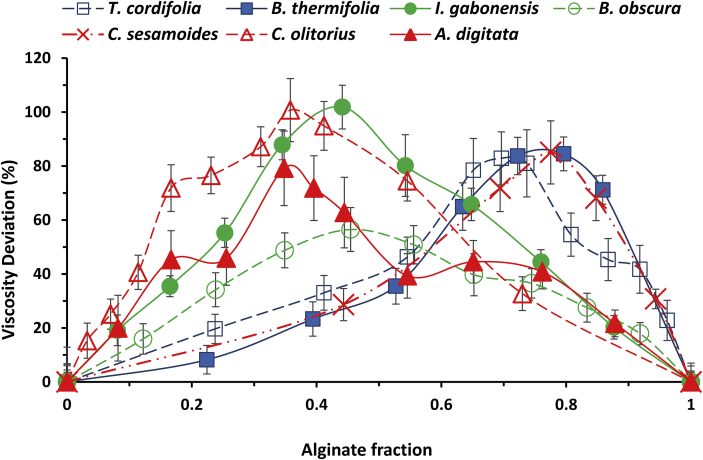
In presence of calcium chloride 2 mM, all the gums show positive synergistic interaction with alginate. For all couples, there exist a clearly separated point of maximum viscosity enhancement which indicates the maximum of cooperative interactions between the polymers involved (Figure 2, Table 3). The gums that showed interactions in distilled water have more pronounced interactions in calcium chloride. Their alginate ratio of maximum viscosity enhancement is the same, in both solvent conditions apart from C. sesamoides gum. This suggests well defined proportions of association with the gums from T. cordifolia, B. obscura and C. olitorius; there could exist some ‘stoichiometry’. Such well-defined optima have been previously observed between xanthan gum and LBG (Goycoolea et al., 1995; Higiro et al., 2006), κ-carrageenan and LBG (Yamazaki et al., 2008), alginate and pectin (Walkenström et al., 2003).
Figure 2.
Percentage deviation of the specific viscosity of alginate-gum mixtures in CaCl2 2 mM with respect to the base line of no interaction (mean ± SD, n = 3).
Yamazaki et al. (2008) observed a synergistic interaction between a fraction of C. olitorius gum and κ-carrageenan. It is, to the best of our knowledge, the only gum of this study for which a report about synergy exists in literature.
Since all gums are negatively charged and all interact with alginate in calcium chloride in a more pronounced way, it shows that the presence of salt favors interactions between alginate and gums. Calcium chloride screens gums charges, reducing electrostatic repulsion. The importance of charges in intermolecular interaction has been emphasized in alginate and pectin interaction where the synergistic gel could only exist at low pH (≈2) where carboxyl groups are protonated, which is not possible at neutral pH because of electrostatic repulsion between gums (Thom et al., 1982; Toft et al., 1986; Walkenström et al., 2003). The higher interaction observed in water is that of B. obscura gum, while in calcium chloride it is the lowest. This is due to the low charge of B. obscura which reduces the repulsions in water and thus allows a good interaction. But when the charges are screened, the interaction is not much increased because the charges are few and the polymer rigidity high. In their work, Pongjanyakul & Puttipipatkhachorn (2007) showed that molecular interactions between alginate and xanthan involve hydrogen bonds between carboxyl, hydroxyl and osidic (ether) groups of alginate and hydroxyl and carboxyl groups of xanthan. The low charge of B. obscura therefore limits the amount of hydrogen bonds that can be formed, and thus the interaction. However, it is seen in each of the two groups of gums (as defined from gums characteristics), that the higher the charge, the lower the interaction surface (Table 4). It can then be concluded that the presence of charges (carboxyl groups) promotes synergy with alginate but when beyond some threshold, they instead create repulsion. This confirms that the interactions are done through hydrogen bonds. Nevertheless, in a potential gel (formed in higher concentrations of calcium), charges will be fully screened and there will be no electrostatic repulsion, and thus a maximum association through hydrogen bonds.
Since calcium chloride concentration of 2 mM corresponds to half saturation of alginate stock solution, when alginate concentration is reduced by half and calcium concentration maintained, alginate is saturated in calcium and starts to gel; but this cannot be observed because of dilution. While moving from pure alginate solution to pure gum solution, the alginate rigid and ordered tridimensional structure is obtained with the highest concentration when alginate solution percentage is 50 % and is diluted from there. The greatest concentration of ordered alginate network is therefore obtained around 50 % solution. The optima of viscosity enhancements occur when alginate solution percentage is between 40 % and 65 % (Table 4), it shows that the highest cooperative interactions occur within alginate ordered network and predicts the formation of homogeneous mixed gels at higher concentrations.
