Graphical abstract
Keywords: Ziziphus mauritiana, Cytotocixicity, Genotoxicity, Allium cepa, Chromosomal aberration
Highlights
-
•
Aim this study is to evaluate cytotoxic & genotoxic Potentials of different solvents extract of Ziziphus mauritiana leaf.
-
•
Mitotic index decreases with increase in dose concentration of these extracts.
-
•
Dose dependent decrease in root tip growth except ethanol extract.
-
•
Effective Concentration of extracts are aqueous (81.30), ethanol (52.01), Ethylacetate (90.68) and hexane (112.30) mg/L.
-
•
Chromosomal aberrations such as vagrant, c-mitosis, bridged anaphase, sticky Telophase were predominant.
-
•
Evaluated parameters in the plant extracts suggest cytotoxic and genotoxic effects.
Abstract
Previous studies showed that Ziziphus mauritiana is ethnomedicinally beneficial against various diseases, however the cytotoxic and genotoxic effects of this plant have not been well investigated. Therefore this study was undertaken to investigate cytotoxic and genotoxic effects of four different solvents extracts of Ziziphus mauritiana (Lam) leaf at different concentrations (20, 40, 60, 80, and 100 mg/L) using Allium cepa model. The cytotoxicity and genotoxicity parameters evaluated were mitotic index, root tip growth length and chromosomal aberration respectively. The result revealed a decrease in mitotic index percentage (%MI) and a dose dependent decrease in root tip length with increase in extracts concentration for all the extracts studied - with the ethanol extract showing the most significant effect in mitotic index. Furthermore, the effective concentrations (EC50) obtained were 81.30, 52.01, 90.68 and 112.30 mg/l for aqueous, ethanol, ethyl acetate and hexane extract respectively. Finally chromosomal aberrations such as vagrant chromosome, c-mitosis, bridged anaphase, sticky telophase were also observed in all four extracts and the percentage chromosomal aberration were observed to decrease with increased concentrations of extracts. Therefore based on the result obtained in this study it may be concluded that the plant (Ziziphus mauritiana (Lam)) extracts are cytotoxic and genotoxic in nature and the observed decrease in percentage chromosomal aberration may be as a result of antimutagenic bioactive principles present in the plant extracts. Hence care must be taken in its consumption and use in folk medicine.
1. Introduction
The use of medicinal plants has always been part of human culture from time immemorial. However, indiscriminate use of herbal preparations in developing countries has become common due to lack of access to better healthcare, affordable orthodox drugs and other factors [1]. In Nigeria a large percentage of the populace are dependent on herbal medicines because orthodox medicines are becoming increasingly expensive and out of reach [2,3]. Plant extracts of various plant parts have been reported to be effective in treating various conditions such as sleeping sickness, wounds, diarrhoea, reproductive and liver problems, circulatory and respiratory problem and parasitic infections with few reports of toxicity [4,5].
Ziziphus is a genus of about 40 species of spiny shrubs and small trees in the buckthorn family, Rhamnaceae, distributed in the warm-temperate and subtropical regions throughout the world [6]. The family contains 50–60 genera and approximately 870–900 species. Ziziphus mauritiana Lam. belongs to one of the Ziziphus genus’ species [7]. It is called jujube tree or Indian jujube [8,9]. The plant is commonly known as magarya in Hausa and whuya in Kilba (Nigeria) [10], Chinese apple or Indian Jujube in English [11]. The leaves of the plant are used in the treatment of diarrhoea, wounds, abscesses, swelling and gonorrhoea [12] also used in the treatment of liver diseases, asthma and fever [11]. The Allium cepa test has been used by many researchers mainly as a bioindicator of environmental pollution [13,14], testing crude extracts of cyanobacteria, as well as to evaluate the genotoxic potential of medicinal plants [[15], [16], [17], [18]]. The Allium cepa test is important since it is an excellent model in vivo, where the roots grow in direct contact with the substance of interest (i.e. effluent or complex medicinal mix being tested), enabling possible damage to the DNA of eukaryotes to be predicted. Therefore, the data can be extrapolated for all animal and plant biodiversity [16]. The analysis of chromosomal alterations is equivalent to the test of mutagenicity mainly for the detection of structural alterations; however, it is possible to observe numerical chromosomal alterations, as well [18]. The aim of this study was to evaluate the cytotoxic and genotoxic effects of solvent extracts (different polarities) of Ziziphus mauritiana leaf using the Allium cepa assay.
