Abstract
Context
α-klotho is an integral membrane protein that serves as a coreceptor for fibroblast growth factor 23 (FGF23) in conjunction with cognate fibroblast growth factor receptors. Proteolytic cleavage sheds the ectodomain of α-klotho (soluble α-klotho) as an endocrine substance into blood, urine, and cerebrospinal fluid.
Objective
To study the relationship of soluble α-klotho to mineral metabolism in the general population with mainly preserved kidney function.
Design
Cross-sectional analysis of the associations between soluble α-klotho with laboratory markers of markers of mineral metabolism in a population-based cohort.
Setting
Three centers in Switzerland including 1128 participants.
Measures
Soluble full-length α-klotho levels by a specific immunoassay and markers of mineral metabolism.
Results
The median serum level of soluble α-klotho was 15.0 pmol/L. Multivariable analyses using α-klotho as the outcome variable revealed a sex-by-PTH interaction: In men, PTH was positively associated with α-klotho levels, whereas this association was negative in women. Plasma phosphate associated with soluble α-klotho levels in an age-dependent manner, changing from a positive association in young adults gradually to a negative association in the elderly. The decline of 1,25 (OH)2 vitamin D3 levels in parallel to the gradual impairment of kidney function was greatly attenuated in the setting of high circulating soluble α-klotho levels.
Conclusions
Soluble α-klotho level is associated with plasma phosphate in an age-dependent manner and with PTH in a sex-dependent manner. Furthermore, our data reveal soluble α-klotho as a modulator of 1,25 (OH)2 vitamin D3 levels in individuals with preserved renal function.
Keywords: Soluble α-klotho, Parathyroid hormone, Phosphate
α-klotho is a single-pass transmembrane protein that is expressed in renal tubular epithelia, the parathyroid gland, the brain choroid plexus, and the hippocampus. The integral membrane protein α-klotho serves as a scaffold with cognate fibroblast growth factor receptors (FGFRs), including FGFR1c, FGFR3c, and FGFR4, and is critical for FGF23 action in FGF23 target tissues (1). Loss of α-klotho in mice results in premature multiorgan failure with reduced lifespan, increased blood pressure from impaired endothelium-mediated vasodilation, and multiple organ defects such as ectopic calcifications, osteoporosis, hypogonadism, neurodegeneration, and lung parenchymal degenerative changes (2). α-klotho-deficient mice exhibit hypercalcemia, hyperphosphatemia, and increased 1,25-(OH)2 vitamin D levels. The phenotype of α-klotho-deficient mice is virtually identical to FGF23 null mice, indicating that both act along similar pathways (3). In humans, homozygous loss-of-function mutations in α-klotho cause a syndrome resembling familial tumoral calcinosis, characterized by ectopic calcifications and severe hypercalcemia and hyperphosphatemia (4).
Proteolytic cleavage of the transmembrane α-klotho by members 10 and 17 of the A disintegrin and metalloproteinase family, and the beta-secretase beta-APP-1, sheds the ectodomain of α-klotho (soluble α-klotho) as an endocrine substance into blood, urine, and cerebrospinal fluid (5, 6). Circulating soluble α-klotho is mainly of renal origin (7, 8). Soluble α-klotho lacks FGFR coreceptor activity and can act independently of FGF23 through Wnt, growth factor, and TGF-β pathways, possibly by modulation of lipid raft signaling, as well as direct action on renal phosphate and calcium transporters via intrinsic glycosidase activity (9–13); an enzymatic activity that is debated (14). The same study provided an alternative view by demonstrating that soluble α-klotho in very high concentrations in vitro can serve as a coreceptor for FGF23 in conjunction with FGFRs suggesting that the pleiotropic effects of soluble α-klotho may also be dependent on FGF23 (14).
Reduced renal α-klotho expression and reduced circulating α-klotho levels were found in acute and chronic kidney disease models, hormonal hypertension, metabolic syndrome, and diabetes in rodents (15–17). Low α-klotho is not a mere biomarker, but pathogenic as restoration of α-klotho in experimental rodent chronic kidney disease (CKD) models ameliorated kidney disease and extrarenal complications in rodents (16, 18). This hoists the significance of α-klotho deficiency in CKD from diagnostic and prognostic to therapeutic.
Relatively little is known about the factors that regulate circulating α-klotho levels in humans, and the available data are conflicting (17, 19–27). Current commercial ELISAs used for the determination of soluble α-klotho in humans have certain limitations, including cross-reactivity with other serum proteins, loss of efficiency after repeated freeze-thawing of samples, and loss of sensitivity with vintage of storage resulting in discrepant results with fresh versus stored samples (28). These limitations likely explain some of the discrepancies reported in the literature. An assay for α-klotho in serum and urine was developed and characterized, based on an immunoprecipitation-immunoblotting assay, which overcomes a number of the limitations of the commercial ELISAs (28). This assay measures the full-length 130 kDa, secreted form of α-klotho specifically. Using this assay, we conducted a cross-sectional analysis in a large, multicenter, population-based cohort to investigate the association of mineral metabolism markers with serum α-klotho levels (29).