3.2.2. Influence of the flexibility and conformational order
Two major groups emerge from gum interactions (Table 4). The random coil gums attain the maximum viscosity enhancement at alginate fractions between 0.65 and 0.80, while those able to adopt a rod-like shape reach their optimum viscosity at fractions between 0.35 and 0.45. In either group, the higher the degree of contraction (that is the flexibility), the higher the interaction surface. A similar observation was made by Menchicchi et al. (2015) in the interaction of mucin with negatively charged polysaccharides. In our case, with flexible gums (degree of contraction higher than 1) the interaction surfaces of the rod-like shape gums is higher than the random coils. In fact, while moving from the pure solution of alginate to the pure solution of a gum, alginate is progressively diluted and rearranged in structure. It moves from the state of simple dimeric egg-box (Goycoolea et al., 1995) to a well-organized tridimensional network which is then progressively dispersed by the solvent. Any added polymer is interacting with ordered molecules in a network in formation, and needs to be both flexible and able to be ordered in conformation. This is another reason why despite its well observed interaction in water, the rigid gum from B. obscura (degree of contraction of 0.7) ended with the lowest surface and the lowest viscosity enhancement in calcium chloride solution. It also explains the higher surface and the lower alginate ratio of highest viscosity enhancement of ordered gums; they form so many junctions with alginate that they can be present in greater amount. The need of some conformational order and flexibility for interacting gums have been previously observed in some other systems. Xanthan and pectin are both able to adopt ordered conformation (Brejnholt, 2010; Sworn, 2010; Walkenström et al., 2003). The flexibility of xanthan has been much documented. In interacting with alginate, it loses its rheological and spectral properties to adopt those of alginate (Pongjanyakul & Puttipipatkhachorn, 2007) while in interacting with LBG, it dictates the conformational order (Goycoolea et al., 2001; Higiro et al., 2006; Walkenström et al., 2003).
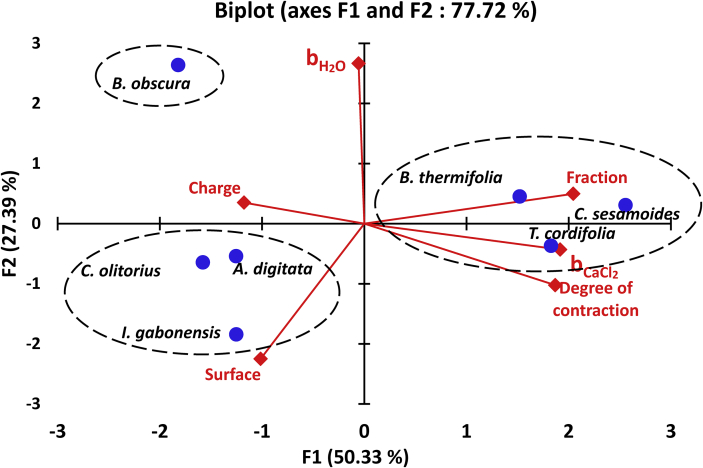
Combining the four key characteristics of our gums (power law coefficients in water and in calcium chloride, charge and degree of contraction) and their two interaction parameters in calcium chloride solution (surface and alginate fraction of maximum viscosity enhancement) confirms the three main gums groups hypothesized from gums characteristics as shown by Principal Component Analysis (PCA) indicating the distribution of gums according to the above characteristics (Figure 3). In this representation, the gums are distributed along F1 axis according to the alginate ratio of maximum viscosity enhancement (30.4 %), the power law coefficient in calcium chloride (26.7 %) and the flexibility (expressed by the degree of contraction 25.4 %), while along F2 axis, the power law coefficient in water (51.6 %) and the surface of interaction (36.8 %) are the determinant factors. The charge does not influence much in this categorization. The random coil gums, that is the gums from T. cordifolia, B. thermifolia and C. sesamoides, constitute the first group, characterized by the greatest values of degree of contraction (>2.2), of power law viscosity coefficients in calcium chloride solution (>1.2), the highest alginate fraction of maximum viscosity enhancement (0.70–0.80) and intermediate interaction surfaces (36–38). The second group is that of the flexible gums able to adopt a rod-like shape (I. gabonensis, C. olitorius and A. digitata); they have the greatest interaction surfaces (>38), intermediate degrees of contraction (1.6–2.2) and intermediate alginate fractions of maximum viscosity enhancement (0.35–0.44). The last group is that of the only non-flexible gum (B. obscura), which has the greatest value of the power law viscosity coefficient in water and the lowest interaction surface. This last group, previously considered as a sub group of rod-like shape gums based on gum characteristics, stands as a totally different group, showing that the low charge and the gelling property of gums totally modify their involvement in interactions with alginate. It can be concluded that for a gum to interact well with alginate, it must be charged and flexible. When these requirements are met, the gums that can be ordered in conformation will have the greater interaction.
