Abstract
Purpose:
Leiomyosarcoma (LMS) and liposarcoma (LPS) are common subtypes of soft tissue sarcoma (STS). Patients with metastatic LMS or dedifferentiated LPS (DDLPS) typically have worse outcomes compared to localized LMS or well-differentiated LPS (WDLPS). A better understanding of genetic changes between primary/metastatic LMS and between WDLPS/DDLPS may provide insight into their genetic evolution.
Experimental Design:
We interrogated whole exome sequencing (WES) from “trios” of normal tissue, primary tumor, and metastatic tumor from individual patients with LMS (n=9), and trios of normal tissue, well-differentiated tumor, and dedifferentiated tumor from individual patients with LPS (n=19). Specifically, we performed mutational, copy number, and tumor evolution analyses on these cohorts and compared patterns among LMS and LPS trios.
Results:
LMS cases harbored shared drivers through a typical parent/child relationship where the metastatic tumor was derived from the primary tumor. In contrast, while all LPS cases shared the characteristic focal chromosome 12 amplicon, most paired LPS cases did not share additional mutations, suggesting a divergent evolutionary pattern from a common precursor. No highly recurrent genomic alterations from WES were identified that could be implicated as driving the progression of disease in either sarcoma subtype.
Conclusions:
From a genomic perspective, LMS metastases contain genetic alterations that are also found in primary tumors. WDLPS and DDLPS, however, appear to divergently evolve from a common precursor harboring 12p amplification, rather than as a transformation to a higher-grade tumor. Further efforts to identify specific drivers of these distinct evolutionary patterns may inform future translational and clinical research in STS.
Keywords: Liposarcoma, leiomyosarcoma, genomic, sarcoma, tumor evolution
Introduction
Sarcomas represent a heterogeneous group of malignant tumors, with over 50 histologic subtypes identified in the most recent World Health Organization Classification of Tumors of Soft Tissue and Bone (1). Leiomyosarcoma (LMS) and liposarcoma (LPS) are two of the most common histologies, accounting for approximately 30% and 25% of all soft tissue sarcomas, respectively.
LMS typically expresses markers of smooth muscle differentiation and can occur at almost any soft tissue anatomic site as a primary tumor. LMS has a notable risk of developing distant metastasis (up to 50% depending on the site and grade of the primary tumor), most frequently to distant sites via hematogenous spread, with local recurrences being less common. Once metastatic, LMS is incurable with current therapeutic approaches (2).
The most frequent form of LPS is represented by a disease spectrum comprising well-differentiated LPS (WDLPS), a non-metastasizing, indolent, but locally recurrent disease with adipocytic differentiation; and dedifferentiated LPS (DDLPS), an aggressive, rapidly growing sarcoma with a significant risk of locoregional recurrence, as well as a 15-20% risk of distant metastatic spread (3). Both the well-differentiated and the dedifferentiated components of LPS can present as the sole component of a tumor or they can co-exist within the same tumor.
For both LMS and LPS, the genomic drivers of oncogenic transformation have been explored, and these include recurrent TP53 mutations in LMS and elements of the focal chromosome 12q amplification (including CDK4 and MDM2) in both WDLPS and DDLPS (4). However, for both LMS and LPS, genomic analysis of patient-matched tumors following progression from localized/indolent to metastatic/aggressive tumors has not yet been described. For LPS, prior studies have implicated additional copy number gains in ASK1/MAP3K5 and JUN in the transition to DDLPS (6) although these studies were not performed in samples obtained from the same patient and/or tumor.
We hypothesized that the genomic evolutionary patterns of localized to metastatic LMS and well-differentiated to dedifferentiated LPS might be distinct and reflect unique biological processes, as manifested by their differing clinical behavior and patterns of spread. To address this hypothesis, we performed genomic analyses using whole exome sequencing (WES) on paired samples from individual patients with early localized and late metastatic LMS tumors (N=9) as well as paired specimens of WDLPS and DDLPS from within individual patients (N=19) to determine the phylogenetic relationship underlying histopathologic differences and disease progression in these tumors.