Materials and Methods
Study population
Swiss Kidney Project on Genes in Hypertension (SKIPOGH) is a multicenter family-based cross-sectional study exploring the role of genes and kidney hemodynamics in blood pressure regulation and kidney function in the general adult population (29). Inclusion criteria were: (i) a minimum age of 18 years; (ii) European ancestry; (iii) having at least 1 and ideally 3 first-degree family members willing to participate; and (iv) a written informed consent. The SKIPOGH study adhered to the Declaration of Helsinki and was approved by the institutional ethical committees of each participating University hospital. Of a total of 1128 SKIPOGH participants, 118 were excluded from the final analysis for the following reasons: (i) primary hyperparathyroidism or history of parathyroidectomy (n = 2); (ii) chronic liver disease (n = 1); (iii) active malignant diseases (n = 10); (iv) pregnancy (n = 1); and (v) intake of the following drugs: over-the-counter or prescribed calcium and/or vitamin D supplements (n = 22), bisphosphonates (n = 2), glucocorticoids or mineralocorticoids (n = 5), antiepileptics (n = 2), loop diuretics (n = 6), thiazide diuretics (n = 63), potassium-sparing diuretics (n = 5), and desmopressin (n = 1). Of the remaining 1010 participants, 98 serum samples were lacking for the analysis of α-klotho, leaving 912 participants from 269 distinct families and 3 study centers available for the final analysis.
Data collection and measurements
Absolute urinary creatinine excretion was used as the criterion for completeness of 24-hour urine collections, using recently published data from the cross-sectional Swiss Salt Study (29, 30). Urine collections were considered as complete if the total 24-hour creatinine excretion was within percentiles 2.5 and 97.5. Fasting morning electrolytes and serum creatinine were measured by standard clinical laboratory methods in each center. Plasma FGF23, PTH, 25-(OH) vitamin D, and 1,25-(OH)2 vitamin D3 were determined centrally for all study participants as single measurements. Plasma FGF23 was measured in the laboratory of TECOmedical AG (Sissach, Switzerland) by the second-generation C-terminal assay (Immutopics, San Clemente, CA) (29, 31). Lower detection limit of the assay is 1.5 RU/mL, intra- and inter-assay coefficients of variation are 1.4% to 2.4% and 2.4% to 4.7%, respectively. Plasma PTH, 25-(OH) vitamin D and 1,25-(OH)2 vitamin D3 were measured in the Center of Laboratory Medicine, Inselspital, Berne: Plasma intact PTH by electrochemiluminescence immunoassay on a Roche Modular E170 (Roche Diagnostics AG, Rotkreuz, Switzerland), plasma 25-(OH) vitamin D by a direct, competitive chemiluminescence-immunoassay on a LIAISON Analyzer (DiaSorin S.p.A., Saluggia [VC], Italy) and plasma 1,25-(OH)2 vitamin D3 by a competitive enzyme immunoassay on a Multilabel Counter Victor 3 (PerkinElmer, Inc., Waltham, MA). Intra- and inter-assay coefficients of variation are 1.1% to 2.0% and 2.5% to 3.4% for plasma PTH, 4.1% to 5.8% and 6.6% to 7.1% for plasma 25-(OH) vitamin D and <8% to <10% for plasma 1,25-(OH)2 vitamin D3, respectively.
For the determination of soluble α-klotho, we used serum samples obtained while fasting in the morning, frozen the day of blood draw, and kept at –80°C thereafter. All serum samples were thawed only once, for determination of serum α-klotho levels. Soluble serum α-klotho levels were determined by a quantitative immunoprecipitation–immunoblotting assay, as described in detail (28, 32). Briefly, 50 μL of serum were diluted with KRH buffer (25 mM HEPES–NaOH [pH 7.4], 120 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 1.3 mM KH2PO4) to a final volume of 0.5 mL and incubated overnight at 4°C with 2 μg of Fab 48 (alias sb106), which recognizes both HEK293 cell–expressed human full-length membrane α-klotho as well as the soluble α-klotho ectodomain. Fab sb48-FLAG–α-klotho complexes were immunoprecipitated with ANTI-FLAG M2 Affinity Agarose Gel beads (Sigma-Aldrich, St. Louis, MO), fractionated by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblotting with anti-α klotho antibody KM2076 (Transgenic Inc., Kobe, Japan) was used to detect the soluble α-klotho ectodomain (~130 kDa). The 130-kDa bands were scanned, and density was compared with internal standards of known amounts of α-klotho (murine klotho isoform, obtained from a CHO cell line that stably expresses the full-length klotho isoform 1; gift from Kyowa Hakko Kogyo Co. Ltd. (33)) to calculate final α-klotho concentration in individual serum samples. Intra- and interassay coefficients of the assay are 8% to 18% and 4.8% to 16.6%, respectively.
The creatinine-based CKD-EPI 2009 equation was used to estimate the glomerular filtration rate (eGFRcr) (34). Tubular maximum reabsorption of phosphorus per glomerular filtration rate (TmP/GFR in mmol/L) was calculated with the algorithm derived by Kenny and Glen (35, 36).
Statistical analysis
All statistical analyses were conducted using the R software, version 3.2.2 (37). The shape of the distribution of each continuous variable was visually inspected and square-root, log, or inverse transformations were applied to better approximate a normal distribution of the residuals for statistical analyses. All statistical tests were 2-sided and a P value < .05 was considered statistically significant, including for 2-way interaction analyses.
We assessed the univariable association between the linear and quadratic terms of explanatory variables of interest with square root–transformed soluble α-klotho as outcome variable, determined as an appropriate transformation. All models were calculated by mixed-effects linear regression with day of blood sampling and family nested within center as the random effect structure. Quadratic terms of the explanatory variables were not found to be significantly associated with the outcome variable. All models were then extended to multivariable analyses by adding the following explanatory variables and their interaction terms as fixed effects: sex, age, body mass index (BMI), eGFRcr, study center, and serum sample freezing time. Fixed effects and statistically significant interaction terms were kept in the final models while keeping hierarchically sound models. Models were validated by graphical analysis for homogeneity of variance, normality of residuals, and highly influential observations. Additional regression analyses were conducted exploring the univariate association between soluble α-klotho, as explanatory variable, with transformed laboratory parameters of mineral metabolism as outcome variables while keeping the random effect structure in all models. Multivariable models were derived thereof by adding sex, age, BMI, eGFRcr, study center, and the serum sample freezing time to soluble α-klotho (as the explanatory variable of interest). All models were created with continuous explanatory variables mean centered at 0 to minimize collinearity problems. Selected multivariable regression models were plotted to illustrate the relationship between the explanatory variable of interest with the outcome variable while holding the effect of all other covariables in the model constant using the statistical R package visreg (38). It should be noted that these plots provide no confidence bands as the created models do not offer predictions about the uncertainty of random effects.