Figure 3.
Distribution of tropical vegetal gums according to their characteristics and interaction parameters with alginate in CaCl2 2 mM.
3.3. Particle size of interacting gums
Viscosity reduction observed when two polymers are mixed in a diluted system is due to the association of polymers into aggregates which can either remain soluble or precipitate (Menchicchi et al., 2014, 2015). Positive synergies are obtained in case of network formation, they can be coupled or not with aggregates formation. The particle size of the alginate - gum mixtures in calcium chloride solution at different alginate fractions are given in Figure 4. Individual particle size has no relationship with the intrinsic viscosities in calcium chloride solution (Table 2). The gum from B. thermifolia, which had the highest intrinsic viscosity now has the same low particle size with I. gabonensis that had the lowest viscosity. This observation simply demonstrates a capability of self-aggregation of the studied gums. The particle size of the four gums which showed interactions with alginate in distilled water is similar to that of alginate alone. In the calcium solution used, alginate forms large aggregates half saturated in calcium, therefore the gums sizes observed correspond to large aggregates. This aggregation in calcium explains the viscosity reduction and the greater solubility observed in presence of calcium (Tables 2 and 3).
Figure 4.
Average diameter of alginate-gum mixtures in calcium chloride 2 mM solution at different alginate mass fractions determined by laser scattering (n = 2; mean ± minimum and maximum values).
The sizes of the mixtures are like intermediate between the gum alone and alginate alone. However, for the three non-gelling gums, the mixtures particle size is lower than alginate alone, meaning either particle contraction in the mixtures, or a destruction of individual aggregates in the formation of the tridimensional network. Since they do not interact directly with alginate, they might be in competition for calcium ions while entering within alginate network to interact, consequently reducing its particle size. However, the gum from I. gabonensis shows an increase of size at the fraction of 0.43, which corresponds to its high viscosity enhancement, it could be the formation of a specific complex. The mixtures with the other gums show higher or equal size with individual gums, it suggests the interactions are done mainly between aggregates. But aggregates constitutions cannot be predicted by the present method. Looking at the particle size at maximum viscosity enhancement, the gums from T. cordifolia, C. sesamoides show a maximum particle size, which imply that the network is better built with aggregates. These, along with the mixture with I. gabonensis gum, would give compact gels, with low porosity at higher concentrations of gums and calcium (Jones and McClements, 2010). But C. sesamoides does not have a fixed fraction for the maximum viscosity enhancement (0.60 in water and 0.78 in calcium chloride solution), demonstrating a possible non-homogeneity of its aggregates, and subsequently a non-homogeneity of the mixed gel formed. T. cordifolia showed first in absence of calcium an ability to aggregate with alginate without network formation and its fraction of maximum viscosity enhancement, reduction and highest particle size remain the same; it is a good candidate for compact mixed gel formation. Although I. gabonensis did not clearly interact in water, its fraction remained constant for maximum viscosity enhancement and highest particle size, it could also be tested for gel formation.
4. Conclusion
The gums from the barks of T. cordifolia and B. thermifolia, the seeds of I. gabonensis and B. obscura, the leaves of C. sesamoides, A. digitata and C. olitorius are all charged and present synergistic interactions with alginate in presence of calcium chloride 2 mM. In distilled water, only the gums from T. cordifolia, B. obscura, C. sesamoides and C. olitorius present a synergy with alginate. In calcium solution, they form large aggregates comparable to alginate half saturated with calcium, demonstrating their ability to self-aggregation which confirms their gelling property. These interactions depend on gums flexibility and their ability to adopt ordered conformation. The rod like shaped gums, that display the higher interaction surfaces when flexible, form the strongest network (maximum viscosity enhancement) at alginate fraction of about 0.40 and the random coils at about 0.75. In this respect, these gums can be potentially used in formulation of encapsulation matrix at higher concentrations. Two gums, those from T. cordifolia and I. gabonensis are likely to give with alginate compact gels for the strongest network they form is built from largest aggregates. In addition, this synergistic dynamic of tropical gums shows also hypothetical potential of synergies among themselves.