Materials and Methods
Patient selection.
Patients with LMS and LPS were identified through retrospective clinical review (A.J.W., S.G., and S.S.). All patients provided written informed consent for research review of medical records and analysis of pathology specimens, including molecular profiling of tumors and germline (DFCI Institutional Review Board approved protocol #05-434). Studies were conducted in accordance with recognized ethical guidelines.
Pathology review.
All samples underwent detailed pathology review to confirm diagnosis by an expert sarcoma surgical pathologist in a single center (B.Q., M.N., S.S., J.L.H.), including delineation of WDLPS and DDLPS features within a given sample.
Molecular profiling.
Whole exome sequencing:
For whole-exome sequencing, 150 bp insert libraries were prepared by Covaris sonication (Covaris, Woburn MA), followed by SPRI size-selection (Agencourt AMPure XP beads) and ligation to molecular barcoded adaptors for multiplexed analysis. Exome hybrid capture was performed using Agilent SureSelect All Exon v2.0 hybrid capture kit as described (7).
Whole genome sequencing:
Library construction was performed as described (7) with some slight modifications. Initial genomic DNA input into shearing was reduced from 3 μg to 80 ng in 50 μL of solution. Genomic DNA was then acoustically sheared using the Covaris E210 to a mean fragment length of 385 bp. Additionally, all enzymatic steps were performed using the Kapa Library preparation kit, while Illumina TruSeq PCR free adapters, containing unique 8 base index sequences embedded within the adapter, were used in adapter ligation. Using the Kapa hifi library amplification kit, eight cycles of PCR amplifications were performed using a 3-minute extension time during each cycle. Following PCR, an AMPure bead cleanup was performed, after which these libraries were subsequently quantified using PicoGreen.
Somatic mutation analysis and Filtering:
Somatic single nucleotide variations and small insertions or deletions were identified using MuTect (8) (v2.4) and Indelocator (http://www.broadinstitute.org/cancer/cga/indelocator), respectively, and were annotated using Oncotator (v1.0.0.0rc27) (http://www.broadinstitute.org/cancer/cga/oncotator). Oxidative DNA damages were removed as described (9). The cross-individual score was determined using the ContEst algorithm (10). ContEst scores of > 4% were excluded. Mean target coverage and contamination scores can be found in Supp. table 1 and Supp. table 4.
Copy Number Analysis:
Somatic Copy Number Alterations (SCNAs) were called from the whole exome sequencing data by using the ReCapSeg tool, (http://gatkforums.broadinstitute.org/gatk/categories/recapseg-documentation; additional details are available at https://www.biorxiv.org/content/10.1101/566505v1). Also, allele-specific analysis was performed using Allelic-Capseg as previously described (11,12).
Phylogenetic analysis:
Cancer cell fractions (CCF) for each mutation and purity estimates for each tumor sample was discovered using the previously published ABSOLUTE algorithm (13,14) A CCF value of 0.7 would suggest that the mutation in question is present in 70 percent all cancer cells. Phylogenetic trees were constructed using the Phylogic algorithm. Phylogic clusters mutations with similar CCF values; this will estimate the number of (sub)clones. Phylogic then uses the number of (sub)clones to infer evolutionary trees. The length of each branch is determined by the number of mutations added between each (sub)clone. CCF values for all mutations and indels can be found in Supp. table 5 and Supp. table 6.