Results
Characteristics of the study population
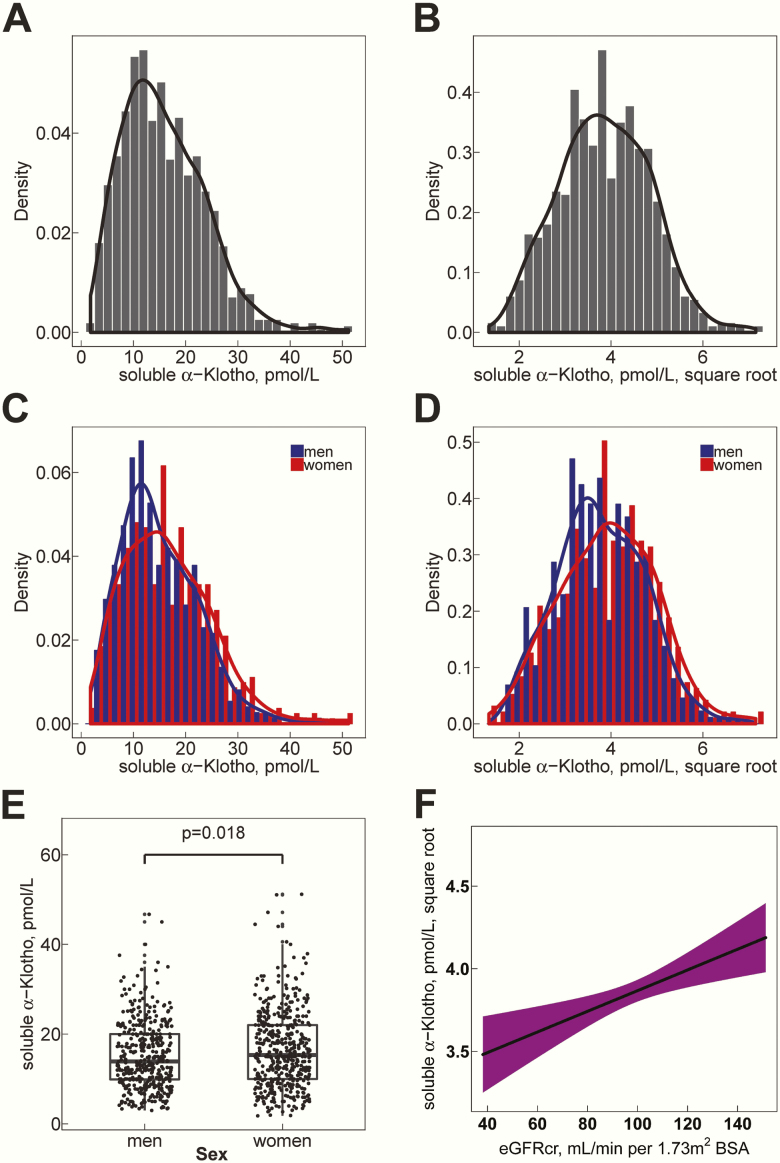
Characteristics of the study population are shown in Table 1. Participants were 18 to 90 years old, with a median age of 45.7 years (25th-75th percentile, 30.2-58.0 years), and 52.3% were women. Median eGFRcr was 98.4 mL/min per 1.73 m2. Of note, only 2.3% of study participants had an eGFRcr <60 mL/min per 1.73 m2. The distribution of soluble α-klotho levels was right skewed with a median serum level of 15.0 pmol/L (25th-75th percentile, 10.0-21.0 pmol/L) (Fig. 1A, C). After square root transformation, soluble α-klotho residual values displayed a near normal distribution, both in the entire study population but also within both sex subgroups (Fig. 1B, D). Soluble α-klotho levels were slightly higher in women (median, 15.3 pmol/L; 25th-75th percentile, 10.0-22.0 pmol/L) than in men (median, 13.9 pmol/L; 25th-75th percentile, 9.9-20.0; P = .018, Welch t-test, Fig. 1E), and higher in individuals aged ≤30 years (median, 16.0 pmol/L; 25th-75th percentile, 10.2-22.0) than in those aged ≥60 years old (median 14.0 pmol/L; 25th-75th percentile, 9.6-20.0; P = .028, Welch t-test). In a simple univariable linear regression analysis, higher soluble α-klotho levels were found at higher eGFRcr (β: 0.0063, P = .00096, Fig. 1F).
Table 1.