Declarations
Author contribution statement
Inès Estelle Nkenmogne Kamdem: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Clément Saidou: Analyzed and interpreted the data; Wrote the paper.
Martin Benoit Ngassoum, Robert Ndjouenkeu: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was financially supported by IFS (International Foundation for Science) and OPCW (Organization for the Prohibition of Chemical Weapons) [grant reference F/5772].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Bonine B.M., Polizelli P.P., Bonilla-Rodriguez G.O. Immobilization of a plant lipase from Pachira aquatica in alginate and alginate/PVA beads. Enzym. Res. 2014;2014:738739. doi: 10.1155/2014/738739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejnholt S.M. Pectin. In: Imeson A., editor. Food Stabilisers, Thickeners and Gelling Agents. Wiley-Blackwell; Oxford: 2010. pp. 237–265. [Google Scholar]
- Dolatabadi-Farahani T., Vasheghani-Farahani E., Mirzadeh H. Swelling behaviour of alginate- N,O Carboxymethyl Chitosan gel beads Coated by Chitosan. Iran. Polym. J. (Engl. Ed.) 2006;15(5):405–415. [Google Scholar]
- Endreß H.-U., Christensen S.H. Pectins. In: Phillips G.O., Williams P.A., editors. Handbook of Hydrocolloids. second ed. Woodhead Publishing Limited; Cambridge: 2009. pp. 274–297. [Google Scholar]
- Foster T., Wolf B. Hydrocolloid gums – their role and interactions in foods. In: Norton I.T., Spyropoulos F., Cox P., editors. Practical Food Rheology an Interpretive Approach. Wiley-Blackwell; Oxford: 2011. pp. 61–84. [Google Scholar]
- Goycoolea F.M., Milas M., Rinaudo M. Associative phenomena in galactomannan-deacetylated xanthan systems. Int. J. Biol. Macromol. 2001;29:181–192. doi: 10.1016/s0141-8130(01)00164-7. [DOI] [PubMed] [Google Scholar]
- Goycoolea F.M., Morris E.R., Gidley M.J. Screening for synergistic interactions in dilute polysaccharide solutions. Carbohydr. Polym. 1995;28:351–358. [Google Scholar]
- Helgerud T., Gåserød O., Fjæreide T., Andersen P.O., Larsen C.K. Alginates. In: Imeson A., editor. Food Stabilisers, Thickeners and Gelling Agents. Wiley-Blackwell; Oxford: 2010. pp. 50–72. [Google Scholar]
- Higiro J., Herald T.J., Alavi S. Rheological study of xanthan and locust bean gum interaction in dilute solution. Food Res. Int. 2006;39:165–175. [Google Scholar]
- Jones O.G., McClements D.J. Functional biopolymer particles: design, fabrication, and applications. Compr. Rev. Food Sci. Food Saf. 2010;9:374–397. doi: 10.1111/j.1541-4337.2010.00118.x. [DOI] [PubMed] [Google Scholar]
- Lai L.S., Chiang H.F. Rheology of decolorized hsian-tsao leaf gum in the dilute domain. Food Hydrocolloids. 2002;16:427–440. [Google Scholar]
- Menchicchi B., Fuenzalida J.P., Bobbili K.B., Hensel A., Swamy M.J., Goycoolea F.M. Structure of Chitosan determines its interactions with mucin. Biomacromolecules. 2014;15:3550–3558. doi: 10.1021/bm5007954. [DOI] [PubMed] [Google Scholar]
- Menchicchi B., Fuenzalida J.P., Hensel A., Swamy M.J., David L., Rochas C., Goycoolea F.M. Biophysical Analysis of the molecular interactions between polysaccharides and mucin. Biomacromolecules. 2015;16:924–935. doi: 10.1021/bm501832y. [DOI] [PubMed] [Google Scholar]
- Ndjouenkeu R., Akingbala J.O., Richardson R.K., Morris E.R. ‘Weak gel’ properties of khan flour thickener from Belschmiedia sp.- a traditional food thickener from tropical West Africa. Food Hydrocolloids. 1995;9(3):165–172. [Google Scholar]
- Ndjouenkeu R., Goycoolea F.M., Morris E.R., Akingbala J.O. Rheology of okra (Hibiscus esculentus L.) and dika nut (Irvingia gabonensis) polysaccharides. Carbohydr. Polym. 1996;29:263–269. [Google Scholar]