2. Materials and methods
2.1. Collection of medicinal plants
The medicinal plant utilized in this study was leaves of Ziziphus mauritiana. They were collected at different locations in the botanical garden of Nigeria Police Academy, Wudil, Nigeria and were taken to Bayero University Kano Herbarium at Plant Biology Department for identification. A voucher [BUKHAN 0233] was allocated and the specimen deposited.
2.2. Preparation of extracts
The extracts were prepared according to the procedure described by Sunmonu [19]. The plant leaf was dried in shade for five (5) days and were then pulverized using an electric blender (Binatone blender with grinder, model BLG-402, China). The powdered material was stocked in plastic containers from which varying amounts were taken and extracted in distilled water, ethanol, ethylacetate and hexane for 72 h at room temperature. They were then filtered using filter paper (Whatman No. 1). The filtrates were concentrated using rotary evaporator (Buchi Labortechnik, Model: R-100) and water bath to give different extracts of the leaf.
2.3. Allium cepa assay
Onion bulbs (Allium cepa, L.) were obtained commercially at Wudil, Nigeria. They were sun dried for 4 days and the dried outer scales were carefully removed and the root were scraped leaving the ring of the primordial root intact to promote the emergence of new roots. These were used for the bioassay according to standard procedures [1,20]. For the root growth inhibition, five concentrations of each extract, viz: 20, 40, 60, 80, and 100 mg/L were considered. Six onion bulbs were utilized for each concentration of each extract and the control (tap water). The base of each of the bulbs was suspended on the extract inside 100 mL beakers in the dark for 72 h. The test extracts were changed daily and the root length was measured at the end of the exposure period, the length of the roots of five onion bulbs with the best growth at each concentration was measured (in cm) with a ruler. Average length for each concentration and the control was obtained, the percentage root growth inhibition in relation to the negative control and the EC50 (the effective concentration where root growth amounts to 50 % of the controls) for each extract were also determined [21]. The effect of each sample on the morphology of growing roots cells was also examined according to the protocol described by Sibhghatulla and his team [22]. Root tips 1–3 cm long were cut and placed in a watch glass, fixed in acetic alcohol (ethanol: glacial acetic acid in 3:1 ratio) for 12 h at room temperature, the root tips were hydrolyzed in 1 N HCL at 60°c for 10 min and stained with Acetocarmine for 20 min, it was then squashed on glass slide under 45 % acetic acid to determine the mitotic index and the presence of chromosomal aberrations by viewing slides under the light microscope (Olympus CX 23) using the 100X objective lens with oil immersion. Percentage Mitotic index were calculated as shown below.
Where: P = prophase, M = metaphase, A = Anaphase, T = Telophase
2.4. Statistical analysis
The data obtained from the studies were represented as Mean ± SEM. The data were analyzed by one-way analysis of variance (ANOVA), ‘P’ value less than 0.05 was considered as statistically significant. Graphpad Instat version 3.05 and Microsoft Excel 2010 were used for statistical analysis and production of tables.
3. Result and discussion
3.1. Results
3.1.1. Cytotoxicity of the plant extracts (Percentage Mitotic Index)
The mitotic indices of the different solvent extracts are summarized in Table 1. A decrease in the percentage mitotic index (% MI) value was observed as the concentration of each of solvent extracts increases. Aqueous extract showed % mitotic index (% MI) of 11.07 + 0.24 (20 mg/L) and 05.73 + 0.13 (100 mg/L), ethanol and ethylacetate extracts revealed similar trend of 04.89 + 0.12 and 09.59 + 0.12 (20 mg/L) and 05.09 + 0.22, 04.89 + 0.22(100 mg/L) and are statistically significant at p value <0.001 while hexane extract showed 08.96 + 0.12 (20 mg/L) and 05.37 + 0.22(100 mg/L) with p value <0.001. At 20 mg/L concentration, comparison between different extracts show there were significant effect between aqueous extract and other extracts while no statistically significant effect was observed between ethylacetate and hexane extracts, at 40 mg/L significant effect was observed between aqueous extract and others extracts but no significant effects between ethanol extract and other extracts (ethylacetate and hexane extracts). At 60 mg/L a significant effect was observed between aqueous extract, ethanol extract and other extracts while no statistically significant effect was observed between ethylacetate and hexane extracts, for 80 mg/L no significant effect was observed between aqueous extract and ethanol extracts. However, a significant effect was observed between aqueous and ethylacetate and hexane extracts. For 100 g/L aqueous extract, a significant difference from other extracts was observed while no statistically significant effect was observed between ethanol, ethylacetate and hexane extracts.