Participants’ Characteristics
| Parameters | N | Median; 25th-75th or Mean ± SD | Range |
|---|---|---|---|
| Women, N (%) | 912 | 477 (52.3) | |
| Age, y | 912 | 45.7; 30.2–58 | 18–90 |
| Body mass index, kg/m2 | 911 | 24; 21.6–27.1 | 15.6–45.8 |
| eGFRcr, mL/min per 1.73 m2 BSA | 906 | 98.4; 87.2–110 | 38.1–151 |
| 38–60 | 21 | 2.3% | |
| 60–70 | 42 | 4.6% | |
| 70–80 | 73 | 8.1% | |
| 80–90 | 136 | 15% | |
| 90–100 | 215 | 23.7% | |
| 100–110 | 194 | 21.4% | |
| 110–120 | 130 | 14.3% | |
| 120–130 | 74 | 8.2% | |
| 130–151 | 21 | 2.3% | |
| Soluble α-klotho, pmol/L | 912 | 15; 10–21 | 1.8–51.2 |
| PTH, pg/mL | 909 | 36.9; 29.6–45 | 8.8–108 |
| 25-OH-vitamin D, nmol/L | 908 | 47; 34–62 | 5.5–163 |
| 1,25-(OH)2 vitamin D3, pmol/L | 846 | 92; 70–116 | 5.5–217 |
| FGF23, RU/mL | 912 | 78.6; 63–103 | 18–1078 |
| Phosphate plasma, mmol/L | 904 | 1.04 ± 0.166 | 0.55–1.66 |
| Calcium plasma, mmol/L | 905 | 2.33; 2.27–2.38 | 2.05–2.69 |
| Phosphate urine mmol/24 h | 890 | 25.3; 20.2–31.9 | 2.77–101 |
| Calcium urine, mmol/24 h | 887 | 3.66; 2.46–5.22 | 0.275–16.4 |
Categorical variables are described by number of participants N (%); continuous variables are described by their mean ± SD and range or by their median; 25th-75th percentile and range.
Abbreviations: eGFRcr, glomerular filtration rate estimated creatinine equation CKD-EPI 2009; FGF23, fibroblast growth factor 23; RU, relative unit.
Figure 1.
Distribution of soluble serum α-klotho in study participants. (A-D) Density plots (y-axes) representing the probability density function for the kernel density estimation. In addition, histograms corresponding to frequencies are overlaid. (A) Kernel density plots of soluble α-klotho serum levels (pmol/L) in entire study population. (B) Distribution after square root transformation in entire study population. (C) Kernel density plots of soluble α-klotho serum levels separated by sex (men: blue, women: red). (D) Distribution after square root transformation separated by sex (men: blue, women: red). (E) Soluble α-klotho levels are higher in women (median, 15.3 pmol/L; 25th-75th percentile, 10.0-22.0 pmol/L) than men (median, 13.9 pmol/L; 25th-75th percentile, 9.9-20.0; P = 0.018). (F) Linear regression function of the associations of eGFRcr with square root transformed soluble serum α-klotho. The shadowed area represents the 95% confidence interval of the regression line. eGFRcr, creatinine-based CKD-EPI 2009 equation to estimate the glomerular filtration rate.
Association analyses with soluble α-klotho as outcome variable
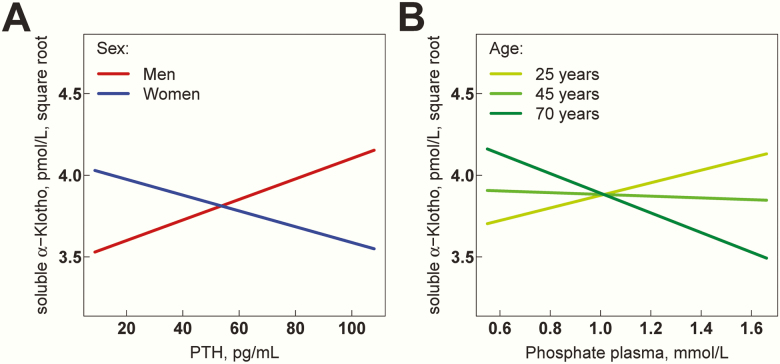
We first performed association analyses between the population characteristics as explanatory (i.e., independent) variables and square root–transformed soluble α-klotho as outcome (i.e., dependent) variable. All models were calculated by mixed-effects linear regression (Table 2). In univariable analyses, positive correlates of soluble α-klotho were female sex (β: 0.1507, P = .0035), eGFRcr (β: 0.0044, P = .0051), and plasma calcium (β: 0.6672, P = .042), whereas age (β: -0.0038, P = .015) and BMI (β: -0.0172, P = .01) were negatively associated with soluble α-klotho levels. In a next step, we conducted multivariable analyses by mixed-effects linear regression using indicated predictor variables and soluble α-klotho as outcome variable. As shown in Table 2, multivariable analyses revealed a significant sex-by-PTH interaction for their association with soluble α-klotho (β: -0.0111, P = .0045). Visualization of this interaction in Fig. 2A reveals that the association of PTH with soluble α-klotho levels is sex-specific, being positive in men and negative in women. Additionally, we also discovered a significant age-dependent association between plasma phosphate and soluble α-klotho (β: -0.0220, P = .011). As depicted in Fig. 2B, in young adults, the association is positive but then becomes progressively negative with increasing age. All other associations with soluble α-klotho as outcome variable remained no longer significant after multivariable adjustment.
Table 2.