Deep assessment of LPS sample quality:
To account for alternate explanations of the observed divergent mutations in paired WDLPS and DDLPS samples, we performed the following additional procedures. First, we explored the potential for tumor-in-normal contamination and applied deTin algorithm (15); the highest contamination estimate was 2%, which is not likely to impact mutation calling (Supplementary Table 2). Next, we accounted for whether FFPE degradation had reduced the quality of our FFPE samples. The difference between two read pair orientations was attributed to the FFPE specific error rate and converted to a Phred-based quality score (FFPE_Q). FFPE_Q values less than 30 reflect the component of sequencing errors arising from FFPE artifacts, while FFPE_Q values exceeding 30 indicate a low contribution of FFPE artifact to the total sequencing error rate in a given DNA sample. (Supplementary Table 3). However, only 10 out of 38 of our samples did not have FFPE_Q scores of > 30. Finally, since low tumor purity could also impact mutation detection power, we assessed tumor purity through ABSOLUTE (13) (Supplementary Table 2) and determined that all tumor samples had purity estimates between 24% and 70%, with a median of 46%. A purity of 24% means that we may miss some subclonal mutations, but it is enough purity to be able to detect clonal events.
Results
Tumor evolution from localized primary to metastatic leiomyosarcoma
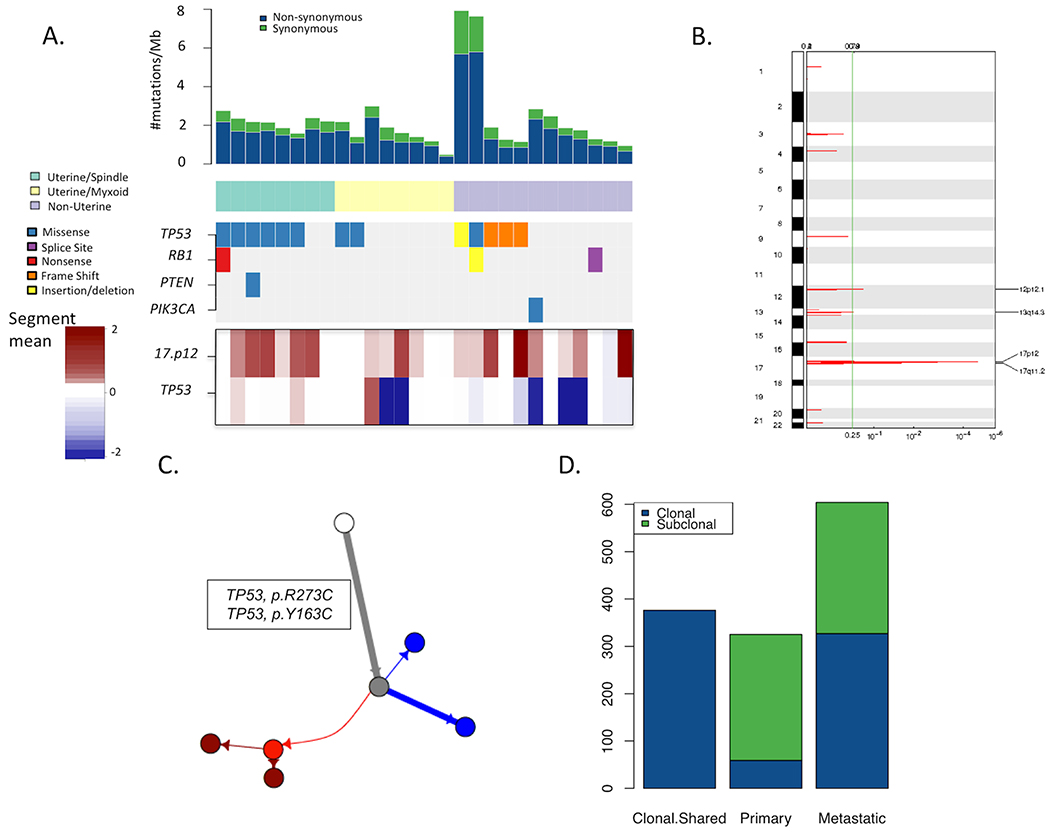
To establish the baseline genomic status of LMS across the exome, WES was performed on 28 pairs of primary LMS tumor and normal blood (Table 1). The germline mean target coverage was 135X [115X-161X] and tumor mean target coverage was 139X [115X-178X] (Supp. Table 1). TP53 alterations (these could be point mutations, heterozygous or homozygous deletions or indels) were present in 17 of 28 (61%) primary LMS (Fig. 1A), consistent with previous studies (16). As previously described, other less common somatic alterations also were noted in RB1, PTEN, and PIK3CA (Fig. 1A). Focal chromosome 17 amplifications, including MAP2K4, were also observed in 16 of 28 (57%) primary LMS (Fig. 1B), consistent with prior studies (1,16). Thus, other than TP53 and focal chromosome 17 gain, no additional recurrent somatic mutations were observed across the exome in this LMS cohort. Genomic abnormalities in uterine (N=16) vs non-uterine LMS (N=12) were generally similar in these patients sampled, and the unique histologic subset of myxoid uterine LMS (N=7) did not appear to have a distinct genomic pattern.