Table 1.
Effect of different solvent extracts of Ziziphus mauritiana on cell division in A. cepa.
| Aqueous |
Ethanol |
Ethylacetate |
Hexane |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Number of Cells | Mitotic Index (%) | Total Aberration (%) |
Number of Cells | Mitotic Index (%) | Total Aberration (%) |
Number of Cells | Mitotic Index (%) | Total Aberration (%) |
Number of Cells | Mitotic Index (%) | Total Aberration (%) |
| Control | 500 | 10.60 + 0.00 a a | 0.00 + 0.00 a a | 500 | 10.60 + 0.00 a a | 0.00 + 0.00 a a | 500 | 10.60 + 0.00 a a | 0.00 + 0.00 a a | 500 | 10.60 + 0.00 a a | 0.00 + 0.00 a a |
| 20 mg/L | 482 | 11.07 + 0.24 b a | 6.36 + 0.12b a | 478 | 04.89 + 0.12 b b | 5.16 + 0.12 b b | 476 | 09.59 + 0.12 b c | 5.18 + 0.12 b b | 465 | 08.96 + 0.12 b c | 5.16 + 0.22 b b |
| 40 mg/L | 472 | 09.87 + 0.21 c a | 5.74 + 0.12 c a |
472 | 08.68+ 0.21 c b | 4.45 + 0.21 c b | 468 | 08.48 + 0.12 c b | 4.20 + 0.12 c b | 461 | 08.17 + 0.14 c b | 4.34 + 0.22 c b |
| 60 mg/L | 476 | 08.26 + 0.21 d a | 4.73 + 0.12 d a | 466 | 06.47+ 0.22 d b | 4.45 + 0.12 c a | 462 | 07.43 + 0.13 d c | 4.04 + 0.13 c b | 457 | 07.15 + 0.13 d c | 4.16 + 0.22 c b |
| 80 mg/L | 466 | 07.08 + 0.22 e a | 4.58 + 0.12 d a | 458 | 06.99+ 0.22 e a | 4.22+ 0.13 c b | 456 | 06.06 + 0.13 e b | 4.46 + 0.13 d b | 453 | 06.40 + 0.22 e b | 3.61 + 0.13 d c |
| 100 mg/L | 450 | 05.73+ 0.13 f a | 3.91 + 0.00 e a | 452 | 05.09+ 0.22 f b | 3.92+ 0.13 c a | 450 | 04.89 + 0.22 f b | 3.33 + 0.22 e b | 447 | 05.37 + 0.22 f b | 3.51 + 0.13 d b |
N = 3, X + SD. First superscript represent comparison along the column (between different concentration) and second superscript represent comparison along the row (between different extracts). All test values with different superscripts across the rows and column are significantly different. P < 0.001.
3.1.2. Percentage aberration
Percentage aberration for each extract is presented in Table 1. In the aqueous extract, a significant increase was observed between the control and 20 mg/L concentration while a significant decrease at 40−100 mg/L concentrations, Ethanol extract showed a similar trend except no statistical difference was observed in 40−100 mg/L concentrations. Ethylacetate extract showed a significant difference between the control and 20 mg/L and a dose dependent decrease in % aberration from 40 to 100 mg/L. For hexane extract a significant % increase was observed between the control and 20 mg/L concentration and other concentrations (40−100 mg/L concentrations) but no significant difference between 40 and 60 mg/L and 80 and 100 mg/L. Also between the extracts, at 20 mg/L there were significant difference between aqueous extract and other extracts while no significant was observed between ethanol, ethylacetate and hexane extracts. For 40 and 100 mg/L there were significant difference between aqueous extract and other extracts while no significant was observed between ethanol, ethylacetate and hexane extracts. For 60 mg/L, there were no significant difference between aqueous and ethanol while a significant a difference was observed in ethylacetate and hexane extracts when compared with aqueous extract. Also, a similar trend was observed in 80 mg/L concentration.