- Ntalikwa W.J. Determination of surface charge density of α-alumina by acid - base titration. Bull. Chem. Soc. Ethiop. 2007;21(1):117–128. [Google Scholar]
- Nwokocha L.M., Williams P.A. Hydrodynamic and rheological properties of Irvingia gabonensis gum. Carbohydr. Polym. 2014;114:352–356. doi: 10.1016/j.carbpol.2014.07.071. [DOI] [PubMed] [Google Scholar]
- Nwokocha L.M., Williams P.A. Rheological properties of a polysaccharide isolated from Adansonia digitata leaves. Food Hydrocolloids. 2016;58:29–34. [Google Scholar]
- Pamies R., Schmidt R.R., Martínez M.d.C.L., de la Torre J.G. The influence of mono and divalent cations on dilute and non-dilute aqueous solutions of sodium alginates. Carbohydr. Polym. 2010;80:248–253. [Google Scholar]
- Pongjanyakul T., Puttipipatkhachorn S. Xanthan–alginate composite gel beads: molecular interaction and in vitro characterization. Int. J. Pharm. 2007;331:61–71. doi: 10.1016/j.ijpharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Rahim M.Z.A., Lee P.M., Lee K.H. Butyl acetate synthesis using immobilized lipase in calcium alginate beads. Malays. J Anal. Sci. 2008;12(3):575–585. [Google Scholar]
- Saidou C., Ndjouenkeu R., Roux D., Tchatchueng J.B., Heyraud A., El Kissi N. Effect of drying conditions on rheological properties of hydrocolloids gums from Triumfetta cordifolia and Bridelia thermifolia barks. Food Nutr. Sci. 2013;4:626–631. [Google Scholar]
- Saidou C., Roux D.C.D., Ndjouenkeu R. Saarbrücken: Presses académiques francophones; 2014. Propriétés des gommes de Triumfetta cordifolia et Bridelia thermifolia. [Google Scholar]
- Saidou C., Tchatchueng J.B., Ndjouenkeu R., Roux D.C.D. Extraction and partial characterisation of hydrocolloid gums from some African legumes. Int. J. Food Eng. 2011;7(3):15. https://www.bepress.com/ijfe/vol7/iss3/art15 [Google Scholar]
- Sworn G. Xanthan gum. In: Imeson A., editor. Food Stabilisers, Thickeners and Gelling Agents. Wiley-Blackwell; Oxford: 2010. pp. 325–342. [Google Scholar]
- Tchoundjeu Z., Atangana A.R. Irvingia gabonensis (Aubry-Lecomte ex O'Rorke) baill. In: van der Vossen H.A.M., Mkamilo G.S., editors. PROTA (Plant Resources of Tropical Africa/Ressources végétales de l'Afrique tropicale). Wageningen, Netherlands. 2007. https://uses.plantnet-project.org/en/Irvingia_gabonensis_(PROTA [Google Scholar]
- Thom D., Dea I.C.M., Morris E.R., Powell D.A. Interchain associations of alginate and pectins. Prog. Food Nutr. Sci. 1982;6:97–108. [Google Scholar]
- Toft K., Grasdalen H., Smidsrød O. Synergistic gelation of alginates and pectins. In: Fishman M.L., Jen J.J., editors. Chemistry and Function of Pectins. American Chemical Society; Washington DC: 1986. pp. 117–132. [Google Scholar]
- Ullah F., Othman M.B.H., Javed F., Ahmad Z., Akil H.M. Classification, processing and application of hydrogels: a review. Mater. Sci. Eng. C. 2015;57:414–433. doi: 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Walkenström P., Kidman S., Hermansson A.M., Rasmussen P.B., Hoegh L. Microstructure and rheological behaviour of alginate/pectin mixed gels. Food Hydrocolloids. 2003;17:593–603. [Google Scholar]
- Woolfe M.L., Chaplid M.F., Otchere G. Studies on the mucilages extracted from okra fruits (Hibiscus esculentus L.) and baobab leaves (Adansonia digitata L.) J. Sci. Food Agric. 1977;28:519–529. [Google Scholar]
- Yamazaki E., Kurita O., Matsumura Y. Hydrocolloid from leaves of Corchorus olitorius and its synergistic effect on κ-carrageenan gel strength. Food Hydrocolloids. 2008;22:819–825. [Google Scholar]
- Yamazaki E., Kurita O., Matsumura Y. High viscosity of hydrocolloid from leaves of Corchorus olitorius L. Food Hydrocolloids. 2009;23:655–660. [Google Scholar]