Associations Between Predictor Variables of interest With Square-Root Transformed Serum Soluble α-Klotho
| Univariable Models | Multivariable Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor Variable | N | β | 95% CI | P value | N | β | 95% CI | P value |
| Age, y | 912 | -0.0038 | -0.0068 to -0.0007 | .015 | 905 | -0.0009 | -0.00560 to .0038 | .70 |
| Sex, women | 912 | 0.1507 | 0.0484–0.2534 | .0035 | 905 | 0.1620 | 0.0586–0.2669 | .0021 |
| BMI, kg/m2 | 911 | -0.0172 | -0.0303 to -0.0041 | .010 | 905 | -0.0084 | -0.022 to 0.0051 | .22 |
| eGFRcr, mL/min per 1.73 m2 BSA | 906 | 0.0044 | 0.0013–0.0075 | .0051 | 905 | 0.0024 | -0.0022 to 0.007 | .31 |
| PTH, pg/mL | 909 | -0.0032 | -0.0074 to 0.001 | .13 | 902 | 0.0063 | 0.0001–0.0125 | .049 |
| Interaction with sex | -0.0111 | -0.0188 to -0.0035 | .0045 | |||||
| Sex, women in the model | 0.1697 | 0.2814–0.9137 | .00129 | |||||
| 25-OH-vitamin D, nmol/L | 908 | 0.0022 | -0.0004 to 0.0049 | .099 | 901 | 0.0014 | -0.0013 to 0.004 | .32 |
| 1,25-(OH)2 vitamin D3, pmol/L | 846 | 0.0000 | -0.0017 to 0.0018 | .99 | 840 | -0.0006 | -0.0023 to 0.0012 | .55 |
| FGF23, RU/mL | 912 | 0.0001 | -0.0005 to 0.0008 | .69 | 905 | 0.0002 | -0.0005 to 0.0008 | .64 |
| Phosphate plasma, mmol/L | 904 | 0.0702 | -0.2671 to 0.4056 | .68 | 903 | -0.0648 | -0.4120 to 0.2797 | .71 |
| Interaction with age | -0.0220 | -0.0387 to -0.0050 | .011 | |||||
| Age in the model | -0.0007 | -0.0054 to 0.0040 | .76 | |||||
| Calcium, mmol/L | 905 | 0.6672 | 0.0265–1.3091 | .042 | 904 | 0.3957 | -0.2358 to 1.0263 | .22 |
| Phosphate urine, mmol/24 h | 890 | -0.0035 | -0.009 to 0.002 | .21 | 883 | -0.0005 | -0.0067 to 0.0057 | .87 |
| Fractional excretion of phosphate, % | 881 | -0.0030 | -0.0146 to 0.0086 | .61 | 880 | 0.0053 | -0.008 to 0.0186 | .44 |
| TmP/GFR, mmol/L | 881 | 0.1165 | -0.1643 to 0.3957 | .41 | 880 | -0.0468 | -0.3531 to 0.2571 | .76 |
| Calcium urine, mmol/24 h | 887 | -0.0173 | -0.0434 to 0.0086 | .19 | 880 | -0.0068 | -0.0326 to 0.019 | .61 |
| Fractional excretion of calcium, % | 876 | -0.0294 | -0.0564 to 0.0482 | .31 | 875 | -0.0123 | -0.0544 to 0.0463 | .66 |
For each predictor variable, the results of an univariable and of a multivariable model are shown. Number of participants (N), beta coefficients (β), 95% confidence intervals (95% CI), and P values are indicated for the predictor variables of interest and in multivariable models also for significant interactions.
Abbreviations: eGFRcr, glomerular filtration rate estimated creatinine equation CKD-EPI 2009; TmP/GFR, tubular maximum reabsorption of phosphorus per glomerular filtration rate.
Figure 2.
Association of PTH and plasma phosphate with soluble serum α-klotho. Multivariable regression models in Table 2 are visualized to illustrate the relationship between the explanatory variable of interest while holding the effect of all other covariables in the model constant. Solid lines represent regression lines. (A) Association of PTH with soluble serum α-klotho depending on sex. (B) Association of plasma phosphate with soluble serum α-klotho depending on age. Study participants were divided into 3 equal-sized groups belonging to the nearest class of age.
Association analyses with soluble α-klotho as predictor variable
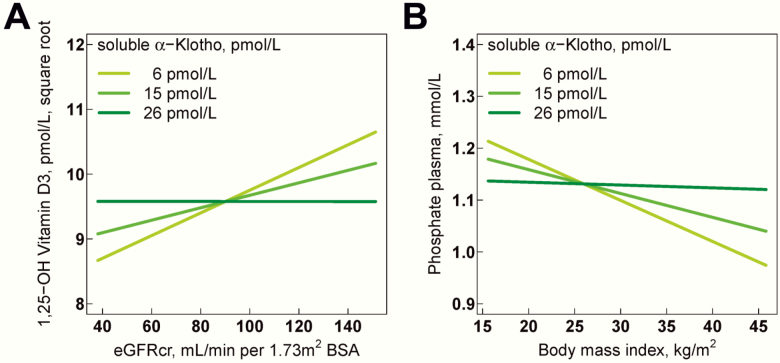
We then performed association analyses with soluble serum α-klotho as predictor variable and laboratory parameters of calcium-phosphate metabolism as outcome variables using mixed-effects linear regression (Table 3). In univariable analyses, eGFRcr (β: 3.47, P = .0018) and plasma calcium (β: 0.0002, P = .036) were positively associated with soluble α-klotho. In multivariable regression analyses (Table 3), soluble α-klotho displayed a significant interaction with eGFRcr for the association with 1,25 (OH)2 vitamin D3 (β: -0.0009, P = .029); for example, the positive association of eGFRcr with 1,25 (OH)2 vitamin D3 was significantly influenced by circulating α-klotho levels (Fig. 3A). Similarly, we observed an inverse association of body mass index with plasma phosphate (β: 0.0004, P = .011) that was dependent on circulating α-klotho levels (Fig. 3B).
Table 3.