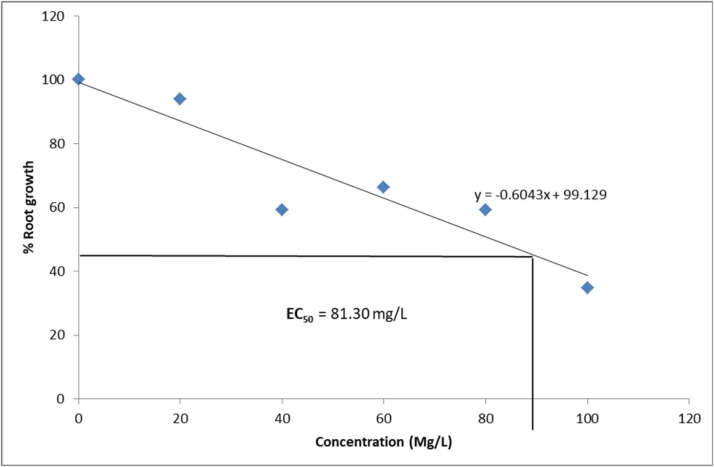
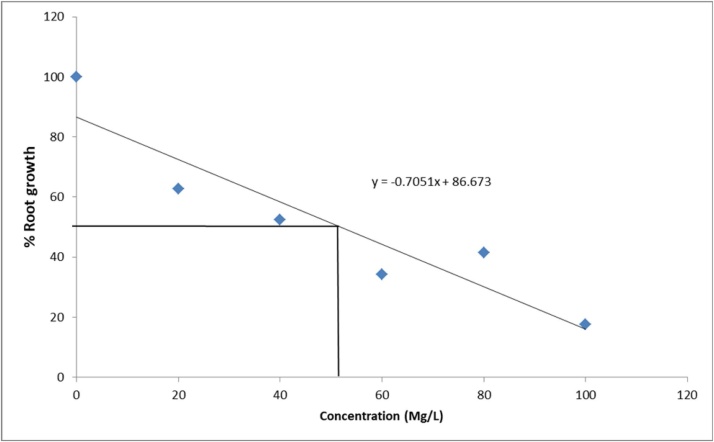
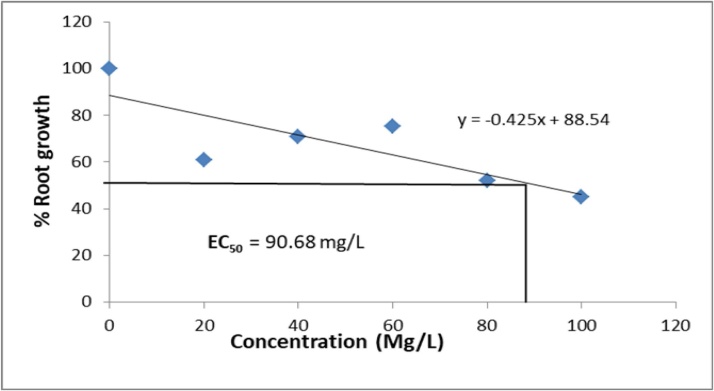
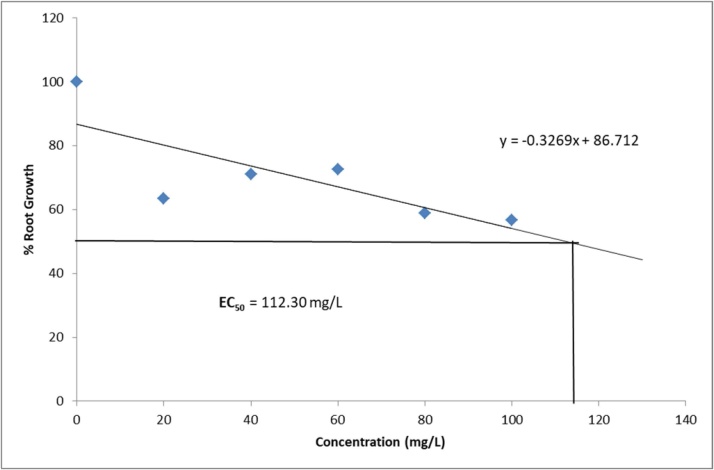
3.1.3. Root growth inhibition and effective concentration
The effect of different concentrations Ziziphus mauritiana leaves extracts on root growth of A. cepa is presented in Table 2. The estimated EC50 (concentration of extracts that produces 50 % inhibitory effects on root length when compared with the control are presented in Fig. 1, Fig. 2, Fig. 3, Fig. 4. The EC ranged from 81.30 mg/L for aqueous extract, 52.01 mg/L for ethanol extract, 90.68 mg/L for ethyl acetate extract to 112.30 mg/L for hexane extract. The extracts showed a significant (p < 0.05) decline in meristematic growth relative to the control as the concentrations of the extracts increased. The most significant decrease in growth was observed in the ethanol extract which also gave the lowest EC50 value.
Table 2.