Table 1. Clinical characteristics of the LMS and LPS cohorts.
Baseline clinical characteristics of the patient cohorts are described in this table, including histologic subtyping when available.
| Histotype | Location/Histology | Number of Patients | Female (%) | Median Age, years (range) |
|---|---|---|---|---|
| Leiomyosarcoma | Uterine/Myxoid | 7 | 100 | 58 years [44-77] |
| Uterine/high grade spindle cell | 8 | 100 | 64.5 [54-73] | |
| Non-uterine/high-grade spindle cell | 13 | 85 | 51.9 [31.2-63.8] | |
| Liposarcoma | Retroperitoneal | 15 | 53 | 59.3 [35.3-78.5] |
| Inguinal | 2 | 0 | 55.6 | |
| Extremity | 2 | 100 | 58.8 | |
Figure 1: Genomic overview of matched primary (localized) and metastatic leiomyosarcomas.
(A) Overview of mutations observed in the 28 primary localized LMS samples, each from a unique patient. Each column represents a sample/patient. Mutation rates in the tumor samples are shown in the top barplot with reference to synonymous and non-synonymous mutation rates. LMS cases were classified into three subtypes of Uterine-Myxoid, Uterine-Spindle cell, and non-Uterine. Mutations in established biologically relevant genes in LMS (TP53, RB1, PTEN, PIK3CA, and BRAF) are shown. TP53 mutations were the most frequent somatic alterations observed. Similarly, the bottom track shows amplifications in chromosome 17p12. (B) Copy number alteration significance in primary LMS samples. Copy number alteration significance through GISTIC (24) in primary LMS samples demonstrate chromosome 17p12 is the only region that is significantly amplified (FDR<0.0001). (C) Phylogenetic tree of the primary and metastatic sample of patient 4. The white circle represents the germline sample, the grey sample shows the inferred parent clone of the tumor samples before there was divergence, the red circles represent the metastatic (sub)clones and the blue circles represent the primary (sub)clones. The length of the branches shows the proportion of mutations accumulated between each clone. (D) Barplot of the frequency of mutations demonstrates how many of the mutations were categorized as shared between primary and metastatic (Shared), exclusive to the primary samples or exclusive to the metastatic samples. The mutations have been also distinguished based on clonality. A considerable percentage of clonal somatic alterations is shared between the primary and metastatic samples.