Associations Between the Predictor Variable Serum Soluble α-Klotho With Laboratory Parameters of Calcium-Phosphate Metabolism
| Univariable Models | Multivariable Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variable | N | β | 95% CI | P value | N | β | 95% CI | P value |
| eGFRcr, mL/min per 1.73 m2 BSA | 906 | 3.474 | 1.274–5.662 | .0018 | 905 | 0.8742 | -0.7328 to 2.4849 | .29 |
| PTH, pg/mL | 909 | -0.0017 | -0.0045 to 0.0012 | .26 | 902 | 0.0004 | -0.0024 to 0.0032 | .80 |
| 25-OH-vitamin D, nmol/L | 908 | 0.0025 | -0.0107 to 0.0158 | .71 | 901 | -0.0022 | -0.0162 to 0.0116 | .75 |
| 1,25-(OH)2 vitamin D3, pmol/L | 846 | -0.0073 | -0.0227 to 0.0082 | .36 | 840 | -0.0067 | -0.0226 to 0.0092 | .41 |
| -0.0009a | -0.0017 to -0.0009a | .029a | ||||||
| 0.0889b | -0.0006 to 0.0184b | .068b | ||||||
| FGF23, RU/mL | 912 | 0.0010 | -0.003 to 0.005 | .63 | 905 | 0.0014 | -0.0029 to 0.0057 | .54 |
| Phosphate plasma, mmol/L | 904 | -0.0005 | -0.0019 to 0.0009 | .49 | 903 | -0.0005 | -0.0019 to 0.0010 | .53 |
| 0.0004#1 | 0.0001–0.0006#1 | .011c | ||||||
| -0.0043d | -0.0068 to -0.0018d | .00066d | ||||||
| Calcium, mmol/L | 905 | 0.0002 | 0–0.0003 | .036 | 904 | 0.0001 | -0.0001 to 0.0002 | .35 |
| Phosphate urine, mmol/24 h | 890 | -0.0028 | -0.0106 to 0.0048 | .47 | 883 | -0.0009 | -0.0083 to 0.0065 | .81 |
| Fractional excretion of phosphate, % | 881 | 0.0003 | -0.0051 to 0.0056 | .92 | 880 | .0012 | -0.0037 to 0.0061 | .62 |
| TmP/GFR, mmol/L | 881 | -0.0002 | -0.0011 to 0.0007 | .67 | 880 | -0.0004 | -0.0012 to 0.0005 | .40 |
| Calcium urine, mmol/24 h | 887 | -0.0042 | -0.0089 to 0.0004 | .075 | 880 | -0.0012 | -0.0061 to 0.0038 | .64 |
| Fractional excretion of calcium, % | 876 | -0.0024 | -0.0056 to 0.0007 | .13 | 875 | -0.0009 | -0.0042 to 0.0025 | .61 |
For each outcome variable, the results of an univariable and of a multivariable model are shown. Number of participants (N), beta coefficients (β), 95% confidence intervals (95% CI), and P values are indicated for serum soluble α-klotho in univariable models and in multivariable models also for significant interactions with serum soluble α-klotho.
Abbreviations: eGFRcr, glomerular filtration rate estimated creatinine equation CKD-EPI 2009; TmP/GFR, tubular maximum reabsorption of phosphorus per glomerular filtration rate.
aFor interaction between soluble serum α-klotho and eGFRcr.
bFor eGFRcr in the model.
cFor interaction between soluble serum α-klotho and BMI.
dFor BMI in the model.
Figure 3.
Influence of soluble serum α-klotho on the association of eGFRcr with 1,25-(OH)2 vitamin D3 and on the association of body mass index with plasma phosphate. Multivariable regression models in Table 3 are visualized to illustrate the relationship between the explanatory variable of interest while holding the effect of all other covariables in the model constant. Solid lines represent regression lines. (A) Influence of soluble serum α-klotho on the association of eGFRcr with 1,25-(OH)2 vitamin D3. (B) Influence of soluble serum α-klotho on the association of body mass index with plasma phosphate. Study participants were divided into 3 equal-sized groups belonging to the nearest class of soluble serum α-klotho levels. BSA, body surface area; eGFRcr, creatinine-based CKD-EPI 2009 equation to estimate the glomerular filtration rate.
Discussion
We quantified soluble α-klotho levels using a specific immunoprecipitation-immunoblotting immunoassay in a well-characterized population-based sample of individuals with largely preserved renal function. Our analysis revealed levels of circulating α-klotho in serum in the double-digit picomolar range (median 15 pmol/L), which is the same order of magnitude as what has been reported previously with this assay (28). In univariable analyses, we observed several significant associations of eGFR and mineral metabolism markers with soluble α-klotho levels, which partially corroborate previous findings (23, 26, 39–41). In contrast to previous small studies, the large data set enabled us to adjust for several potential confounders in multivariable analysis. We found a sex-specific association of α-klotho with PTH in the general adult population, with a negative association in women and a positive one in men. In mice, androgens increase and estrogens decrease α-klotho mRNA and protein expression in the kidney (42, 43), but to our knowledge, the impact of sex hormones on soluble α-klotho has not been studied in rodents or humans.
Parathyroid glands express α-klotho, but mice with parathyroid-specific deletion of α-klotho have a normal phenotype and survival, normal serum PTH and calcium, and unaltered expression of the PTH gene in parathyroid tissue (44). However, combined deletion of α-klotho and the calcium-sensing receptor (CASR) in parathyroid glands results in significantly higher serum PTH, FGF23, and 1,25 (OH)2 vitamin D3 levels compared with mice with a parathyroid-specific deletion of the CASR alone, indicating that α-klotho expressed in the parathyroid gland is a negative regulator of PTH synthesis that works synergistically with CASR (45). Another model indicates that α-klotho directly interacts with the PTH receptor and thereby attenuates PTH signaling in target tissues (46). Although target organ resistance to PTH would be consistent with a positive association of circulating α-klotho levels with PTH, an inverse correlation between soluble α-klotho and PTH would be expected if α-klotho acts as a suppressor of PTH secretion in vivo. Our data indicate that the association of PTH and α-klotho is complex and modified by sex-related factors. The cause of the sex-specific association observed in the cohort cannot be answered through cross-sectional analyses like our study and remains to be tested using longitudinal data.