Inhibitory Effect of different solvent extracts of Ziziphus mauritiana leaf on Allium cepa meristemic root growth.
| Concentration (mg/L) | Aqueous (cm) | Ethanol (cm) | Ethylacetate (cm) | Hexane (cm) |
|---|---|---|---|---|
| Control | 4.82 + 0.17 a (0%) |
4.82 + 0.17 a (0 %) |
4.82 + 0.17 a (0 %) |
4.82 + 0.17 a (0 %) |
| 20 | 4.53 + 0.34a (06.00 %) |
3.02 + 0.14 b (37.33 %) |
2.93 + 0.34 b (39.21 %) |
3.05 + 0.37b (36.72 %) |
| 40 | 2.85 + 0.32 b (40.87 %) |
2.53 + 0.35 b (47.51 %) |
3.42 + 0.26 b (29.11 %) |
3.42 + 0.47 b (29.05 %) |
| 60 | 3.20 + 0.41 c (33.61 %) |
1.65 + 0.10 c (65.77 %) |
3.62 + 0.51 b (24.90 %) |
3.50 + 0.58 b (27.39 %) |
| 80 | 2.85 + 0.17 b (40.87 %) |
2.00 +0.16 b (58.51 %) |
2.50 + 0.33 b (48.13 %) |
2.83 + 0.32b (41.29 %) |
| 100 | 1.68 + 0.12 b (65.10 %) |
0.85 + 0.15 c (82.37 %) |
2.17 + 0.30 c (54.98 %) |
2.73 + 0.48 c (43.36 %) |
Note: Value in bracket indicate percentage decrease in root length of Allium cepa.
N = 3, X + SEM. All test values with different superscripts across the rows are significantly different. P < 0.001.
Fig. 1.
Growth inhibition of Allium cepa roots exposed to Aqueous extract of Ziziphus mauritiana Leaves.
Fig. 2.
Growth inhibition of Allium cepa roots exposed to Ethanol extract of Ziziphus mauritiana Leaves.
Fig. 3.
Growth inhibition of Allium cepa roots exposed to Ethylacetate extract of Ziziphus mauritiana Leaves.
Fig. 4.
Growth inhibition of Allium cepa roots exposed to Hexane extract of Ziziphus mauritiana Leaves.
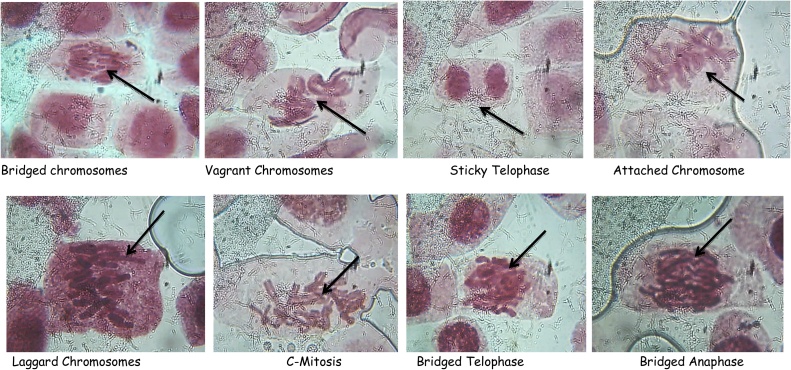
3.1.4. Chromosomal aberration
The different types of chromosomal aberrations observed in the A. cepa assay with different solvents extracts of Z. mauritiana leaves, are presented in photomicrographs in Figs. 5, The result reveals aberrations such as vagrant, c-mitosis, bridged anaphase, sticky telophase and attached chromosomes.
Fig. 5.
micrograph showing different chromosomal aberrations of Allium cepa due to exposure of different extract of Z. mauritiana leaves extracts.