Building upon the baseline LMS genomic data, whole exomes of matched “trios” of normal blood, primary tumor, and available metastatic tumor samples from nine of the 28 patients were sequenced to enable phylogenetic analyses between primary and metastatic LMS arising within each individual. A representative phylogenetic tree from the LMS cohort (Patient 4; Figure 1C) revealed truncal somatic alterations, including clonal mutations in TP53, that were shared between the primary and metastatic tumors. The phylogenies of the eight other LMS patient sample trios showed similar patterns (Supp. Fig. 1). Across the nine LMS primary-metastasis matched cases, 83.4% (332/398) of clonal mutations in the primary were also observed in the metastasis of the same individual (Fig. 1D), including all putative driver mutations (e.g. TP53), consistent with genomic tumor evolution models for other cancer types (17,18). Mutations in genes exclusive to the metastases were not recurrent and were unique to the individual tumors (Supp. Table 2), and some involved putative tumor suppressor genes/oncogenes, including TP53, CREBBP, PTEN, FGFR1, and PLAG1. Therefore, LMS exhibits a typical Parent/Child evolutionary relationship with shared clonal drivers and additional clonal mutations between matched primary and metastatic tumors, without clearly identifiable recurrent changes that correlate with development of metastases.
Characterization of well- and dedifferentiated LPS somatic genomics
To examine the genomic differences between WDLPS and DDLPS, and to compare with LMS patient tumors, 19 tumors that harbored both WDLPS and DDLPS components were identified through pathology review (Table 1). DNA was extracted from the regions of WDLPS and DDLPS and then subjected to WES (Fig 2A–C, Supp. Table 3). The germline mean target coverage was 149X [105X-183X] and tumor mean target coverage was 151X [90X-187X] in these cases (Supp. Table 4).
Figure 2: Patient-matched WDLPS and DDLPS showing a distinct genomic pattern of tumor evolution.
(A) Gross resection specimen of a representative LPS sample highlights WD and DD components of a mixed liposarcoma. Histology images of WDLPS (B) and DDLPS (C) from patient 11 are shown. (D) Overlap of copy number changes in WD and DDLPS in chromosome 12 of patient 11 demonstrate high concordance between segmentations at those loci. Breakpoints occur in the same locations in both samples. CDK4 and MDM2 are highlighted. (E) Phylogenetic tree of the WD and DDLPS sample of patient 11. The white circle represents the germline sample, the grey sample shows the inferred parent clone of the tumor samples before there was divergence, the red circles represent the DD (sub)clones and the blue circles represent the WD (sub)clones. The length of the branches shows the proportion of mutations accumulated between each clone. (F) Rearrangements shared between the WD and DDLPS samples in patient 11. (G) Rearrangements unique to WDLPS. (H) Rearrangements unique to DDLPS.
In one representative case (Patient 11), genomic analysis across the whole exome of WDLPS and DDLPS from a single tumor from one patient revealed a high degree of overlap of copy number changes in chromosome 12 (Fig. 2D, Supp. Fig. 3). It also showed a short truncal component of the phylogenetic tree with only one shared nonsynonymous point mutation of uncertain functional significance (CDK4 P40S) (Fig. 2E). To further examine this finding, whole genome sequencing was subsequently performed on a trio of specimens including WDLPS, DDLPS, and normal tissue (mean coverage ~ 19x) from this patient. The tumors, but not the normal sample, had nearly complete overlap between breakpoints in the chromosome 12 region that included CDK4 and MDM2 (Supp. Fig. 2) indicative of an initial genome-wide rearrangement event in a precursor cell, consistent with reported models of neochromosome formation in LPS (19). However, beyond the amplification and rearrangements involving chromosome 12, the WDLPS and DDLPS samples shared only one other rearrangement (Fig. 2F–H), again suggesting genomic divergence from an early precursor event.
Expanding the investigation across the entire LPS cohort, focal chromosome 12 amplifications were observed in all WDLPS and DDLPS samples (Fig. 3A–B). There was significant intra-patient correlation between chromosome 12 copy ratios (r2 = 0.87, p-value < 0.001, Pearson; Fig. 2D and Supp. Fig. 3), and all cases manifested similar divergent mutation-based phylogenies with short phylogenic trunks and a paucity of overlap in shared mutations (Supp. Fig. 4). In fact, only 14.8% (92/620) of clonal mutations were shared between WDLPS and DDLPS within the same tumor (Fig. 3D), sharply contrasting with the genomic evolutionary model inferred from the comparative study of primary versus metastatic LMS tumors (Fig. 1D). Mutational divergence was observed across the cohorts even before correcting for tumor purity, filtering for formalin fixation/paraffin embedding artifact, and performing tumor-in-normal deconvolution analyses to investigate potential alternative explanations for these discordant matched WDLPS/DDLPS mutation sets.