The lack of an association with GFR contrasts with a previous study in a small group of healthy volunteers and patients with much more severe reductions of GFR (CKD stages I-V) using the same immunoassay that concluded that there was a gradual decrease of α-klotho in serum with declining GFR (28). Importantly, there is considerable overlap of serum α-klotho levels between healthy volunteers and CKD stages I and II. Also, because of the small number of individuals, adjustment for potential confounders was not possible in that study. In univariable analyses, we similarly observed a significant association of GFR with soluble α-klotho even within the small and mostly normal range of GFR, but the association was not significant after multivariable adjustment. The most important point is that the individuals in our cohort had normal renal function, and only an extremely small fraction (2.3%) had a GFR <60 mL/min per 1.73 m2. Hence, it is entirely possible that circulating α-klotho levels indeed decline marginally in CKD I and II but can be buried with the other determinants, but in CKD stages III, IV, and V, the association remains significant even after adjustment for multiple confounders.
As expected for a circulating factor involved in phosphate homeostasis, plasma phosphate correlated with circulating α-klotho levels in our cohort (8). The association was strongly age-dependent (positive in young and negative in older adults) and not paralleled by correlations of soluble α-klotho levels with tubular maximum reabsorption of phosphorus/GFR or fractional excretion of phosphate. Thus, soluble α-klotho-mediated differences in renal phosphate handling are unlikely to account for this association. Rather, the result suggests that plasma phosphate influences soluble α-klotho levels, possibly by affecting its synthesis in the kidney. Phosphate loading in rodents consistently reduced circulating serum α-klotho but this was not observed in humans (47). A recent interventional study demonstrated an increase in circulating α-klotho levels in healthy volunteers under a high phosphate diet (32). Alternatively, soluble α-klotho may affect extrarenal handling of phosphate in bone or gut in an age-dependent manner.
In a previous analysis of this cohort, we discovered that the decline of 1,25 (OH)2 vitamin D3 with falling GFR is closely paralleled and significantly associated by a rise in FGF23, a negative regulator of 1,25 (OH)2 vitamin D3 synthesis in the proximal tubule (29). Our data now demonstrate that the well-known GFR dependence of 1,25 (OH)2 vitamin D3 levels is significantly influenced by soluble α-klotho. In the setting of highly soluble α-klotho, the GFR-dependent decline of 1,25 (OH)2 vitamin D3 is greatly attenuated. This finding suggests that α-klotho antagonizes the FGF23-mediated downregulation of 1,25 (OH)2 vitamin D3 in early CKD. Because soluble α-klotho and 1,25 (OH)2 vitamin D3 are both kidney tubule–derived substances, the finding could also be explained by differences in tubular function at an individual level of GFR: Preserved tubular function despite reduction in GFR would be associated with normal or near normal circulating α-klotho and 1,25 (OH)2 vitamin D3 levels. On the other hand, circulating α-klotho and 1,25 (OH)2 vitamin D3 levels may be reduced because of tubular dysfunction despite preserved GFR. Quantification of circulating α-klotho in different CKD subgroups (predominant tubule-interstitial vs isolated glomerular disease) could shed more light on the interaction between α-klotho and 1,25 (OH)2 vitamin D3. Ultimately, however, interventional studies will be needed to understand the underlying mechanisms.
Our study reproduces the previous observation of an inverse association of plasma phosphate with BMI (48). Both increased PTH and FGF23 levels have been reported in individuals with increased fat mass and adiposity (49–51). Our results indicate that the negative association of BMI with plasma phosphate is modulated by circulating α-klotho, possibly by either affecting PTH/FGF23 signalling at target organs or alternatively by altering PTH/FGF23 secretion.
Our study has several limitations. The cross-sectional design only allows us to infer associations but not causal relationships. Also, no direct GFR measurements based on exogenous filtration markers were available. In addition, although the IB-IP assay is very specific, it is not as quantitative as ELISA. Hence, it is possible that we missed factors weakly associated with soluble α-klotho in the current study.
In summary, using a specific novel immunoassay for soluble α-klotho, our study reveals that plasma phosphate—in an age-dependent manner—and parathyroid hormone—in a sex-dependent manner—are associated with soluble α-klotho levels in humans. Furthermore, our data reveal soluble α-klotho as a modulator of 1,25 (OH)2 vitamin D3 levels in individuals with preserved renal function.
Acknowledgments
We thank research nurses Marie-Odile Levy, Guler Gök-Sogüt, Ulla Schüpbach, and Dominique Siminski for data collection and Sandrine Estoppey for her invaluable help in logistics and database management.
Financial Support: SKIPOGH was supported by a grant from the Swiss National Science Foundation (grant #FN 33CM30-124087 to M.B.). D.G.F. was supported by the Swiss National Centre of Competence in Research NCCR TransCure and the Swiss National Science Foundation (grants #31003A_135503, 31003A_152829, and 33IC30_166785/1). O.W.M. was supported by the National Institutes of Health (O’Brien Kidney Research Center P30 DK-079328; R01 DK081423; R01 DK091392), the American Heart Foundation, the Charles Pak Foundation Innovative Research Award, and the Seldin-Pak Fund for Human Metabolic Research.