4. Discussion
In this study, toxic effect of Z. mauritiana leaf extracts were evaluated by analyzing percentage mitotic index (%MI), root tip growth inhibition, Effective concentration and root cell morphology to identify the potential cytotoxic and genotoxic effect of the different solvent extracts of Z. mauritiana leaves. According to Chukwujekwu and van Staden, the degree of cytotoxicity of an agent can be determined by the increase or decrease in the MI [24] therefore observed decrease in mitotic indexes with increasing concentration of the extracts indicates inhibition of cell division activity (MI), and proportions of division phases (phase index) [24,25]. The dose dependent reduction in mitotic indexes may be due to delay in cell division and alteration of cell cycle activities imposed by the different solvent plant extracts exposed to the A. cepa roots. The result obtained is in agreement with previous research findings [25,1,31]. This may be an indication of inhibitory and mitodepressive effects elicited by the extracts. Inhibition in root growth is a general indication of adverse effect of xenobiotics (chemical substance or plant extract) which are used as an indicator of cytotoxicity [23,26]. The observed dose dependent decrease in root length in all extracts in this study may be an indication of growth inhibition and adverse effect on mitotic division in cell cycle activity. This observation is in agreement with the work of Udo et al. [27], which reported cytotoxic effect of different plant extracts. Comparatively, the effective concentration [EC50] [Fig. 1, Fig. 2, Fig. 3, Fig. 4] showed that the ethanol extract was most toxic with indication of mitodepressive effect on the meristematic growth of the A. cepa root, this is also corroborated by the report of Akinboro and Bakare (1) which evaluated the effect of different extract of A. indica, M. lucida, C. citratus, M. indica, C. medica and C. papaya on Allium cepa root. External stimuli (mutagens (synthetic and biological compounds) which are usually phytoconstituents in plant or synthetic chemical compound (monosodium glutamate) [28,29] can block cellular progress in one of the phases of the cell cycle or cell division (mito-inhibition) resulting in chromosomal aberrations which includes chromosome and/or chromatid fragments, interchromatid or subchromatid connections, nucleoplasmic bridges, heteromorphic chromosomes, dicentric or ring chromosomes, and micronuclei which are observed as c-mitosis, bridges, vagrants, sticky and attached chromosomes, multipolar anaphase [30]. Also these aberrations could occur due to spindle failure orchestrated by the interaction of phytochemical constituents with the spindle apparatus [31]. Studies on have shown evidence of inhibition of mitosis and also binding to tubulin, thus, preventing the formation/progression of mitotic processes [32]. Increase in chromosomal aberration with a decrease in mitotic index is logically an indication of cytotoxicity [1], However, this study observed a decrease in chromosomal aberration percentage with a concomitant decrease in mitotic index. This may be due to presence of mitogenic agents which could act to overcome intracellular braking mechanisms that block cell cycle progression (mitostimulatory) or may be due to antioxidants present in Ziziphus mauritiana (Lam) extracts which have been reported by other research works to be present in the leave extracts [33,34]. Antioxidants have been shown to improve DNA repair as well as modulate toxicity in different independent studies [35,36].
5. Conclusion
From the results obtained from this research work, it is concluded that the various solvent extract of Ziziphus mauritiana (Lam) leaves may have potential cytotoxic and genotoxic effect which may be more significant with the ethanol extract. Thus, care must be exercised in the use of these extracts for their pharmacological effects.
Author comments
All comments by the reviewer and editor have been taken care of in this revision.
Funding/financial support
This research work did not receive any fund or research grant from any institution.
Declaration of Competing Interest
The authors declared that there is no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We will like to say a big thank you to Mr. Obu Francis of the department of cell biology and genetic of University of Lagos, Nigeria for his technical support.
Contributor Information
Tajudeen A. Owolarafe, Email: taowolarafe@gmail.com, taowolarafe@polac.edu.ng.
Kailani Salawu, Email: kailani.salawu@polac.edu.ng.
Godwin O. Ihegboro, Email: goihegboro@polac.edu.ng.
Chimaobi J. Ononamadu, Email: ononamaducj0016@gmail.com.
Adamu J. Alhassan, Email: ajalhassan.bch@buk.edu.ng.
Alhasan M. Wudil, Email: amwudil.bch@buk.edu.ng.