Figure 3: Genomic overview of the 19 matched WD and DD liposarcoma samples.
(A) An overview of mutations in the 19 WD and DDLPS samples highlight mutation rate (top), established biologically relevant genes in LPS (middle) and focal amplifications involving MDM2/CDK4 (bottom). GISTIC copy number analysis demonstrates focal amplification in chromosome 12 in WD (B) and DD (C) LPS samples. (D) Overview of the frequencies of subclonal and clonal mutations that are either shared between primary and metastatic samples from the same individual or that are exclusive to either cohort. (E) Rare (~1%) point mutations are shared by the WD and DDLPS samples from the same patient.
While no highly recurrent genomic driver distinguishing WDLPS and DDLPS was observed, somatic CDK4 point mutations were detected at low frequency in 4/19 (21%) DDLPS tumors (Fig. 3E), and in only one case was the CDK4 mutation also observed in the matched WDLPS component (patient 11). None of these patients had received CDK4 inhibitors prior to resection of their tumors. The observed CDK4 mutations have not previously been described (20) and are of unknown functional significance. Broadly, WDLPS and DDLPS exhibit nearly private clonal mutations indicative of early divergence from a common 12q amplified precursors, rather than transition from WDLPS to DDLPS within a given tumor.
Conclusions/Discussion
Genomic evaluation of tumors longitudinally and in a multi-regional fashion has provided molecular insights into tumor evolution patterns across a variety of malignancies (13,18). While genomic characterizations of LMS and LPS have previously been performed (1,4,16,21), a comprehensive assessment of changes between early and advanced tumor states in individual patients has not been described and may identify molecular features related to this progression.
We have demonstrated that primary and metastatic LMS from individual patients share a significant number of mutations, providing genomic evidence that a subclone of the primary tumor shed cells that disseminated, and thus the metastasis is presumed to be the progeny of the primary tumor. Although additional mutations were seen in the metastases that were not present in the primary tumor, no clearly recurring genomic alteration in an individual gene defined the metastatic event across samples from multiple patients. The presence of shared clonal mutations in both the primary and metastatic samples indicate that therapeutic development geared towards these alterations, if possible, may be an effective strategy to impact all sites of tumor within a patient.
In contrast, the study of different elements co-existing in LPS tumors suggests an evolution that differs from the LMS pattern. While all analyzed components of tumors shared highly concordant chromosome 12 amplification, this event appears to have occurred early in oncogenesis and was followed by divergent clonal evolution, rather than a straightforward serial transition from WDLPS to DDLPS which instead would have been reflected by subsets of mutations shared in both regions of the tumor, as illustrated in the primary-to-metastasis examples of LMS. This observation challenges the previously established view that dedifferentiated liposarcomas arise in a transformation event from well-differentiated liposarcomas. Rather, our data demonstrate the novel observation that these tumors arise from a shared precursor cell and diverge early through mechanisms not observable in the sequenced exome.
Notably, in neither LMS nor LPS were we able to identify a recurrent alteration exclusive to the aggressive or advanced tumors when compared to the patient-matched primary or well-differentiated tumors. It is possible that the driving lesions for these clinical phenotypes occur in non-coding regions or are the product of epigenetic changes. For example, miRNAs were found to be differentially expressed in subtypes of liposarcoma and may contribute to clinical behavior (22). Additionally, HDAC1 mutations were reported at a low frequency in liposarcoma (4). Similar alterations were not found in any of the 19 cases that we analyzed this cohort, which may be the result of limited sample size. The role of chromatin modification or chromosomal instability (e.g. chromoplexy) in the development or maintenance of liposarcoma remains unknown; however, given the relative paucity of mutations in the exome, further evaluation of mechanisms other than genomic sequence alterations is warranted.