Glossary
Abbreviations
- BMI
body mass index
- CASR
calcium-sensing receptor
- CKD
chronic kidney disease
- eGFRcr
creatinine-based CKD-EPI 2009 equation to estimate the glomerular filtration rate
- FGFR
fibroblast growth factor receptor
- SKIPOGH
Swiss Kidney Project on Genes in Hypertension
Additional Information
Author Contributions: N.A.D., B.V., O.W.M., M.B., and D.G.F. conceived and planned the study. M.P., B.P., D.A., G.E., I.G., A.P.B., P.Y.M., and M.B. recruited patients into the study. A.B.L., O.D., J.P., and G.M.F. performed laboratory analyses. N.A.D. and D.G.F. performed statistical analyses. N.A.D., O.W.M., M.B., and D.G.F. wrote the manuscript with input from all authors. All authors approved the final version of the manuscript.
Disclosure Summary: D.G.F. has served as a consultant for Otsuka Pharmaceuticals and received unrestricted research funding from Novartis, Abbvie, and Otsuka Pharmaceuticals. O.W.M. served on Scientific or Medical Advisory Boards for Allena, Ardelyx, Genzyme-Sanofi, Alnylam, and Triceda.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 2. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 3. Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7(4):318–319. [PubMed] [Google Scholar]
- 5. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104(50):19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583(19):3221–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. Faseb J. 2010;24(9):3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105(28):9805–9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. [DOI] [PubMed] [Google Scholar]
- 12. Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310(5747):490–493. [DOI] [PubMed] [Google Scholar]
- 13. Dalton G, An SW, Al-Juboori SI, et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci U S A. 2017;114(4):752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Liu Y, Goetz R, et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553(7689):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haruna Y, Kashihara N, Satoh M, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A. 2007;104(7):2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54(4):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280(4):1015–1020. [DOI] [PubMed] [Google Scholar]
- 18. Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akimoto T, Kimura T, Watanabe Y, et al. The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc. 2013;45(1):134–136. [DOI] [PubMed] [Google Scholar]
- 20. Ponte B, Trombetti A, Hadaya K, et al. Acute and long term mineral metabolism adaptation in living kidney donors: a prospective study. Bone. 2014;62:36–42. [DOI] [PubMed] [Google Scholar]
- 21. Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137(3):479–485. [DOI] [PubMed] [Google Scholar]
- 22. Carpenter TO, Insogna KL, Zhang JH, et al. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab. 2010;95(11):E352–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seiler S, Rogacev KS, Roth HJ, et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol. 2014;9(6):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seiler S, Wen M, Roth HJ, et al. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int. 2013;83(1):121–128. [DOI] [PubMed] [Google Scholar]
- 25. Oh HJ, Nam BY, Lee MJ, et al. Decreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Perit Dial Int. 2015;35(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim HR, Nam BY, Kim DW, et al. Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis. 2013;61(6):899–909. [DOI] [PubMed] [Google Scholar]
- 27. Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398(3):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barker SL, Pastor J, Carranza D, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhayat NA, Ackermann D, Pruijm M, et al. Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int. 2016;90(3):648–657. [DOI] [PubMed] [Google Scholar]
- 30. Forni Ogna V, Ogna A, Vuistiner P, et al. ; Swiss Survey on Salt Group New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohammad J, Scanni R, Bestmann L, Hulter HN, Krapf R. A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol. 2018;29(8):2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1-3):143–147. [DOI] [PubMed] [Google Scholar]
- 34. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bijvoet OL, Morgan DB, Fourman P. The assessment of phosphate reabsorption. Clin Chim Acta. 1969;26(1):15–24. [DOI] [PubMed] [Google Scholar]
- 36. Kenny AP, Glen AC. Tests of phosphate reabsorption. Lancet. 1973;2(7821):158. [DOI] [PubMed] [Google Scholar]
- 37. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/. Accessed August 30, 2019. [Google Scholar]
- 38. Breheny P, Burchett W.. Visualization of regression models using visreg. R package. 2013:1–15. [Google Scholar]
- 39. Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. Plos One. 2013;8(2):e56695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akimoto T, Yoshizawa H, Watanabe Y, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pavik I, Jaeger P, Ebner L, et al. Secreted Klotho and FGF23 in chronic kidney disease stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013;28(2):352–359. [DOI] [PubMed] [Google Scholar]
- 42. Oz OK, Hajibeigi A, Howard K, et al. Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice. J Bone Miner Res. 2007;22(12):1893–1902. [DOI] [PubMed] [Google Scholar]
- 43. Hsu SC, Huang SM, Lin SH, et al. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J. 2014;464(2):221–229. [DOI] [PubMed] [Google Scholar]
- 44. Olauson H, Lindberg K, Amin R, et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. Plos Genet. 2013;9(12):e1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan Y, Liu W, Bi R, et al. Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci U S A. 2018;115(16):E3749–E3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takenaka T, Inoue T, Miyazaki T, Hayashi M, Suzuki H. Xeno-Klotho inhibits parathyroid hormone signaling. J Bone Miner Res. 2016;31(2):455–462. [DOI] [PubMed] [Google Scholar]
- 47. Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26(6):1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Billington EO, Gamble GD, Bristow S, Reid IR. Serum phosphate is related to adiposity in healthy adults. Eur J Clin Invest. 2017;47(7):486–493. [DOI] [PubMed] [Google Scholar]
- 49. Zaheer S, de Boer IH, Allison M, et al. Fibroblast growth factor 23, mineral metabolism, and adiposity in normal kidney function. J Clin Endocrinol Metab. 2017;102(4):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bolland MJ, Grey AB, Ames RW, Horne AM, Gamble GD, Reid IR. Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone. 2006;38(3):317–321. [DOI] [PubMed] [Google Scholar]
- 51. Grethen E, Hill KM, Jones R, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab. 2012;97(5):1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]