References
- 1.Akinboro A., Bakare A.A. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J. Ethnopharmacol. 2007;112:470–475. doi: 10.1016/j.jep.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Fasola T.R., Egunyomi A. Nigerian usage of bark in phytomedicine. J. Plants People Appl. Res. 2005 73’77. [Google Scholar]
- 3.Obi R.K., Iroagba I.I., Ojiako O.A. Virucidal potential of some edible Nigerian vegetables. Afr. J. Biotechnol. 2006;5:1785–1788. [Google Scholar]
- 4.Ajiboye T.O. In vivo antioxidant potentials of Piliostigma thonningii (Schum) leaves: studies on hepatic marker enzyme, antioxidant system, drug detoxifying enzyme and lipid peroxidation. Hum. Exp. Toxicol. 2011;30 doi: 10.1177/0960327110366785. 55’62. [DOI] [PubMed] [Google Scholar]
- 5.Bulus T., Atawodi S.E., Mamman M. Acute toxicity effect of the aqueous extract of terminalia avicennioides on white albino rats. Sci. World J. 2011;6(2):1–4. [Google Scholar]
- 6.Patel B.H., Upadhyay V.R., Muralidharan C.M., Judal G.S. Effect of various insecticides on honey bee, Apis florea Fabricius in ‘ber’ (Zizyphus mauritiana Lamk) Curr. Sci. 1988;57(21):1199–1200. [Google Scholar]
- 7.Wunderlin R.P., Hansen B.F. In: Atlas of Florida Vascular Plants. Landry S.M., Campbell K.N., editors. Florida Center for Community Design and Research.) Institute for Systematic Botany, University of South; Florida,Tampa: 2008. http://www.plantatlas.usf.edu/ (application development) [Google Scholar]
- 8.Morton J. Indian Jujube. In: Morton J.F., editor. Fruits of Warm Climates. 1987. pp. 272–275.http://www.IndianJujube.htm Miami, Florida. Last updated: 23/4/2004. Accessed on 7/6/2017 at. [Google Scholar]
- 9.Michel C.G., Nesseem D.I., Ismail M.F. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. J. Ethnopharmacol. 2011;133:53–62. doi: 10.1016/j.jep.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Babatunde B.B., Bakare A.A. Genotoxicity screening of wastewaters from Agbara Industrial estate Nigeria evaluated with the Allium test. Pollut. Res. 2006;25 227’234. [Google Scholar]
- 11.Heuze V., Tran G., Boval M., Lebas F. 2007. Indian jujube (Ziziphus mauritiana). Feedipedia, a programme by INRA, CIRD, AFZ and FAO. [Google Scholar]
- 12.Bagatini M.D., Fachinetto J.M., Silva A.C.F., Tedesco S.B. Cytotoxic effects of infusions (tea) of Solidago microglossa DC. (Asteraceae) on the cell cycle of Allium cepa. Braz. J. Pharmacogn. 2009;19(2B):632–636. ISSN: 0102695X. [Google Scholar]
- 13.Leme D.M., Marin-Morales M.A. Allium cepa test in environmental monitoring: a review on its application. Mutat. Res. 2020;682 doi: 10.1016/j.mrrev.2009.06.002. pp. 71’81, ISSN 0027-5107. [DOI] [PubMed] [Google Scholar]
- 14.Camparoto M.L., Teixeira R.O., Mantovani M.S., Vicentini V.E.P. Effects of Maytenus ilicifolia Mart. and Bauhinia candicans Benth infusions on onion root-tip and rat bone-marrow cells. Genet. Mol. Biol. 2002;25:85–89. ISSN 1415-4757. [Google Scholar]
- 15.Lubini G., Fachinetto J.M., Laughinghouse H.D., IV, Paranhos J.T., Silva A.C.F., Tedesco S.D. Extracts affecting mitotic division in root-tip meristematic cells. Biologia. 2008;63:647–651. [Google Scholar]
- 16.Fachinetto J.M., Bagatini M.D., Silva A.C.F., Tedesco S.D. Efeito antiproliferativo das infusões de Achyrocline satureioides DC (Asteraceae) sobre o ciclo celular de Allium cepa. Rev. Bras. Farmacogn. 2009;17(1):49–54. ISSN: 0102695X. [Google Scholar]
- 17.Fachinetto J.M., Tedesco S.B. Atividade antiproliferative mutagênica dos extratos aquosos de Baccharis trimera (Less.) A. P. de Candolle e Baccharis articulata (Lam.) Pers. (Asteraceae) sobre o sistema teste deAllium cepa. Rev. Bras. Pl. Med. 2009;11(4):360–4367. ISSN 1516-0572. [Google Scholar]
- 18.Sunmonu T.O., Oloyede O.B., Owolarafe T.A., Yakubu M.T., Dosunmu O.O. Toxicopathological evaluation of Picralima nitida seed aqueous extract in Wistar rats Turk. J. Biochem. 2014;39(2) 119’125. [Google Scholar]