Of note, in 21% of tumors we identified novel mutations in CDK4, a gene known to be highly amplified in most liposarcomas. The functional significance of the amino acid substitutions observed in our study is unknown, and their prevalence should be explored in a larger cohort of samples. Similarly, an increase in CDK4 copy number potentially could contribute to the development of DDLPS; however, the absolute CDK4 copy number was qualitatively similar in the whole genome patient trio, and the correlation between WDLPS and DDLPS lesions in chromosome 12 was strong (see Supplementary Data).The ability to determine amount of focality (e.g. 25 copies vs. 50 copies) from bulk whole exome sequencing data is confounded by many technical (purity, sequencing depth) and biological (e.g. spatial heterogeneity) effects that make it not reliably quantifiable and limits the ability to assess changes in CDK4 copy number from this dataset.
CDK4 inhibitors are FDA approved for treatment of metastatic breast carcinoma and have been studied in patients with advanced WDLPS/DDLPS (23). In liposarcoma the radiographic response rate to the CDK4 inhibitor palbociclib is low, although minor prolongation of disease control compared to historical values was reported. The CDK4 mutational status of tumors from patients participating in these studies was not reported and thus it is unclear whether there is any correlation between the CDK4 genotype and response or resistance to inhibitors.
Finally, this study highlights the utility of serial patient-matched tumor profiling to inform tumor evolution trends, especially as applied to uncommon tumor types with unique clinical features. In this context, patient-matched sample “trio” analyses enabled the observation of tumor evolution patterns that sharply contrast among two common mesenchymal tumors. These results may have implications for understanding the origins of LPS and open possibilities for other factors that influence transition to either WDLPS or DDLPS within an individual (i.e. epigenetic). Furthermore, given the phylogenetic relationship between primary and metastatic LMS, this study highlights the need to sample metastatic sites in this context as the genomic profile may be related but not identical to the primary tumors, although the primary drivers of metastasis in these diseases remains to be defined.
Supplementary Material
TRANSLATIONAL RELEVANCE:
This study identifies heterogeneity of genomic evolution in leiomyosarcoma and liposarcoma. Whereas leiomyosarcomas demonstrate a typical pattern of progression to metastatic disease, liposarcomas exhibit early divergence between well- and dedifferentiated foci. These findings provide insights into the clinical evolution of LMS and LPS, and more generally, progression patterns in cancers.
ACKNOWLEDGEMENTS/FINANCIAL SUPPORT
Catherine England Leiomyosarcoma Fund (S. George), Paul C. Zamecnik Chair in Oncology at the Massachusetts General Hospital Cancer Center (G. Getz), Ludwig Center at Harvard (G.D. Demetri), Erica’s Entourage of the Pan Mass Challenge (G.D. Demetri), Fasseas Fund for Liposarcoma Research (A.J. Wagner and G.D. Demetri), Damon Runyon Foundation (E.M. Van Allen), Liddy Shriver Sarcoma Initiative (A.J. Wagner)
CONFLICTS OF INTEREST
SG is a consultant to Blueprint Medicines, Deciphera, AstraZeneca, and has received research support to her institution from Blueprint, Deciphera, Pfizer, Bayer, Novartis, and Ariad. GG receives research funds from IBM and Pharmacyclics. G.G. is an inventor on patent applications related to MuTect, MutSig and ABSOLUTE. Disclosures for GDD are listed in supplemental document. LG is an employee and shareholder of Eli Lilly, is co-founder and equity holder of Tango Therapeutics, and was co-founder and equity holder in Foundation Medicine. EMV is a consultant for Tango Therapeutics, Genome Medical, Invitae, Foresite Capital, and Illumina. EMV received research support from Novartis and BMS, as well as travel support from Roche/Genentech. EMV is an equity holder of Syapse, Tango Therapeutics, Genome Medical. AJW has received honoraria from Novartis and is a consultant for Eli Lilly, Daiichi-Sankyo, and Five Prime Therapeutics. AJW has received research funding to his institution from Eli Lilly, Daiichi-Sankyo, Plexxikon, Karyopharm, Five Prime Therapeutics, and AADi.