- 19.Dahiru D., William E.T., Nadro M.S. Protective effect of Ziziphus mauritiana leaf extract on carbon tetrachloride-induced liver injury. Afr. J. Biotechnol. 2005;4(10):177–1179. [Google Scholar]
- 20.Fiskesjo G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102 doi: 10.1111/j.1601-5223.1985.tb00471.x. 99’ 112. [DOI] [PubMed] [Google Scholar]
- 21.Chukwujekwu J.C., Van Staden J. Cytotoxic and genotoxic effects of water extract of Distephanus angulifolius on Allium cepa Linn. South Afr. J. Bot. 2014;92:147–150. [Google Scholar]
- 22.Sibhghatulla S., Nazia N., Mohammad I.L., Waseem A. Dichlorophen and Dichlorovos mediated genotoxic and cytotoxic assessment on root meristem cells of Allium cepa. Sci. Diliman. 2012;24(1):13–22. [Google Scholar]
- 23.Ślusarczyk J., Dudek M., Wierzbicka M., Suchocki P., Kuraś M. Antimitotic effect of Selol and sodium selenate (IV) on Allium test cells. Caryologia. 2014;67(3):250–259. [Google Scholar]
- 24.Burim R.V., Candle R., Lopes J.L.S., Takahashi C.S. Genotoxic action of the sesquiterpene lactone glaucolide B on mammalian cells in vitro and in vivo. Genet. Mol. Biol. 1999;22:401–406. [Google Scholar]
- 25.Celik T.A., Aslantruk O.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium cepa test. J. Biomed. Biotechnol. 2020:8. doi: 10.1155/2010/189252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udo I.J., Akpan G.A., Esenowo I.K. Cytotoxic effects of (5) medicinal plants on mitosis in Allium cepa root tips. Curr. Res. J. Biol. Sci. 2014;6(2):71–75. [Google Scholar]
- 27.Tedesco S.B., Laughinghouse H.D. In: Bioindicator of Genotoxicity: The Allium Cepa Test, Environmental Contamination. Srivastava Jatin., editor. InTech; 2012. http://www.intechopen.com/books/environmentalcontamination/bioindicator-of-genotoxicitythe-allium-cepa-test ISBN: 978-953-51-0120-8, Available from: [Google Scholar]
- 28.Ganachari M.S., Shiv K., Bhat K.G. Effect of Ziziphus jujube leaves extract on phagocytosis by human neutrophils. J. Nat. Remedies. 2004;41:47–51. [Google Scholar]
- 29.Oyenike A.A., Ayomide E.F. Genotoxic and cytotoxic effects of food flavor enhancer, monosodium glutamate (MSG) using Allium cepa assay. Afr. J. Biotechnol. 2013;12(13):1459–1466. doi: 10.5897/AJB12.2927. [DOI] [Google Scholar]
- 30.Azam-Ali S., Bonkoungou E., Bowe C., deKock C., Godara A., Williams J.T. Ber and other jujubes, Ziziphus species. Fruits for Gupta et al. IJPSR. 2012;3(3):818–821. ISSN: 0975-8232 Available online on www.ijpsr.com 821. [Google Scholar]
- 31.Ihegboroa G.O., Alhassan A.J., Ononamadu C.J., Owolarafe T.A., Sule M.S. Evaluation of the biosafety potentials of methanol extracts/fractions of Tapinanthus bangwensis and Moringa oleifera leaves using Allium cepa model. Toxicol. Rep. 2020;7:671–679. doi: 10.1016/j.toxrep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khakdan F., Piri K. Cytotoxic and genotoxic effects of aqueous root extract of Arctium lappa on Allium cepa root tip cells. Int. J. Agron. Plant. Prod. 2012;3(2):630–637. [Google Scholar]
- 33.Seong H.K., Seong W.C., Sang K.Y., Angho S.Y., Hyun S.K., Myung H.C. Comparison of antioxidant activities of seventy herbs that have been used in Korean traditional medicine. Nutr. Res. Pract. 2008;2(3):143–151. doi: 10.4162/nrp.2008.2.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalidoss A., Krishnamoorthy P. Antioxidant efficacy of endocarp with kernel of Ziziphus mauritiana Lam. in p-dimethylaminoazobenzene induced hepatocarcinoma in Rattus norvigicus. Indian J. Nat. Prod. Resour. 2011;2(3) 387–314. [Google Scholar]
- 35.Ihegboro G.O., Ononamadu C.J., Owolarafe A.T., Afor E., Zaharadeen I.K. Antioxidants in plant extracts may contribute to the modulation of their toxicity: an insight with Allium cepa model. NISEB J. 2018;18(2):92–104. [Google Scholar]
- 36.Khan S., Anas M., Malik A. Mutagenicity and genotoxicity evaluation of textile industry wastewater using bacterial and plant bioassays. Toxicol. Rep. 2019;6:193–201. doi: 10.1016/j.toxrep.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]