References
- 1.Barretina J, Taylor BS, Shantanu B, Ramos AH, Lagos-Quintana M, DeCarolis PL, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nature Genetics 42, 715–721 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano C, George S Leiomyosarcoma In: Wagner AJ, editor. Hematology/Oncology Clinics of North America: Sarcoma. Philadelphia: Elsevier, Inc; 2013. p. 957–974. [DOI] [PubMed] [Google Scholar]
- 3.Henze J, Bauer S, Liposarcomas In: Wagner AJ, editor. Hematology/Oncology Clinics of North America: Sarcoma. Philadelphia: Elsevier, Inc; 2013. p. 939–955. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BS, DeCarolis PL, Angeles CV, Brenet F, Schultz N, Antonescu CR, et al. Frequent alterations and epigenetic silencing of differentiation pathway genes in structurally rearranged liposarcomas. Cancer Discovery 1, 587–597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chibon F, Mariani O, Derré J, Mairal A, Coindre JM, Guillou L et al. ASK1 (MAP3K5) as a potential therapeutic target in malignant fibrous histiocytomas with 12q14-q15 and 6q23 amplifications. Genes, Chromosomes & Cancer 40, 32–37 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Mariani O, Brennetot C, Coindre JM, Gruel N, Ganem C, Delattre O, Stern MH et al. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell 11, 361–374 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delorey TM, et al. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biology 12, R1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnology 31, 213–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello M, Pugh TJ, Fennell TJ, Stewart C, Lichtenstein L, Meldrim JC, et al. Discovery and characterization of artifactual mutations in deep coverage targeted capture sequencing data due to oxidative DNA damage during sample preparation. Nucleic Acids Research 41, e67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cibulskis K, McKenna A, Fennell T, Banks E, DePristo M, Getz G et al. ContEst: estimating cross-contamination of human samples in next-generation sequencing data. Bioinformatics 27, 2601–2602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152, 714–726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger JA, Landau DA, Taylor-Weiner A, Bozic I, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nature Communications, 7, 11589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, et al. Absolute quantification of somatic DNA alterations in human cancer. Nature Biotechnology 30, 413–421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherniack AD, Shen H, Walter V, Stewart C, et al. Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell 31, 411–423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor-Weiner A, Stewart C, et al. DeTiN : Overcoming Tumor in Normal Contamination. Nature Methods 15, 531–534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AH, Lee CH, Witten DM, Gleason BC, Edris B, Espinosa I, et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene 29, 845–854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachler MD, Taylor-Weiner A, Peng S, McKenna A, Agoston AT, Odze RD et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nature Genetics 47, 1047–1055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discovery 5, 1164–1177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garsed DW, Marshall OJ, Corbin VD, Hsu A, Di Stefano L, Schröder J, et al. The architecture and evolution of cancer neochromosomes. Cancer Cell 26, 653–667 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makinen N, Aavikko M, Heikkinen T, Taipale M, Taipale J, Koivisto-Korander R, et al. Exome sequencing of uterine leiomyosarcomas identifies frequent mutations in TP53, ATRX, and MED12. PLoS Genetics 12, e1005850 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gits CM, Van Kuijk PF, Jonkers MB, Boersma AW, Smid M, Van Ijcken WF, et al. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. International Journal of Cancer 135, 348–361 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Dickson MA, Schwartz GK, Keohan ML, D’Angelo SP, Gounder MM, Chi P, et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncology 2, 937–940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G, et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biology 12, R41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.